Abstract
Although atopic dermatitis (AD) is characterized by cytokine production predominantly mediated by T helper (Th) 2 cells, AD pathogenesis also involves innate immune and Th1 cells. To optimize the cytokine milieu required for accurate reproduction of AD-related gene expression profile in vitro, we evaluated the expression pattern of CCL22, CCL17, IL5, IL13, IL33, IL25, TSLP, FLG, and LOR in human lesional AD skin and cytokine-stimulated HaCaT cells. An increase in Th2 mediators (IL5, IL13, CCL22, CCL17, IL25, IL33, and TSLP) and a decrease in genes related to cornified cell envelope (filaggrin and loricrin) were observed in human AD lesions. Innate (tumor necrosis factor-α) and/or Th1/Th2 adaptive cytokines (interferon-γ/IL-4) were required for inducing these inflammatory changes in HaCaT cells, implying that a complex network of innate, Th1, and Th2 cytokines drives AD-like changes. Therefore, stimulation with various combinations of cytokines, beyond Th2 polarization, is necessary when HaCaT cell line is used to study genetic changes implicated in AD pathogenesis.
Keywords: Atopic dermatitis, Cytokine, In vitro stimulation
INTRODUCTION
Atopic dermatitis (AD) has been considered as a T helper (Th) cell type 2 disease characterized by predominant Th2-mediated cytokine production, including IL-4, IL-5, and IL-13, elevated serum IgE, and eosinophilia (1,2). Thymic stromal lymphopoietin (TSLP), IL-25, and IL-33 are mainly produced by epithelial cells, and have important functions of inducing Th2-type adaptive responses and group 2 innate lymphoid cells, which contribute to AD phenotype (3,4). However, Th1 cells and innate inflammatory cytokines are also involved in the pathogenesis of AD. Interferon-γ (IFN-γ) and IL-12 are also expressed in chronic AD lesions. The expression of IFN-γ, but not IL-4, in skin correlates with the clinical course of AD and is known to be downregulated with AD improvement (5). AD patients have elevated levels of CC chemokine receptor 4 ligands, including thymus and activation-regulated chemokine (TARC) and macrophage-derived chemokine (MDC), which mediate preferential Th2 recruitment (6). Additionally, CXC chemokine receptor 3 ligands, which induce Th1 polarization, such as monokine induced by IFN-γ, are also elevated in AD patients compared to normal controls (6). In addition to inflammatory microenvironment imbalance, important barrier-related proteins including filaggrin (FLG) and loricrin (LOR) are decreased in AD (7). In primary keratinocytes, tumor necrosis factor (TNF)-α downregulates FLG and LOR through c-Jun N-terminal kinase (8). These results suggest that complex inflammatory networks, involving innate, Th1, and Th2 responses, orchestrate AD pathogenesis. The aim of this study was to investigate the effects of innate, Th1, and Th2 inflammatory cytokines on the expression of genes implicated in the pathogenesis of AD using human keratinocyte cell line HaCaT.
MATERIALS AND METHODS
HaCaT cell culture and cytokine stimulation
HaCaT cells were grown to 100% confluence and starved for 24 hours in DMEM without fetal bovine serum, followed by their stimulation with various combinations of innate (TNF-α, 10 ng/ml; PeptroTech, Rocky Hill, NJ, USA), Th1 (IFN-γ, 10 ng/ml; PeptroTech), and Th2 (IL-4, 50 ng/ml; PeptroTech) cytokines for 24 hours (9,10,11). The morphological changes in cells were examined using a phase-contrast microscope (Olympus CX41; Olympus, Tokyo, Japan).
Cell viability assay
The effects of cytokine stimulation on HaCaT cell growth were determined using a water-soluble tetrazolium salt assay kit according to the manufacturer's instructions (EZ-Cytox cell viability assay kit; ITSBio, Seoul, Korea).
Human sample
Skin specimens from six patients with AD and 12 healthy controls were collected under the approval of the Institutional Review Board at Gachon University Gil Medical Center (GBIRB2016-082). All 6 AD patients received no treatment for at least 4 weeks and had chronic disease (average duration 6.83±4.40 years) with a recent exacerbation in 1–3 months AD (SCORAD between 20 and 70, all with elevated IgE) (Table 1).
