Abstract
Eicosanoids, including prostaglandins (PGs) and thromboxanes, are broadly bioactive lipid mediators and increase colon tumorigenesis possibly through chronic inflammatory mechanisms. Epidemiological and experimental data suggest that acetylsalicylic acid (ASA) helps prevent colorectal cancer (CRC), possibly through cyclooxygenase (COX)-mediated suppression of eicosanoid, particularly PGE2, formation. Recent studies suggest that statins prevent CRC and improve survival after diagnosis. We identified patients on ASA and/or statin treatment undergoing routine colonoscopy and measured eicosanoid levels in colonic mucosa with targeted metabolomics technology (LC-MS/MS). ASA-treated individuals (n = 27) had significantly lower tissue eicosanoid levels of most COX-derived metabolites than untreated individuals (n = 31). In contrast, COX-derived lipid metabolites tended to be higher in patients with statin treatment (n = 7) as compared with those not receiving statins (n = 24). This effect was not discernible in subjects treated with ASA and statins (n = 11): Individuals treated with both drugs showed a pronounced suppression of COX-derived eicosanoids in colon tissue, even compared with subjects treated with ASA alone. Our data from a routine clinical setting support the hypothesis that ASA and statins could inhibit CRC development via lipid mediator modification. Further studies should directly investigate the effect of dual ASA and statin treatment on colon tumorigenesis in humans.
Keywords: colorectal cancer, lipidomics, cyclooxygenase, arachidonic acid, acetylsalicylic acid
Colorectal cancer (CRC) is the third most diagnosed cancer worldwide (1). Numerous epidemiological and observational studies show that long-term low-dose acetylsalicylic acid (ASA) intake reduces the incidence of CRC and that postdiagnosis ASA therapy exerts positive effects on CRC overall survival (2, 3). Prostaglandin (PG)E2, one of the main cyclooxygenase (COX) products suppressed by the COX-inhibitor, ASA, has a well-established pro-tumorigenic role in CRC development (4–6) and oral treatment with ASA was found to decrease colonic tissue PGE2 levels in individuals suffering from CRC (7). Several studies have shown that treatment with ASA or other COX-inhibitors (8, 9) can suppress the development of colonic neoplasia by suppressing the formation of colon polyps (10–12), which are neoplastic precursor lesions leading to most CRCs (13, 14). The preventive effect of ASA might thus occur within normal (neither neoplastic nor inflamed) colon mucosa. The protection from CRC in individuals treated with aspirin becomes visible in the rates of CRC incidence 8–10 years after the beginning of aspirin administration (15). This observation also supports the thesis of an early effect of aspirin treatment on healthy colonic mucosa.
In addition, a role in colon tumorigenesis chemoprevention has been postulated for statins (hydroxymethylglutaryl-CoA reductase inhibitors), which are widely used for the reduction of serum cholesterol levels in the prevention of cardiovascular or cerebrovascular events. A large population-based case-control study demonstrated a relative risk reduction for CRC development for statin treatment over 5 years (16). Most recently, these findings were confirmed in different cohorts, including a large cohort of patients with inflammatory bowel disease (17, 18). Furthermore, statin use was found to be associated with a reduced incidence of advanced colorectal adenoma, suggesting a preventive role in the malignant transformation of adenomatous tissue (19, 20). Besides the effect in colon tumorigenesis prevention, several studies indicate positive effects on CRC-specific mortality and survival (21–23). The effect of statin treatment on eicosanoid formation is controversial, with studies demonstrating increased eicosanoid formation due to statin use (24–27) as compared with other data indicating suppression of eicosanoid formation (28–30).
Both drugs can modify the product spectrum of COX-2: While COX-2 acetylation by aspirin blocks prostanoid and 11-H(p)ETE formation, acetylated COX-2 still leads to the formation of 15-HETE (31–34). Interestingly, statins were shown to modulate COX-2 activity by increasing inducible nitric oxide synthase and S-nitrosylation of COX-2 (35). 15-HETE is a pathway marker of anti-inflammatory and pro-resolving lipoxins (36–39). Similarly, acetylated COX-2 enhances formation of EPA-derived 18-hydroxy eicosapentaenoic acid (HEPE) and DHA-derived 17-hydroxy (H)DHA, anti-inflammatory compounds and precursors of potent specialized pro-resolving mediators (40–42). The induction of those metabolites by aspirin or statin treatment could therefore contribute to a low-grade anti-inflammatory effect in the colon mucosa.
In this context, the current study was designed to investigate the profile of eicosanoids and other oxylipins in healthy colonic mucosa biopsies from individuals without or with ASA and/or statin treatment.
MATERIALS AND METHODS
Sample acquisition
Patients (n = 58) were randomly selected from a cohort of walk-in patients scheduled for screening or control colonoscopy in the Department of Gastroenterology, Sana Klinikum Lichtenberg, Berlin, Germany. Bowel preparation was performed with MoviPrep® [ascorbic acid (4.7 g)/potassium chloride (1.015 g)/polyethylene glycol 3350 (100 g)/sodium ascorbate (5.9 g)/sodium chloride (2.691 g)/sodium sulfate (7.5 g) per 123 g] according to the manufacturer’s instructions on the day before and on the day of the procedure. Colonoscopy was performed with propofol sedation according to national guidelines. Each patient underwent biopsies of macroscopically intact colonic mucosa to test for hidden inflammation. All samples were immediately quick frozen on dry ice and then stored at −80°C. Patients were asked for their medication intake on the day of colonoscopy and were defined as ASA or statin users, if the corresponding drug was taken regularly over the last 6 months. ASA and statin medication was also taken on the day of the colonoscopy. Patients with a history of CRC within the last 10 years, inflammatory bowel disease, or diseases related to a systemic inflammatory state (e.g., rheumatic diseases) were excluded from the study.
