Abstract
Empathy is crucial for successful interpersonal interactions, and it is impaired in many psychiatric and neurological disorders. Action-perception matching, or action simulation mechanisms, has been suggested to facilitate empathy by supporting the simulation of perceived experience in others. However, this remains unclear, and the involvement of the action simulation circuit in cognitive empathy (the ability to adopt another’s perspective) vs emotional empathy (the capacity to share and react affectively to another’s emotional experience) has not been quantitatively compared. Presently, healthy adults completed a classic cognitive empathy task (false belief), an emotional empathy task and an action simulation button-pressing task during functional magnetic resonance imaging. Conjunction analyses revealed common recruitment of the inferior frontal gyrus (IFG), thought to be critical for action-perception matching, during both action simulation and emotional, but not cognitive, empathy. Furthermore, activation was significantly greater in action simulation regions in the left IFG during emotional vs cognitive empathy, and activity in this region was positively correlated with mean feeling ratings during the emotional empathy task. These findings provide evidence for greater involvement of action simulation mechanisms in emotional than cognitive empathy. Thus, the action simulation circuit may be an important target for delineating the pathophysiology of disorders featuring emotional empathy impairments.
Keywords: cognitive empathy, emotional empathy, simulation, mirror neuron system, fMRI, theory of mind
Introduction
Empathy, a crucial component of interpersonal interactions, is impaired in many psychiatric and neurological disorders (Blair, 2005; Kraemer et al., 2013; Green et al., 2015; Oliver et al., 2015). Some advocate that empathy may be facilitated through the embodied simulation or internal representation of perceived experience in others (Gallese, 2001; Preston and de Waal, 2002; Carr et al., 2003). The discovery of mirror neurons in macaques, which fire during both action observation and execution, provided a potential neural basis for such an action-perception matching mechanism (di Pellegrino et al., 1992; Fogassi et al., 2005). Evidence suggests that a similar system exists in humans, subserved by analogous circuitry, including the ventral premotor cortex (vPMC) into inferior frontal gyrus (IFG), and inferior parietal lobule (IPL; Caspers et al., 2010; Molenberghs et al., 2012). The posterior superior temporal sulcus (pSTS), though not believed to contain observation-execution matching neurons, is thought to provide visual input to these two critical action simulation regions (Iacoboni et al., 2001; Keysers and Perrett, 2004). Increased activity in critical frontal and parietal areas of this ‘action simulation circuit’ has been demonstrated during the observation and imitation of emotional expressions (Carr et al., 2003; Leslie et al., 2004; Montgomery and Haxby, 2008), suggesting that these regions may generate a motor representation of the emotional or mental states of others. Although there is some support for the suggestion that empathy may rely on action simulation mechanisms, this remains unsettled, and is further complicated by evidence that empathy is a multidimensional construct, including cognitive and emotional facets (Eslinger, 1998; Shamay-Tsoory, 2011).
Cognitive empathy, often used interchangeably with theory of mind (Blair, 2005), refers to the capacity to adopt another individual’s perspective and infer their mental or emotional state. Emotional empathy involves the ability to share and react affectively to the emotional experience of another. Though these facets undoubtedly interact, evidence from behavioural (Jones et al., 2010), lesion (Shamay-Tsoory et al., 2009) and neuroimaging (Bzdok et al., 2012) studies suggest they are dissociable. Neural areas typically implicated in cognitive empathy include the medial prefrontal cortex (mPFC), temporoparietal junction (TPJ), STS, precuneus, and sometimes the IFG and temporal poles (Carrington and Bailey, 2009; Schurz et al., 2014). In contrast, evidence suggests that the anterior insula, anterior cingulate cortex, IFG and sometimes the amygdala are involved in emotional empathy (Dziobek et al., 2011; Shamay-Tsoory, 2011; Hillis, 2013). Given their differential impairment in different psychiatric disorders (Blair, 2008), determining the relative contribution of action simulation mechanisms to these facets of empathy is of particular interest.
The action simulation circuit has been suggested to interact with more typical cognitive empathy regions via the pSTS/TPJ, rapidly providing goal-related information from perceived actions (Van Overwalle, 2009; Tramacere and Ferrari, 2016). Indeed, early evidence suggested that the action simulation circuit was sensitive to the intended goal of actions vs merely actions themselves (Iacoboni et al., 2005; Kaplan and Iacoboni, 2006). Activity in putative action simulation regions has also been observed during mental state inference tasks (Lawrence et al., 2006; Pineda and Hecht, 2009; Zaki et al., 2009). However, a conjunction analysis between a cognitive empathy animation task and an action observation task has revealed overlap in the pSTS, but not the IFG or IPL (Ohnishi et al., 2004). Accordingly, many functional magnetic resonance imaging (fMRI) investigations of cognitive empathy fail to find significant activation in reputed critical action simulation areas (Van Overwalle and Baetens, 2009).
It has also been proposed that the action simulation circuit may underlie emotional empathy, influencing areas of the limbic system, such as the amygdala, via the IFG and the insula (Carr et al., 2003). Indeed, there is some support for greater involvement of the action simulation circuit in emotional empathy. Questionnaire scores of emotional, but not cognitive, empathy have been positively correlated with IFG activation during the observation and imitation of emotional expressions (Pfeifer et al., 2008), white matter integrity in a tract connecting inferior frontal and temporoparietal regions (Parkinson and Wheatley, 2014), and right IFG and IPL gray matter volume (Cheng et al., 2009). Further, an fMRI investigation has interpreted increased activity in the anterior PMC during emotional vs cognitive empathy as evidence for greater engagement of the action simulation circuit (Nummenmaa et al., 2008).
Thus, though there is some support for a greater role of the action simulation circuit in emotional than cognitive empathy, this remains contentious. Prior studies have largely provided evidence via correlations between empathy questionnaire scores and activity in action simulation regions, which do not provide insight into online empathic responding. Evidence also comes from activation during empathy tasks in vast regions broadly accepted as being part of the action simulation circuit, rather than functionally defined regions of interest. Critically, the involvement of the action simulation circuit in cognitive vs emotional empathy has never been statistically compared. Accordingly, the objective of this study was to delineate the involvement of the action simulation circuit in cognitive and emotional empathy utilizing fMRI and behavioural indices. This marks the first time that functional localizers will be used to determine the correspondence between brain areas identified as having action simulation properties, and those recruited during cognitive and emotional empathy within the same sample. Our central hypothesis is that regions within the action simulation circuit critical for action-perception matching (i.e. vPMC into IFG and the IPL) will be preferentially involved in emotional vs cognitive empathy. Behavioural indices of emotional empathy are also expected to correlate more strongly with action simulation circuit activity than cognitive empathy performance.
Materials and methods
Participants
Thirty-six healthy, right-handed individuals (19 females and 17 males) with a mean age of 21.5 years (range 18–26, s.d. = 2.2) took part in the experiment. All participants were in good health and had no history of psychiatric problems, neurological disease or head injury. All participants granted informed consent and were compensated for their participation. This study was approved by the Health Sciences Research Ethics Board at the University of Western Ontario, London, Ontario, Canada.
fMRI tasks
Participants underwent fMRI while performing four randomly presented tasks, three of which were utilized for the present investigation. Prior to scanning, participants completed a practice version of the tasks. Tasks were programmed using E-Prime (Schneider et al., 2002).
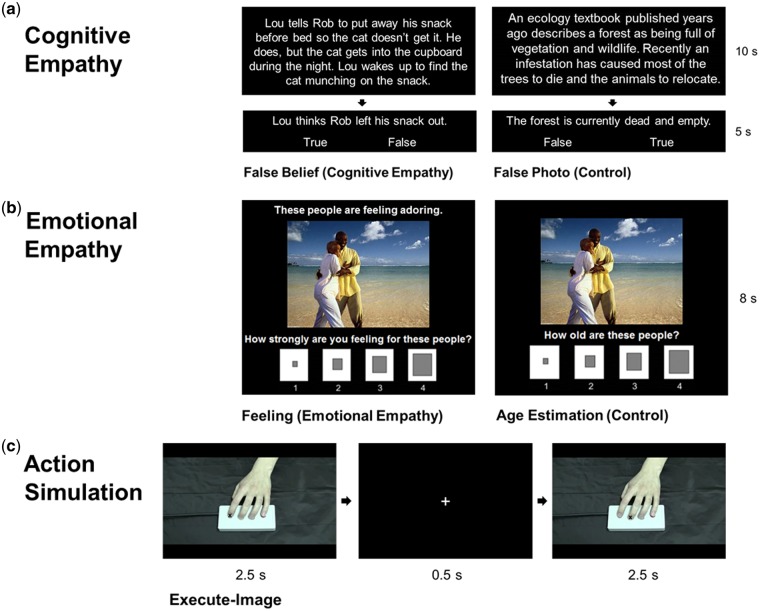
Cognitive empathy (Figure 1a). The False Belief Task (Dodell-Feder et al., 2011; Dufour et al., 2013) was used to localize brain regions recruited for cognitive empathy. Participants viewed false belief short stories, which described someone’s false belief and their resultant actions, requiring belief inference, and false photo stories, which described outdated, or no longer true, maps or pictures. False photo and false belief stories are matched for causal structure and difficulty, and both require the representation of false content (Saxe and Kanwisher, 2003). For each trial, stories were presented visually, then participants responded ‘true’ or ‘false’ via button press to a relevant statement. The task included 24 stories per condition, presented in 3 runs of 4 blocks per condition. An 18 s inter-block interval showing a fixation cross followed each block. Accuracy for the cognitive empathy task was calculated as the proportion correct of the false belief and false photo questions for each participant. Paired t-tests demonstrated no significant difference between false belief and false photo accuracy, confirming the conditions were matched in difficulty (false belief: M = 82.9%, s.d. = 14%, range 42–100%; false photo: M = 83.4%, s.d. = 11%, range 54–100%).