Table 1. Characteristics of patients with atopic dermatitis.
| Patient No. | Sex/age | Duration (yr) | Aggravation (mon) | Co-morbidities |
|---|---|---|---|---|
| 1 | M/20 | 10 | 3 | Asthma |
| 2 | M/19 | 12 | 2 | Allergic rhinitis |
| 3 | M/21 | 2 | 2 | Allergic rhinitis |
| 4 | M/13 | 5 | 2 | Urticaria |
| 5 | M/25 | 10 | 1 | - |
| 6 | M/46 | 2 | 3 | - |
Real-time PCR validation
Total RNA from skin specimens and HaCaT cells was extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany). The viable HaCaT cells were harvested with density centrifugation using 40% Percoll (Sigma-Aldrich, St. Louis, MO, USA) and used for RNA extraction. The mRNA level of CCL22 (encoding MDC), CCL17 (encoding TARC), IL5, IL13, FLG, LOR, IL25, TSLP, and IL33 in cytokine-stimulated HaCaT cells was evaluated by real-time PCR and compared with the expression profiles examined in the lesional skin of AD patients. Primer sequences are listed in Table 2.
Table 2. Primer sequences for real-time PCR.
| Target gene | Primer sequence |
|---|---|
| CCL22 | Forward: 5′-GAA GCC TGT GCC AAC TCT CT-3′ |
| Reverse: 5′-GGG AAT CGC TGA TGG GAA CA-3′ | |
| CCL17 | Forward: 5′-CGG ACC CCA ACA ACA AGA GA-3′ |
| Reverse: 5′-CTC CCT CAC TGT GGC TCT TC-3′ | |
| IL5 | Forward: 5′-TCT ACT CAT CGA ACT CTG CTG A-3′ |
| Reverse: 5′-CCC TTG CAC AGT TTG ACT CTC-3′ | |
| IL13 | Forward: 5′-TGT TTG TCA CCG TTG GGG AT-3′ |
| Reverse: 5′-TGA GTC TCT GAA CCC TTG GC-3′ | |
| FLG | Forward: 5′-TGA AGC CTA TGA CAC CAC TGA-3′ |
| Reverse: 5′-TCC CCT ACG CTT TCT TGT CCT-3′ | |
| LOR | Forward: 5′-GAG GTG TTT TCC AGG GGC A-3′ |
| Reverse: 5′-TGG GGT TGG GAG GTA GTT GTA-3′ | |
| IL33 | Forward: 5′-TGT CAC ATT GGG CAA AGT T-3′ |
| Reverse: 5′-CAG TAA GCA GTG TTA TCA GGA A-3′ | |
| IL25 | Forward: 5′- TTG TTT GTT TAC TCA TCA CTC AG-3′ |
| Reverse: 5′- TCC TCC TCA GAA TCA TCC A-3′ | |
| TSLP | Forward: 5′-TCC TCT GAA GAC CTG ACC-3′ |
| Reverse: 5′-TCT CCT TTC TCC CTA ATC CTC-3′ | |
| GAPDH | Forward: 5′-CTG GGC TAC ACT GAG CAC C-3′ |
| Reverse: 5′-AAG TGG TCG TTG AGG GCA ATG-3′ |
Statistical analysis
The data are presented as the mean±standard error of the mean (SEM). All data are representatives of 2 or more independent experiments. When necessary, a 2-group comparison was performed using a Student's t-test. A p-value <0.05 was considered statistically significant.
