Abstract
Most of the cholesterol in plasma is in an esterified form that is generated in potentially cardioprotective HDLs. Cholesteryl ester transfer protein (CETP) mediates bidirectional transfers of cholesteryl esters (CEs) and triglycerides (TGs) between plasma lipoproteins. Because CE originates in HDLs and TG enters the plasma as a component of VLDLs, activity of CETP results in a net mass transfer of CE from HDLs to VLDLs and LDLs, and of TG from VLDLs to LDLs and HDLs. As inhibition of CETP activity increases the concentration of HDL-cholesterol and decreases the concentration of VLDL- and LDL-cholesterol, it has the potential to reduce atherosclerotic CVD. This has led to the development of anti-CETP neutralizing monoclonal antibodies, vaccines, and antisense oligonucleotides. Small molecule inhibitors of CETP have also been developed and four of them have been studied in large scale cardiovascular clinical outcome trials. This review describes the structure of CETP and its mechanism of action. Details of its regulation and nonlipid transporting functions are discussed, and the results of the large scale clinical outcome trials of small molecule CETP inhibitors are summarized.
Keywords: lipid transfers, atherosclerosis, lipoproteins
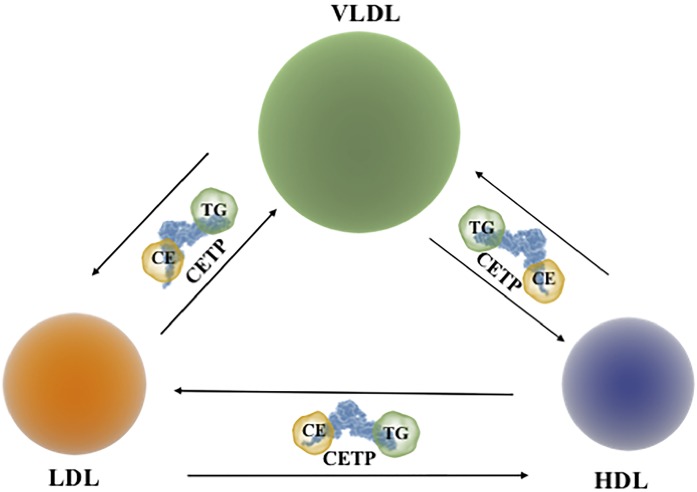
Cholesteryl ester transfer protein (CETP) is a hydrophobic glycoprotein that is present in the plasma of humans, nonhuman primates, rabbits, and hamsters, but not in most other animal species (1). It is a 74 kDa member of the lipid transfer protein/lipopolysaccharide binding protein (LTP/LBP) gene family (2). CETP mediates bidirectional transfers (and thus an equilibration) of cholesteryl esters (CEs) and triglycerides (TGs) between plasma lipoprotein particles (3). Because most of the CE originates in the HDL fraction in a reaction catalyzed by the enzyme, LCAT, and most of the TG enters plasma as a component of VLDLs, activity of CETP results in a net mass transfer of CE from HDLs to VLDLs and LDLs (Fig. 1). Activity of CETP also results in a net mass transfer of TG from VLDLs to LDLs and HDLs (Fig. 1).
Fig. 1.
CETP-mediated transfers of neutral lipids between different lipoproteins. CETP transfers CEs and TGs between HDLs, VLDLs, and LDLs.
Inhibition of CETP activity reduces these lipid transfers and thus increases the concentration of HDL CE and decreases the concentration of CE in VLDLs and LDLs. The concentration of HDL-cholesterol (HDL-C) is a negative risk factor for atherosclerotic CVD (ASCVD), while the concentration of cholesterol in the non-HDL fractions is a positive risk factor. As humans that are CETP deficient have high plasma HDL-C levels and decreased non-HDL-C levels and are reported to be at decreased risk of developing ASCVD, it follows that inhibiting the activity of CETP may translate into a reduction in cardiovascular risk. Several approaches that inhibit CETP activity and increase plasma HDL-C levels have been proposed and tested. However, as inhibition of CETP activity also decreases apoB and non-HDL-C levels, any reduction in ASCVD risk that is mediated by these agents cannot be attributed to an increase in HDL-C levels alone.
Approaches for inhibiting CETP include anti-CETP neutralizing antibodies (4–8), antisense CETP oligonucleotides (9), and an anti-CETP vaccine (10, 11). The anti-CETP vaccine reduced atherosclerosis in the New Zealand White rabbits (10) and was effective in a phase I clinical trial in humans (11), but has not proceeded to clinical development. The neutralizing antibodies and the antisense oligonucleotide also did not proceed to clinical development.
Several small molecule CETP inhibitors that reduce atherosclerosis in animal models extremely effectively have also been developed (12, 13). Four of these inhibitors (torcetrapib, dalcetrapib, evacetrapib, and anacetrapib) have been evaluated in large-scale randomized cardiovascular clinical outcome trials. While the trials with torcetrapib, dalcetrapib, and evacetrapib failed to show any cardiovascular benefit of CETP inhibition, treatment with anacetrapib significantly decreased major coronary events (14). However, as the manufacturers of anacetrapib recently decided to suspend development of the drug, the future of CETP inhibition as a potential therapeutic option for reducing major cardiovascular events is currently uncertain.
This review is concerned with the structure, function, and regulation of CETP and its inhibitors. It also outlines functions of CETP that are distinct from its lipid transfer activities, summarizes preclinical studies of CETP inhibition in animal models, and presents details of the outcomes of the randomized clinical outcome trials of the aforementioned small molecule CETP inhibitors.
STRUCTURE OF CETP
The structure of CETP has been the focus of numerous investigations. The LTP/LBP gene family, of which CETP is a member, includes several proteins, such as LBP, bactericidal permeability-increasing protein (BPI), and phospholipid transfer protein (PLTP), all of which have a high degree of structural similarity (15). Early structural models of CETP that were based on the crystal structure of BPI (2) identified CETP as a boomerang-shaped molecule with a hydrophobic lipid binding pocket at each end of the concave side (16, 17). CETP is a highly flexible molecule that undergoes a twisting motion when it binds to neutral lipids. This rotating motion enables CETP to bind to the surface of lipoproteins that vary widely in size and surface curvature and is a very important aspect of its mechanism of action.
The assumption of a “boomerang” structure for CETP was confirmed by Qiu et al. (18) who reported the first crystal structure of CETP at 3.5 Å resolution. That study identified the presence of a continuous central tunnel within the CETP molecule, which is unique among members of the LTP/LBP gene family. Two lipid binding pockets in the N- and C-terminal domains, and an amphipathic helix, helix X, located in the C-terminal domain of CETP have also been reported (18, 19). The central CETP tunnel can accommodate two CE molecules, one CE and one TG molecule, or two TG molecules (18). These structural features of CETP have been confirmed in atomistic and coarse-grained simulation studies (20), and by cryo-electron microscopy (21). Evidence that structural integrity of the central CETP tunnel is essential for the transfer activity of CETP was established by mutating selected polar amino acid residues that are located in the tunnel into hydrophobic residues. This altered the tunnel architecture and reduced the transfer activity of CETP (18).
MECHANISM OF ACTION OF CETP
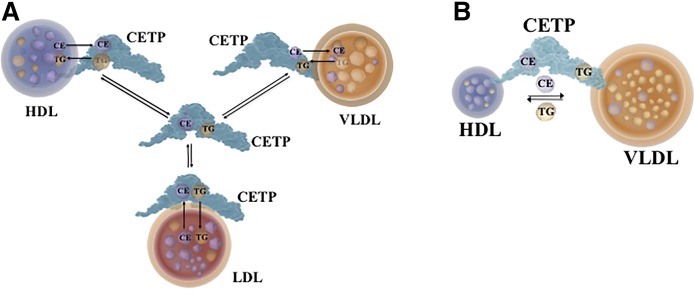
CETP transfers CE and TG between different lipoproteins by two mechanisms (Fig. 2). The first mechanism is a “shuttle” process (Fig. 2A) that involves random collisions of CETP with HDLs, LDLs, and VLDLs. This leads to the formation of complexes that facilitate bidirectional exchanges of CE and TG between each of the lipoproteins and CETP. The complexes subsequently dissociate from the lipoproteins where they were generated, and remain in the circulation until they randomly collide with another lipoprotein and participate in a further round of CE and TG exchanges. This process is repeated multiple times (3, 4). The crystal structure of CETP supports the shuttle mechanism and is consistent with the interaction of CETP with only one lipoprotein particle at a time (18).
Fig. 2.
Mechanism of action of CETP. CETP transfers CEs and TGs between HDLs and other lipoproteins by a shuttle mechanism (A) or by forming a bridging complex between HDL and another lipoprotein (VLDL is shown) (B).
The second mechanism of action of CETP involves the formation of a bridge between CETP and two lipoprotein particles to form a ternary complex (Fig. 2B) (21, 22). Neutral lipids move in both directions between the two lipoproteins through the tunnel in CETP. Evidence consistent with ternary complex formation comes from cryo-electron microscopy studies with anti-CETP polyclonal antibodies and atomistic molecular dynamics simulations (21). The results of these studies support the penetration of the N-terminal domain of CETP into the surface of an HDL particle together with a concomitant interaction of the C-terminal domain of CETP with an LDL or VLDL particle. Additional analyses have indicated that the transfer of CEs between HDLs and LDLs, or HDLs and VLDLs, by this mechanism is dependent on conformational changes in the N- and the C-terminal domains of CETP that increase tunnel continuity and improve neutral lipid accessibility (19, 21).