Fig. 1.
(a) Trial structure for the False Belief Task (Dodell-Feder et al., 2011; Dufour et al., 2013), including examples of false belief and false photo conditions. (b) Trial structure for the emotional empathy task (adapted from Dziobek et al., 2011), including examples of feeling and age estimation conditions. (c) Trial structure for the action simulation circuit localizer task (based on Iacoboni et al., 1999), including an example of the execute-image condition.
Emotional empathy (Figure 1b). The emotional empathy task was based on the Multifaceted Empathy Test (Dziobek et al., 2011), including only emotional empathy and control conditions. For feeling trials (emotional empathy), participants were presented with emotionally charged naturalistic social images and asked how strongly they are feeling for people in the image. A tagline explicitly stated the emotional state of the individuals in the images to minimize the need for cognitive empathy. Responses were made on a 4-point Likert scale from ‘not at all’ to ‘very strongly’ using a button box. During age estimation trials (control), the same images were presented without a tagline, and participants were asked, ‘How old is this person?’ Responses were made on a 4-point Likert scale from ‘very young’ to ‘very old’. Stimuli included 36 images (18 positive and 18 negative), taken from the Multifaceted Empathy Test (Dziobek et al., 2008) and the International Affective Picture System (Lang et al., 2008). Images were presented with the relevant question and response scale. The task included the same 36 images per condition, presented in 3 runs of 4 blocks per condition. Each block began with a 4 s instruction slide indicating the condition, and each was followed by an 18 s inter-block interval. Mean feeling ratings were determined across all feeling trials for each participant (M = 3.06, s.d. = 0.51, range 1.94–3.97).
Action simulation (Figure 1c). The action simulation circuit localizer task was based on the paradigm used by Iacoboni et al. (1999). Participants were presented with images and videos developed by the laboratory of a hand pressing a button, and asked to observe or execute a button press in response. Videos from 24 actors (12 females and 12 males) used in the task feature left hands with either the index or middle finger pressing a button on the same button box used by participants in the scanner. Still-frames were also taken from these videos (1 image per finger from each actor), and each one included a symbolic cue (‘X’) to indicate the pressing finger. Task conditions included observe-image (‘Just watch the image’), execute-image (‘Raise and lower the finger labeled X to press the button’), observe-video (‘Just watch the video’) and imitate-video (‘Imitate the finger movement in the video to press the button’). For each trial, an image or video was presented, followed by a fixation cross. The task included the same 48 images and 48 videos (24 index finger and 24 middle finger each) for both of the image and video conditions, presented in 3 runs of 2 blocks per condition. Each block began with a 4 s instruction screen indicating the condition and a short description (shown above), and each was followed by an 18 s inter-block interval.
MRI data acquisition
Participants were scanned in a single session using a 3 T Siemens Prisma scanner with a 32-channel head coil at Robarts Research Institute. fMRI images were taken with a T2*-gradient echo-planar imaging sequence [repetition time (TR): 3000 ms; echo time (TE): 30 ms; field of view (FOV): 20 cm; 80 × 80 matrix]. These parameters were chosen to optimize the signal-to-noise ratio for the amygdala (Robinson et al., 2004; Morawetz et al., 2008) with whole-brain coverage. For functional scans, 50 contiguous slices of 2.5 × 2.5 mm in-plane with a slice thickness of 2.5 mm (2.5 × 2.5 × 2.5 mm voxels) were obtained. At the midway point of the scanning session, after completion of the second task, a high-resolution, T1-weighted, anatomical scan was obtained with whole-brain coverage (TR: 2300 ms; TE: 2.98 ms; FOV: 25.6 cm; 256 × 240 matrix; 192 axial slices; 1 × 1 × 1 mm voxels).
fMRI analysis
Analyses of fMRI data were conducted using the Analysis of Functional NeuroImages (AFNI) software (Cox, 1996). Prior to performing analyses, all volumes of a given task were registered to a functional volume adjacent to the anatomical scan. Within task runs, volumes and the preceding volume were also censored if the derivatives of the six generated motion parameters had a Euclidean norm greater than 2.0 mm (Siegel et al., 2014). All data were spatially smoothed using a 4 mm full width at half maximum isotropic Gaussian kernel. The time series data were normalized such that each time point within a voxel was represented as a percent change from the mean voxel intensity. For each task, regressors were created for each condition by convolving the blocked stimulus events with a gamma-variate hemodynamic response function. The blood-oxygen-level dependent (BOLD) response was fitted to each regressor to conduct linear regression modelling for each task for each participant. To account for voxel-wise correlated drifting, a baseline plus linear drift and quadratic trend were modeled to the time series of each voxel, as well. This produced a beta coefficient and t-statistic for each voxel at each regressor. Regression coefficients represented the percentage signal change from the mean activity. Group analyses involved transforming each participant’s data into the standard space of Talairach and Tournoux (1988).
Additional regressors were made to model in-block instructions for the emotional empathy and action simulation tasks. Regressors of no interest were also created to model error-laden blocks from the action simulation task, where instructions were not followed on at least five of eight trials. A participant’s data were included for a specific condition if at least four of six blocks were usable. This was not the case for six participants, due to a lack of attention paid to the instruction presented in a block or missed trials due to this in combination with the speed of the task. Accordingly, data from these participants were excluded from the action simulation task analysis, and another due to computer error. For the False Belief Task, data from one participant was excluded due to a failure to understand the task.
t-Tests were conducted in AFNI to investigate within-task effects. These compared the whole-brain BOLD response to false belief vs false photo stories, feeling vs age estimation questions and imitate-video vs execute-image conditions for action simulation. As in previous investigations (e.g. Iacoboni et al., 1999; Koski et al., 2003; Cross et al., 2013), action simulation regions were identified using a contrast of action imitation vs action execution in response to a symbolic cue. This contrast was implemented on the basis that action simulation areas are activated during action observation and execution, and maximally active during imitation as it involves both visual encoding of the action and execution (Iacoboni, 2009). This contrast also served to control for motor plan initiation. Whole-brain contrasts were thresholded at P < 0.005 and corrected for multiple comparisons to P < 0.05 (16 contiguous voxels) using AFNI’s updated 3dClustSim, a spatial clustering operation with 10 000 Monte Carlo simulations on the whole brain echo-planar imaging matrix. A threshold of P < 0.005 was reasoned to be sufficiently conservative given our interest in the regions of overlap identified in the conjunction analyses, and reducing Type II errors as a result (Lieberman and Cunningham, 2009; Lin et al., 2016).
Conjunction analyses were performed using the minimum statistic compared to the conjunction null (Nichols et al., 2005) to identify overlap between regions engaged during cognitive empathy (false belief > false photo) and localized action simulation regions (imitate-video > execute-image), and emotional empathy (feeling > age estimation) and action simulation regions, as well as across cognitive and emotional empathy, and all three conditions. The contrasts of interest were individually thresholded at P < 0.005 and corrected for multiple comparisons to P < 0.05, then overlapping areas of significant activation were determined.
Paired t-tests with Bonferroni correction were also conducted in SPSS to examine differences in percent BOLD signal change during cognitive vs emotional empathy. To restrict the number of comparisons, this was only interrogated in localized action simulation clusters that incorporated traditional regions of the action simulation circuit, or mirror neuron system, including the IFG, PMC, IPL and/or pSTS (Grezes et al., 2003). Lastly, correlational analyses with Bonferroni correction were utilized to identify relationships between behavioural performance on the empathy tasks and activation within the same subset of action simulation clusters, as well as activity in regions of overlap identified using conjunction analyses between cognitive and emotional empathy, and the action simulation circuit. Data points falling ±3 s.d.s from the mean were identified as outliers and omitted from the analyses.
