Abstract
The detailed kinetics of the cytomegalovirus (CMV)-specific T cell response in hematopoietic stem cell transplant (HCT) recipients have not yet been fully assessed. We evaluated these kinetics of CMV-specific T cell response and factors associated with high CMV-specific T cell responses 1 year after HCT. In HCT recipients, CMV pp65 and IE1-specific ELISPOT assay were performed before HCT (D0), and at 30 (D30), 90 (D90), 180 (D180), and 360 (D360) days after HCT. Of the 51 HCT recipients with donor-positive (D+)/recipient-positive (R+) serology, 26 (51%) developed CMV infections after HCT. The patterns of post-transplantation reconstitution for CMV-specific T cell response were classified into 4 types: 1) an initial decrease at D30 followed by gradual T cell reconstitution without CMV infection (35%), 2) an initial decrease at D30 followed by gradual T cell reconstitution preceded by CMV infection (35%), 3) failure of gradual or constant T cell reconstitution (26%), and 4) no significant T cell reconstitution (4%). There was no significant difference between ELISPOT counts of D360 and those of D0. High CMV-specific T cell responses at D360 were not associated with high CMV-specific T cell response at D0, CMV infection, ganciclovir therapy, graft versus host disease (GVHD), and immunosuppressant use. In conclusion, there are 4 distinct patterns of reconstitution of the CMV-specific T cell response after HCT. In addition, reconstituted donor-origin CMV-specific T cell responses appeared to be constant until day 360 after HCT, regardless of the level of the pre-transplant CMV-specific T cell response, CMV infection, and immunosuppressant use.
Keywords: Cytomegalovirus, ELISPOT, Hematopoietic stem cell transplantation
INTRODUCTION
Cytomegalovirus (CMV) infection is a major cause of morbidity and mortality in hematopoietic stem cell transplant (HCT) recipients (1,2). Although advances in preemptive treatment based on monitoring viral load determined by pp65 antigenemia assays and/or quantitative PCR for CMV DNA have reduced the incidence of CMV viremia and subsequent disease, breakthrough CMV disease and drug-related toxicity is still a problem (3,4,5). The T cell system plays a crucial role in controlling CMV infection, and the deficiency of this immune system after HCT creates a risk of CMV replication and shedding (6,7). Several previous studies have reported that the ELISPOT assay for measuring CMV-specific T cell responses is useful for predicting the development of CMV infection and disease after HCT (8,9,10,11). Immunological monitoring of CMV-specific T cells might allow more targeted use of antiviral agents. However, the detailed kinetics of CMV-specific T cell response in HCT recipients have not yet been fully assessed. In addition, it has been reported that the long-term immunity after HCT varies according to the pathogen and may decrease over the years (12,13). We therefore evaluated these kinetics of CMV-specific T cell response and factors associated with high CMV-specific T cell responses at 1 year after HCT.
MATERIALS AND METHODS
Study population and design
This study was performed at Asan Medical Center, a 2,700 bed tertiary care hospital in Seoul, Korea. All adult patients (≥16 years old) who were scheduled to undergo allogeneic HCT between April 2014 and April 2015 were invited to participate in the study. The exclusion criteria were refusal of written informed consent, as well as donor-negative (D−) and/or recipient-negative (R−) CMV serology. Tests for CMV IgG were performed in the HCT recipients and donors before HCT. CMV pp65 antigenemia assays or PCR test were monitored weekly from day 21 to day 100 and monthly for 1 year post-transplantation. Patients who developed viremia received preemptive therapy with ganciclovir or valganciclovir until negative conversion for CMV antigenemia. All drug dosages were adjusted for renal impairment. The study protocol was approved by the Institutional Review Board of Asan Medical Center (approval number: 2014-0103).