However, it should be noted that these observations are not supported by other electron microscopy studies in which HDLs were shown to bind to the N- as well as the C-terminal domain of CETP (23). It should be noted, however, that no interactions of CETP with LDLs, or formation of HDL-CETP-LDL complexes, were observed in that study, and that monoclonal antibodies targeted toward the N- and C-terminal domains of CETP did not prevent the penetration of CETP into the HDL surface or affect CETP activity (23). When taken together, these findings do not support the formation of a ternary complex as a major mechanism of action of CETP. There are, by contrast, multiple reports of anti-CETP antibodies inhibiting CETP-mediated transfers of CE and TG between HDLs and other lipoproteins (5, 24). These discrepant findings highlight a potential dependency of CETP-mediated neutral lipid transfers on the antibodies that are used to target the N- and C-terminal domains of the CETP molecule. For example, Zhang et al. (21) used polyclonal antibodies that recognized a large area of the CETP molecule, whereas the more recent studies of Lauer et al. (23) were undertaken with monoclonal antibodies that recognize specific epitopes within the protein.
REGULATION OF CETP
CETP gene transcription
Transcription of the CETP gene is under the control of extrinsic and intrinsic factors. For example, dietary cholesterol upregulates CETP expression in mice transgenic for human CETP (25–27). Plasma cholesterol levels also correlate with CETP mass in human plasma (28). Studies of transgenic mice have established that induction of human CETP gene expression in response to cholesterol is a consequence of transactivation of a nuclear receptor binding site in the promoter region of the gene by the transcription factors, liver X receptor (LXR) and retinoid X receptor (29, 30). These results are supported by studies of LXR agonists that increase CETP expression in mice transgenic for human CETP, and in mice with LXRα deficiency in which CETP expression is not increased by administration of an LXR agonist (31). The human CETP gene is also regulated by SREBP-1, a transcription factor that transactivates sterol regulatory-like elements in the promoter region of the gene (32).
Lifestyle factors
Light to moderate, but not heavy, alcohol consumption is generally considered to decrease CETP mass and activity, increase HDL-C levels, and decrease CVD risk. However, investigations into this relationship have produced conflicting results. Some investigators have confirmed the association (33), while others have found that the alcohol-mediated increase in HDL-C levels is independent of CETP activity (34, 35) and unrelated to effects on genes that regulate HDL levels (36).
Physical activity in the form of endurance exercise also increases HDL-C levels, decreases plasma CETP levels, and reduces CVD risk in humans (37). However, aerobic exercise has been reported not to affect CETP activity in mice transgenic for the human CETP gene (38) or plasma CETP levels in humans (39, 40).
HUMAN GENETIC STUDIES
Loss-of-function mutations in the CETP gene (CETP deficiency)
The first report of a loss-of-function mutation in the CETP gene was in a Japanese population with a G-to-A substitution in the 5′-splice donor site of intron 14 (Int 14A) (41). Homozygosity for this mutation is associated with very low or undetectable CETP activity, markedly elevated plasma HDL-C, apoA-I, and apoE levels, a moderate reduction in VLDL-cholesterol, LDL-cholesterol (LDL-C), and apoB levels, a low incidence of atherosclerosis, and increased life span compared with unaffected family members (41, 42). HDLs isolated from people homozygous for this mutation, as well as compound heterozygotes, also have HDLs that are larger than the HDLs in unaffected individuals (41, 43). In addition, people with CETP deficiency have LDLs that are small and polydisperse relative to people with a normal level of CETP activity (44).
Several other mutations associated with CETP deficiency have been reported (45–47). A missense mutation of Asp to Gly at codon 442 in exon 15 of the CETP gene (Asp442Gly) that is associated with abnormally high levels of HDL-C has been reported in the Japanese population and in Japanese Americans (48, 49). People homozygous for a nonsense mutation in the CETP gene at codon 309 in exon 10 and a G-to-T substitution at codon 181 of exon 6 (G181X) have elevated plasma concentrations of HDL-C and apoA-I (45, 46). A nonsense T-to-G mutation at codon 57 of exon 2 that is associated with high HDL-C levels has also been reported (47).
Human CETP gene polymorphisms
Results from small studies of CETP gene polymorphisms in humans have not been conclusive. The results of larger genetic studies are, however, more consistent and have led to the conclusion that CETP is pro-atherogenic and that its inhibition is potentially anti-atherogenic.
In a large meta-analysis of 92 studies involving 113,833 participants, it was concluded that CETP gene polymorphisms that are associated with decreased CETP activity and mass are associated with high HDL-C levels, low LDL-C levels, and a significantly decreased risk of having a coronary event (50). A similar conclusion emerged from a study of 18,245 healthy Americans in the Women’s Genome Health Study, where 20 SNPs in the CETP gene that had genome-wide effects on HDL-C levels were identified (51). In particular, the Taq1B polymorphism at rs708272 in the CETP gene was associated with a per-allele increase in HDL-C levels of 3.1 mg/dl and a 24% lower risk of future myocardial infarction (51). This conclusion was further supported by another meta-analysis in which a common variant in the CETP gene was accompanied by increased HDL-C levels, decreased LDL-C levels, and a reduced risk of myocardial infarction comparable to that reported in the earlier meta-analysis (52).
Perhaps the most compelling genetic evidence in favor of CETP activity being pro-atherogenic comes from the Copenhagen City Heart Study (53) and from a study that examined the effect of protein-truncating variants of the CETP gene (54). In the Copenhagen City Heart Study, 10,261 people were followed for up to 34 years (53). More than 3,000 of these people had a cardiovascular event and 3,807 died. In this study, two common CETP gene polymorphisms known to be associated with low CETP activity were also associated with significant reductions in the risk of ischemic heart disease, myocardial infarction, ischemic cerebrovascular disease, and ischemic stroke. People with these polymorphisms also had increased longevity, with no evidence of adverse effects.
In a study of protein-truncating variants in the CETP gene, it was found that the HDL-C level was 22.6 mg/dl higher and the LDL-C level was 12.2 mg/dl lower than in those without the variants (54). These lipoprotein changes were accompanied by a significant 30% lower risk of having a coronary event (54). Collectively, these human genetic studies support the proposition that CETP inhibition is potentially anti-atherogenic.
CETP AND ANIMAL MODELS OF ATHEROSCLEROSIS
CETP exists in the plasma of only few species, including humans, rabbits, and hamsters, but not in rodents, which have a low susceptibility to atherosclerotic lesion development (1). As mice are naturally deficient in CETP, rendering them transgenic with the human CETP gene means that studies can be undertaken in the absence of the confounding effects of endogenous CETP activity. However, the results from mice transgenic for CETP are model-dependent, with some studies suggesting that CETP is pro-atherogenic (55–57). Other studies in mice transgenic for human CETP and LCAT, by contrast, have indicated that CETP is anti-atherogenic (58). An anti-atherogenic role for CETP has also been suggested for the db/db mouse model of type 2 diabetes when it is made transgenic for human CETP (59), and for hypertriglyceridemic CETP transgenic mice (60). In contrast to mice, rabbits have approximately twice as much CETP activity in their plasma as humans (1) and they are very susceptible to diet-induced atherosclerosis (61), which is reduced by inhibiting CETP (12).
NON-LIPID TRANSPORT FUNCTIONS OF CETP
CETP and innate immunity
From the available evidence, CETP appears to have a beneficial role in reducing the inflammatory response to bacterial endotoxins through interaction with the innate immune system and the sequestration of pro-inflammatory lipopolysaccharide (LPS). The innate immune system detects highly conserved components of micro-organisms, termed pathogen-associated molecular patterns (PAMPs), by pattern recognition receptors (PRRs), including members of the toll-like receptor (TLR) family. Detection of PAMPs by PRRs is the first line of defense against a nonself pathogen, which leads to activation of the innate immune system and a cascade of inflammatory responses (62, 63). If not effectively regulated, the inflammatory responses that are activated when PRRs detect PAMPs can result in sepsis with shock, end-organ damage, and, ultimately, death of the host. Beyond antimicrobial therapy, treatments for septic shock are limited (64).
LPS is a component of the gram-negative bacteria cell wall and the ligand for TLR4 (63, 65). It potently stimulates the innate immune system and is largely responsible for the septic response to gram-negative bacteremia (66). Effective sequestration and excretion of LPS is required to curtail the inflammatory response. LPS binds to circulating HDLs, LDLs, and VLDLs, making it unavailable for stimulation of the innate immune system (67–69). Following early cessation of the trial of the CETP inhibitor, torcetrapib, in the ILLUMINATE trial, the role of CETP in the immune response came under scrutiny because of an excess of deaths related to infection (70, 71). This was not an issue in cardiovascular clinical outcome trials of other CETP inhibitors.
Although CETP has an intrinsically weak ability to bind LPS compared with LBP or BPI (72), it is associated with resilience to sepsis. Mice transgenic for human CETP have improved mortality following LPS administration compared with wild-type mice (73, 74). This is likely due, at least in part, to an increase in LPS sequestration by HDLs and LDLs and increased uptake of LPS by the liver (73). Conversely, PTLP knockout mice have increased endotoxin-associated mortality, delayed uptake of LPS by lipoproteins, and decreased LPS clearance (75).
The mechanism of LPS clearance is not well-understood. CETP facilitates the transfer of LPS from HDLs to LDLs (73), and LDL receptor-mediated uptake of LDL-associated LPS by the liver has been reported (76). Hepatic uptake of HDL CEs by scavenger receptor B1 has also been implicated in LPS clearance (77).
In addition to facilitating LPS sequestration and excretion, mice expressing CETP and mice with the cecal ligation/puncture model of polymicrobial sepsi, have reduced production of the pro-inflammatory cytokines, TNF-α and interleukin (IL)-6, in response to LPS administration (73, 74). Furthermore, LPS decreases TNF-α production in macrophages from mice transgenic for human CETP relative to macrophages from wild-type mice (73). Incubation of RAW 264.7 murine macrophages with LPS and human CETP also induces a dose-dependent decrease in TNF-α production (73), possibly due to reduced expression of TLR4. TLR4 and IL-6 secretion are both reduced and survival is improved in mice transgenic for human CETP compared with wild-type mice following LPS administration (74). LPS-stimulated peritoneal macrophages from mice transgenic for human CETP also have reduced TLR4 expression, LPS uptake, nuclear factor (NF)-κB activation, and IL-6 production compared with peritoneal macrophages from wild-type mice (74). The reduced IL-6 production and increased resistance to sepsis in human CETP transgenic mice is consistent with evidence that IL-6 levels are correlated with the risk of death and that this is ameliorated by anti-IL-6 monoclonal antibody treatment in humans (78).