The study was approved by the local ethics committee of Charité Universitätsmedizin Berlin and procedures were in accord with the Declaration of Helsinki. All subjects provided written informed consent.
Sample extraction and analyses
Tissue samples were homogenized in 300 μl of methanol with a 5 mm metal bead using a ball mill (5–10 min, 15–20 Hz; Retsch, Haan, Germany) following addition of internal standards [10 μl of 100 nM 2H4-6-keto-PGF1α, 2H4-PGE2, 2H4-PGD2, 2H4-thromboxane (Tx)B2, 2H4-leukotriene (LT)B4, 2H4-9- HODE, 2H8-5-HETE, 2H8-12-HETE, 2H6-20-HETE, 2H11-14,15-dihydroxyeicosatrienoic acid, 2H11-14(15)-epoxyeicosatrienoic acid, 2H4-9(10)-epoxyoctadecenoic acid, and 2H4-9,10-dihydroxyoctadecenoic acid] and 10 μl of an antioxidant/inhibitor solution [0.2 mg/ml EDTA, 0.2 mg/ml butylated hydroxytoluene, 100 μM indomethacin, 100 μM 1-(1-methylsulfonyl-piperidin-4-yl)-3-(4-trifluoromethoxy-phenyl)-urea (sEH inhibitor) in methanol/water (50/50, v/v)]. Extraction was carried out on anion exchange BondElut Certify II cartridges (3 ml, 200 mg; Agilent, Waldbronn, Germany) preconditioned with one column volume of methanol and one column volume of 0.1 M sodium acetate buffer (pH 6.0)/methanol (95/5, v/v). Samples were centrifuged (10 min, 4°C, 20,000 g) and supernatants were diluted with 2,700 μl of 1 M sodium acetate buffer (pH 6.0) and loaded onto the preconditioned SPE cartridges. Cartridges were washed with one column volume of water and methanol/water (50/50, v/v) and dried by low vacuum (∼200 mbar) for 20 min. Analytes were eluted with 2 ml 75/25 (v/v) ethyl acetate/n-hexane with 1% acetic acid in glass tubes containing 6 μl of 30% glycerol in methanol. Utilizing a Speedvac (Christ, Osterode, Germany), the extract was evaporated to dryness until only the glycerol plug was left. The residue was dissolved in 50 μl methanol. Oxylipin quantification by LC-MS/MS was carried out as described previously (43).
Statistical analysis
Only metabolites detectable above their limit of quantitation (LOQ) were included in the statistical analysis: Metabolites were excluded from the analysis and categorized as “below detectable levels” whenever 30% or more of the examined samples in one group showed lipid metabolite concentrations below the LOQ (supplemental Table S1). If single tissue samples were found with metabolite levels below their respective LOQ, we used the corresponding LOQ for calculation of the mean and further analysis (supplemental Table S1). Prism 5 software (GraphPad, San Diego, CA) was used for statistical analyses. Summary statistics are presented as mean ± SEM.
Mann-Whitney testing was used to assess significance of differences between subjects treated with ASA and those without ASA and, in a separate analysis, to test for differences between subjects receiving statin treatment and those without in the subset of patients without ASA treatment. To correct for the multiple comparisons performed (54 metabolites analyzed), we used Bonferroni correction with an α of 5%, leading us to assume significance at a level of P < 0.00092.
Subsequently, two-way ANOVA was used to test for interaction between ASA and statin medication in the ASA-treated patients; results of this testing are shown in Fig. 3 and supplemental Table S2. Categorical data for the comparison of clinical characteristics of the study groups were analyzed by Chi-square test.
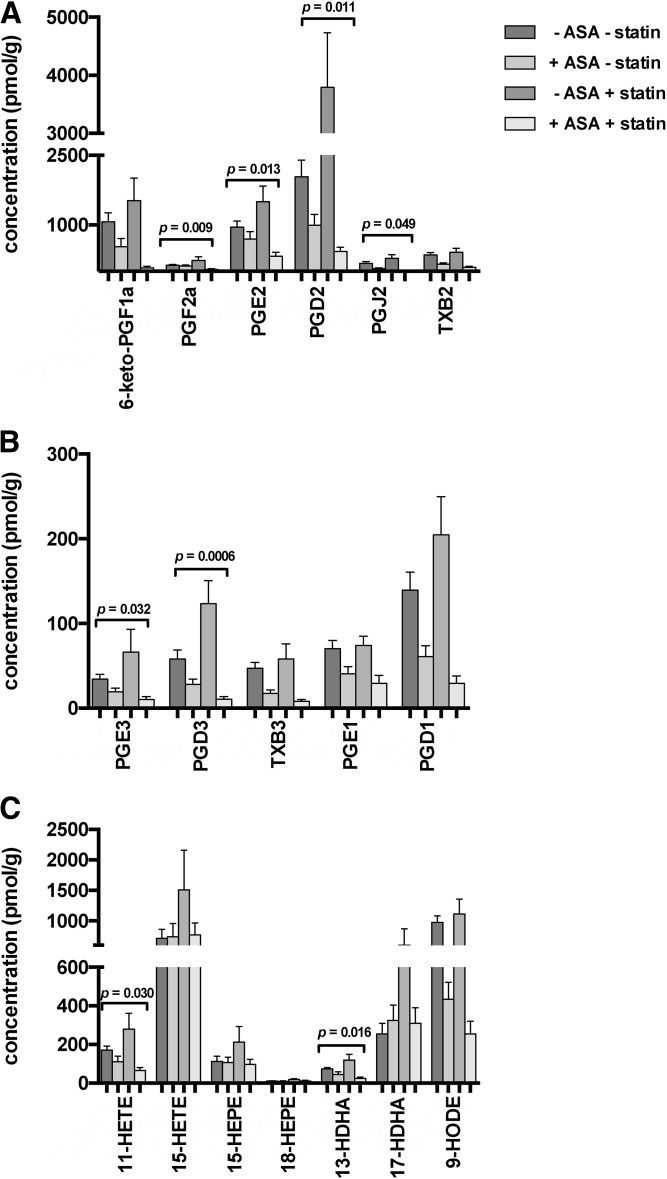
Fig. 3.