Virological monitoring
The CMV antigenemia assay was performed as previously described (3). EDTA-treated whole blood samples were fractionated by dextran sedimentation and lysis of erythrocytes, and the granulocytes were centrifuged to prepare a cytospin slide. The cells were then fixed with formaldehyde and sequentially immunostained with monoclonal antibodies C10/C11 (Clonab CMV; Biotest, Dreieich, Germany). Counts are expressed as positive cells per 200,000 leukocytes. CMV DNA quantitation was performed with a Qiagen Artus CMV RGQ MDx kit (Qiagen, Doncaster, Australia) on a Rotor-Gene Q platform (Qiagen) following DNA extraction with a NucliSens easyMAG nucleic acid extraction system (bioMérieux, Lyon, France).
Immunological monitoring
CMV pp65- and IE1-specific ELISPOT assays were performed before HCT (D0), and 30 (D30), 90 (D90), 180 (D180), 360 (D360) days after HCT. A peripheral venous blood sample (up to 8 ml) was collected from each patient to detect T cells producing interferon (IFN)-gamma (i.e., T-track CMV; Lophius Biosciences, Regensburg, Germany). Briefly, peripheral blood mononuclear cells (PBMCs) were immediately (within 30 min) separated and collected. The collected cells were resuspended at 2.0×106 cells/ml, placed (2.0×105 cells/well) in wells pre-coated with anti-human IFN-gamma antibody. The PBMC were stimulated with phytohemagglutinin (positive control), pp65, IE1 and medium only (negative control) and incubated for 18 h. The resulting spots were counted with an automated microscope (ELiSpot 04 HR; Autoimmune Diagnostika GmbH, Strassberg, Germany). Background counts, obtained in the negative control wells, were subtracted. The results are expressed as spot-forming cells (SFC)/2.0×105 cells.
Assessment of outcomes
CMV infection was defined as positive pp65 antigenemia (≥1 cell per 200,000 cells) or positive PCR (≥500 copies/ml) as evidence of CMV DNAemia. CMV disease was defined as evidence of localized CMV infection (cells with CMV inclusions, in situ detection of CMV antigen by immunohistochemistry, or DNA) in a biopsy or other appropriate specimen (e.g., bronchoalveolar lavage, cerebrospinal fluid) and symptoms of organ dysfunction.
Statistical analysis
Continuous variables were compared using paired t-test, while categorical variables were examined with the χ2 or Fisher's exact test. Repeated measures ANOVA were used for trend analysis. To analyze the correlations between the different variables selected and the dependent variable, linear regression analysis was performed. All p-values were 2-tailed, and p<0.05 was considered statistically significant. SPSS Statistics, version 19.0 (IBM Corp., Armonk, NY, USA), was used for analyses.
RESULTS
Clinical and demographic characteristics of HCT recipients
During the study period, a total of 140 patients underwent allogeneic HCT. Of these, 52 (37%) refused informed consent and 4 (3%) with D−/recipient-positive (R+) CMV serology were excluded. Of the remaining 84 (60%) HCT recipients with donor-positive (D+)/R+ serology, 30 died and 3 were lost to follow-up by 1 year after HCT. Only the 51 patients who survived 12 months post-HCT were eligible for the final analysis. Their demographic and clinical characteristics are summarized in Table 1. Acute myeloid leukemia was the most common cause of HCT, followed by myelodysplastic syndrome, and aplastic anemia. All HCT recipients received stem cell grafts of donor' peripheral blood. As most individuals in Korea have CMV IgG, only D+/R+ cases were included in the study. Of the 51 patients, 26 (50%) developed ≥1 episode of CMV infection, and 10 (21%) had relapsing CMV infections (2 or more episodes of CMV infections). The median time for onset of CMV infection post-HCT was 35 days after transplantation (range, 14–115 days). Three (6%) patients suffered CMV disease, comprising CMV retinitis, esophagitis, and gastritis. Two of the episodes of CMV esophagitis and gastritis developed in patients with low or undetectable ELISPOT counts, whereas in the third episode the patient experienced retinitis after documented CMV-specific immune restoration.