Reverse cholesterol transport, the process whereby excess cholesterol from peripheral tissues is transported to the liver for excretion, is also inhibited in sepsis (79), and CETP protein and mRNA levels are both decreased in hamsters and human CETP transgenic mice in response to LPS (80, 81). This is in line with the outcome of a small study in humans in which an association of increased mortality with the magnitude of CETP reduction in septic hospitalized patients was reported (82).
INHIBITION OF CETP
Targeted inhibition of CETP with neutralizing antibodies
Treatment of New Zealand White rabbits with neutralizing monoclonal antibodies to CETP increases HDL-C levels and reduces atherosclerosis (8). In another study of mice transgenic for human CETP, suppression of plasma CETP activity with an anti-CETP monoclonal antibody increased liver CETP mRNA levels. This unexpected finding was presumably due to increased plasma cholesterol levels in these mice (83). Anti-CETP monoclonal antibodies have also been shown to inhibit CETP activity by 70–80% and increase HDL-C levels by 33% in chow- and cholesterol-fed male Golden Syrian hamsters (84).
Targeted inhibition of CETP with antisense oligonucleotides
Antisense oligonucleotides to CETP that target and degrade CETP mRNA levels and decrease hepatic CETP protein levels have been reported to increase HDL-C levels by 32% and reduce atherosclerosis in cholesterol-fed Japanese White rabbits (9). Similarly, administration of antisense oligonucleotides to LDL receptor-deficient mice transgenic for human CETP inhibits CETP activity by 81% and increases plasma HDL-C levels by 38% (85). Enhanced macrophage reverse cholesterol transport and decreased accumulation of aortic cholesterol have also been reported in these mice relative to mice treated with a control antisense oligonucleotide (85).
Targeted inhibition of CETP with vaccines
Human CETP contains a hydrophobic 26 amino acid residue sequence in the C-terminal domain that is essential for neutral lipid transfer (5, 24). Immunization of cholesterol-fed New Zealand White rabbits with a peptide that targets this region of CETP generates neutralizing antibodies that inhibit CETP activity, increase plasma HDL-C levels, and decrease atherosclerotic lesion area (10). In another study, immunization of high-fat high-cholesterol-fed New Zealand White rabbits with a vaccine in which rabbit IgG-Fc was conjugated to a 26 amino acid C-terminal epitope of CETP increased plasma HDL-C and apoA-I levels. This vaccine also decreased the plasma level of oxidized LDL, as well as atherosclerosis and nonalcoholic hepatic steatosis, in New Zealand White rabbits (86, 87). A similar increase in plasma HDL-C levels and a reduction in atherosclerotic lesion area were observed in New Zealand White rabbits immunized subcutaneously with a heat shock protein-65-CETP vaccine (88). Intranasal administration with the combined heat shock protein-65-CETP vaccine also decreased atherosclerotic lesion area and serum total cholesterol and LDL-C levels, but did not affect TG or HDL-C levels in rabbits (89). Vaccination of cholesterol-fed New Zealand White rabbits with a tetanus toxoid-CETP peptide, by contrast, was associated with only a modest reduction in CETP activity, a modest increase in HDL-C levels, and no effect on atherosclerosis (10, 90). To date only one vaccine, CETi-1, has progressed to a phase I human clinical trial (11). Repeated administration of this vaccine in healthy adults generated variable anti-CETP antibody titers, and did not significantly inhibit CETP activity or increase plasma HDL-C levels (11).
Targeted inhibition of CETP with small molecule inhibitors
Use of small molecule inhibitors of CETP activity mimics the lipid profile changes that occur in humans and animals with CETP deficiency (12, 91). In most cases these inhibitors bind to and inactivate the CETP that is associated with HDLs (92). In doing so they prevent neutral lipid transfers between HDLs and TG-rich lipoproteins, including VLDLs. This results in the retention of CEs in HDLs (93). As discussed in detail below, four small molecule inhibitors that were developed to pharmacologically inhibit CETP activity have been tested in large cardiovascular clinical outcome trials.
SMALL MOLECULE CETP INHIBITORS
Torcetrapib
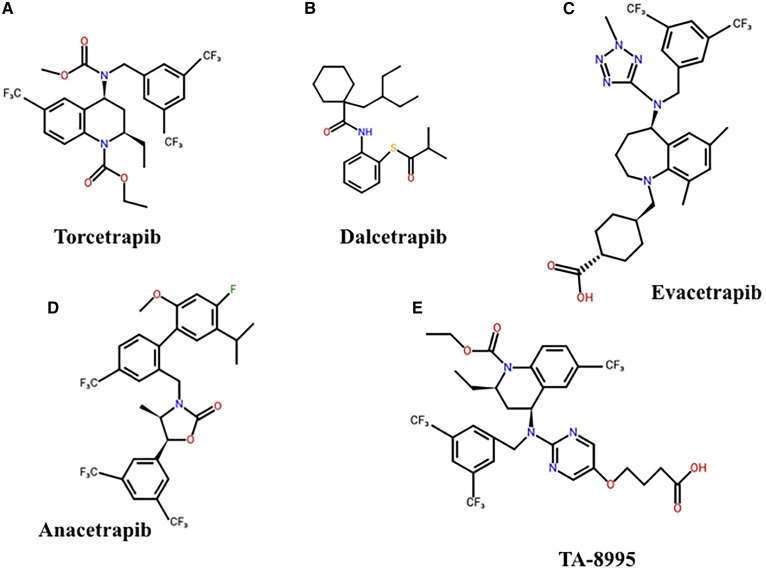
Torcetrapib is a small lipophilic tetrahydroquinoline derivative (Fig. 3A) that forms a tight complex between HDLs and CETP. This impedes the exchange of CE and TG between HDLs and other lipoproteins (94). Torcetrapib increases plasma HDL-C levels 3-fold and reduces atherosclerosis by 60% in cholesterol-fed rabbits (12). Treatment with torcetrapib also increases plasma HDL-C levels in hamsters, which, in turn, increases macrophage cholesterol efflux (95).
Fig. 3.
Structures of small molecule CETP inhibitors. Chemical structures for torcetrapib (A), dacetrapib (B), evacetrapib (C), anacetrapib (D), and TA-8995 (E) are shown.
Dalcetrapib
Dalcetrapib (formerly JTT-705) is a thioaniline inhibitor that binds to CETP via cysteine residue-13 (Fig. 3B) (96). It inhibits the transfer of CE between HDLs, VLDLs, and LDLs, but does not inhibit CE transfers between different HDL particles (96). Treatment of mildly hyperlipidemic cholesterol-fed rabbits with dalcetrapib increases HDL-C levels and significantly decreases atherosclerotic lesion progression (13). Dalcetrapib is, however, without effect on atherosclerosis in severely hypercholesterolemic rabbits (97).
Evacetrapib
Evacetrapib is a benzazepine-based CETP inhibitor (Fig. 3C) that dose dependently inhibits CETP activity and increases HDL-C levels by up to 130% in mice transgenic for human CETP and apoA-I (98, 99). It also increases macrophage-to-feces reverse cholesterol transport in CETP transgenic mice and improves the net efflux of cholesterol from macrophages to HDLs (100).
Anacetrapib
Anacetrapib is structurally similar to torcetrapib (Fig. 3D) (99). It also increases HDL-C levels, which leads to enhanced macrophage cholesterol efflux and reverse cholesterol transport in dyslipidemic hamsters (101). In a dose escalation study with APOE*3 Leiden.CETP transgenic mice, anacetrapib, either as a monotherapy or in combination with atorvastatin, promoted a dose-dependent increase in HDL-C levels, improved lesion stability, and reduced atherosclerosis (102).
Anacetrapib has a number of additional cardioprotective functions. Treatment of normocholesterolemic New Zealand White rabbits with endothelial denudation of the abdominal aorta with the anacetrapib analog, des-fluoro-anacetrapib, which has one less fluorine atom than the parent compound, improves endothelial repair and endothelial function (103), increases angiogenesis in New Zealand White rabbits with hind limb ischemia (104), and reduces neointimal hyperplasia in New Zealand White rabbits with endothelial denudation of the iliac artery and stent deployment (105).
RANDOMIZED CLINICAL TRIALS OF CETP INHIBITORS IN HUMANS
ILLUMINATE trial with torcetrapib
The ILLUMINATE trial (Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events) (ClinicalTrials.gov number NCT00134264) was performed in 15,067 high-risk statin-treated people randomized in a double-blind design to receive torcetrapib or placebo (70). The primary endpoint was the time to first occurrence of a major cardiovascular event, a composite that included four components: death from coronary heart disease (defined as fatal myocardial infarction excluding procedure-related events, fatal heart failure, sudden cardiac death, or other cardiac death), nonfatal myocardial infarction (excluding procedure-related events), stroke, and hospitalization for unstable angina. Treatment with torcetrapib increased HDL-C levels by 72% and decreased LDL-C levels by 25%. This trial was terminated after 18 months because of a statistically significant excess of deaths (93 vs. 59) in those treated with torcetrapib. There was also a statistically significant 25% increase in ASCVD events in the participants that received torcetrapib.
The explanation for the harm caused by torcetrapib is not known with certainty, but it may have been the consequence of serious off-target adverse effects of the drug (70), including increased blood pressure, increased synthesis and secretion of aldosterone, and an increase in endothelin-1 levels in the artery wall. Given these off-target effects of torcetrapib that are unrelated to CETP inhibition, it was not possible to draw conclusions from the ILLUMINATE trial regarding the potential cardiovascular benefits of CETP inhibition.