Interaction between ASA and statin treatment. Tissue levels of AA-derived (A) and EPA- and DGLA-derived (B) PGs and Txs, and AA-, EPA-, and DHA-derived monohydroxy lipid metabolites (C) in colon tissue samples of individuals without ASA/statin medication (n = 24 subjects), with ASA medication (n = 16 subjects), with statin medication alone (n = 7), and with dual ASA and statin medication (n = 11). Statistical analysis was performed using two-way ANOVA. P values for interaction are indicated whenever P < 0.05. Values are presented as mean ± SEM.
RESULTS
Clinical characteristics of study population
There were no significant differences in the number of female/male patients and BMI between the four study groups. Patients in the study group without ASA and statin medication were younger than individuals in the ASA-treated study group (P < 0.01). The number of patients suffering from type 2 diabetes and the number of smokers did not differ significantly between the groups (Table 1). Twenty-six out of 27 individuals under ASA treatment were on a daily dose of 100 mg ASA, while one individual took 250 mg ASA per day. Statin medication included treatment with simvastatin 10 mg (n = 2), simvastatin 20 mg (n = 6), simvastatin 30 mg (n = 1), simvastatin 40 mg (n = 4), atorvastatin 20 mg (n = 1), atorvastatin 40 mg (n = 1), and pravastatin 20 mg (n = 2). Two patients under simvastatin treatment were on a comedication with ezetimibe. The number of patients suffering from arterial hypertension and taking cardiac medication (including angiotensin-converting-enzyme inhibitors, angiotensin II receptor antagonists, diuretics, and β blockers) was higher in the study groups with ASA, statin, or dual therapy. The number of individuals with a history of colorectal adenoma did not differ between the four groups (Table 1).
TABLE 1.
Patient characteristics
| Variable | −ASA −statin (n = 24) | +ASA (n = 16) | +Statin (n = 7) | +ASA +Statin (n = 11) | P |
| Age (years) | 69.29± 1.59 | 77.25± 1.83 | 74.86± 1.63 | 74.27± 2.37 | 0.011 |
| BMI (kg/m2) | 28.58± 0.98 | 30.22± 1.40 | 29.89± 1.45 | 30.88± 1.18 | 0.542 |
| Gender (male:female) | 15:9 | 9:7 | 5:2 | 6:5 | 0.880 |
| Smoking | 8/24 | 3/16 | 2/7 | 3/11 | 0.795 |
| Type 2 diabetes mellitus | 7/24 | 2/16 | 0/7 | 4/11 | 0.188 |
| Oral antidiabetics | 5/24 | 2/16 | 0/7 | 3/11 | 0.440 |
| Arterial hypertension | 14/24 | 14/16 | 7/7 | 10/11 | 0.027 |
| Cardiac medication | 14/24 | 14/16 | 6/7 | 10/11 | 0.074 |
| History of colorectal adenoma | 12/24 | 5/16 | 3/7 | 6/11 | 0.597 |
Variables represent frequencies (n/total) or mean ± SEM. P values represent statistical differences as calculated by one-way ANOVA followed by Tukey’s multiple comparison test for age and BMI and by Chi-square test for categorical data.