Table 1. Baseline demographic and clinical characteristics of 51 recipients of HCTs.
| Characteristic | Total (n=51) | |
|---|---|---|
| Median age (range, yr) | 41 (20–64) | |
| Male | 32 (63) | |
| Underlying disease | ||
| Acute myeloid leukemia | 32 (63) | |
| Myelodysplastic syndrome | 8 (16) | |
| Aplastic anemia | 6 (12) | |
| Acute lymphocytic leukemia | 2 (4) | |
| Chronic myeloid leukemia | 1 (2) | |
| Non-Hodgkin's lymphoma | 1 (2) | |
| Hemophagocytic lymphohistiocytosis | 1 (2) | |
| Transplant type | ||
| Full allogeneic | 10 (20) | |
| Non-myeloablative allogeneic | 41 (80) | |
| Stem cell source | ||
| Peripheral blood | 51 (100) | |
| Cord blood or bone marrow | 0 (0) | |
| HLA matching | ||
| Matched related | 19 (37) | |
| Matched unrelated | 16 (31) | |
| Mismatched related | 13 (25) | |
| Mismatched unrelated | 3 (6) | |
| CMV serostatus | ||
| Donor positive/recipient positive | 51 (100) | |
| Remission before HCT | 27 (53) | |
| Acute GVHD | 13 (25) | |
| Chronic GVHD | 13 (25) | |
| Preemptive ganciclovir therapy | 17 (33) | |
| Corticosteroid use before HCT | 11 (22) | |
| Corticosteroid use after HCT | 46 (90) | |
| GVHD prophylaxis regimen | ||
| Cyclosporine | 51 (100) | |
| Methotrexate | 43 (84) | |
| Tacrolimus or mycophenolate | 7 (14) | |
| CMV infection | 26 (51) | |
| Relapsing CMV infection | 10 (20) | |
| CMV disease | 3 (6) | |
Values are number (%) unless otherwise indicated. HLA, human leukocyte antigen.
A total of 25 patients experienced non-CMV infection. Fifteen cases were identified in the non-survivor group and 10 in the survivor group (Supplementary Table 1). Among the survivors, there was no significant difference in the opportunistic infection (OI) incidence in the failed recovery pattern C and D (13% [2/15]) compared to the recovered pattern A and B (22% [8/36]; p=0.700). However, there was a significant difference in OI incidence between survivors and non-survivors (20% [10/51] vs. 50% [15/30]; p=0.004). During the period, 30 patients died and were excluded from the analysis. The cause of mortality in 30 patients is summarized in Supplementary Table 2. In the mortality cases, failed immune reconstitution patterns such as pattern C and D were observed more frequently than in the survivor group (70% [21/30] vs. 29% [15/51]; p<0.001). However, ELISPOT counts between mortality cases and survivors were not significantly different except for pp65-specific response at D180 (median, 113 SFC/2.0×105 cells vs. 14 SFC/2.0×105 cells; p=0.034).
CMV T cell specific immune recovery over a period of 1 year post-transplantation
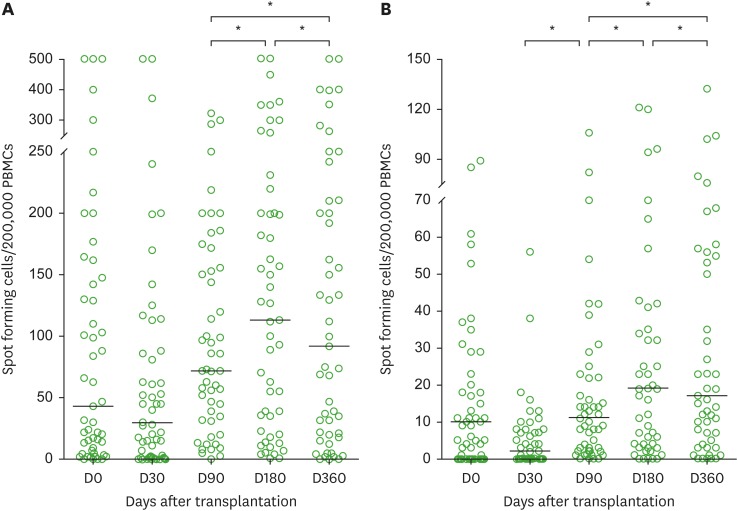
The development of CMV-specific T cell responses over the period are shown in Fig. 1. In the majority of cases (70%) the CMV-specific T cell response decreased initially at D30, then recovered progressively with or without a CMV infection. The trend analysis using repeated measures ANOVA showed an overall time-dependent increase of immune responses during the period from D30 to D360 (Supplementary Table 3). The CMV-specific T cell responses at D360 to pp65 (median, 92 SFC/2.0×105 cells vs. 43 SFC/2.0×105 cells; p=0.886) and to IE1 (median, 17 SFC/2.0×105 cells vs. 10 SFC/2.0×105 cells; p=0.457) showed no significant differences from those at D0. Factors including a high CMV-specific T cell response at D0, CMV infection after HCT, preemptive ganciclovir therapy, graft versus host disease (GVHD) (including acute and/or chronic form), and prolonged immunosuppressant use were not associated with the ELISPOT count at D360 (Supplementary Table 4).