The dal-OUTCOMES trial with dalcetrapib
The dal-OUTCOMES trial (ClinicalTrials.gov number NCT00658515) included 15,871 participants recruited soon after an acute coronary syndrome (ACS) event. All patients were treated with a statin and were randomized in a double-blind design to receive either dalcetrapib or placebo (106). The primary end point was a composite of death from coronary heart disease, nonfatal myocardial infarction, ischemic stroke, unstable angina, or cardiac arrest with resuscitation. Treatment with dalcetrapib increased the concentration of HDL-C by about 30%, but its effect on LDL-C and apoB levels was minimal. Treatment with dalcetrapib did not reduce ASCVD events (106).
The absence of benefit in the dal-OUTCOMES trial may have been because dalcetrapib did not reduce the level of LDL-C. However, it may also have been because this trial was conducted in patients soon after an ACS event, at a time when HDL function is likely to be compromised. This explanation was supported by the observation in the placebo group in the dal-OUTCOMES trial in which the concentration of HDL-C was unrelated to the risk of having an ASCVD event (106). This is in contrast to what occurs in people with stable ASCVD, where there is an inverse relationship between HDL-C levels and the risk of having an ASCVD event. As was the case in the ILLUMINATE trial, dal-OUTCOMES did not test the hypothesis that CETP inhibition may reduce ASCVD events in people with stable coronary artery disease. As loss of the cardioprotective functions of HDLs after an ACS is likely to be temporary, it is possible that a meaningful reduction of cardiovascular events may have been observed in this trial if the median intervention had been extended beyond 31 months.
ACCELERATE trial with evacetrapib
The ACCELERATE (Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition with Evacetrapib in Patients at a High-Risk for Vascular Outcomes) trial (ClinicalTrials.gov number NCT01687998) included approximately 12,500 high-risk statin-treated patients randomized in a double-blind design to receive evacetrapib or placebo (107). The primary endpoint was the first occurrence of any component of the composite endpoint of cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization. The planned follow-up was 3 years. Evacetrapib reduced the level of LDL-C by 37% and increased HDL-C levels by 132% compared with placebo. This trial was terminated after just over 2 years when it became apparent that there would not be a positive outcome if it continued to its planned 3 year follow-up. There was no evidence that evacetrapib caused harm. The reason for the failure of evacetrapib to impact on the primary endpoint is not known, but it is possible that the trial was too short to detect benefit. If the REVEAL trial (see below) had stopped at the same time as ACCELERATE, a similar lack of efficacy would have been found, thus emphasizing the fact that cardiovascular events are unlikely to be decreased in the short term by interventions that increase plasma HDL-C levels and lower plasma LDL-C levels.
DEFINE trial with anacetrapib
The DEFINE (Determining the Efficacy and Tolerability of CETP Inhibition with Anacetrapib) trial (ClinicalTrials.gov number NCT00685776) was an 18 month intervention designed to assess the lipid efficacy and safety of anacetrapib. The trial included 1,623 high-risk statin-treated patients who were randomized in a double-blind design to receive anacetrapib or placebo (108). Anacetrapib decreased the concentration of non-HDL-C by 32% and increased HDL-C levels by 138%. Anacetrapib had no effect on blood pressure or on plasma electrolyte or aldosterone levels. DEFINE showed that treatment with anacetrapib had favorable effects on plasma lipid levels by decreasing LDL-C levels and increasing HDL-C levels. It also showed that anacetrapib had an acceptable side-effect profile and, within the limits of the power of the study, did not have any of the adverse effects that were observed with torcetrapib. In a long-term follow-up of participants in the DEFINE trial, it was found that anacetrapib accumulated in adipose tissue and remained detectable in the body for two or more years after the last dose of the drug (109). There was no evidence, however, that retention of anacetrapib was associated with adverse effects. Despite the tendency for anacetrapib to be retained in the body, a decision was made to proceed with the REVEAL trial.
REVEAL trial with anacetrapib
The REVEAL (Randomized Evaluation of the Effects of Anacetrapib through Lipid Modification) trial (ClinicalTrials.gov number NCT01252953) included more than 30,000 high-risk statin-treated people who were randomized to receive anacetrapib or placebo. The planned follow-up was 4 years (14). The primary endpoint was the first major coronary event, a composite of coronary death, myocardial infarction, or coronary revascularization.
The participants in REVEAL, who were treated intensively with atorvastatin prior to randomization, had a low baseline mean LDL-C level of 61 mg/dl, a mean non-HDL-C level of 92 mg/dl, and a mean HDL-C level of 40 mg/dl. Treatment with anacetrapib increased the level of HDL-C by 104% and decreased non-HDL-C levels by 18%. During the median 4.1 years of follow-up, the primary outcome was reduced from 11.8% in the placebo group to 10.8% in those treated with anacetrapib (rate ratio, 0.91; 95% confidence interval, 0.85–0.97; P = 0.004). The magnitude of this benefit was consistent with that observed for comparable reductions in non-HDL-C levels in statin trials (14). Participants with a baseline LDL-C level in the upper tertile (>66 mg/dl) had a statistically significant reduction in the primary endpoint of 13%. In those with a baseline non-HDL-C level in the upper tertile (>101 mg/dl), the reduction in the primary endpoint was a statistically significant 17%. There were no significant between-group differences in the risk of death, cancer, or other serious adverse events in this trial.
The reduction in coronary events in those treated with anacetrapib did not become apparent until after 2 years of treatment (14). During the third year, however, the reduction in coronary events was 13%, while coronary events occurring beyond 4 years of treatment were reduced by a statistically significant 17%. This delay in benefit highlights the possibility that the failure of evacetrapib to reduce cardiovascular events in ACCELERATE may have been related to termination of the trial after only 2 years of follow-up.
Treatment with anacetrapib in the REVEAL trial also reduced the risk of developing diabetes from 6.0% in those on statin alone to 5.3% in those treated with a statin plus anacetrapib. A positive effect of CETP inhibition on glycemic control (a reduction in plasma glucose levels and HbA1c) was also observed in participants in the torcetrapib arm of the ILLUMINATE trial (110) and in the evacetrapib arm of the ACCELERATE trial (111).
The mechanism by which CETP inhibition improves glycemic control and reduces the risk of new onset diabetes is uncertain, but may be related to the increased levels of HDL-C and apoA-I. Both HDLs and apoA-I increase the synthesis and secretion of insulin in pancreatic β cells (112, 113). They also enhance glucose uptake by skeletal muscle (114–116) and, thus, improve insulin sensitivity. An increase in either, or both, of these HDL functions in people treated with a CETP inhibitor could explain the improvement in glycemic control and decreased risk of developing diabetes that was observed in these studies.
NEW CETP INHIBITORS
TA-8995 is another novel tetrahydroquinoline derivative CETP inhibitor (Fig. 3E) (117). A phase I dose-escalating study of healthy subjects treated with single or multiple doses of TA-8995 (30–150 mg daily) or placebo, confirmed that TA-8995 is well-tolerated and does not adversely affect blood pressure, aldosterone levels, or serum electrolyte concentrations (117). In that study, TA-8995 inhibited CETP activity by 92–99%, increased HDL-C levels by 140%, and decreased LDL-C levels by 53% at the 10 mg/day dose (117).
In the 12 week randomized double-blind parallel-group phase II TULIP (TA-8995 in Patients with Mild Dyslipidaemia) trial (ClinicalTrials.gov number NCT01970215), subjects with mild dyslipidemia were randomly assigned to receive placebo, TA-8995 as monotherapy (1–10 mg/day), 10 mg/day TA-8995 with atorvastatin (20 mg) or with rosuvastatin (10 mg), or statin alone (118). TA-8995 dose-dependently inhibited CETP activity, increased HDL-C and apoA-I levels by up to 179% and 63%, respectively, and decreased LDL-C and apoB levels by 45% and 34%, respectively (118). In combination with atorvastatin, TA-8995 increased HDL-C levels by 152% and reduced LDL-C levels by 68%, while in combination with rosuvastatin, HDL-C levels increased by 157% and LDL-C levels decreased by 63% (118). This makes TA-8995 one of the most potent CETP inhibitors available to date (118, 119). Although TA-8995 did not affect plasma TG and total cholesterol levels, it did increase the ability of HDLs to promote cholesterol efflux at the 10 mg/day dose (111).
CONCLUSIONS
Our understanding of the structure and function of CETP has progressed rapidly in recent years. There is clear evidence from the REVEAL trial that inhibition of CETP significantly reduces the risk of having a coronary event in statin-treated patients. There is also evidence that CETP inhibition improves glycemic control and reduces new onset diabetes, an effect that has the potential to counteract the increase in new onset diabetes associated with statin treatment. There is thus a compelling case for using the combination of a statin plus a CETP inhibitor in people at high cardiovascular risk that are treated with a statin and are at risk of developing diabetes. This will not only reduce the risk of having a coronary event beyond that achieved by a statin alone, but it will also counteract the statin-induced development of diabetes. Whether new CETP inhibitors, such as TA-8995, will be investigated in outcome trials in this population remains to be seen. Further investigations are awaited with interest.
Footnotes
Abbreviations:
- ACS
- acute coronary syndrome
- ASCVD
- atherosclerotic CVD
- BPI
- bactericidal permeability-increasing protein
- CE
- cholesteryl ester
- CETP
- cholesteryl ester transfer protein
- HDL-C
- HDL-cholesterol
- IL
- interleukin
- LDL-C
- LDL-cholesterol
- LPS
- lipopolysaccharide
- LTP/LBP
- lipid transfer protein/lipopolysaccharide binding protein
- LXR
- liver X receptor
- PAMP
- pathogen-associated molecular pattern
- PRR
- pattern recognition receptor
- TG
- triglyceride
- TLR
- toll-like receptor
This work was supported by National Health and Medical Research Council Grant 1037903 to K-A.R. and P.J.B. Additional support was provided by an Australian Postgraduate Award to S.S.