Effect of ASA treatment on colon tissue eicosanoid levels
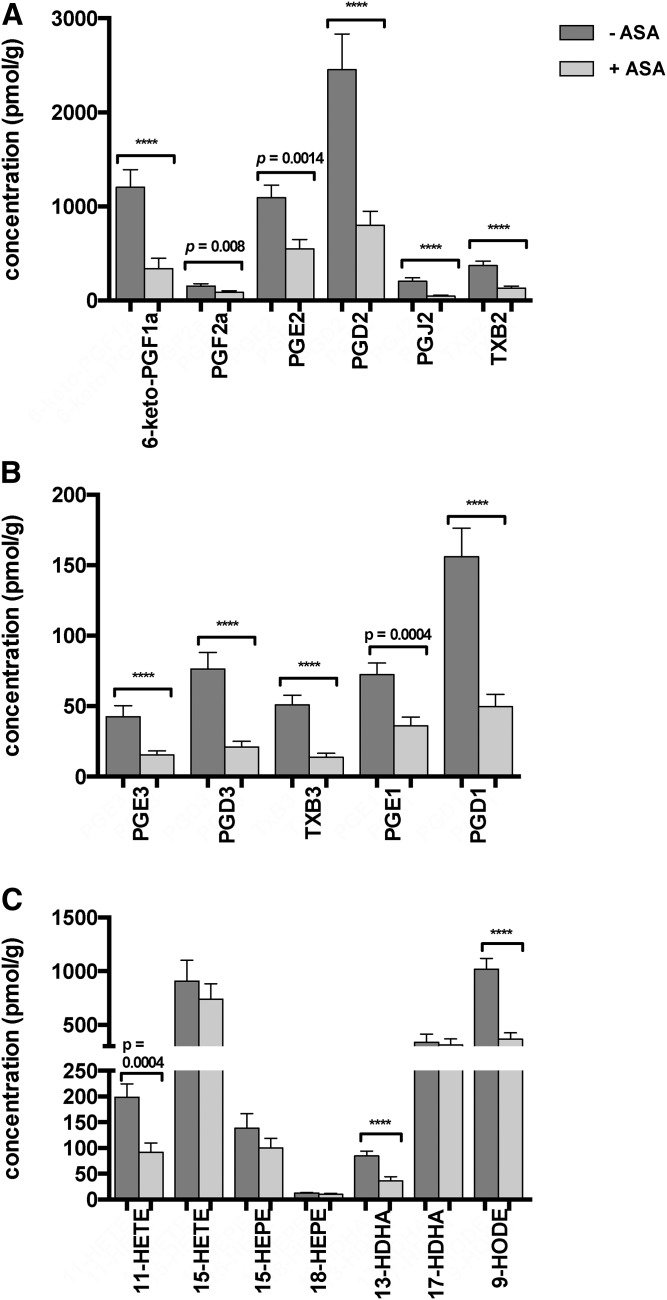
The most abundant PGs in human colorectal tissue samples were PGD2, 6-keto-PGF1α, and PGE2. Levels of LTB4 were below detectable levels in 41 of 58 individuals. ASA-treated individuals showed significantly lower tissue levels of 6-keto-PGF1α, PGF2a, PGD2, PGJ2, and TxB2 than individuals without ASA medication. However, after correcting for multiple testing using the Bonferroni approach the difference in PGE2 levels failed to reach significance (Fig. 1A). The EPA-derived metabolites, PGE3, PGD3, and TxB3, were also significantly lower in ASA-treated individuals compared with untreated subjects, as were dihommo γ-linolenic acid (DGLA)-derived metabolites, PGE1 and PGD1 (Fig. 1B). Monohydroxy oxylipins were also assessed, and while levels of the arachidonic acid (AA)-derived COX-metabolite 11-HETE were significantly lower in ASA-treated patients compared with untreated subjects, 15-HETE as well as EPA-derived HEPEs, 15-HEPE and 18-HEPE, did not show differences in tissue levels between patients treated with ASA and those without ASA. Similarly, DHA-derived 17- HDHA was not increased in ASA-treated individuals, while 13-HDHA showed significant differences with ASA-treated subjects having lower 13-HDHA tissue levels than untreated ones. The most abundant hydroxy PUFA in human colorectal tissue samples was the linoleic acid (LA)-derived metabolite, 9-HODE. 9-HODE levels were significantly lower in tissue samples of individuals treated with ASA in comparison to untreated individuals (Fig. 1C).
Fig. 1.
Effect of ASA treatment on colon tissue lipid metabolite levels. Tissue levels of AA-derived (A) and EPA- and DGLA-derived (B) PGs and Txs, and AA-, EPA-, and DHA-derived monohydroxy lipid metabolites (C) in healthy colonic tissue samples of individuals without ASA medication (n = 31 subjects), as compared with those with ASA medication (n = 27 subjects). Statistical analyses were performed using Mann-Whitney testing. P values are included when below 0.05; ****P < 0.0001: Using Bonferroni correction as indicated in the Materials and Methods, we assume P < 0.00092 as significant after controlling for multiple testing. Values are presented as mean ± SEM.
Effect of statin treatment on colon tissue eicosanoid levels
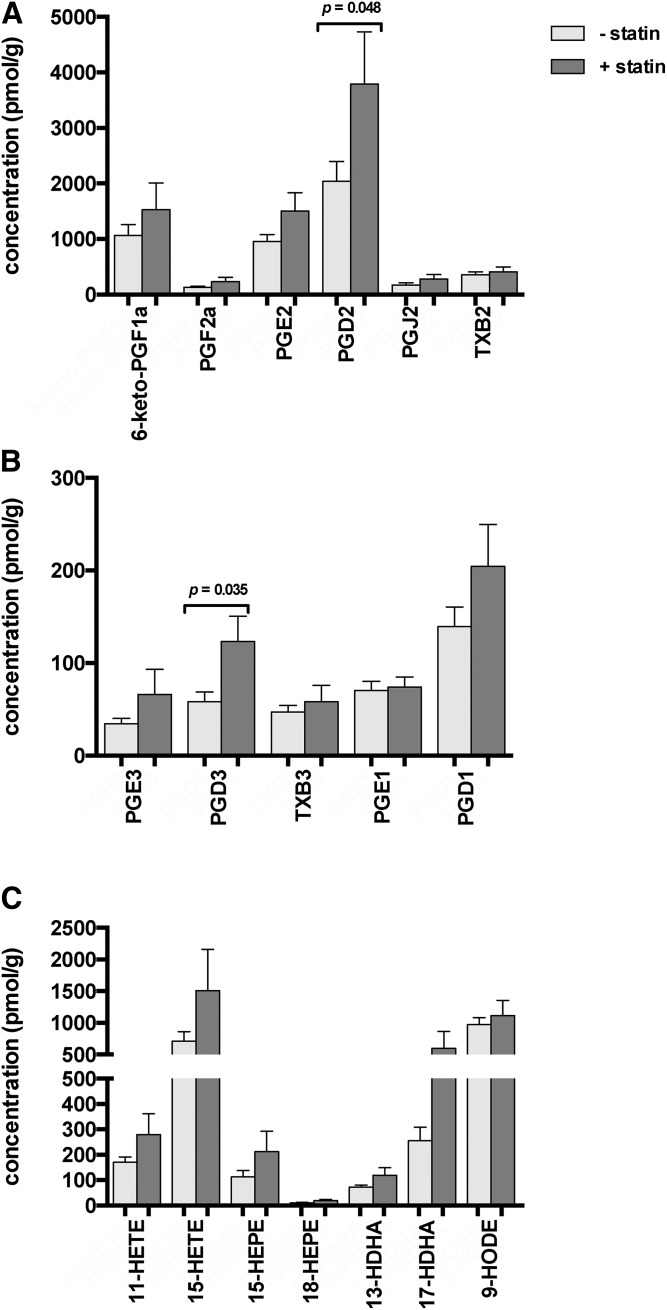
Next, we analyzed whether statin treatment had an effect on the assayed PG and monohydroxy metabolites in the subset of patients not treated with ASA. Colon tissue from statin-treated patients had higher levels of these PGs and Txs in comparison to untreated patients. These differences only reached significance for PGD2 and PGD3 when assuming a significance level of P < 0.05 (Fig. 2A, B) for the individual tests (i.e., before Bonferroni correction for the multiple testing performed). There was also a trend toward higher monohydroxy metabolites derived from AA, EPA, DHA, and LA, such as 11-HETE, 15-HEPE, 18-HEPE, and 9-HODE. While these higher amounts were most notable for 15-HETE and 17-HDHA, even these differences were not significant (Fig. 2C).