Results
Within-task effects
Cognitive empathy
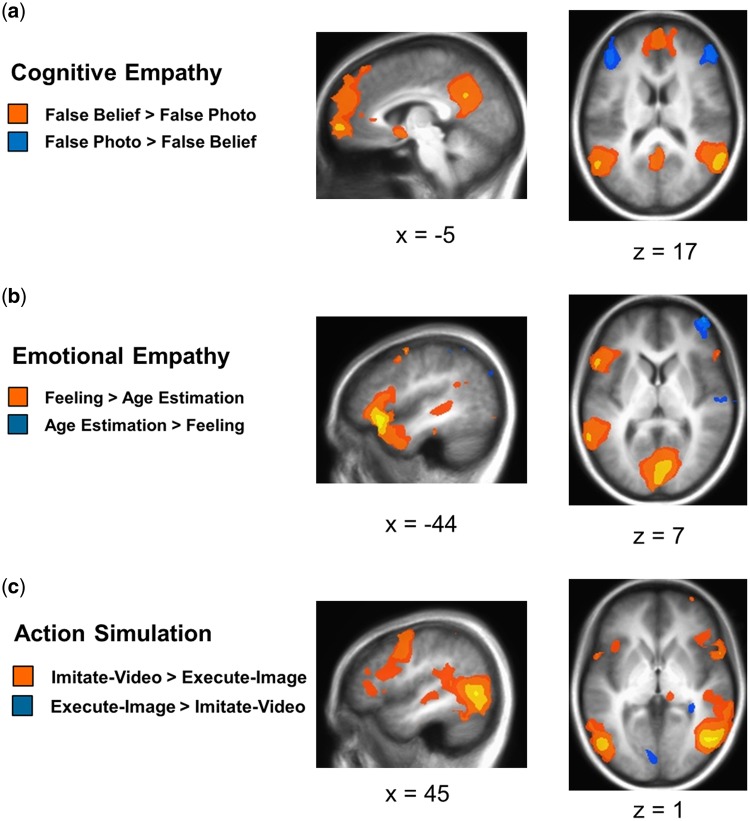
The contrast of false belief trials vs false photo trials for cognitive empathy revealed significantly greater activation in bilateral temporal pole, STS and TPJ, dorsal to ventromedial PFC, and posterior cingulate into precuneus, consistent with expectations (Table 1 and Figure 2).
Table 1.
Whole-brain within-task contrasts
| Region | L/R | BA | x | y | z | t-Value | Voxels |
|---|---|---|---|---|---|---|---|
| Cognitive empathy | |||||||
| False belief > false photo | |||||||
| Temporal pole/STS/TPJ | R | 38/21/22/39/40 | 54.3 | 2.1 | –19.2 | 9.75 | 2414 |
| Temporal pole/STS/TPJ | L | 38/21/22/39 | –56.8 | –56.4 | 20.1 | 8.30 | 1946 |
| mPFC | L/R | 9/10 | –8.8 | 54 | 31.4 | 6.52 | 1605 |
| Precuneus/posterior cingulate cortex | L/R | 7/31 | –1.3 | –59.9 | 39 | 10.45 | 1217 |
| Pyramis (cerebellum) | L | –24 | –74.5 | –38.6 | 5.77 | 83 | |
| Subgenual cingulate cortex/caudate | L | 25 | –6.3 | 4.2 | –7.2 | 4.86 | 56 |
| Middle frontal gyrus/PMC | R | 6 | 39.1 | 1.6 | 47.7 | 3.73 | 21 |
| Anterior cingulate cortex | L | 24 | –3.8 | 26.7 | 8.2 | 4.01 | 17 |
| Middle frontal gyrus | R | 8 | 21.5 | 24.8 | 48.9 | 3.53 | 16 |
| False photo > false belief | |||||||
| IPL/postcentral gyrus | L | 40/2 | –56.8 | –36.7 | 40.2 | –6.24 | 601 |
| Dorsolateral PFC | L | 46 | –41.7 | 28.2 | 30.1 | –5.97 | 470 |
| Mid/inferior temporal gyrus | L | 20/37 | –59.3 | –41.9 | –15.8 | –7.08 | 389 |
| Dorsolateral PFC | R | 46 | 49.2 | 36.4 | 22.3 | –5.40 | 321 |
| IPL/postcentral gyrus | R | 40/2 | 41.7 | –50.1 | 50.4 | –5.14 | 273 |
| Mid/inferior temporal gyrus | R | 20/37 | 56.8 | –39.2 | –18.7 | –5.39 | 122 |
| IFG | L | 9 | –51.8 | 7.4 | 34.4 | –4.91 | 64 |
| IFG | R | 9/44 | 49.2 | 0.1 | 25.8 | –4.60 | 50 |
| Medial frontal gyrus/SMA | R | 6 | 6.3 | –14.7 | 65.9 | –4.62 | 36 |
| Paracentral lobule/SMA | L | 4/6 | –8.8 | –32.6 | 62.2 | –3.87 | 26 |
| Medial postcentral gyrus | R | 2/5 | 11.4 | –48.5 | 69.5 | –4.60 | 22 |
| Precuneus | L | 7 | –11.4 | –83.3 | 43.2 | –3.88 | 20 |
| Middle frontal gyrus | L | 10/46 | –36.6 | 37.6 | –5.3 | –4.26 | 19 |
| Emotional empathy | |||||||
| Feeling > age estimation | |||||||
| Cuneus/lingual gyrus/R cerebellum | L/R | 17/18/19 | 11.4 | –73.7 | 2.9 | 10.91 | 3025 |
| IFG/anterior insula/temporal pole/STS/TPJ | L | 44/45/47/13/38/ 21/22/39/40 | –46.7 | 20.3 | –21.2 | 7.74 | 2346 |
| Dorsomedial PFC | L | 6/8/9 | –6.3 | 5.7 | 69.7 | 5.85 | 717 |
| Temporal pole/IFG | R | 38/47 | 39.1 | 15 | –18.5 | 5.31 | 239 |
| IFG | R | 45/47 | 49.2 | 34.8 | 0.5 | 4.29 | 62 |
| Middle frontal gyrus/PMC | L | 6 | –44.2 | –4.2 | 61 | 3.76 | 46 |
| Inferior occipital gyrus | L | 18/19 | –31.6 | –88.5 | –12.6 | 3.82 | 43 |
| Mid-STS | R | 22 | 44.2 | –29.7 | –0.2 | 5.34 | 38 |
| Dorsomedial PFC | R | 9 | 11.4 | 51.3 | 34 | 4.02 | 30 |
| Parahippocampal gyrus/fusiform gyrus | L | 20/36 | –39.1 | –26.2 | –20.9 | 4.02 | 22 |
| Middle frontal gyrus/PMC | L | 6 | –41.7 | 4.1 | 50.5 | 4.15 | 21 |
| Anterior cingulate cortex | R | 24 | 8.8 | 15.1 | 34.8 | 3.65 | 19 |
| Medial/superior frontal gyrus | R | 9 | 24 | 38.4 | 33.3 | 3.37 | 17 |
| Anterior cingulate cortex | L | 32 | –8.8 | 12.3 | 40.1 | 4.1 | 16 |
| Age estimation > feeling | |||||||
| IPL/precuneus | R | 40/39/19 | 46.7 | –55.5 | 55.6 | –7.84 | 1853 |
| Dorsolateral PFC | R | 8/9/46 | 26.5 | 6.1 | 61.5 | –8.03 | 1331 |
| IPL/precuneus | L | 40/19 | –36.6 | –77.8 | 35.3 | –6.18 | 524 |
| Mid/inferior temporal gyrus | R | 20/37 | 56.8 | –23.9 | –14.8 | –6.82 | 378 |
| Precuneus | L/R | 7/31 | 6.3 | –64.9 | 36 | –4.43 | 268 |
| Posterior cingulate cortex | L/R | 31 | 3.8 | –39.3 | 40.1 | –5.9 | 209 |
| Superior/mid frontal gyrus/PMC | L | 6 | –21.5 | 6.4 | 56.1 | –5.55 | 121 |
| Mid/inferior temporal gyrus | L | 20/21 | –59.3 | –36.7 | –15.5 | –5.55 | 75 |
| Subgenual cingulate cortex | L | 25/32 | –11.4 | 17.3 | –12.4 | –4.35 | 41 |
| Frontal pole | R | 10 | 24 | 62.5 | 15.5 | –3.93 | 21 |
| Inferior semilunar lobule (cerebellum) | L/R | –6.3 | –79 | –53.7 | –4.24 | 20 | |
| Inferior temporal gyrus | R | 37 | 64.4 | –49.7 | –13.3 | –4.23 | 20 |
| Primary motor cortex | R | 4 | 61.9 | –18.6 | 38.5 | –3.99 | 18 |
| Orbitofrontal cortex/ventromedial PFC | R | 11 | 6.3 | 35.6 | –17.3 | –5.1 | 17 |
| Action simulation | |||||||
| Imitate-video > execute-image | |||||||
| Mid-posterior STS/TPJ/IPL/mid-inferior temporal | R | 22/37/19/39/40 | 51.8 | –63.1 | –2.2 | 13.7 | 2628 |
| gyrus/mid occipital gyrus/fusiform gyrus | |||||||
| PMC/mid frontal gyrus/IFG/anterior insula | R | 6/45/47/13 | 39.1 | –6.9 | 63.6 | 5.59 | 1168 |
| Posterior STS/TPJ/IPL/mid temporal gyrus/mid | L | 22/19/39/40 | –54.3 | –53.2 | 6.7 | 6.81 | 928 |
| occipital gyrus/fusiform gyrus | |||||||
| Precuneus/superior-mid occipital gyrus | R | 7/19 | 21.5 | –73.3 | 49.2 | 5.55 | 797 |
| Medial-superior frontal gyrus/SMA | R | 6 | 16.4 | 3 | 72.3 | 5.56 | 422 |
| Pyramis/uvula (cerebellum) | L | –16.4 | –66.6 | –41.1 | 6.66 | 225 | |
| Superior-mid frontal gyrus/PMC | L | 6 | –29 | –7.3 | 71.7 | 4.92 | 209 |
| Dorsolateral PFC | L | 46 | –49.2 | 41.4 | 25.3 | 4.49 | 108 |
| Anterior insula | L | 13 | –39.1 | 19.1 | 5.1 | 4.44 | 53 |
| Declive (cerebellum) | L | –46.7 | –51.7 | –28.3 | 4.16 | 44 | |
| Lateral mid frontal gyrus | L | 10 | –44.2 | 52.3 | 12.3 | 4.04 | 42 |
| Culmen (cerebellum) | L | –29 | –56.7 | –31.6 | 4.94 | 32 | |
| Precuneus | R | 7 | 26.5 | –49.7 | 42.3 | 3.67 | 32 |
| Pre/cuneus | L | 7 | –18.9 | –80.6 | 40.6 | 4.57 | 31 |
| Fusiform gyrus | R | 20 | 41.7 | –20.8 | –26.5 | 4.45 | 29 |
| Lateral mid frontal gyrus | R | 10 | 34.1 | 43.6 | 33.6 | 4.04 | 29 |
| IFG | L | 9/6 | –44.2 | 2.6 | 26 | 4.29 | 28 |
| IFG | L | 47/45/13 | –49.2 | 16.9 | –3.5 | 4 | 26 |
| IPL | L | 40 | –59.3 | –26.3 | 38.1 | 3.91 | 25 |
| Medial-superior frontal gyrus/SMA | L | 6 | –8.8 | –14.9 | 68.6 | 3.94 | 25 |
| Orbitofrontal gyrus | R | 11 | 21.5 | 25.4 | –20.9 | 4.19 | 22 |
| Superior frontal gyrus | L | 9 | –34.1 | 48.5 | 39.3 | 4.08 | 22 |
| Superior parietal lobule/precuneus | L | 7 | –16.4 | –73.4 | 51.9 | 4.05 | 18 |
| Execute-image > imitate-video | |||||||
| Cuneus | L/R | 17/18 | –16.4 | –79 | 5.4 | –4.76 | 178 |
| Caudate/hippocampus | R | 29 | –34.7 | –3.5 | –5 | 38 | |
Notes. Thresholded at P < 0.005; P < 0.05 corrected. Table displays region (STS, TPJ, PMC, PFC, SMA and IFG), hemisphere (L, left; R, right), BA, montreal neurological institute (MNI) coordinates (x, y, z), maximum neural activity for the peak of that cluster (t-value) and cluster size in voxels.