REFERENCES
- 1.Ha Y. C., and Barter P. J.. 1982. Differences in plasma cholesteryl ester transfer activity in sixteen vertebrate species. Comp. Biochem. Physiol. B. 71: 265–269. [DOI] [PubMed] [Google Scholar]
- 2.Beamer L. J., Carroll S. F., and Eisenberg D.. 1997. Crystal structure of human BPI and two bound phospholipids at 2.4 angstrom resolution. Science. 276: 1861–1864. [DOI] [PubMed] [Google Scholar]
- 3.Barter P. J., Hopkins G. J., and Calvert G. D.. 1982. Transfers and exchanges of esterified cholesterol between plasma lipoproteins. Biochem. J. 208: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hesler C. B., Tall A. R., Swenson T. L., Weech P. K., Marcel Y. L., and Milne R. W.. 1988. Monoclonal antibodies to the Mr 74,000 cholesteryl ester transfer protein neutralize all of the cholesteryl ester and triglyceride transfer activities in human plasma. J. Biol. Chem. 263: 5020–5023. [PubMed] [Google Scholar]
- 5.Swenson T. L., Hesler C. B., Brown M. L., Quinet E., Trotta P. P., Haslanger M. F., Gaeta F. C., Marcel Y. L., Milne R. W., and Tall A. R.. 1989. Mechanism of cholesteryl ester transfer protein inhibition by a neutralizing monoclonal antibody and mapping of the monoclonal antibody epitope. J. Biol. Chem. 264: 14318–14326. [PubMed] [Google Scholar]
- 6.Yen F. T., Deckelbaum R. J., Mann C. J., Marcel Y. L., Milne R. W., and Tall A. R.. 1989. Inhibition of cholesteryl ester transfer protein activity by monoclonal antibody. Effects on cholesteryl ester formation and neutral lipid mass transfer in human plasma. J. Clin. Invest. 83: 2018–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukasawa M., Arai H., and Inoue K.. 1992. Establishment of anti-human cholesteryl ester transfer protein monoclonal antibodies and radioimmunoassaying of the level of cholesteryl ester transfer protein in human plasma. J. Biochem. 111: 696–698. [DOI] [PubMed] [Google Scholar]
- 8.Whitlock M. E., Swenson T. L., Ramakrishnan R., Leonard M. T., Marcel Y. L., Milne R. W., and Tall A. R.. 1989. Monoclonal antibody inhibition of cholesteryl ester transfer protein activity in the rabbit. Effects on lipoprotein composition and high density lipoprotein cholesteryl ester metabolism. J. Clin. Invest. 84: 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugano M., Makino N., Sawada S., Otsuka S., Watanabe M., Okamoto H., Kamada M., and Mizushima A.. 1998. Effect of antisense oligonucleotides against cholesteryl ester transfer protein on the development of atherosclerosis in cholesterol-fed rabbits. J. Biol. Chem. 273: 5033–5036. [DOI] [PubMed] [Google Scholar]
- 10.Rittershaus C. W., Miller D. P., Thomas L. J., Picard M. D., Honan C. M., Emmett C. D., Pettey C. L., Adari H., Hammond R. A., Beattie D. T., et al. 2000. Vaccine-induced antibodies inhibit CETP activity in vivo and reduce aortic lesions in a rabbit model of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 20: 2106–2112. [DOI] [PubMed] [Google Scholar]
- 11.Davidson M. H., Maki K., Umporowicz D., Wheeler A., Rittershaus C., and Ryan U.. 2003. The safety and immunogenicity of a CETP vaccine in healthy adults. Atherosclerosis. 169: 113–120. [DOI] [PubMed] [Google Scholar]
- 12.Morehouse L. A., Sugarman E. D., Bourassa P. A., Sand T. M., Zimetti F., Gao F., Rothblat G. H., and Milici A. J.. 2007. Inhibition of CETP activity by torcetrapib reduces susceptibility to diet-induced atherosclerosis in New Zealand White rabbits. J. Lipid Res. 48: 1263–1272. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto H., Yonemori F., Wakitani K. Minowa T., Maeda K., and Shinkai H.. 2000. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature. 406: 203–207. [DOI] [PubMed] [Google Scholar]
- 14.HPS3/TIMI55-REVEAL Collaborative Group, Bowman L., Hopewell J. C., Chen F., Wallendszus K., Stevens W., Collins R., Wiviott S. D., Cannon C. P., Braunwald E., et al. 2017. Effects of anacetrapib in patients with atherosclerotic vascular disease. N. Engl. J. Med. 377: 1217–1227. [DOI] [PubMed] [Google Scholar]
- 15.Bingle C. D., and Craven C. J.. 2004. Meet the relatives: a family of BPI- and LBP-related proteins. Trends Immunol. 25: 53–55. [DOI] [PubMed] [Google Scholar]
- 16.Guyard-Dangremont V., Tenekjian V., Chauhan V., Walter S., Roy P., Rassart E., and Milne A. R.. 1999. Immunochemical evidence that cholesteryl ester transfer protein and bactericidal/permeability-increasing protein share a similar tertiary structure. Protein Sci. 8: 2392–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruce C., Beamer L. J., and Tall A. R.. 1998. The implications of the structure of the bactericidal/permeability-increasing protein on the lipid-transfer function of the cholesteryl ester transfer protein. Curr. Opin. Struct. Biol. 8: 426–434. [DOI] [PubMed] [Google Scholar]
- 18.Qiu X., Mistry A., Ammirati M. J., Chrunyk B. A., Clark R. W., Cong Y., Culp J. S., Danley D. E., Freeman T. B., Geoghegan K. F., et al. 2007. Crystal structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nat. Struct. Mol. Biol. 14: 106–113. [DOI] [PubMed] [Google Scholar]
- 19.Chirasani V. R., Revanasiddappa P. D., and Senapati S.. 2016. Structural plasticity of cholesteryl ester transfer protein assists the lipid transfer activity. J. Biol. Chem. 291: 19462–19473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koivuniemi A., Vuorela T., Kovanen P. T., Vattulainen I., and Hyvonen M. T.. 2012. Lipid exchange mechanism of the cholesteryl ester transfer protein clarified by atomistic and coarse-grained simulations. PLOS Comput. Biol. 8: e1002299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L., Yan F., Zhang S., Lei D., Charles M. A., Cavigiolio G., Oda M., Krauss R. M., Weisgraber K. H., Rye K. A., et al. 2012. Structural basis of transfer between lipoproteins by cholesteryl ester transfer protein. Nat. Chem. Biol. 8: 342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ihm J., Quinn D. M., Busch S. J., Chataing B., and Harmony J. A.. 1982. Kinetics of plasma protein-catalyzed exchange of phosphatidylcholine and cholesteryl ester between plasma lipoproteins. J. Lipid Res. 23: 1328–1341. [PubMed] [Google Scholar]
- 23.Lauer M. E., Graff-Meyer A., Rufer A. C., Maugeais C., von der Mark E., Matile H., D’Arcy B., Magg C., Ringler P., Muller S. A., et al. 2016. Cholesteryl ester transfer between lipoproteins does not require a ternary tunnel complex with CETP. J. Struct. Biol. 194: 191–198. [DOI] [PubMed] [Google Scholar]
- 24.Wang S., Deng L., Milne R. W., and Tall A. R.. 1992. Identification of a sequence within the C-terminal 26 amino acids of cholesteryl ester transfer protein responsible for binding a neutralizing monoclonal antibody and necessary for neutral lipid transfer activity. J. Biol. Chem. 267: 17487–17490. [PubMed] [Google Scholar]
- 25.Masucci-Magoulas L., Plump A., Jiang X. C., Walsh A., Breslow J. L., and Tall A. R.. 1996. Profound induction of hepatic cholesteryl ester transfer protein transgene expression in apolipoprotein E and low density lipoprotein receptor gene knockout mice. A novel mechanism signals changes in plasma cholesterol levels. J. Clin. Invest. 97: 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinet E. M., Agellon L. B., Kroon P. A., Marcel Y. L., Lee Y. C., Whitlock M. E., and Tall A. R.. 1990. Atherogenic diet increases cholesteryl ester transfer protein messenger RNA levels in rabbit liver. J. Clin. Invest. 85: 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang X. C., Agellon L. B., Walsh A., Breslow J. L., and Tall A.. 1992. Dietary cholesterol increases transcription of the human cholesteryl ester transfer protein gene in transgenic mice. Dependence on natural flanking sequences. J. Clin. Invest. 90: 1290–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin L. J., Connelly P. W., Nancoo D., Wood N., Zhang Z. J., Maguire G., Quinet E., Tall A. R., Marcel Y. L., and McPherson R.. 1993. Cholesteryl ester transfer protein and high density lipoprotein responses to cholesterol feeding in men: relationship to apolipoprotein E genotype. J. Lipid Res. 34: 437–446. [PubMed] [Google Scholar]
- 29.Luo Y., and Tall A. R.. 2000. Sterol upregulation of human CETP expression in vitro and in transgenic mice by an LXR element. J. Clin. Invest. 105: 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gautier T., de Haan W., Grober J., Ye D., Bahr M. J., Claudel T., Nijstad N., Van Berkel T. J., Havekes L. M., Manns M. P., et al. 2013. Farnesoid X receptor activation increases cholesteryl ester transfer protein expression in humans and transgenic mice. J. Lipid Res. 54: 2195–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honzumi S., Shima A., Hiroshima A., Koieyama T., Ubukata N., and Terasaka N.. 2010. LXRalpha regulates human CETP expression in vitro and in transgenic mice. Atherosclerosis. 212: 139–145. [DOI] [PubMed] [Google Scholar]