Fig. 2.
Effect of statin treatment on colon tissue lipid metabolite levels. Tissue levels of selected AA-derived (A) and EPA- and DGLA-derived (B) PGs and Txs, and AA-, EPA-, and DHA-derived monohydroxy lipid metabolites (C) in healthy colonic tissue samples of patients without statin medication (n = 24 subjects), as compared with those with statin medication (n = 7 subjects). Statistical analyses were performed using Mann-Whitney testing. P values are included when below 0.05: Using Bonferroni correction as indicated in the Materials and Methods, we assume P < 0.00092 as significant after controlling for multiple testing. Values are presented as mean ± SEM.
Effect of ASA and statin treatment on colon eicosanoid levels
Given the finding of an oxylipin-lowering effect of ASA in contrast to a trend toward an increase of oxylipins in colon tissue from statin-treated patients, we then decided to analyze the subset of patients with ASA-only treatment versus those receiving ASA and statin medication. Interestingly, we observed markedly lower tissue PG levels in individuals simultaneously receiving ASA and statin treatment, as compared with untreated individuals or subjects receiving ASA monotherapy. Statistical testing for interaction between the effects of ASA and statin medication yielded P values <0.05 for the metabolites, PGF2a, PGE2, PGD2, PGJ2, PGE3, PGD3, 11-HETE, and 13-HDHA (Fig. 3), pointing toward the possibility of a synergistic effect of ASA and statin medication on PG suppression.
Effect of ASA and statin treatment on other oxylipins
There were no significant differences in colon tissue epoxy- and dihydroxy-fatty acid levels between the four groups, but levels of epoxy- and dihydroxy-fatty acids tended to be higher in statin-treated subjects (supplemental Table S2).
DISCUSSION
This study examined differences in eicosanoid and other oxylipin levels in human colonic tissue from individuals receiving aspirin and/or statin treatment, two widely prescribed drugs with an attributed protective role in colorectal carcinogenesis.
Aspirin-treated individuals had significantly lower PG and Tx tissue levels than untreated subjects, reflecting the ASA-mediated COX inhibition. PGs exert pro-inflammatory and anti-inflammatory effects in different pathophysiological settings and different tissue types. The pro-tumorigenic role of PGE2 in colon tumorigenesis is well-established (5, 44, 45). In a more general context, PGE2 has been shown to help tumor cells escape immune control (46). Nevertheless, the significance of PGE2 for colonic mucosal homeostasis is rather complex, as it was also found to mediate regeneration of inflamed colon tissue in colitis (47). Furthermore, recent studies have indicated a protective role for PGD2 in colorectal carcinogenesis (48). Our data provide insight into colonic lipid metabolism under the influence of ASA medication in healthy humans and supports the widely hypothesized anti-tumorigenic effect due to aspirin’s effect on PG suppression.
Some studies in CRC cell lines indicated that COX-2 inhibition results in an activation of the 5- lipoxygenase (LOX) pathway (49, 50). At least in healthy colon tissue, our findings do not support this hypothesis, as 5-LOX-derived eicosanoid levels remained essentially unaffected upon ASA treatment.
With respect to changes in the hydroxy-PUFAs formed as side products by COX-2 (51), ASA-treated subjects had significantly lower levels of 11-HETE, 9-HODE, and 13-HDHA compared with untreated individuals. In vitro data shows that ASA-acetylated COX-2 forms (hydroperoxy-)15-HETE and thereby induces the formation of 15-HETE-derived anti-inflammatory metabolites (33, 52, 53). Correspondingly, the n3-PUFA-derived metabolites, 17-HDHA and 18-HEPE, were shown to be formed by acetylated COX-2 and function as precursors of highly potent specialized pro-resolving lipid mediators (40). A redirection of COX-2 metabolism toward 15-HETE and its anti-inflammatory lipoxin derivates upon ASA treatment has been demonstrated in rat hepatocytes (54) and human lung cancer cell lines (55). Furthermore, aspirin treatment led to the production of biologically active lipoxins in colon tissue of patients suffering from ulcerative colitis in vitro (56).