RESULTS
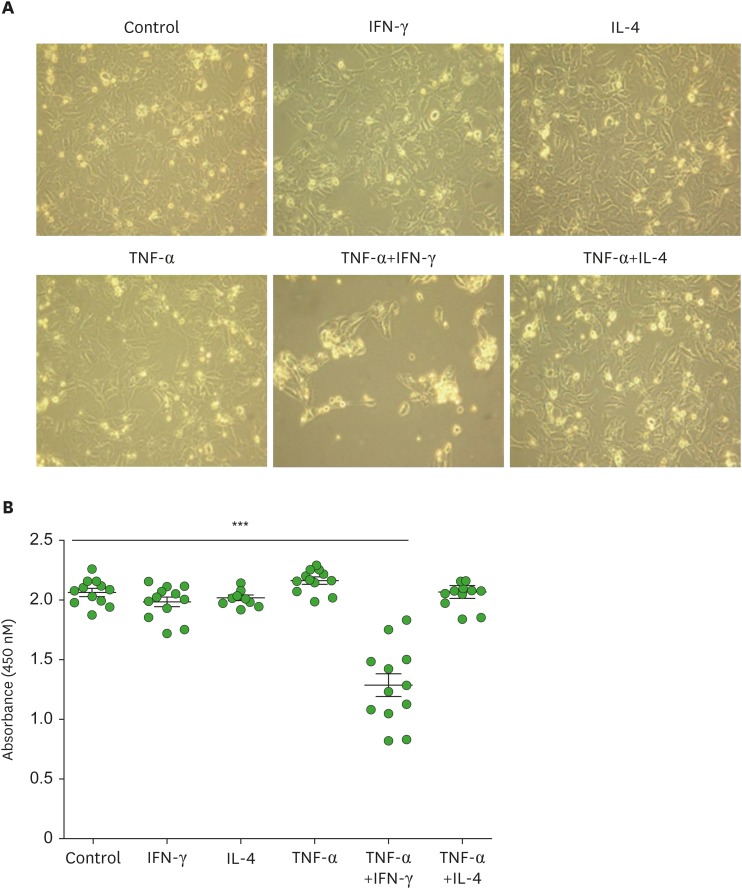
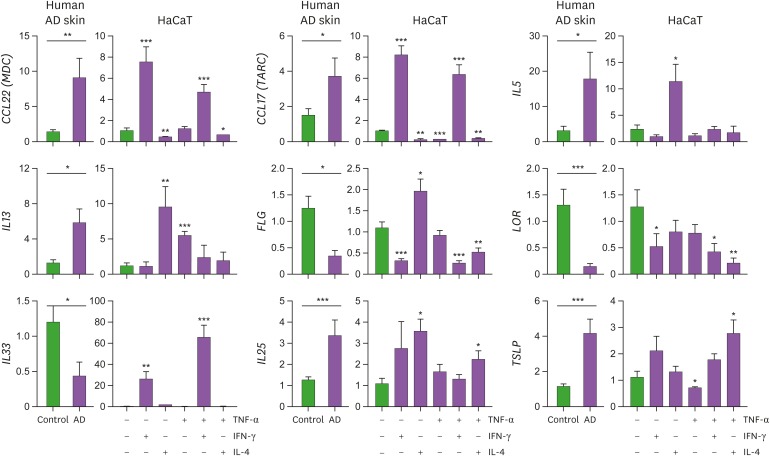
The viability of HaCaT cells was unaltered following treatment with TNF-α, IFN-γ, or IL-4 alone or the combination of TNF-α and IL-4 (Fig. 1). Moreover, combinatorial treatment of IFN-γ and IL-4 did not affect the viability of HaCaT cells (data now shown). However, treatment of HaCaT cells with the combination of TNF-α and IFN-γ resulted in a significant reduction in the cell viability (Fig. 1). This decrease in the viability of cells was likely attributable to the synergistic cytotoxic effects of IFN-γ and TNF-α. Next, we compared the gene expression profiles of HaCaT cells stimulated with various combinations of cytokines with those observed in human AD skin samples (Fig. 2). Consistent with previous reports (1,2,5,7), the expression of genes encoding Th2 chemokines (CCL22 and CCL17) and Th2 cytokines (IL5, IL13, IL25, and TSLP) was increased in human AD skin lesions, while the expression of genes related to the cornified cell envelope (FLG and LOR) was reduced. The expression of IL-33, a recently described Th2-linked cytokine (12), was significantly decreased in AD skin samples. The gene expression profiles observed for HaCaT cells were similar upon their stimulation with TNF-α and/or IFN-γ but not IL-4. Treatment with IFN-γ alone or TNF-α and IFN-γ combination augmented the expression of CCL22 and CCL17 and decreased the expression of FLG and LOR. Expression of IL-25 and TSLP was increased by combined stimulation with IL-4 and TNF-α. IL-4 treatment inhibited the expression of CCL22 and CCL17 induced by TNF-α and IFN-γ in HaCaT cells, as previously reported (13,14). Furthermore, FLG expression increased upon treatment of HaCaT cells with IL-4 alone but decreased in the presence of the combination of IL-4 and TNF-α. These results imply that the innate and Th1 inflammatory cytokines are required to reproduce AD-like features in HaCaT cells. Stimulation of HaCaT cells with TNF-α and IFN-γ significantly upregulated the expression of IL33 gene. The expression of classical Th2 cytokines IL-5, IL-13, and IL-25 was markedly upregulated in HaCaT cells following treatment with IL-4.