- 32.Chouinard R. A. Jr., Luo Y., Osborne T. F., Walsh A., and Tall A. R.. 1998. Sterol regulatory element binding protein-1 activates the cholesteryl ester transfer protein gene in vivo but is not required for sterol up-regulation of gene expression. J. Biol. Chem. 273: 22409–22414. [DOI] [PubMed] [Google Scholar]
- 33.Hannuksela M. L., Liinamaa M. J., Kesaniemi Y. A., and Savolainen M. J.. 1994. Relation of polymorphisms in the cholesteryl ester transfer protein gene to transfer protein activity and plasma lipoprotein levels in alcohol drinkers. Atherosclerosis. 110: 35–44. [DOI] [PubMed] [Google Scholar]
- 34.Riemens S. C., van Tol A., Hoogenberg K., van Gent T., Scheek L. M., Sluiter W. J., and Dullaart R. P.. 1997. Higher high density lipoprotein cholesterol associated with moderate alcohol consumption is not related to altered plasma lecithin:cholesterol acyltransferase and lipid transfer protein activity levels. Clin. Chim. Acta. 258: 105–115. [DOI] [PubMed] [Google Scholar]
- 35.Ito T., Nishiwaki M., Ishikawa T., and Nakamura H.. 1995. CETP and LCAT activities are unrelated to smoking and moderate alcohol consumption in healthy normolipidemic men. Jpn. Circ. J. 59: 541–546. [DOI] [PubMed] [Google Scholar]
- 36.Marques-Vidal P., Bochud M., Paccaud F., Waterworth D., Bergmann S., Preisig M., Waeber G., and Vollenweider P.. 2010. No interaction between alcohol consumption and HDL-related genes on HDL cholesterol levels. Atherosclerosis. 211: 551–557. [DOI] [PubMed] [Google Scholar]
- 37.Seip R. L., Moulin P., Cocke T., Tall A., Kohrt W. M., Mankowitz K., Semenkovich C. F., Ostlund R., and Schonfeld G.. 1993. Exercise training decreases plasma cholesteryl ester transfer protein. Arterioscler. Thromb. 13: 1359–1367. [DOI] [PubMed] [Google Scholar]
- 38.Rocco D. D., Okuda L. S., Pinto R. S., Ferreira F. D., Kubo S. K., Nakandakare E. R., Quintao E. C., Catanozi S., and Passarelli M.. 2011. Aerobic exercise improves reverse cholesterol transport in cholesteryl ester transfer protein transgenic mice. Lipids. 46: 617–625. [DOI] [PubMed] [Google Scholar]
- 39.Tiainen S., Luoto R., Ahotupa M., Raitanen J., and Vasankari T.. 2016. 6-mo aerobic exercise intervention enhances the lipid peroxide transport function of HDL. Free Radic. Res. 50: 1279–1285. [DOI] [PubMed] [Google Scholar]
- 40.Brites F., Verona J., De Geitere C., Fruchart J. C., Castro G., and Wikinski R.. 2004. Enhanced cholesterol efflux promotion in well-trained soccer players. Metabolism. 53: 1262–1267. [DOI] [PubMed] [Google Scholar]
- 41.Brown M. L., Inazu A., Hesler C. B., Agellon L. B., Mann C., Whitlock M. E., Marcel Y. L., Milne R. W., Koizumi J., Mabuchi H., et al. 1989. Molecular basis of lipid transfer protein deficiency in a family with increased high-density lipoproteins. Nature. 342: 448–451. [DOI] [PubMed] [Google Scholar]
- 42.Inazu A., Brown M. L., Hesler C. B., Agellon L. B., Koizumi J., Takata K., Maruhama Y., Mabuchi H., and Tall A. R.. 1990. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N. Engl. J. Med. 323: 1234–1238. [DOI] [PubMed] [Google Scholar]
- 43.Asztalos B. F., Horvath K. V., Kajinami K., Nartsupha C., Cox C. E., Batista M., Schaefer E. J., Inazu A., and Mabuchi H.. 2004. Apolipoprotein composition of HDL in cholesteryl ester transfer protein deficiency. J. Lipid Res. 45: 448–455. [DOI] [PubMed] [Google Scholar]
- 44.Yamashita S., Matsuzawa Y., Okazaki M., Kako H., Yasugi T., Akioka H., Hirano K., and Tarui S.. 1988. Small polydisperse low density lipoproteins in familial hyperalphalipoproteinemia with complete deficiency of cholesteryl ester transfer activity. Atherosclerosis. 70: 7–12. [DOI] [PubMed] [Google Scholar]
- 45.Arai T., Yamashita S., Sakai N., Hirano K., Okada S., Ishigami M., Maruyama T., Yamane M., Kobayashi H., Nozaki S., et al. 1996. A novel nonsense mutation (G181X) in the human cholesteryl ester transfer protein gene in Japanese hyperalphalipoproteinemic subjects. J. Lipid Res. 37: 2145–2154. [PubMed] [Google Scholar]
- 46.Gotoda T., Kinoshita M., Shimano H., Harada K., Shimada M., Ohsuga J., Teramoto T., Yazaki Y., and Yamada N.. 1993. Cholesteryl ester transfer protein deficiency caused by a nonsense mutation detected in the patient’s macrophage mRNA. Biochem. Biophys. Res. Commun. 194: 519–524. [DOI] [PubMed] [Google Scholar]
- 47.Ritsch A., Drexel H., Amann F. W., Pfeifhofer C., and Patsch J. R.. 1997. Deficiency of cholesteryl ester transfer protein. Description of the molecular defect and the dissociation of cholesteryl ester and triglyceride transport in plasma. Arterioscler. Thromb. Vasc. Biol. 17: 3433–3441. [DOI] [PubMed] [Google Scholar]
- 48.Zhong S., Sharp D. S., Grove J. S., Bruce C., Yano K., Curb J. D., and Tall A. R.. 1996. Increased coronary heart disease in Japanese-American men with mutation in the cholesteryl ester transfer protein gene despite increased HDL levels. J. Clin. Invest. 97: 2917–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inazu A., Jiang X. C., Haraki T., Yagi K., Kamon N., Koizumi J., Mabuchi H., Takeda R., Takata K., Moriyama Y., et al. 1994. Genetic cholesteryl ester transfer protein deficiency caused by two prevalent mutations as a major determinant of increased levels of high density lipoprotein cholesterol. J. Clin. Invest. 94: 1872–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson A., Di Angelantonio E., Sarwar N., Erqou S., Saleheen D., Dullaart R. P., Keavney B., Ye Z., and Danesh J.. 2008. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA. 299: 2777–2788. [DOI] [PubMed] [Google Scholar]
- 51.Ridker P. M., Pare G., Parker A. N., Zee R. Y., Miletich J. P., and Chasman D. I.. 2009. Polymorphism in the CETP gene region, HDL cholesterol, and risk of future myocardial infarction: genomewide analysis among 18 245 initially healthy women from the Women’s Genome Health Study. Circ Cardiovasc Genet. 2: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niu W., and Qi Y.. 2015. Circulating cholesteryl ester transfer protein and coronary heart disease: Mendelian randomization meta-analysis. Circ Cardiovasc Genet. 8: 114–121. [DOI] [PubMed] [Google Scholar]
- 53.Johannsen T. H., Frikke-Schmidt R., Schou J., Nordestgaard B. G., and Tybjaerg-Hansen A.. 2012. Genetic inhibition of CETP, ischemic vascular disease and mortality, and possible adverse effects. J. Am. Coll. Cardiol. 60: 2041–2048. [DOI] [PubMed] [Google Scholar]
- 54.Nomura A., Won H. H., Khera A. V., Takeuchi F., Ito K., McCarthy S., Emdin C. A., Klarin D., Natarajan P., Zekavat S. M., et al. 2017. Protein-truncating variants at the cholesteryl ester transfer protein gene and risk for coronary heart disease. Circ. Res. 121: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westerterp M., van der Hoogt C. C., de Haan W., Offerman E. H., Dallinga-Thie G. M., Jukema J. W., Havekes L. M., and Rensen P. C.. 2006. Cholesteryl ester transfer protein decreases high-density lipoprotein and severely aggravates atherosclerosis in APOE*3-Leiden mice. Arterioscler. Thromb. Vasc. Biol. 26: 2552–2559. [DOI] [PubMed] [Google Scholar]
- 56.Plump A. S., Masucci-Magoulas L., Bruce C., Bisgaier C. L., Breslow J. L., and Tall A. R.. 1999. Increased atherosclerosis in ApoE and LDL receptor gene knock-out mice as a result of human cholesteryl ester transfer protein transgene expression. Arterioscler. Thromb. Vasc. Biol. 19: 1105–1110. [DOI] [PubMed] [Google Scholar]
- 57.Marotti K. R., Castle C. K., Boyle T. P., Lin A. H., Murray R. W., and Melchior G. W.. 1993. Severe atherosclerosis in transgenic mice expressing simian cholesteryl ester transfer protein. Nature. 364: 73–75. [DOI] [PubMed] [Google Scholar]
- 58.Föger B., Chase M., Amar M. J., Vaisman B. L., Shamburek R. D., Paigen B., Fruchart-Najib J., Paiz J. A., Koch C. A., Hoyt R. F., et al. 1999. Cholesteryl ester transfer protein corrects dysfunctional high density lipoproteins and reduces aortic atherosclerosis in lecithin cholesterol acyltransferase transgenic mice. J. Biol. Chem. 274: 36912–36920. [DOI] [PubMed] [Google Scholar]
- 59.MacLean P. S., Bower J. F., Vadlamudi S., Osborne J. N., Bradfield J. F., Burden H. W., Bensch W. H., Kauffman R. F., and Barakat H. A.. 2003. Cholesteryl ester transfer protein expression prevents diet-induced atherosclerotic lesions in male db/db mice. Arterioscler. Thromb. Vasc. Biol. 23: 1412–1415. [DOI] [PubMed] [Google Scholar]