Fig. 2.
Whole-brain analyses were conducted at a threshold of P < 0.005, and corrected to a family-wise error rate of P < 0.05. (a) The false belief > false photo contrast revealed greater activity in areas including bilateral STS and TPJ, medial PFC, and posterior cingulate into precuneus. False photo > false belief showed greater activity in areas including bilateral dorsolateral PFC. (b) The feeling > age estimation contrast showed greater activity in regions including bilateral IFG, anterior insula, and temporal pole, and left posterior STS and TPJ. Age estimation > feeling revealed greater activity in regions including right dorsolateral PFC. (c) The imitate-video > execute-image contrast showed greater activity in areas including bilateral posterior STS into IPL, IFG and PMC, and anterior insula. Execute-image > imitate-video showed greater activity in bilateral cuneus and right hippocampus.
Emotional empathy
The feeling vs age estimation contrast for emotional empathy showed significantly heightened activity in areas including bilateral IFG and dorsomedial PFC, as well as left PMC, anterior insula and a region extending from the temporal pole to the pSTS and TPJ.
Action simulation
The contrast of imitate-video vs execute-image conditions from the action simulation circuit localizer task revealed greater activity in bilateral pSTS into IPL and mid occipital gyrus, bilateral IFG and PMC, as well as bilateral anterior insula, supplementary motor area (SMA) and precuneus.
Conjunction analyses
Cognitive empathy and action simulation
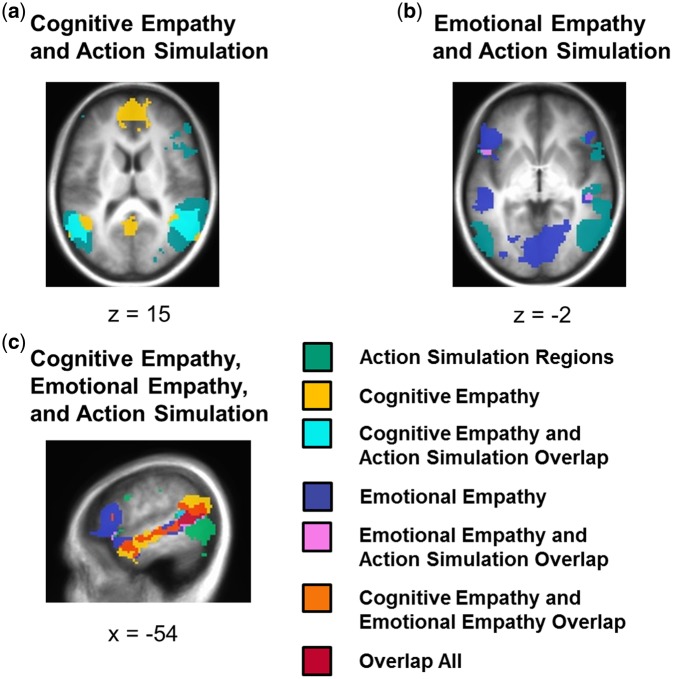
A conjunction of regions activated by the action simulation circuit localizer and cognitive empathy identified common activation in the right mid/posterior STS and TPJ, left pSTS and TPJ, and bilateral precuneus (Table 2 and Figure 3).
Table 2.
Conjunction analyses
| Region | L/R | BA | x | y | z | Voxels |
|---|---|---|---|---|---|---|
| Cognitive empathy and action simulation | ||||||
| Mid/posterior STS/TPJ | R | 21/22/39 | 46.7 | –18.9 | –11.5 | 591 |
| Posterior STS/TPJ | L | 22/39 | –59.3 | –42.8 | 4.5 | 199 |
| Precuneus | L/R | 7 | 3.8 | –55 | 44.7 | 18 |
| Emotional empathy and action simulation | ||||||
| Posterior STS/TPJ | L | 22/39 | –59.3 | –47.7 | –1.3 | 274 |
| Pre/cuneus | R | 18/31 | 26.5 | –79.2 | 10.8 | 103 |
| Superior frontal gyrus/SMA | R | 6 | 11.4 | 6 | 64.3 | 27 |
| Medial frontal gyrus/SMA | L/R | 6 | –1.3 | 1.1 | 58.6 | 26 |
| Mid-STS | R | 21/22 | 46.7 | –24.4 | –2.9 | 21 |
| IFG | L | 47/45/13 | –54.3 | 19.6 | –6.3 | 16 |
| Cognitive empathy and emotional empathy | ||||||
| Temporal pole/STS/TPJ | L | 38/21/22/39 | –41.7 | 10.7 | –39.6 | 804 |
| Dorsomedial PFC | L | 9 | –11.4 | 46.5 | 25.6 | 272 |
| Temporal pole | R | 38 | 54.3 | 12.9 | –30.5 | 162 |
| Mid-STS | R | 21/22 | 44.2 | –26.8 | –6 | 37 |
| Dorsomedial PFC | R | 9 | 8.8 | 56.6 | 31.6 | 27 |
| Cognitive empathy, emotional empathy, and action simulation | ||||||
| Posterior STS/TPJ | L | 22/39 | –59.3 | –42.8 | 4.5 | 158 |
| Mid-STS | R | 21/22 | 46.7 | –24.4 | –2.9 | 21 |
Notes. Table displays region (STS, TPJ, PMC, PFC, SMA and IFG,), hemisphere (L, left; R, right), BA, MNI coordinates (x, y, z) and cluster size in voxels.
Fig. 3.
(a) The conjunction of cognitive empathy and the action simulation circuit revealed overlap in areas including bilateral posterior STS and TPJ, and bilateral precuneus. (b) The conjunction of emotional empathy and the action simulation circuit showed overlap in regions including right mid STS and left IFG. (c) The conjunction of cognitive empathy, emotional empathy, and the action simulation circuit showed overlap in areas including left posterior STS.
Emotional empathy and action simulation
A second conjunction analysis revealed brain areas that were significantly activated both during the action simulation circuit localizer and emotional empathy, including the left pSTS and TPJ, two regions in the SMA, the right mid STS and left IFG.
Cognitive and emotional empathy
A conjunction of regions significantly activated during both cognitive empathy and emotional empathy identified areas including the left temporal pole into STS and TPJ, bilateral dorsomedial PFC, and right temporal pole and mid STS.
Cognitive empathy, emotional empathy, and action simulation
The left pSTS and right mid STS were identified as regions activated by the action simulation circuit localizer, and both cognitive and emotional empathy.