Figure 1.
Assessment of HaCaT cell viability following their stimulation with various combinations of Th1 (IFN-γ, 10 ng/ml or TNF-α, 10 ng/ml) and Th2 (IL-4, 50 ng/ml) cytokines for 24 hours. (A) Evaluation of the morphological changes in cytokine-stimulated HaCaT cells using phase-contrast microscopy. Original magnification ×20. (B) The effects of cytokine stimulation on the growth of HaCaT cells were measured using a water-soluble tetrazolium salt assay and the cell viability was determined by measuring the absorbance at 450 nm wavelength. Graphs show the mean±standard error of the mean.
***p<0.001 (Student's t-test).
Figure 2.
Correlation between AD-related gene expression in lesional skin and that observed in cytokine-stimulated HaCaT cells. HaCaT cells were cultured in the presence of IFN-γ (10 ng/ml), TNF-α (10 ng/ml), and/or IL-4 (50 ng/ml) for 24 hours. The expression of CCL22 (encoding macrophage-derived chemokine), CCL17 (encoding thymus and activation-regulated chemokine), IL5, IL13, FLG (filaggrin), LOR (loricrin), IL33, IL25, and TSLP was evaluated by real-time PCR as a fold change normalized to the expression of GAPDH in skin samples from AD patients (left) and cytokine-stimulated HaCaT cells (right). mRNA expression of each gene in cytokine-stimulated HaCaT cells was compared with non-stimulated control. Graphs show the mean±standard error of the mean.
MDC, macrophage-derived chemokine.
*p<0.05, **p<0.01, ***p<0.001 (Student's t-test).
DISCUSSION
AD is a complex inflammatory skin condition, which is incompletely understood. Epidermal barrier defects and dysregulated Th2 immune responses have crucial roles in the pathogenesis of AD. However, it has been reported that human keratinocytes are more sensitive to Th1-derived lymphocytes than those derived from Th2 in terms of chemokine release (15). In addition, IL-4 influences Th1-type responses, including antigen-induced arthritis, autoimmune uveoretinitis, and T cell transfer model of colitis (16,17,18). TNF-α along with Th2 cytokines plays an important role to induce AD-like features in epidermal differentiation proteins (19). Our observations have indicated that innate and Th1-type cytokines induce pathogenic changes implicated in the development of AD in HaCaT cells. Although primary human epidermal keratinocytes could be a primary choice to study molecular characteristics of skin, immortalized keratinocytes such as HaCaT cells may be an alternative model, considering their usefulness for in vitro assays (20). HaCaT cells stimulated with cytokines successfully reproduced inflammatory changes, except for IL-33 expression, observed in skin lesions of AD patients (Fig. 2). IL-33, IL-25, and TSLP are known to drive type 2 innate lymphoid cell response (3,4). Despite their similarities, IL-25 and IL-33 have distinct features. IL-25 is constitutively expressed in cellular compartments of epithelial cells and released upon exposure to allergen proteases, whereas IL-33 is a nuclear protein, which has regulatory proteins in its nuclear localization (21), and acts as an alarming signal once it is released by damaged epithelial cells (22). Because the number of mast cells is also increased in AD, overproduction of mast cell chymase could degrade IL-33 and affect its balance in AD (23). In addition, IFN-γ is another key molecule which is reported to upregulate IL-33 levels in keratinocytes of AD patients (9,24). Since most AD patients in this study exhibited chronic disease with recent exacerbation and decreased IFNG expression (data not shown), it is plausible that disease status affects the cytokine expression profiles in the lesion of AD. Therefore, cytokine-stimulated HaCaT cell line could be an appropriate tool for the demonstration of mixed chronic and aggravated status of AD. This hypothesis is further supported by the remarkable decrease in cell viability of HaCaT cells upon stimulation with TNF-α and IFN-γ (Fig. 1) and increased expression of IL-33 from damaged HaCaT cells. Taken together, our data suggest that Th1 and Th2 cytokines do not function dichotomously and that a complicated inflammatory network drives AD-like changes. Therefore, future in vitro experiments using HaCaT cells should employ various cytokine combinations beyond Th2 polarization to observe optimal expression of AD-related genes.