- 60.Hayek T., Masucci-Magoulas L., Jiang X., Walsh A., Rubin E., Breslow J. L., and Tall A. R.. 1995. Decreased early atherosclerotic lesions in hypertriglyceridemic mice expressing cholesteryl ester transfer protein transgene. J. Clin. Invest. 96: 2071–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bocan T. M., Mueller S. B., Mazur M. J., Uhlendorf P. D., Brown E. Q., and Kieft K. A.. 1993. The relationship between the degree of dietary-induced hypercholesterolemia in the rabbit and atherosclerotic lesion formation. Atherosclerosis. 102: 9–22. [DOI] [PubMed] [Google Scholar]
- 62.Medzhitov R. 2013. Pattern recognition theory and the launch of modern innate immunity. J. Immunol. 191: 4473–4474. [DOI] [PubMed] [Google Scholar]
- 63.Kumagai Y., and Akira S.. 2010. Identification and functions of pattern-recognition receptors. J. Allergy Clin. Immunol. 125: 985–992. [DOI] [PubMed] [Google Scholar]
- 64.Dellinger R. P., Levy M. M., Rhodes A., Annane D., Gerlach H., Opal S. M., Sevransky J. E., Sprung C. L., Douglas I. S., Jaeschke R., et al. ; Surviving Sepsis Campaign Guidelines Committee. 2013. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 39: 165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maldonado R. F., Sa-Correia I., and Valvano M. A.. 2016. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 40: 480–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bone R. C. 1993. Gram-negative sepsis: a dilemma of modern medicine. Clin. Microbiol. Rev. 6: 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wurfel M. M., and Wright S. D.. 1997. Lipopolysaccharide-binding protein and soluble CD14 transfer lipopolysaccharide to phospholipid bilayers: preferential interaction with particular classes of lipid. J. Immunol. 158: 3925–3934. [PubMed] [Google Scholar]
- 68.Flegel W. A., Baumstark M. W., Weinstock C., Berg A., and Northoff H.. 1993. Prevention of endotoxin-induced monokine release by human low- and high-density lipoproteins and by apolipoprotein A-I. Infect. Immun. 61: 5140–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harris H. W., Grunfeld C., Feingold K. R., and Rapp J. H.. 1990. Human very low density lipoproteins and chylomicrons can protect against endotoxin-induced death in mice. J. Clin. Invest. 86: 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J., Komajda M., Lopez-Sendon J., Mosca L., Tardif J. C., Waters D. D., et al. ; ILLUMINATE Investigators. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122. [DOI] [PubMed] [Google Scholar]
- 71.Di Bartolo B. A., Duong M., and Nicholls S. J.. 2016. Clinical trials with cholesteryl ester transfer protein inhibitors. Curr. Opin. Lipidol. 27: 545–549. [DOI] [PubMed] [Google Scholar]
- 72.Clark R. W., Cunningham D., Cong Y., Subashi T. A., Tkalcevic G. T., Lloyd D. B., Boyd J. G., Chrunyk B. A., Karam G. A., Qiu X., et al. 2010. Assessment of cholesteryl ester transfer protein inhibitors for interaction with proteins involved in the immune response to infection. J. Lipid Res. 51: 967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cazita P. M., Barbeiro D. F., Moretti A. I., Quintao E. C., and Soriano F. G.. 2008. Human cholesteryl ester transfer protein expression enhances the mouse survival rate in an experimental systemic inflammation model: a novel role for CETP. Shock. 30: 590–595. [DOI] [PubMed] [Google Scholar]
- 74.Venancio T. M., Machado R. M., Castoldi A., Amano M. T., Nunes V. S., Quintao E. C., Camara N. O., Soriano F. G., and Cazita P. M.. 2016. CETP lowers TLR4 expression which attenuates the inflammatory response induced by LPS and polymicrobial sepsis. Mediators Inflamm. 2016: 1784014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gautier T., Klein A., Deckert V., Desrumaux C., Ogier N., Sberna A. L., Paul C., Le Guern N., Athias A., Montange T., et al. 2008. Effect of plasma phospholipid transfer protein deficiency on lethal endotoxemia in mice. J. Biol. Chem. 283: 18702–18710. [DOI] [PubMed] [Google Scholar]
- 76.Topchiy E., Cirstea M., Kong H. J., Boyd J. H., Wang Y., Russell J. A., and Walley K. R.. 2016. Lipopolysaccharide is cleared from the circulation by hepatocytes via the low density lipoprotein receptor. PLoS One. 11: e0155030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cai L., Ji A., de Beer F. C., Tannock L. R., and van der Westhuyzen D. R.. 2008. SR-BI protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. J. Clin. Invest. 118: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Riedemann N. C., Neff T. A., Guo R. F., Bernacki K. D., Laudes I. J., Sarma J. V., Lambris J. D., and Ward P. A.. 2003. Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J. Immunol. 170: 503–507. [DOI] [PubMed] [Google Scholar]
- 79.Feingold K. R., and Grunfeld C.. 2010. The acute phase response inhibits reverse cholesterol transport. J. Lipid Res. 51: 682–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Masucci-Magoulas L., Moulin P., Jiang X. C., Richardson H., Walsh A., Breslow J. L., and Tall A.. 1995. Decreased cholesteryl ester transfer protein (CETP) mRNA and protein and increased high density lipoprotein following lipopolysaccharide administration in human CETP transgenic mice. J. Clin. Invest. 95: 1587–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hardardóttir I., Moser A. H., Fuller J., Fielding C., Feingold K., and Grunfeld C.. 1996. Endotoxin and cytokines decrease serum levels and extra hepatic protein and mRNA levels of cholesteryl ester transfer protein in syrian hamsters. J. Clin. Invest. 97: 2585–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grion C. M., Cardoso L. T., Perazolo T. F., Garcia A. S., Barbosa D. S., Morimoto H. K., Matsuo T., and Carrilho A. J.. 2010. Lipoproteins and CETP levels as risk factors for severe sepsis in hospitalized patients. Eur. J. Clin. Invest. 40: 330–338. [DOI] [PubMed] [Google Scholar]
- 83.Oliveira H. C., Ma L., Milne R., Marcovina S. M., Inazu A., Mabuchi H., and Tall A. R.. 1997. Cholesteryl ester transfer protein activity enhances plasma cholesteryl ester formation. Studies in CETP transgenic mice and human genetic CETP deficiency. Arterioscler. Thromb. Vasc. Biol. 17: 1045–1052. [DOI] [PubMed] [Google Scholar]
- 84.Gaynor B. J., Sand T., Clark R. W., Aiello R. J., Bamberger M. J., and Moberly J. B.. 1994. Inhibition of cholesteryl ester transfer protein activity in hamsters alters HDL lipid composition. Atherosclerosis. 110: 101–109. [DOI] [PubMed] [Google Scholar]
- 85.Bell T. A. 3rd, Graham M. J., Lee R. G., Mullick A. E., Fu W., Norris D., and Crooke R. M.. 2013. Antisense oligonucleotide inhibition of cholesteryl ester transfer protein enhances RCT in hyperlipidemic, CETP transgenic, LDLr-/- mice. J. Lipid Res. 54: 2647–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liaw Y. W., Lin C. Y., Lai Y. S., Yang T. C., Wang C. J., Whang-Peng J., Liu L. F., Lin C. P., Nieh S., Lu S. C., et al. 2014. A vaccine targeted at CETP alleviates high fat and high cholesterol diet-induced atherosclerosis and non-alcoholic steatohepatitis in rabbit. PLoS One. 9: e111529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mao D., Kai G., Gaofu Q., Zheng Z., Li Z., Jie W., Jingjing L., and Rongyue C.. 2006. Intramuscular immunization with a DNA vaccine encoding a 26-amino acid CETP epitope displayed by HBc protein and containing CpG DNA inhibits atherosclerosis in a rabbit model of atherosclerosis. Vaccine. 24: 4942–4950. [DOI] [PubMed] [Google Scholar]
- 88.Gaofu Q., Jun L., Xin Y., Wentao L., Jie W., Xiuyun Z., and Jingjing L.. 2005. Vaccinating rabbits with a cholesteryl ester transfer protein (CETP) B-Cell epitope carried by heat shock protein-65 (HSP65) for inducing anti-CETP antibodies and reducing aortic lesions in vivo. J. Cardiovasc. Pharmacol. 45: 591–598. [DOI] [PubMed] [Google Scholar]
- 89.Jun L., Jie L., Dongping Y., Xin Y., Taiming L., Rongyue C., Jie W., and Jingjing L.. 2012. Effects of nasal immunization of multi-target preventive vaccines on atherosclerosis. Vaccine. 30: 1029–1037. [DOI] [PubMed] [Google Scholar]
- 90.Aghebati T., Badiee A., Mohammadpour A. H., Afshar M., Jaafari M. R., Abnous K., Issazadeh S., Hashemzadeh S., Zareh M., Hashemizadeh H., et al. 2016. Anti-atherosclerosis effect of different doses of CETP vaccine in rabbit model of atherosclerosis. Biomed. Pharmacother. 81: 468–473. [DOI] [PubMed] [Google Scholar]
- 91.Brousseau M. E., Schaefer E. J., Wolfe M. L., Bloedon L. T., Digenio A. G., Clark R. W., Mancuso J. P., and Rader D. J.. 2004. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N. Engl. J. Med. 350: 1505–1515. [DOI] [PubMed] [Google Scholar]