Between-task effects
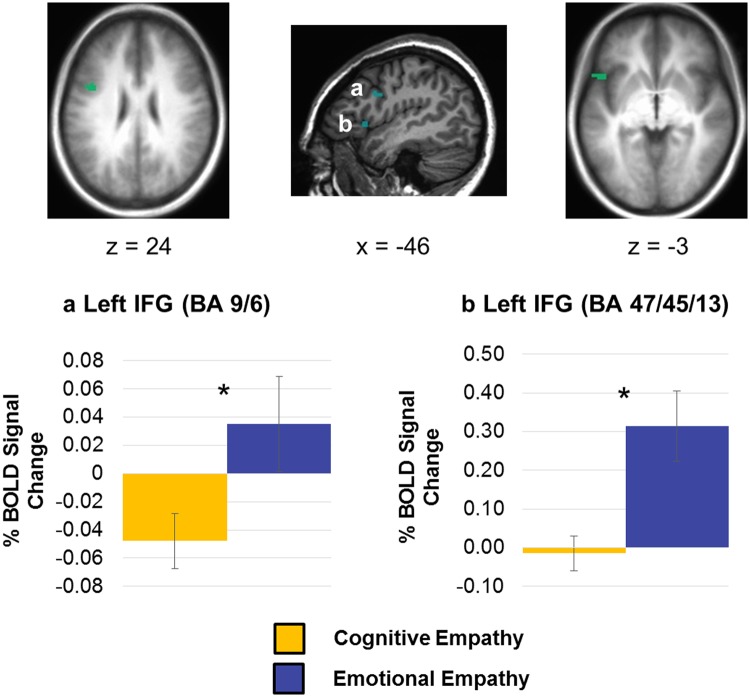
Following examination of the whole brain data, we took an ROI approach next. Paired t-tests were utilized to determine whether action simulation regions were significantly more active during emotional vs cognitive empathy. This was done only in identified action simulation clusters that included our a priori regions of interest (the IFG, PMC, STS and/or IPL; see Table 1), of which there were seven. Of these regions, paired t-tests revealed that activation was significantly greater during emotional vs cognitive empathy in two clusters in the left IFG. One of these regions was located in Brodmann area (BA) 9/6 [t(34) = 3.02, corrected P = 0.035; Figure 4a] and the other was in BA 47/45/13 [t(34) = 3.17, corrected P = 0.021; Figure 4b]. Notably, none of these action simulation clusters of interest showed significantly greater activity for cognitive relative to emotional empathy.
Fig. 4.
Mean percent BOLD signal change during cognitive empathy and emotional empathy in clusters identified using the action simulation circuit localizer task, including (a) a cluster in BA 9/6 in the left IFG and (b) a cluster in BA 47/45/13 in the left IFG. Error bars represent standard error of the mean; *Bonferroni corrected P < 0.05.
Correlational analyses with task performance
Correlations with activation in action simulation regions of interest
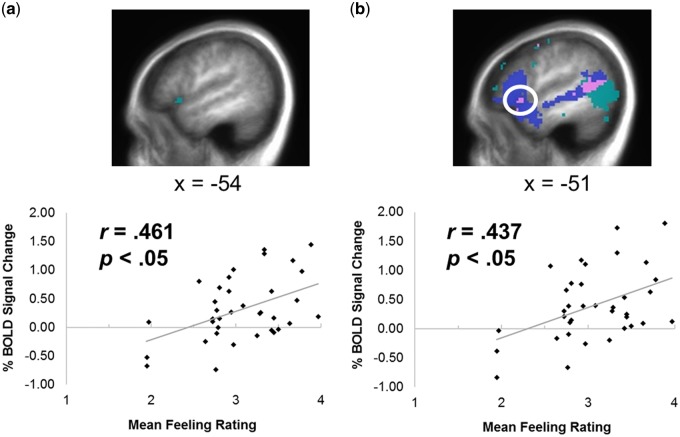
Correlational analyses between task performance and corresponding neural activity during the corresponding task were conducted in the same seven action simulation clusters of interest (see Table 3). For cognitive empathy, accuracy on the false belief questions was not significantly correlated with activation during the cognitive empathy task in any of the action simulation clusters of interest. For emotional empathy, a positive association was identified between mean feeling ratings and activation during the emotional empathy task in the cluster identified in BA 47/45/13 of the left IFG (Figure 5a).
Table 3.
Correlations between task performance and corresponding neural activity in significant clusters identified using the action simulation circuit localizer including a priori regions of interest (IFG, PMC, STS and/or IPL)
|
Activation during cognitive empathy (false belief > false photo) |
|||||||
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | Cluster 6 | Cluster 7 | |
| False belief question accuracy | 0.121 | 0.040 | 0.063 | –0.018 | –0.029 | 0.109 | –0.045 |
|
Activation during emotional empathy (feeling > age estimation) |
|||||||
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | Cluster 6 | Cluster 7 | |
| Mean feeling ratings | 0.297 | 0.129 | 0.333 | 0.293 | 0.111 | 0.461* | 0.274 |
Notes. Cluster 1: R mid-posterior STS/TPJ/IPL/mid-inferior temporal gyrus/mid occipital gyrus/fusiform gyrus. Cluster 2: R PMC/mid frontal gyrus/IFG/anterior insula. Cluster 3: L posterior STS/TPJ/IPL/mid temporal gyrus/mid occipital gyrus/fusiform gyrus. Cluster 4: L superior-mid frontal gyrus/PMC. Cluster 5: L IFG (BA 9/6). Cluster 6: L IFG (BA 47/45/13). Cluster 7: L IPL.
Bonferroni corrected P < 0.05.
Fig. 5.
Correlations between percent BOLD signal change during emotional empathy and mean feeling ratings on the emotional empathy task in (a) the cluster in BA 47/45/13 in the left IFG identified using the action simulation circuit localizer task, and (b) the region of overlap (pink) in the left IFG identified in the conjunction of emotional empathy and the action simulation circuit.
Fisher z-transformation (Fisher, 1915) was also performed to determine whether the correlations differed significantly from one another. Within the action simulation cluster identified in BA 47/45/13 of the left IFG, there was a trend towards a stronger association between mean feeling ratings and emotional empathy activation than between cognitive empathy accuracy and activation (z = 1.57, P = 0.058 one-sided).
Correlations with activation in regions of overlap
Correlations were also utilized to determine whether task performance was associated with activation in regions of overlap identified in the conjunction analyses. Specifically, false belief accuracy was not significantly associated with activation during cognitive empathy in any of the three identified regions recruited during both action simulation and cognitive empathy. However, mean feeling ratings were positively associated with activity during emotional empathy in the region of the left IFG (BA 47/45/13) recruited by both action simulation and emotional empathy (r = .437, corrected P = 0.048; Figure 5b). Activation in the other five areas of overlap between emotional empathy and action simulation was not associated with emotional empathy performance.
Discussion
The present study uniquely utilized tasks tapping the action simulation circuit, cognitive empathy, and emotional empathy during fMRI in healthy adults, allowing for a quantitative comparison of action simulation circuit involvement in each facet of empathy. As predicted, conjunction analyses revealed common recruitment of the IFG, a region thought to be critical for action-observation matching, during both action simulation and emotional, but not cognitive, empathy. Critically, action simulation regions in the left IFG were also recruited to a greater degree during emotional vs cognitive empathy. Further, within the region of overlap between emotional empathy and action simulation in the left IFG, activation during the emotional empathy task was positively associated with mean feeling ratings. Thus, our results suggest that action simulation areas thought to be critical for observation-execution matching are preferentially involved in emotional empathy.
Action simulation and cognitive empathy
Consistent with expectations, action simulation regions commonly engaged during cognitive empathy included bilateral pSTS extending into the TPJ, and bilateral precuneus. Studies typically identify activation in the pSTS during both action simulation (Grezes et al., 2003) and cognitive empathy (Mar, 2011; Molenberghs et al., 2016) tasks. It has also been suggested that this region may act as a link between these systems (Van Overwalle, 2009). Notably, though the pSTS is considered part of the action simulation circuit, it is not engaged during action execution, and instead provides visual input to action-perception matching regions (Iacoboni et al., 2001; Keysers and Perrett, 2004). Thus, the absence of common recruitment of the IFG, PMC or IPL demonstrated presently does not provide support for the involvement of critical action simulation regions in cognitive empathy as it is defined by false belief inference. This coincides with demonstrated overlap in bilateral STS activation from another fMRI investigation that directly compared neural activity during cognitive empathy and action simulation (Ohnishi et al., 2004).
Despite the lack of evidence provided here for a role for the action simulation circuit in cognitive empathy, it is important to consider that action simulation could be implicated in certain aspects of this. Specifically, prior work suggests that cognitive empathy tasks involving movement perception, emotional facial or bodily expressions and action-based intention understanding in particular may engage the action simulation circuit (Kaplan and Iacoboni, 2006; Gobbini et al., 2007; Pineda and Hecht, 2009; Van Overwalle, 2009; Schurz et al., 2014). Indeed, transcranial magnetic stimulation over the IFG, but not control sites, has been found to disrupt recognition of deception from actions (Tidoni et al., 2013), and inference of genuine amusement from videos of observed smiles (Paracampo et al., 2017). Further, recent studies have demonstrated greater involvement of typical cognitive empathy brain areas when considering why an action or emotional expression is made (cognitive empathy) vs how it is done, which produces more activity in action simulation regions (Spunt et al., 2010; Spunt et al., 2011; Spunt and Adolphs, 2014). Future studies incorporating different varieties of cognitive empathy tasks, and patients with acquired lesions, would be beneficial in elucidating which neural regions make critical contributions to cognitive empathy across task types and stimuli.