In this study, we found no suppression of 15-HETE, 17- HDHA, and 18-HEPE levels in the colon mucosa of ASA-treated individuals compared with untreated subjects, while 11-HETE, 9-HODE, and 13-HDHA levels were significantly lower, comparable to the observed decrease in prostanoid levels (Fig. 2). Compared with other studies (54, 55, 57, 58), we found an unchanged concentration and did not see higher levels of the acetylated-COX-2 hydro(pero)xy-PUFA in aspirin-treated patients compared with untreated patients. This might be due to the low levels of COX-2 activity that has been described for the normal noninflamed colon mucosa (59) that was analyzed here. As the analysis of COX-1/COX-2 expression in tissue samples was impossible in this study due to lack of sufficient amounts of sample material for oxylipin and protein analysis, this should be addressed in future studies. Furthermore, we believe the question of relative contribution of COX-1 and COX-2 activity to the PGE2 formation to be critical for the assessment of aspirin’s anti-tumor effect in different stages of prevention, i.e., in the context of normal noninflamed mucosa (as in this study), or in the context of adenoma progression toward colorectal carcinoma. In this regard, recent observations in an established murine CRC model impressively revealed the significance of the tumor status on the time of treatment initiation for the efficacy of chemopreventive agents against adenoma formation (60).
With regard to the impact of statins on the AA cascade, effective COX-2 mRNA suppression has been shown in the small nonpolyp intestine of multiple intestinal neoplasia mice upon oral pitavastatin treatment (61). This is not in line with our findings, as we detected a trend to increased PG and Tx levels in colon tissue of statin-treated individuals compared with untreated subjects, but changes did not reach statistical significance. In agreement with our findings, investigations in rat myocardial tissue and human umbilical venous endothelial cells described an upregulation of COX-2 upon atorvastatin treatment leading to increased levels of 15d-PGJ2, a product of PGD2 (62). As we were able to detect slightly higher levels of the COX-2 byproducts, 15-HETE, 13-HDHA, and 17-HDHA, in individuals under statin treatment compared with untreated or ASA-treated subjects (Fig. 2), there could be a combined effect of statin treatment to induce COX-2 expression and activation by S-nitrosylation leading to increased formation of 15-HETE, 13-HDHA, and 17-HDHA (36, 38, 39). More detailed studies in human colon tissue are now necessary to test these hypotheses.
Surprisingly, colon tissue levels of most COX-derived metabolites were notably lower in individuals under dual ASA and statin treatment compared with untreated subjects or subjects on ASA medication alone. The investigation of interaction between ASA and statin medication revealed remarkably small P values, which could suggest a synergistic effect of ASA and statins on lowering colonic tissue PG levels. It should be noted that COX products from different PUFAs and their metabolites showed the described tissue level differences consistently, whereas LOX- and CYP-pathway product concentrations remained unaffected by ASA treatment. To our knowledge, this potentially synergistic effect of aspirin and statins on eicosanoid level suppression in human colonic mucosa has not been described before. Cell culture experiments and investigations in animal models of colorectal carcinogenesis showed that comedication with COX-inhibitors and statins has synergistic effects on colon tumorigenesis prevention and induction of apoptosis, the underlying mechanisms are still unknown (63–66). While a large population-based case-control study did not provide evidence of an interaction between statins and COX inhibitors (16), another study detected a stronger CRC risk reduction for the combination of both drugs (67).
A limitation of the data presented here is that underlying medical conditions leading to the prescription of either ASA and/or statins may affect colon tissue lipid metabolite levels themselves. Furthermore, individuals without ASA and/or statin treatment were significantly younger than individuals receiving these treatments. The effects on colonic oxylipin levels of both, underlying medical conditions and age, could not be determined within this study design. This cross-sectional study did not allow for a comparison of lipid metabolite levels pre/post pharmaceutical treatment, which can be considered a study limitation. However, the robust in vivo effects observed here in a group of routine “walk-in” colonoscopy patients add credibility to the observed differences.
Our study is the first to quantify a wide range of eicosanoids and other oxylipins in human colon biopsy samples of individuals under aspirin and statin treatment, indicating that both drugs mainly affect the levels of COX-derived metabolites. While persons taking aspirin had significantly lower PG and Tx tissue levels (Fig. 1), statin-treated individuals showed a trend toward increased tissue levels of these metabolites (Fig. 2). Patients under dual treatment, however, presented with a pronounced decrease of many PG and Tx levels, even in comparison to individuals under ASA monotherapy, possibly indicating a synergistic effect of both drugs (Fig. 3).
Our data add convincing human data from a routine clinical setting to support the hypotheses put forward in many studies arguing that lipid mediator modification effects might contribute to the chemopreventive effect of ASA (and statins) in the context of colon tumorigenesis. Further studies are now necessary to directly investigate how dual ASA and statin treatment affects colon tumorigenesis in humans.