- 92.Ranalletta M., Bierilo K. K., Chen Y., Milot D., Chen Q., Tung E., Houde C., Elowe N. H., Garcia-Calvo M., Porter G., et al. 2010. Biochemical characterization of cholesteryl ester transfer protein inhibitors. J. Lipid Res. 51: 2739–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Krauss R. M., Wojnooski K., Orr J., Geaney J. C., Pinto C. A., Liu Y., Wagner J. A., Luk J. M., Johnson-Levonas A. O., Anderson M. S., et al. 2012. Changes in lipoprotein subfraction concentration and composition in healthy individuals treated with the CETP inhibitor anacetrapib. J. Lipid Res. 53: 540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clark R. W., Ruggeri R. B., Cunningham D., and Bamberger M. J.. 2006. Description of the torcetrapib series of cholesteryl ester transfer protein inhibitors, including mechanism of action. J. Lipid Res. 47: 537–552. [DOI] [PubMed] [Google Scholar]
- 95.Tchoua U., D’Souza W., Mukhamedova N., Blum D., Niesor E., Mizrahi J., Maugeais C., and Sviridov D.. 2008. The effect of cholesteryl ester transfer protein overexpression and inhibition on reverse cholesterol transport. Cardiovasc. Res. 77: 732–739. [DOI] [PubMed] [Google Scholar]
- 96.Niesor E. J., Magg C., Ogawa N., Okamoto H., von der Mark E., Matile H., Schmid G., Clerc R. G., Chaput E., Blum-Kaelin D., et al. 2010. Modulating cholesteryl ester transfer protein activity maintains efficient pre-beta-HDL formation and increases reverse cholesterol transport. J. Lipid Res. 51: 3443–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang Z., Inazu A., Nohara A., Higashikata T., and Mabuchi H.. 2002. Cholesteryl ester transfer protein inhibitor (JTT-705) and the development of atherosclerosis in rabbits with severe hypercholesterolaemia. Clin. Sci. (Lond.). 103: 587–594. [DOI] [PubMed] [Google Scholar]
- 98.Cao G., Beyer T. P., Zhang Y., Schmidt R. J., Chen Y. Q., Cockerham S. L., Zimmerman K. M., Karathanasis S. K., Cannady E. A., Fields T., et al. 2011. Evacetrapib is a novel, potent, and selective inhibitor of cholesteryl ester transfer protein that elevates HDL cholesterol without inducing aldosterone or increasing blood pressure. J. Lipid Res. 52: 2169–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu S., Mistry A., Reynolds J. M., Lloyd D. B., Griffor M. C., Perry D. A., Ruggeri R. B., Clark R. W., and Qiu X.. 2012. Crystal structures of cholesteryl ester transfer protein in complex with inhibitors. J. Biol. Chem. 287: 37321–37329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Simic B., Mocharla P., Crucet M., Osto E., Kratzer A., Stivala S., Kuhnast S., Speer T., Doycheva P., Princen H. M., et al. 2017. Anacetrapib, but not evacetrapib, impairs endothelial function in CETP-transgenic mice in spite of marked HDL-C increase. Atherosclerosis. 257: 186–194. [DOI] [PubMed] [Google Scholar]
- 101.Castro-Perez J., Briand F., Gagen K., Wang S. P., Chen Y., McLaren D. G., Shah V., Vreeken R. J., Hankemeier T., Sulpice T., et al. 2011. Anacetrapib promotes reverse cholesterol transport and bulk cholesterol excretion in Syrian golden hamsters. J. Lipid Res. 52: 1965–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kühnast S., van der Tuin S. J., van der Hoorn J. W., van Klinken J. B., Simic B., Pieterman E., Havekes L. M., Landmesser U., Luscher T. F., Willems van Dijk K., et al. 2015. Anacetrapib reduces progression of atherosclerosis, mainly by reducing non-HDL-cholesterol, improves lesion stability and adds to the beneficial effects of atorvastatin. Eur. Heart J. 36: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu B. J., Shrestha S., Ong K. L., Johns D., Hou L., Barter P. J., and Rye K. A.. 2015. Cholesteryl ester transfer protein inhibition enhances endothelial repair and improves endothelial function in the rabbit. Arterioscler. Thromb. Vasc. Biol. 35: 628–636. [DOI] [PubMed] [Google Scholar]
- 104.Wu B. J., Shrestha S., Ong K. L., Johns D., Dunn L. L., Hou L., Barter P. J., and Rye K. A.. 2015. Increasing HDL levels by inhibiting cholesteryl ester transfer protein activity in rabbits with hindlimb ischemia is associated with increased angiogenesis. Int. J. Cardiol. 199: 204–212. [DOI] [PubMed] [Google Scholar]
- 105.Wu B. J., Li Y., Ong K. L., Sun Y., Shrestha S., Hou L., Johns D., Barter P. J., and Rye K. A.. 2017. Reduction of In-Stent Restenosis by Cholesteryl Ester Transfer Protein Inhibition. Arterioscler. Thromb. Vasc. Biol. 37: 2333–2341. [DOI] [PubMed] [Google Scholar]
- 106.Schwartz G. G., Olsson A. G., Abt M., Ballantyne C. M., Barter P. J., Brumm J., Chaitman B. R., Holme I. M., Kallend D., Leiter L. A., et al. ; dal-OUTCOMES Investigators. 2012. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367: 2089–2099. [DOI] [PubMed] [Google Scholar]
- 107.Lincoff A. M., Nicholls S. J., Riesmeyer J. S., Barter P. J., Brewer H. B., Fox K. A. A., Gibson C. M., Granger C., Menon V., Montalescot G., et al. ; ACCELERATE Investigators. 2017. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N. Engl. J. Med. 376: 1933–1942. [DOI] [PubMed] [Google Scholar]
- 108.Cannon C. P., Shah S., Dansky H. M., Davidson M., Brinton E. A., Gotto A. M., Stepanavage M., Liu S. X., Gibbons P., Ashraf T. B., et al. ; Determining the Efficacy and Tolerability Investigators. 2010. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N. Engl. J. Med. 363: 2406–2415. [DOI] [PubMed] [Google Scholar]
- 109.Gotto A. M. Jr., Cannon C. P., Li X. S., Vaidya S., Kher U., Brinton E. A., Davidson M., Moon J. E., Shah S., Dansky H. M., et al. ; DEFINE Investigators. 2014. Evaluation of lipids, drug concentration, and safety parameters following cessation of treatment with the cholesteryl ester transfer protein inhibitor anacetrapib in patients with or at high risk for coronary heart disease. Am. J. Cardiol. 113: 76–83. [DOI] [PubMed] [Google Scholar]
- 110.Barter P. J., Rye K. A., Tardif J. C., Waters D. D., Boekholdt S. M., Breazna A., and Kastelein J. J.. 2011. Effect of torcetrapib on glucose, insulin, and hemoglobin A1c in subjects in the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial. Circulation. 124: 555–562. [DOI] [PubMed] [Google Scholar]
- 111.Menon V., Lincoff A. M., Nicholls S. J., Barter P. J., Brewer H. B., Fox K. A. A., Gibson C. M., Granger C., Montalescot G., Rader D., et al. 2017. Impact of cholesteryl ester transfer protein inhibition with evacetrapib in patients with diabetes mellitus: results from the ACCELERATE trial. Eur. Heart J. 38: 5355. [DOI] [PubMed] [Google Scholar]
- 112.Fryirs M. A., Barter P. J., Appavoo M., Tuch B. E., Tabet F., Heather A. K., and Rye K. A.. 2010. Effects of high-density lipoproteins on pancreatic beta-cell insulin secretion. Arterioscler. Thromb. Vasc. Biol. 30: 1642–1648. [DOI] [PubMed] [Google Scholar]
- 113.Cochran B. J., Bisoendial R. J., Hou L., Glaros E. N., Rossy J., Thomas S. R., Barter P. J., and Rye K. A.. 2014. Apolipoprotein A-I increases insulin secretion and production from pancreatic beta-cells via a G-protein-cAMP-PKA-FoxO1-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 34: 2261–2267. [DOI] [PubMed] [Google Scholar]
- 114.Cochran B. J., Ryder W. J., Parmar A., Tang S., Reilhac A., Arthur A., Charil A., Hamze H., Barter P. J., Kritharides L., et al. 2016. In vivo PET imaging with [(18)F]FDG to explain improved glucose uptake in an apolipoprotein A-I treated mouse model of diabetes. Diabetologia. 59: 1977–1984. [DOI] [PubMed] [Google Scholar]
- 115.Stenkula K. G., Lindahl M., Petrlova J., Dalla-Riva J., Goransson O., Cushman S. W., Krupinska E., Jones H. A., and Lagerstedt J. O.. 2014. Single injections of apoA-I acutely improve in vivo glucose tolerance in insulin-resistant mice. Diabetologia. 57: 797–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Domingo-Espín J., Lindahl M., Nilsson-Wolanin O., Cushman S. W., Stenkula K. G., and Lagerstedt J. O.. 2016. Dual actions of apolipoprotein A-I on glucose-stimulated insulin secretion and insulin-independent peripheral tissue glucose uptake lead to increased heart and skeletal muscle glucose disposal. Diabetes. 65: 1838–1848. [DOI] [PubMed] [Google Scholar]
- 117.Ford J., Lawson M., Fowler D., Maruyama N., Mito S., Tomiyasu K., Kinoshita S., Suzuki C., Kawaguchi A., Round P., et al. 2014. Tolerability, pharmacokinetics and pharmacodynamics of TA-8995, a selective cholesteryl ester transfer protein (CETP) inhibitor, in healthy subjects. Br. J. Clin. Pharmacol. 78: 498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hovingh G. K., Kastelein J. J., van Deventer S. J., Round P., Ford J., Saleheen D., Rader D. J., Brewer H. B., and Barter P. J.. 2015. Cholesterol ester transfer protein inhibition by TA-8995 in patients with mild dyslipidaemia (TULIP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 386: 452–460. [DOI] [PubMed] [Google Scholar]
- 119.van Capelleveen J. C., Kastelein J. J., Zwinderman A. H., van Deventer S. J., Collins H. L., Adelman S. J., Round P., Ford J., Rader D. J., and Hovingh G. K.. 2016. Effects of the cholesteryl ester transfer protein inhibitor, TA-8995, on cholesterol efflux capacity and high-density lipoprotein particle subclasses. J. Clin. Lipidol. 10: 1137–1144.e3. [DOI] [PubMed] [Google Scholar]