Action simulation and emotional empathy
In the case of emotional empathy, overlap with localized action simulation areas was observed in the left pSTS, right middle STS, right precuneus/cuneus, bilateral SMA, and the left IFG. Notably, the IFG represents a critical region for observation-execution matching, based on single-cell recording in anatomically corresponding areas in the macaque brain (di Pellegrino et al., 1992; Gallese et al., 1996), and fMRI data in humans including action perception and execution studies (Caspers et al., 2010; Molenberghs et al., 2012) and fMRI adaptation paradigms (Kilner et al., 2009; de la Rosa et al., 2016). Further, impaired imitation has been observed following transient lesions of bilateral IFG (Heiser et al., 2003), and acquired IFG lesions are associated with human action encoding deficits (Fazio et al., 2009). Although IFG activation has been reported during emotional empathy tasks (Lamm et al., 2011; Shamay-Tsoory, 2011; Bzdok et al., 2012), such studies tend not to directly compare identified clusters with those activated during action simulation tasks. Similar conclusions have been drawn about the anterior PMC without comparing this region to coordinates identified in prior studies of action simulation (Nummenmaa et al., 2008). Crucially, here we provide unique confirmation of this by demonstrating overlap in recruitment of the IFG between emotional empathy and action simulation tasks in the same sample.
Action simulation and emotional vs cognitive empathy
These results provide compelling evidence that the action simulation circuit is preferentially involved in emotional empathy, and that the IFG may be a particularly important action simulation region for emotional empathic responding. Thus, two action simulation clusters in the left IFG were recruited to a greater degree during emotional than cognitive empathy. Correlational analyses also revealed a positive association between activation during emotional empathy and mean feeling ratings on the emotional empathy task within one of these clusters in the left IFG (BA 47/45/13), supporting its functionality in emotional empathic behaviour. Further, this correlation in the left IFG was stronger than that between activity during cognitive empathy in this region and false belief accuracy. Importantly, this association was also observed within the region of the left IFG that was commonly recruited during both emotional empathy and action simulation. This marks the first time that a link has been demonstrated between emotional empathic responding and activity in an independently localized action simulation region also recruited during emotional empathy. These findings corroborate prior imaging studies demonstrating correlations between questionnaire measures of emotional empathy, and indices of IFG function (Pfeifer et al., 2008) and structure (Cheng et al., 2009), as well as work showing impaired emotional empathy, but intact cognitive empathy, in patients with IFG lesions (Shamay-Tsoory et al., 2009).
Mechanisms of action simulation in emotional empathy
Although our findings implicate action simulation regions in emotional empathy, it remains unclear how they are involved in its elicitation. The anterior insula has been suggested to act as a critical link between action representation in the action simulation circuit and emotion representation in the limbic system to modulate empathic experience (Preston and de Waal, 2002; Carr et al., 2003; Molnar-Szakacs, 2011). The insula is situated between the IFG and regions of the limbic system, such as the amygdala, and anatomical data suggest that its dysgranular field is connected to the inferior frontal, posterior parietal, and superior temporal cortices, as well as the limbic system (Augustine, 1996). Further, Granger causality has suggested effective connectivity from BA 45 of the IFG to the anterior insula during the observation of emotional expressions (Jabbi and Keysers, 2008). This coincides with the present findings, as the area of the IFG commonly engaged during action simulation and emotional empathy was localized on the boundaries of BA 45 and the anterior insula. During emotional empathy, activation was also elicited in the anterior insula, and the anterior cingulate cortex of the limbic system.
This account is also particularly interesting given that humans show an unconscious drive to imitate facial expressions (Dimberg et al., 2000), perhaps reflecting an overt behavioural form of emotional resonance, parallel to this more covert neural matching mechanism (Jabbi and Keysers, 2008). Indeed, stronger facial mimicry in response to emotional scenes has been linked with heightened shared emotional experience (Van der Graaff et al., 2016), and greater emotional empathy questionnaire scores (Balconi et al., 2013). Further, a positive correlation has been shown between imitation accuracy and activation in both the insula and PMC during imitation of emotional faces, as well as between empathy scores and premotor activity during imitation (Braadbaart et al., 2014).
Additional regions of overlap
Notably, the left pSTS and right STS were the only regions commonly recruited during action simulation, cognitive empathy, and emotional empathy. A recent meta-analysis confirms that the pSTS is commonly engaged by theory of mind, social perception, and action observation paradigms, positing that it is involved in the temporal integration of social cues and decoding basic intention from behaviour (Yang et al., 2015). Overlap in bilateral SMA was also identified for action simulation and emotional empathy, which may reflect internal action simulation and resultant priming of associated responses (Lamm et al., 2007). Lastly, the precuneus was commonly engaged during action simulation and cognitive empathy, and action simulation and emotional empathy, which is interesting to consider based on its suggested involvement in self-other representations (Uddin et al., 2007).
Implications
The present findings suggest that the action simulation circuit may be of particular interest in disorders featuring emotional empathy impairments. Interestingly, diminished spontaneous activity in action simulation regions, including the vPMC, during the observation of social hand interactions has been demonstrated in individuals with psychopathy (Meffert et al., 2013). Reduced activation in action simulation regions during motor and social tasks has also been associated with schizophrenia (Mehta et al., 2014). Nevertheless, more work is needed to establish a causal link between action simulation abnormalities and specific symptoms in these populations related to emotional empathy before action simulation regions can be considered a viable novel treatment target (Iacoboni and Mazziotta, 2007).
Limitations and future directions
It is important to note that task differences outside of the parameters of interest may have influenced our results. For example, although our empathy tasks included behavioural measures, it is possible that the difference between correlations with these indices and activation was a result of feeling ratings being a better metric of their target cognitive process than false belief accuracy, thereby complicating our interpretation. However, the spread of feeling ratings on the emotional empathy task was similar to cognitive empathy performance (see Methods for details). Notably, biological motion was featured only in the action simulation task, and thus, differences in the relative role of the IFG in emotional vs cognitive empathy cannot be explained by biological motion. However, whereas the emotional empathy and action simulation tasks involve pictorial biological stimuli, the False Belief Task utilizes linguistic stimuli. Nevertheless, both the cognitive and emotional empathy tasks include carefully designed conditions to control for content and isolate the target cognitive operations. Indeed, theory of mind tasks of different media forms appear sensitive to the target process, reliably activating a similar set of core neural regions (e.g. Carrington and Bailey, 2009). A recent fMRI meta-analysis utilized overlap analyses to demonstrate that reasoning about mental states activated this core circuit irrespective of the utilized task and stimulus formats (Schurz et al., 2014). Still, there is no single gold-standard for identifying circuits involved in cognitive empathy, and the extent to which facets of empathy overlap with action simulation may vary as a function of the type of stimuli used and the aspect of cognitive empathy elicited, as mentioned (Kaplan and Iacoboni, 2006; Pineda and Hecht, 2009; Spunt and Lieberman, 2012). Though there are tasks tapping cognitive and emotional empathy that are more similar in nature, we selected the False Belief Task because it is a classic test of theory of mind, and is a reliable, well-validated localizer (Dodell-Feder et al., 2011; Dufour et al., 2013). Interestingly, it has also been argued that cognitive empathy can be further divided into cognitive (inference of beliefs and intentions) and affective (inference of feelings) components (Shamay-Tsoory and Aharon-Peretz, 2007). However, whereas the latter construct may overlap with emotional empathy, the False Belief Task more clearly excludes affective aspects, allowing for a fine distinction between cognitive and emotional empathy components.
Similarly, we opted to use localizers to identify voxels of interest within our specific study sample, but it should also be noted that the identified regions are reflective of, and limited by, the nature of the chosen tasks. Furthermore, though critical action simulation regions were recruited during emotional empathy, we cannot confirm that the computations performed by these overlapping regions were identical across tasks. Future work should continue to examine the degree to which the target cognitive processes are elicited regardless of the stimuli used with the inclusion of clinical populations to clarify functional relevance. Lastly, despite evidence presented here and elsewhere supporting the distinguishability of cognitive and emotional empathy, it is important to note that these facets can be elicited in response to similar situations. Indeed, recent work demonstrates that cognitive and emotional empathy can flexibly interact (Christov-Moore and Iacoboni, 2016; Kanske et al., 2016). For example, increased connectivity between regions in these two circuits was found to be associated with reduced prosocial behaviour (Christov-Moore and Iacoboni, 2016). Accordingly, although the current results suggest a closer relationship between action simulation and emotional relative to cognitive empathy, these aspects of empathy likely influence one another, contributing to the behavioural expression and experience of empathy (Kerem et al., 2001; Shamay-Tsoory, 2011; Cox et al., 2012; Decety and Svetlova, 2012; Hawco et al., 2017).