Supplementary Material
Footnotes
Abbreviations:
- AA
- arachidonic acid
- ASA
- acetylsalicylic acid
- COX
- cyclooxygenase
- CRC
- colorectal cancer
- DGLA
- dihommo γ-linolenic acid
- HDHA
- hydroxydocosahexaenoic acid
- HEPE
- hydroxyeicosapentaenoic acid
- LA
- linoleic acid
- LOQ
- limit of quantitation
- LOX
- lipoxygenase
- LT
- leukotriene
- PG
- prostaglandin
- Tx
- thromboxane
This work was supported by Deutsche Forschungsgemeinschaft Grants WE 2908 and SCHE 1801. The authors declare no conflicts of interest.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D. M., Forman D., and Bray F.. 2015. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell P. M., Wilson M., Elwin C. E., Norrving B., Algra A., Warlow C. P., and Meade T. W.. 2010. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 376: 1741–1750. [DOI] [PubMed] [Google Scholar]
- 3.Li P., Wu H., Zhang H., Shi Y., Xu J., Ye Y., Xia D., Yang J., Cai J., and Wu Y.. 2015. Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: a meta-analysis. Gut. 64: 1419–1425. [DOI] [PubMed] [Google Scholar]
- 4.Wang D., and Dubois R. N.. 2006. Prostaglandins and cancer. Gut. 55: 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., and DuBois R. N.. 2013. An inflammatory mediator, prostaglandin E2, in colorectal cancer. Cancer J. 19: 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rigas B., Goldman I. S., and Levine L.. 1993. Altered eicosanoid levels in human colon cancer. J. Lab. Clin. Med. 122: 518–523. [PubMed] [Google Scholar]
- 7.Frommel T. O., Dyavanapalli M., Oldham T., Kazi N., Lietz H., Liao Y., and Mobarhan S.. 1997. Effect of aspirin on prostaglandin E2 and leukotriene B4 production in human colonic mucosa from cancer patients. Clin. Cancer Res. 3: 209–213. [PubMed] [Google Scholar]
- 8.Wang Y., Zhang F. C., and Wang Y. J.. 2015. The efficacy and safety of non-steroidal anti-inflammatory drugs in preventing the recurrence of colorectal adenoma: a meta-analysis and systematic review of randomized trials. Colorectal Dis. 17: 188–196. [DOI] [PubMed] [Google Scholar]
- 9.Thompson P. A., Ashbeck E. L., Roe D. J., Fales L., Buckmeier J., Wang F., Bhattacharyya A., Hsu C. H., Chow S. H., Ahnen D. J., et al. 2016. Celecoxib for the prevention of colorectal adenomas: results of a suspended randomized controlled trial. J. Natl. Cancer Inst. 108: doi:10.1093/jnci/djw151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benamouzig R., Uzzan B., Deyra J., Martin A., Girard B., Little J., and Chaussade S.; Association pour la Prévention par l’Aspirine du Cancer Colorectal Study Group (APAAC). 2012. Prevention by daily soluble aspirin of colorectal adenoma recurrence: 4-year results of the APACC randomised trial. Gut. 61: 255–261. [DOI] [PubMed] [Google Scholar]
- 11.Baron J. A., Cole B. F., Sandler R. S., Haile R. W., Ahnen D., Bresalier R., McKeown-Eyssen G., Summers R. W., Rothstein R., Burke C. A., et al. 2003. A randomized trial of aspirin to prevent colorectal adenomas. N. Engl. J. Med. 348: 891–899. [DOI] [PubMed] [Google Scholar]
- 12.Sandler R. S., Halabi S., Baron J. A., Budinger S., Paskett E., Keresztes R., Petrelli N., Pipas J. M., Karp D. D., Loprinzi C. L., et al. 2003. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N. Engl. J. Med. 348: 883–890. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien M. J., Winawer S. J., Zauber A. G., Gottlieb L. S., Sternberg S. S., Diaz B., Dickersin G. R., Ewing S., Geller S., Kasimian D., et al. 1990. The National Polyp Study. Patient and polyp characteristics associated with high-grade dysplasia in colorectal adenomas. Gastroenterology. 98: 371–379. [PubMed] [Google Scholar]
- 14.Zauber A. G., Winawer S. J., O’Brien M. J., Lansdorp-Vogelaar I., van Ballegooijen M., Hankey B. F., Shi W., Bond J. H., Schapiro M., Panish J. F., et al. 2012. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl. J. Med. 366: 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burn J., Gerdes A. M., Macrae F., Mecklin J. P., Moeslein G., Olschwang S., Eccles D., Evans D. G., Maher E. R., Bertario L., et al. 2011. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 378: 2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poynter J. N., Gruber S. B., Higgins P. D., Almog R., Bonner J. D., Rennert H. S., Low M., Greenson J. K., and Rennert G.. 2005. Statins and the risk of colorectal cancer. N. Engl. J. Med. 352: 2184–2192. [DOI] [PubMed] [Google Scholar]
- 17.Liu J. C., Hao W. R., Hsu Y. P., Sung L. C., Kao P. F., Lin C. F., Wu A. T., Yuan K. S., and Wu S. Y.. 2016. Statins dose-dependently exert a significant chemopreventive effect on colon cancer in patients with chronic obstructive pulmonary disease: a population-based cohort study. Oncotarget. 7: 65270–65283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ananthakrishnan A. N., Cagan A., Cai T., Gainer V. S., Shaw S. Y., Churchill S., Karlson E. W., Murphy S. N., Liao K. P., and Kohane I.. 2016. Statin use is associated with reduced risk of colorectal cancer in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 14: 973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siddiqui A. A., Nazario H., Mahgoub A., Pandove S., Cipher D., and Spechler S. J.. 2009. The long-term use of statins is associated with a decreased incidence of adenomatous colon polyps. Digestion. 79: 17–22. [DOI] [PubMed] [Google Scholar]