Figure 1.
Kinetics of (A) pp65- and (B) IE1-specific ELISPOT assays over the first year after transplantation in hematopoietic stem cell recipients. Bars indicate median values. Only significant p-values are shown.
PBMC, peripheral blood mononuclear cell.
*p<0.05.
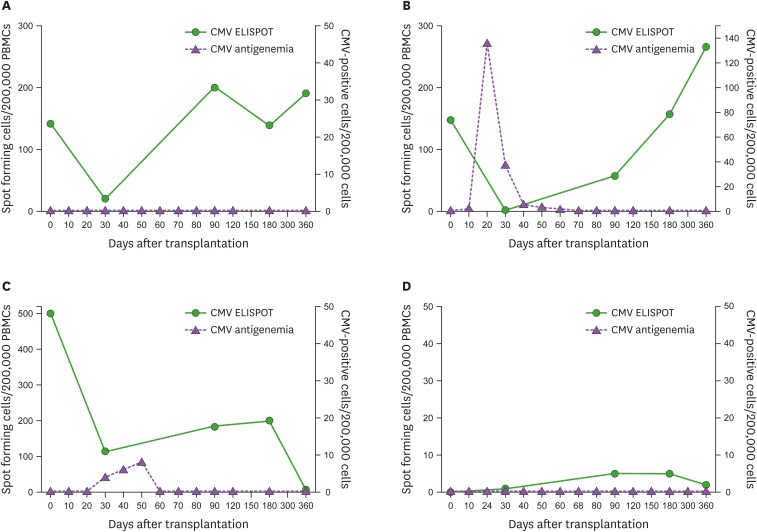
The 4 patterns of immune reconstitution
We discerned 4 patterns of post-transplant CMV-specific immune reconstitution as follows (Fig. 2): 1) an initial decrease at D30 followed by spontaneous T cell reconstitution without CMV viremia (18 [35%], Fig. 2A), 2) a low or absent T cell response at D30 followed by gradual T cell reconstitution boosted by a preceding CMV infection (18 [35%], Fig. 2B), 3) failure of gradual or sustained T cell reconstitution (13 [26%], Fig. 2C), and 4) absence of any detectible significant T cell reconstitution (2 [4%], Fig. 2D). Grouping by presence of immune reconstitution, there was a significant difference in ELISPOT counts between pattern A, B and pattern C, D. pp65-specific ELISPOT count of pattern A, B at D90 (median, 91 SFC/2.0×105 cells vs. 47 SFC/2.0×105 cells; p=0.028), D180 (median, 153 SFC/2.0×105 cells vs. 35 SFC/2.0×105 cells; p=0.007), and D360 (median, 159 SFC/2.0×105 cells vs. 5 SFC/2.0×105 cells; p<0.001) were higher than those of pattern C, D and IE1-specific ELISPOT count of the former at D360 (median, 21 SFC/2.0×105 cells vs. 7 SFC/2.0×105 cells; p=0.038) were higher than those of the latter (Supplementary Fig. 1). There was no significant difference of clinical characteristics between 2 groups (Supplementary Table 5).
Figure 2.