Funding
This research was supported by funding to D.G.V.M. from the Natural Sciences and Engineering Research Council of Canada (Grant #: R3994A04001). Special thanks to Amber McCallum for assistance with the compilation of data.
Conflict of interest. None declared.
References
- Augustine J.R. (1996). Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research Brain Research Reviews, 22(3), 229–44. [DOI] [PubMed] [Google Scholar]
- Balconi M., Bortolotti A., Crivelli D. (2013). Self-report measures, facial feedback, and personality differences (BEES) in cooperative vs. noncooperative situations: contribution of the mimic system to the sense of empathy. International Journal of Psychology, 48(4), 631–40. [DOI] [PubMed] [Google Scholar]
- Blair R.J.R. (2005). Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Consciousness and Cognition, 14(4), 698–718. [DOI] [PubMed] [Google Scholar]
- Blair R.J.R. (2008). Fine cuts of empathy and the amygdala: dissociable deficits in psychopathy and autism. Quarterly Journal of Experimental Psychology, 61(1), 157–70. [DOI] [PubMed] [Google Scholar]
- Braadbaart L., de Grauw H., Perrett D.I., Waiter G.D., Williams J.H. (2014). The shared neural basis of empathy and facial imitation accuracy. Neuroimage, 84, 367–75. [DOI] [PubMed] [Google Scholar]
- Bzdok D., Schilbach L., Vogeley K., et al. (2012). Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Structure & Function, 217(4), 783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr L., Iacoboni M., Dubeau M.C., Mazziotta J.C., Lenzi G.L. (2003). Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of Sciences of the United States of America, 100(9), 5497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington S.J., Bailey A.J. (2009). Are there theory of mind regions in the brain? A review of the neuroimaging literature. Human Brain Mapping, 30(8), 2313–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S., Zilles K., Laird A.R., Eickhoff S.B. (2010). ALE meta-analysis of action observation and imitation in the human brain. Neuroimage, 50(3), 1148–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Chou K.H., Decety J., et al. (2009). Sex differences in the neuroanatomy of human mirror-neuron system: a voxel-based morphometric investigation. Neuroscience, 158(2), 713–20. [DOI] [PubMed] [Google Scholar]
- Christov-Moore L., Iacoboni M. (2016). Self-other resonance, its control and prosocial inclinations: brain-behavior relationships. Human Brain Mapping, 37(4), 1544–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C.L., Uddin L.Q., Di Martino A., Castellanos F.X., Milham M.P., Kelly C. (2012). The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain's intrinsic functional dynamics. Social Cognitive and Affective Neuroscience, 7(6), 727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–73. [DOI] [PubMed] [Google Scholar]
- Cross K.A., Torrisi S., Losin E.A.R., Iacoboni M. (2013). Controlling automatic imitative tendencies: interactions between mirror neuron and cognitive control systems. Neuroimage, 83, 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rosa S., Schillinger F.L., Bulthoff H.H., Schultz J., Uludag K. (2016). fMRI adaptation between action observation and action execution reveals cortical areas with mirror neuron properties in human BA 44/45. Frontiers in Human Neuroscience, 10, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J., Svetlova M. (2012). Putting together phylogenetic and ontogenetic perspectives on empathy. Developmental Cognitive Neuroscience, 2(1), 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino G., Fadiga L., Fogassi L., Gallese V., Rizzolatti G. (1992). Understanding motor events: a neurophysiological study. Experimental Brain Research, 91(1), 176–80. [DOI] [PubMed] [Google Scholar]
- Dimberg U., Thunberg M., Elmehed K. (2000). Unconscious facial reactions to emotional facial expressions. Psychological Science, 11(1), 86–9. [DOI] [PubMed] [Google Scholar]
- Dodell-Feder D., Koster-Hale J., Bedny M., Saxe R. (2011). fMRI item analysis in a theory of mind task. Neuroimage, 55(2), 705–12. [DOI] [PubMed] [Google Scholar]
- Dufour N., Redcay E., Young L., et al. (2013). Similar brain activation during false belief tasks in a large sample of adults with and without autism. PLoS One, 8(9), e75468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziobek I., Preißler S., Grozdanovic Z., Heuser I., Heekeren H.R., Roepke S. (2011). Neuronal correlates of altered empathy and social cognition in borderline personality disorder. Neuroimage, 57(2), 539–48. [DOI] [PubMed] [Google Scholar]
- Dziobek I., Rogers K., Fleck S., et al. (2008). Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the Multifaceted Empathy Test (MET). Journal of Autism and Developmental Disorders, 38(3), 464–73. [DOI] [PubMed] [Google Scholar]
- Eslinger P.J. (1998). Neurological and neuropsychological bases of empathy. European Neurology, 39(4), 193–9. [DOI] [PubMed] [Google Scholar]
- Fazio P., Cantagallo A., Craighero L., et al. (2009). Encoding of human action in Broca's area. Brain, 132(Pt 7), 1980–8. [DOI] [PubMed] [Google Scholar]
- Fisher R.A. (1915). Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika, 10(4), 507–21. [Google Scholar]
- Fogassi L., Ferrari P.F., Gesierich B., Rozzi S., Chersi F., Rizzolatti G. (2005). Parietal lobe: from action organization to intention understanding. Science, 308(5722), 662–7. [DOI] [PubMed] [Google Scholar]
- Gallese V. (2001). The ′shared manifold′ hypothesis – from mirror neurons to empathy. Journal of Consciousness Studies, 8(5-7), 33–50. [Google Scholar]
- Gallese V., Fadiga L., Fogassi L., Rizzolatti G. (1996). Action recognition in the premotor cortex. Brain, 119(2), 593–609. [DOI] [PubMed] [Google Scholar]
- Gobbini M.I., Koralek A.C., Bryan R.E., Montgomery K.J., Haxby J.V. (2007). Two takes on the social brain: a comparison of theory of mind tasks. Journal of Cognitive Neuroscience, 19(11), 1803–14. [DOI] [PubMed] [Google Scholar]
- Green M.F., Horan W.P., Lee J. (2015). Social cognition in schizophrenia. Nature Reviews Neuroscience, 16(10), 620–31. [DOI] [PubMed] [Google Scholar]
- Grezes J., Armony J.L., Rowe J., Passingham R.E. (2003). Activations related to “mirror” and “canonical” neurones in the human brain: an fMRI study. Neuroimage, 18(4), 928–37. [DOI] [PubMed] [Google Scholar]
- Hawco C., Kovacevic N., Malhotra A.K., et al. (2017). Neural activity while imitating emotional faces is related to both lower and higher-level social cognitive performance. Scientific Reports, 7(1), 1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiser M., Iacoboni M., Maeda F., Marcus J., Mazziotta J.C. (2003). The essential role of Broca's area in imitation. European Journal of Neuroscience, 17(5), 1123–8. [DOI] [PubMed] [Google Scholar]
- Hillis A.E. (2013). Inability to empathize: brain lesions that disrupt sharing and understanding another's emotions. Brain, 137(Pt 4), 981–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M. (2009). Imitation, empathy, and mirror neurons. Annual Review of Psychology, 60, 653–70. [DOI] [PubMed] [Google Scholar]
- Iacoboni M., Koski L.M., Brass M., et al. (2001). Reafferent copies of imitated actions in the right superior temporal cortex. Proceedings of the National Academy of Sciences of the United States of America, 98(24), 13995–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M., Mazziotta J.C. (2007). Mirror neuron system: basic findings and clinical applications. Annals of Neurology, 62(3), 213–8. [DOI] [PubMed] [Google Scholar]
- Iacoboni M., Molnar-Szakacs I., Gallese V., Buccino G., Mazziotta J.C., Rizzolatti G. (2005). Grasping the intentions of others with one's own mirror neuron system. Plos Biology, 3(3), e79–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M., Woods R.P., Brass M., Bekkering H., Mazziotta J.C., Rizzolatti G. (1999). Cortical mechanisms of human imitation. Science, 286(5449), 2526–8. [DOI] [PubMed] [Google Scholar]
- Jabbi M., Keysers C. (2008). Inferior frontal gyrus activity triggers anterior insula response to emotional facial expressions. Emotion, 8(6), 775–80. [DOI] [PubMed] [Google Scholar]
- Jones A.P., Happe F.G., Gilbert F., Burnett S., Viding E. (2010). Feeling, caring, knowing: different types of empathy deficit in boys with psychopathic tendencies and autism spectrum disorder. Journal of Child Psychology and Psychiatry, 51(11), 1188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P., Bockler A., Trautwein F.M., Parianen Lesemann F.H., Singer T. (2016). Are strong empathizers better mentalizers? Evidence for independence and interaction between the routes of social cognition. Social Cognitive and Affective Neuroscience, 11(9), 1383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J.T., Iacoboni M. (2006). Getting a grip on other minds: mirror neurons, intention understanding, and cognitive empathy. Social Neuroscience, 1(3-4), 175–83. [DOI] [PubMed] [Google Scholar]
- Kerem E., Fishman N., Josselson R. (2001). The experience of empathy in everyday relationships: cognitive and affective elements. Journal of Social and Personal Relationships, 18(5), 709. [Google Scholar]
- Keysers C., Perrett D.I. (2004). Demystifying social cognition: a Hebbian perspective. Trends in Cognitive Sciences, 8(11), 501–7. [DOI] [PubMed] [Google Scholar]