ACKNOWLEDGEMENTS
The authors thank Eun-Hui Lee and Jinsun Jang (Gachon University, Korea) for their technical assistance. This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A1A09916492).
Abbreviations
- AD
atopic dermatitis
- FLG
filaggrin
- IFN
interferon
- LOR
loricrin
- Th
T helper
- TNF
tumor necrosis factor
- TSLP
thymic stromal lymphopoietin
Footnotes
Conflict of Interest: The authors have no conflict of interest to declare.
Author Contributions: Conceptualization: Kim HJ, Jung Y; Data curation: Kim HJ, Baek J, Lee JR; Formal analysis: Kim HJ, Roh JY, Jung Y; Supervision: Roh JY, Jung Y; Writing - original draft: Kim HJ; Writing - review & editing: Roh JY, Jung Y.
References
- 1.Hamid Q, Naseer T, Minshall EM, Song YL, Boguniewicz M, Leung DY. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol. 1996;98:225–231. doi: 10.1016/s0091-6749(96)70246-4. [DOI] [PubMed] [Google Scholar]
- 2.Leung DY. Pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 1999;104:S99–S108. doi: 10.1016/s0091-6749(99)70051-5. [DOI] [PubMed] [Google Scholar]
- 3.Bartemes KR, Kita H. Dynamic role of epithelium-derived cytokines in asthma. Clin Immunol. 2012;143:222–235. doi: 10.1016/j.clim.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang LC, Johnson D, Scanlon ST, McKenzie AN, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grewe M, Gyufko K, Schöpf E, Krutmann J. Lesional expression of interferon-gamma in atopic eczema. Lancet. 1994;343:25–26. doi: 10.1016/s0140-6736(94)90879-6. [DOI] [PubMed] [Google Scholar]
- 6.Shimada Y, Takehara K, Sato S. Both Th2 and Th1 chemokines (TARC/CCL17, MDC/CCL22, and Mig/CXCL9) are elevated in sera from patients with atopic dermatitis. J Dermatol Sci. 2004;34:201–208. doi: 10.1016/j.jdermsci.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Guttman-Yassky E, Suárez-Fariñas M, Chiricozzi A, Nograles KE, Shemer A, Fuentes-Duculan J, Cardinale I, Lin P, Bergman R, Bowcock AM, et al. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J Allergy Clin Immunol. 2009;124:1235–1244.e58. doi: 10.1016/j.jaci.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Kim BE, Howell MD, Guttman-Yassky E, Gilleaudeau PM, Cardinale IR, Boguniewicz M, Krueger JG, Leung DY. TNF-α downregulates filaggrin and loricrin through c-Jun N-terminal kinase: role for TNF-α antagonists to improve skin barrier. J Invest Dermatol. 2011;131:1272–1279. doi: 10.1038/jid.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savinko T, Matikainen S, Saarialho-Kere U, Lehto M, Wang G, Lehtimäki S, Karisola P, Reunala T, Wolff H, Lauerma A, et al. IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J Invest Dermatol. 2012;132:1392–1400. doi: 10.1038/jid.2011.446. [DOI] [PubMed] [Google Scholar]
- 10.Omori-Miyake M, Yamashita M, Tsunemi Y, Kawashima M, Yagi J. In vitro assessment of IL-4- or IL-13-mediated changes in the structural components of keratinocytes in mice and humans. J Invest Dermatol. 2014;134:1342–1350. doi: 10.1038/jid.2013.503. [DOI] [PubMed] [Google Scholar]