Four patterns of immune reconstitution in hematopoietic stem cell recipients. (A) Spontaneous recovery without CMV infection. (B) T cell reconstitution with a preceding CMV infection. (C) Failure of gradual or sustained T cell reconstitution. (D) Absence of T cell reconstitution.
PBMC, peripheral blood mononuclear cell.
DISCUSSION
CMV remains one of the most problematic pathogens in HCT recipients (1,7). Since T cell compartments are critical for immune control of CMV, a variety tools have been adopted to measure cell-mediated immunity against CMV for immunological monitoring (14). The ELISPOT assay is a sensitive and simple method for counting antigen-specific CD4+ and/or CD8+ lymphocyte to assess cell-mediated immune responses to stimulating antigens (15). It has been reported that the ELISPOT can identify patients at risk of CMV disease (8,9,10,11). However, there are limited data on the detailed kinetics of the CMV-specific T cell response especially on long-term follow-up of HCT recipients.
In present study, the median ELISPOT count at day 360 did not differ from baseline count and an overall time-dependent increasing trend of immune responses was observed during the period from D30 to D360 after HCT. These findings suggest that restoration of T cell-mediated immunity from the graft lasts for at least a year. In detail, recovery of CMV-specific immunity was maintained over that time in the majority (70%) of immune reconstitution patterns. Interestingly, none of the factors tested, including pre-transplant CMV-specific T cell response, CMV infection after HCT, ganciclovir use, GVHD, and immunosuppressant use, was found to be associated with a high CMV-specific T cell responses at D360. It seems likely that the two thirds of HCT recipients who recover CMV-specific T cell responses by D360 can prevent CMV reactivation by that time.
We found 4 different patterns of CMV-specific immune, in partial agreement with a previous study that reported heterogeneous patterns of CMV-specific immune restoration in 31 pediatric allogeneic HCT recipients (11). The authors described 2 patterns in the D+/R+ setting. However, they did not specify the relative frequencies of the patterns. In another study assessing cell-mediated immunity in partially matched-related donor transplantations, both CMV-specific CD8+ T cell counts and ELISPOT responses recovered gradually by day 90 after HCT and then tended to decrease (16). However, those results should be interpreted with caution because differences in donor/recipient serologic status, immunosuppressive regimen, and presence of GVHD may affect patterns of CMV-specific immune reconstitution.
Our data provided us important insight on long-term CMV-specific T cell response in HCT recipients. About two thirds of HCT recipients with or without the evidence of CMV infection restored CMV-specific T cell response. The remaining one third of HCT recipients did not restore CMV-specific T cell response even after the evidence of CMV infection. We thus assume that one third of HCT recipients who cannot ultimately restore CMV-specific T cell response may need long-term surveillance of CMV infection or prolonged antiviral use may be helpful in these patients. In addition, we can roughly estimate the test burden of CMV ELISPOT assays in terms of how long and how often these new assays should be performed in HCT recipients. Actually, the German researchers are conducting “AlloProtectCMV” study which will evaluate whether CMV-specific T cell response after CMV reactivation in HCT recipients can predict secondary CMV reactivation in the future (clinical trials No. NCT02156479 in ClinicalTrials.gov). This study will give us more detailed information on the development of subsequent CMV infection in HCT recipients who cannot restore CMV-specific T cell response.
This study has several limitations. First, only HCT recipients with D+/R+ serology were enrolled. Therefore, it is difficult to extrapolate our data to recipients with other serologies. Second, some may argue that evaluating the IFN-gamma releasing T cell response gives only limited information about CMV infection. Recent studies have revealed that polyfunctional T cell responses are predictive of protection against CMV risk after lung transplantation (17). Hence, evaluation of the polyfunctionality of CMV-specific T cell responses may provide more useful information on protection against CMV infection after HCT. Further studies are needed in this area.
In conclusion, our results reveal 4 distinct patterns of CMV-specific T cell responses after HCT. They also showed that by D360 donor-origin CMV-specific T cell responses appear to be constantly restored to the level of the recipient-origin CMV-specific T cell responses before HCT and that this outcome is unrelated to pre-transplant CMV-specific T cell responses, CMV infection, and immunosuppressant use. Regular monitoring of CMV-specific immune response may allow more sophisticated treatment to prevent CMV infection, and further study is needed to elucidate the usefulness of measuring cell-mediated immunity by the ELISPOT assay.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant No. HI15C1763).