- Kilner J.M., Neal A., Weiskopf N., Friston K.J., Frith C.D. (2009). Evidence of mirror neurons in human inferior frontal gyrus. Journal of Neuroscience, 29(32), 10153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski L., Iacoboni M., Dubeau M.C., Woods R.P., Mazziotta J.C. (2003). Modulation of cortical activity during different imitative behaviors. Journal of Neurophysiology, 89(1), 460–71. [DOI] [PubMed] [Google Scholar]
- Kraemer M., Herold M., Uekermann J., et al. (2013). Theory of mind and empathy in patients at an early stage of relapsing remitting multiple sclerosis. Clinical Neurology and Neurosurgery, 115(7), 1016–22. [DOI] [PubMed] [Google Scholar]
- Lamm C., Batson C.D., Decety J. (2007). The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience, 19(1), 42–58. [DOI] [PubMed] [Google Scholar]
- Lamm C., Decety J., Singer T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage, 54(3), 2492–502. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. (2008) International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Technical Report A-7. Gainesville, FL, University of Florida. [Google Scholar]
- Lawrence E.J., Shaw P., Giampietro V., Surguladze S., Brammer M.J., David A.S. (2006). The role of ′shared representations′ in social perception and empathy: an fMRI study. Neuroimage, 29(4), 1173–84. [DOI] [PubMed] [Google Scholar]
- Leslie K.R., Johnson-Frey S.H., Grafton S.T. (2004). Functional imaging of face and hand imitation: towards a motor theory of empathy. Neuroimage, 21(2), 601–7. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. (2009). Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience, 4(4), 423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N., Yu X., Zhao Y., Zhang M. (2016). Functional anatomy of recognition of chinese multi-character words: convergent evidence from effects of transposable nonwords, lexicality, and word frequency. PLoS One, 11(2), e0149583.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar R.A. (2011). The neural bases of social cognition and story comprehension. Annual Review of Psychology, 62, 103–34. [DOI] [PubMed] [Google Scholar]
- Meffert H., Gazzola V., den Boer J.A., Bartels A.A., Keysers C. (2013). Reduced spontaneous but relatively normal deliberate vicarious representations in psychopathy. Brain, 136(8), 2550–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta U.M., Thirthalli J., Aneelraj D., Jadhav P., Gangadhar B.N., Keshavan M.S. (2014). Mirror neuron dysfunction in schizophrenia and its functional implications: a systematic review. Schizophrenia Research, 160(1-3), 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P., Cunnington R., Mattingley J.B. (2012). Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neuroscience and Biobehavioral Reviews, 36(1), 341–9. [DOI] [PubMed] [Google Scholar]
- Molenberghs P., Johnson H., Henry J.D., Mattingley J.B. (2016). Understanding the minds of others: a neuroimaging meta-analysis. Neuroscience & Biobehavioral Reviews, 65, 276–91. [DOI] [PubMed] [Google Scholar]
- Molnar-Szakacs I. (2011). From actions to empathy and morality – a neural perspective. Journal of Economic Behavior & Organization, 77(1), 76–85. [Google Scholar]
- Montgomery K.J., Haxby J.V. (2008). Mirror neuron system differentially activated by facial expressions and social hand gestures: a functional magnetic resonance imaging study. Journal of Cognitive Neuroscience, 20(10), 1866–77. [DOI] [PubMed] [Google Scholar]
- Morawetz C., Holz P., Lange C., et al. (2008). Improved functional mapping of the human amygdala using a standard functional magnetic resonance imaging sequence with simple modifications. Magnetic Resonance Imaging, 26(1), 45–53. [DOI] [PubMed] [Google Scholar]
- Nichols T., Brett M., Andersson J., Wager T., Poline J.B. (2005). Valid conjunction inference with the minimum statistic. Neuroimage, 25(3), 653–60. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L., Hirvonen J., Parkkola R., Hietanen J.K. (2008). Is emotional contagion special? An fMRI study on neural systems for affective and cognitive empathy. Neuroimage, 43(3), 571–80. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Moriguchi Y., Matsuda H., et al. (2004). The neural network for the mirror system and mentalizing in normally developed children: an fMRI study. Neuroreport, 15(9), 1483–7. [DOI] [PubMed] [Google Scholar]
- Oliver L.D., Mitchell D.G., Dziobek I., et al. (2015). Parsing cognitive and emotional empathy deficits for negative and positive stimuli in frontotemporal dementia. Neuropsychologia, 67, 14–26. [DOI] [PubMed] [Google Scholar]
- Paracampo R., Tidoni E., Borgomaneri S., di Pellegrino G., Avenanti A. (2017) Sensorimotor network crucial for inferring amusement from smiles. Cerebral Cortex, 27, 5116–29. [DOI] [PubMed] [Google Scholar]
- Parkinson C., Wheatley T. (2014). Relating anatomical and social connectivity: white matter microstructure predicts emotional empathy. Cerebral Cortex, 24(3), 614–25. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.H., Iacoboni M., Mazziotta J.C., Dapretto M. (2008). Mirroring others' emotions relates to empathy and interpersonal competence in children. Neuroimage, 39(4), 2076–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda J.A., Hecht E. (2009). Mirroring and mu rhythm involvement in social cognition: are there dissociable subcomponents of theory of mind? Biological Psychology, 80(3), 306–14. [DOI] [PubMed] [Google Scholar]
- Preston S.D., de Waal F.B.M. (2002). Empathy: its ultimate and proximate bases. Behavioral and Brain Sciences, 25(1), 1–71. [DOI] [PubMed] [Google Scholar]
- Robinson S., Windischberger C., Rauscher A., Moser E. (2004). Optimized 3 T EPI of the amygdalae. Neuroimage, 22(1), 203–10. [DOI] [PubMed] [Google Scholar]
- Saxe R., Kanwisher N. (2003). People thinking about thinking people – the role of the temporo-parietal junction in “theory of mind”. Neuroimage, 19(4), 1835–42. [DOI] [PubMed] [Google Scholar]
- Schneider W., Eschman A., Zuccolotto A. (2002) E-Prime Reference Guide,. Pittsburgh, PA: Psychology Software Tools, Inc. [Google Scholar]
- Schurz M., Radua J., Aichhorn M., Richlan F., Perner J. (2014). Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neuroscience and Biobehavioral Reviews, 42, 9–34. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G. (2011). The neural bases for empathy. The Neuroscientist, 17(1), 18–24. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Aharon-Peretz J. (2007). Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia, 45(13), 3054–67. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Aharon-Peretz J., Perry D. (2009). Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain, 132(3), 617–27. [DOI] [PubMed] [Google Scholar]
- Siegel J.S., Power J.D., Dubis J.W., et al. (2014). Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Human Brain Mapping, 35(5), 1981–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt R.P., Adolphs R. (2014). Validating the why/how contrast for functional MRI studies of theory of mind. Neuroimage, 99, 301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt R.P., Falk E.B., Lieberman M.D. (2010). Dissociable neural systems support retrieval of how and why action knowledge. Psychological Science, 21(11), 1593–8. [DOI] [PubMed] [Google Scholar]
- Spunt R.P., Lieberman M.D. (2012). Dissociating modality-specific and supramodal neural systems for action understanding. Journal of Neuroscience, 32(10), 3575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt R.P., Satpute A.B., Lieberman M.D. (2011). Identifying the what, why, and how of an observed action: an fMRI study of mentalizing and mechanizing during action observation. Journal of Cognitive Neuroscience, 23(1), 63–74. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. (1988) Co-Planar Stereotaxic Atlas of the Human Brain,. Stuttgart, New York, Georg Thieme. [Google Scholar]
- Tidoni E., Borgomaneri S., di Pellegrino G., Avenanti A. (2013). Action simulation plays a critical role in deceptive action recognition. Journal of Neuroscience, 33(2), 611–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramacere A., Ferrari P.F. (2016). Faces in the mirror, from the neuroscience of mimicry to the emergence of mentalizing. Journal of Anthropological Science, 94, 13–26. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Iacoboni M., Lange C., Keenan J.P. (2007). The self and social cognition: the role of cortical midline structures and mirror neurons. Trends in Cognitive Sciences, 11(4), 153–7. [DOI] [PubMed] [Google Scholar]
- Van der Graaff J., Meeus W., de Wied M., et al. (2016). Motor, affective and cognitive empathy in adolescence: interrelations between facial electromyography and self-reported trait and state measures. Cognition & Emotion, 30(4), 745–61. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. (2009). Social cognition and the brain: a meta-analysis. Human Brain Mapping, 30(3), 829–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F., Baetens K. (2009). Understanding others' actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage, 48(3), 564–84. [DOI] [PubMed] [Google Scholar]
- Yang D.Y., Rosenblau G., Keifer C., Pelphrey K.A. (2015). An integrative neural model of social perception, action observation, and theory of mind. Neuroscience & Biobehavioral Reviews, 51, 263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J., Weber J., Bolger N., Ochsner K. (2009). The neural bases of empathic accuracy. Proceedings of the National Academy of Sciences of the United States of America, 106(27), 11382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]