- 11.Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, DeBenedetto A, Schneider L, Beck LA, Barnes KC, Leung DY. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2009;124:R7–R12. doi: 10.1016/j.jaci.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Kakinuma T, Nakamura K, Wakugawa M, Yano S, Saeki H, Torii H, Komine M, Asahina A, Tamaki K. IL-4, but not IL-13, modulates TARC (thymus and activation-regulated chemokine)/CCL17 and IP-10 (interferon-induced protein of 10kDA)/CXCL10 release by TNF-alpha and IFN-gamma in HaCaT cell line. Cytokine. 2002;20:1–6. doi: 10.1006/cyto.2002.1965. [DOI] [PubMed] [Google Scholar]
- 14.Xiao T, Kagami S, Saeki H, Sugaya M, Kakinuma T, Fujita H, Yano S, Mitsui H, Torii H, Komine M, et al. Both IL-4 and IL-13 inhibit the TNF-alpha and IFN-gamma enhanced MDC production in a human keratinocyte cell line, HaCaT cells. J Dermatol Sci. 2003;31:111–117. doi: 10.1016/s0923-1811(02)00149-4. [DOI] [PubMed] [Google Scholar]
- 15.Albanesi C, Scarponi C, Sebastiani S, Cavani A, Federici M, Sozzani S, Girolomoni G. A cytokine-to-chemokine axis between T lymphocytes and keratinocytes can favor Th1 cell accumulation in chronic inflammatory skin diseases. J Leukoc Biol. 2001;70:617–623. [PubMed] [Google Scholar]
- 16.Fort M, Lesley R, Davidson N, Menon S, Brombacher F, Leach M, Rennick D. IL-4 exacerbates disease in a Th1 cell transfer model of colitis. J Immunol. 2001;166:2793–2800. doi: 10.4049/jimmunol.166.4.2793. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs MJ, van den Hoek AE, van Lent PL, van de Loo FA, van de Putte LB, van den Berg WB. Role of IL-2 and IL-4 in exacerbations of murine antigen-induced arthritis. Immunology. 1994;83:390–396. [PMC free article] [PubMed] [Google Scholar]
- 18.Ramanathan S, de Kozak Y, Saoudi A, Goureau O, Van der Meide PH, Druet P, Bellon B. Recombinant IL-4 aggravates experimental autoimmune uveoretinitis in rats. J Immunol. 1996;157:2209–2215. [PubMed] [Google Scholar]
- 19.Danso MO, van Drongelen V, Mulder A, van Esch J, Scott H, van Smeden J, El Ghalbzouri A, Bouwstra JA. TNF-α and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J Invest Dermatol. 2014;134:1941–1950. doi: 10.1038/jid.2014.83. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann B. HaCaT cell line as a model system for vitamin D3 metabolism in human skin. J Invest Dermatol. 1997;108:78–82. doi: 10.1111/1523-1747.ep12285640. [DOI] [PubMed] [Google Scholar]
- 21.Divekar R, Kita H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr Opin Allergy Clin Immunol. 2015;15:98–103. doi: 10.1097/ACI.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cevikbas F, Steinhoff M. IL-33: a novel danger signal system in atopic dermatitis. J Invest Dermatol. 2012;132:1326–1329. doi: 10.1038/jid.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy A, Ganesh G, Sippola H, Bolin S, Sawesi O, Dagälv A, Schlenner SM, Feyerabend T, Rodewald HR, Kjellén L, et al. Mast cell chymase degrades the alarmins heat shock protein 70, biglycan, HMGB1, and interleukin-33 (IL-33) and limits danger-induced inflammation. J Biol Chem. 2014;289:237–250. doi: 10.1074/jbc.M112.435156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seltmann J, Werfel T, Wittmann M. Evidence for a regulatory loop between IFN-γ and IL-33 in skin inflammation. Exp Dermatol. 2013;22:102–107. doi: 10.1111/exd.12076. [DOI] [PubMed] [Google Scholar]