Abbreviations
- CMV,
cytomegalovirus
- GVHD
graft versus host disease
- HCT
hematopoietic stem cell transplant
- IFN
interferon
- OI
opportunistic infection
- SFC
spot-forming cells
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
Author Contributions: Conceptualization: Lee SO, Choi SH, Kim SH; Data curation: Kim SM, Kang YA, Lee YS; Formal analysis: Kim SM, Kang YA, Lee YS; Investigation: Bae S, Jung J, Chong YP, Sung H, Lee JH; Supervision: Kim YS, Woo JH, Lee KH; Validation: Chong YP, Sung H, Lee JH; Writing - original draft: Bae S, Jung J, Lee SO, Choi SH, Kim YS, Woo JH, Lee KH, Kim SH; Writing - review & editing: Bae S, Jung J, Kim SM, Kang YA, Lee YS, Chong YP, Sung H, Lee SO, Choi SH, Kim YS, Woo JH, Lee JH, Lee JH, Lee KH, Kim SH.
Supplementary Materials
Patients with infections other than CMV
Cause of mortality in 30 non-survivor group
The trend analysis of ELISPOT counts from D30 to D360 by using repeated measures analysis of variances
Associations of the ELISPOT count at D360 with multiple variables by univariate linear regression analyses
Comparison of clinical and demographic characteristics in patients with or without CMV-specific T cell immune reconstitution
Different kinetics of (A) pp65- and (B) IE1-specific ELISPOT assays in 2 groups according to immune reconstitution patterns over the first year after HCT. Only significant p-values are shown.
References
- 1.Reusser P, Riddell SR, Meyers JD, Greenberg PD. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78:1373–1380. [PubMed] [Google Scholar]
- 2.Barron MA, Gao D, Springer KL, Patterson JA, Brunvand MW, McSweeney PA, Zeng C, Barón AE, Weinberg A. Relationship of reconstituted adaptive and innate cytomegalovirus (CMV)-specific immune responses with CMV viremia in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49:1777–1783. doi: 10.1086/648423. [DOI] [PubMed] [Google Scholar]
- 3.Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88:4063–4071. [PubMed] [Google Scholar]
- 4.Einsele H, Hebart H, Kauffmann-Schneider C, Sinzger C, Jahn G, Bader P, Klingebiel T, Dietz K, Löffler J, Bokemeyer C, et al. Risk factors for treatment failures in patients receiving PCR-based preemptive therapy for CMV infection. Bone Marrow Transplant. 2000;25:757–763. doi: 10.1038/sj.bmt.1702226. [DOI] [PubMed] [Google Scholar]
- 5.Green ML, Leisenring W, Stachel D, Pergam SA, Sandmaier BM, Wald A, Corey L, Boeckh M. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:1687–1699. doi: 10.1016/j.bbmt.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li CR, Greenberg PD, Gilbert MJ, Goodrich JM, Riddell SR. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994;83:1971–1979. [PubMed] [Google Scholar]
- 7.Ciáurriz M, Zabalza A, Beloki L, Mansilla C, Pérez-Valderrama E, Lachén M, Bandrés E, Olavarría E, Ramírez N. The immune response to cytomegalovirus in allogeneic hematopoietic stem cell transplant recipients. Cell Mol Life Sci. 2015;72:4049–4062. doi: 10.1007/s00018-015-1986-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hebart H, Daginik S, Stevanovic S, Grigoleit U, Dobler A, Baur M, Rauser G, Sinzger C, Jahn G, Loeffler J, et al. Sensitive detection of human cytomegalovirus peptide-specific cytotoxic T-lymphocyte responses by interferon-gamma-enzyme-linked immunospot assay and flow cytometry in healthy individuals and in patients after allogeneic stem cell transplantation. Blood. 2002;99:3830–3837. doi: 10.1182/blood.v99.10.3830. [DOI] [PubMed] [Google Scholar]
- 9.Ohnishi M, Sakurai T, Heike Y, Yamazaki R, Kanda Y, Takaue Y, Mizoguchi H, Kawakami Y. Evaluation of cytomegalovirus-specific T-cell reconstitution in patients after various allogeneic haematopoietic stem cell transplantation using interferon-gamma-enzyme-linked immunospot and human leucocyte antigen tetramer assays with an immunodominant T-cell epitope. Br J Haematol. 2005;131:472–479. doi: 10.1111/j.1365-2141.2005.05800.x. [DOI] [PubMed] [Google Scholar]
- 10.Avetisyan G, Aschan J, Hägglund H, Ringdén O, Ljungman P. Evaluation of intervention strategy based on CMV-specific immune responses after allogeneic SCT. Bone Marrow Transplant. 2007;40:865–869. doi: 10.1038/sj.bmt.1705825. [DOI] [PubMed] [Google Scholar]
- 11.Abate D, Cesaro S, Cofano S, Fiscon M, Saldan A, Varotto S, Mengoli C, Pillon M, Calore E, Biasolo MA, et al. Diagnostic utility of human cytomegalovirus-specific T-cell response monitoring in predicting viremia in pediatric allogeneic stem-cell transplant patients. Transplantation. 2012;93:536–542. doi: 10.1097/TP.0b013e31824215db. [DOI] [PubMed] [Google Scholar]
- 12.Ljungman P, Lewensohn-Fuchs I, Hammarström V, Aschan J, Brandt L, Bolme P, Lönnqvist B, Johansson N, Ringdén O, Gahrton G. Long-term immunity to measles, mumps, and rubella after allogeneic bone marrow transplantation. Blood. 1994;84:657–663. [PubMed] [Google Scholar]
- 13.Spoulou V, Giannaki M, Vounatsou M, Bakoula C, Grafakos S. Long-term immunity to measles, mumps and rubella after MMR vaccination among children with bone marrow transplants. Bone Marrow Transplant. 2004;33:1187–1190. doi: 10.1038/sj.bmt.1704476. [DOI] [PubMed] [Google Scholar]
- 14.Lacey SF, Diamond DJ, Zaia JA. Assessment of cellular immunity to human cytomegalovirus in recipients of allogeneic stem cell transplants. Biol Blood Marrow Transplant. 2004;10:433–447. doi: 10.1016/j.bbmt.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann PV, Zhang W. Unique strengths of ELISPOT for T cell diagnostics. Methods Mol Biol. 2012;792:3–23. doi: 10.1007/978-1-61779-325-7_1. [DOI] [PubMed] [Google Scholar]
- 16.Luo XH, Huang XJ, Li D, Liu KY, Xu LP, Liu DH. Immune reconstitution to cytomegalovirus following partially matched-related donor transplantation: impact of in vivo T-cell depletion and granulocyte colony-stimulating factor-primed peripheral blood/bone marrow mixed grafts. Transpl Infect Dis. 2013;15:22–33. doi: 10.1111/j.1399-3062.2012.00722.x. [DOI] [PubMed] [Google Scholar]
- 17.Snyder LD, Chan C, Kwon D, Yi JS, Martissa JA, Copeland CA, Osborne RJ, Sparks SD, Palmer SM, Weinhold KJ. Polyfunctional T-Cell signatures to predict protection from cytomegalovirus after lung transplantation. Am J Respir Crit Care Med. 2016;193:78–85. doi: 10.1164/rccm.201504-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patients with infections other than CMV
Cause of mortality in 30 non-survivor group
The trend analysis of ELISPOT counts from D30 to D360 by using repeated measures analysis of variances
Associations of the ELISPOT count at D360 with multiple variables by univariate linear regression analyses
Comparison of clinical and demographic characteristics in patients with or without CMV-specific T cell immune reconstitution
Different kinetics of (A) pp65- and (B) IE1-specific ELISPOT assays in 2 groups according to immune reconstitution patterns over the first year after HCT. Only significant p-values are shown.