- 20.Jung Y. S., Park C. H., Eun C. S., Park D. I., and Han D. S.. 2016. Statin use and the risk of colorectal adenoma: a meta-analysis. J. Gastroenterol. Hepatol. 31: 1823–1830. [DOI] [PubMed] [Google Scholar]
- 21.Cardwell C. R., Hicks B. M., Hughes C., and Murray L. J.. 2014. Statin use after colorectal cancer diagnosis and survival: a population-based cohort study. J. Clin. Oncol. 32: 3177–3183. [DOI] [PubMed] [Google Scholar]
- 22.Mace A. G., Gantt G. A., Skacel M., Pai R., Hammel J. P., and Kalady M. F.. 2013. Statin therapy is associated with improved pathologic response to neoadjuvant chemoradiation in rectal cancer. Dis. Colon Rectum. 56: 1217–1227. [DOI] [PubMed] [Google Scholar]
- 23.Shao Y. Y., Hsu C. H., Yeh K. H., Chen H. M., Yeh Y. C., Lai C. L., Lin Z. Z., Cheng A. L., and Lai M. S.. 2015. Statin use is associated with improved prognosis of colorectal cancer in Taiwan. Clin. Colorectal Cancer. 14: 177–184.e4. [DOI] [PubMed] [Google Scholar]
- 24.Dolkart O., Amar E., Shapira S., Marmor S., Steinberg E. L., and Weinbroum A. A.. 2015. Protective effects of rosuvastatin in a rat model of lung contusion: Stimulation of the cyclooxygenase 2-prostaglandin E-2 pathway. Surgery. 157: 944–953. [DOI] [PubMed] [Google Scholar]
- 25.Chen J. C., Huang K. C., Wingerd B., Wu W. T., and Lin W. W.. 2004. HMG-CoA reductase inhibitors induce COX-2 gene expression in murine macrophages: role of MAPK cascades and promoter elements for CREB and C/EBPbeta. Exp. Cell Res. 301: 305–319. [DOI] [PubMed] [Google Scholar]
- 26.Heeba G. H., Hassan M. K., and Amin R. S.. 2009. Gastroprotective effect of simvastatin against indomethacin-induced gastric ulcer in rats: role of nitric oxide and prostaglandins. Eur. J. Pharmacol. 607: 188–193. [DOI] [PubMed] [Google Scholar]
- 27.Dolkart O., Liron T., Chechik O., Somjen D., Brosh T., Maman E., and Gabet Y.. 2014. Statins enhance rotator cuff healing by stimulating the COX2/PGE2/EP4 pathway: an in vivo and in vitro study. Am. J. Sports Med. 42: 2869–2876. [DOI] [PubMed] [Google Scholar]
- 28.Habib A., Shamseddeen I., Nasrallah M. S., Antoun T. A., Nemer G., Bertoglio J., Badreddine R., and Badr K. F.. 2007. Modulation of COX-2 expression by statins in human monocytic cells. FASEB J. 21: 1665–1674. [DOI] [PubMed] [Google Scholar]
- 29.Santodomingo-Garzón T., Cunha T. M., Verri W. A. Jr., Valério D. A., Parada C. A., Poole S., Ferreira S. H., and Cunha F. Q.. 2006. Atorvastatin inhibits inflammatory hypernociception. Br. J. Pharmacol. 149: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martín-Ventura J. L., Blanco-Colio L. M., Gómez-Hernández A., Muñoz-García B., Vega M., Serrano J., Ortega L., Hernández G., Tuñón J., and Egido J.. 2005. Intensive treatment with atorvastatin reduces inflammation in mononuclear cells and human atherosclerotic lesions in one month. Stroke. 36: 1796–1800. [DOI] [PubMed] [Google Scholar]
- 31.Rowlinson S. W., Crews B. C., Goodwin D. C., Schneider C., Gierse J. K., and Marnett L. J.. 2000. Spatial requirements for 15-(R)-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid synthesis within the cyclooxygenase active site of murine COX-2. Why acetylated COX-1 does not synthesize 15-(R)-hete. J. Biol. Chem. 275: 6586–6591. [DOI] [PubMed] [Google Scholar]
- 32.Schneider C., Boeglin W. E., Prusakiewicz J. J., Rowlinson S. W., Marnett L. J., Samel N., and Brash A. R.. 2002. Control of prostaglandin stereochemistry at the 15-carbon by cyclooxygenases-1 and -2. A critical role for serine 530 and valine 349. J. Biol. Chem. 277: 478–485. [DOI] [PubMed] [Google Scholar]
- 33.Lecomte M., Laneuville O., Ji C., DeWitt D. L., and Smith W. L.. 1994. Acetylation of human prostaglandin endoperoxide synthase-2 (cyclooxygenase-2) by aspirin. J. Biol. Chem. 269: 13207–13215. [PubMed] [Google Scholar]
- 34.Mancini J. A., O’Neill G. P., Bayly C., and Vickers P. J.. 1994. Mutation of serine-516 in human prostaglandin G/H synthase-2 to methionine or aspirin acetylation of this residue stimulates 15-R-HETE synthesis. FEBS Lett. 342: 33–37. [DOI] [PubMed] [Google Scholar]
- 35.Atar S., Ye Y., Lin Y., Freeberg S. Y., Nishi S. P., Rosanio S., Huang M. H., Uretsky B. F., Perez-Polo J. R., and Birnbaum Y.. 2006. Atorvastatin-induced cardioprotection is mediated by increasing inducible nitric oxide synthase and consequent S-nitrosylation of cyclooxygenase-2. Am. J. Physiol. Heart Circ. Physiol. 290: H1960–H1968. [DOI] [PubMed] [Google Scholar]
- 36.Birnbaum Y., Ye Y., Lin Y., Freeberg S. Y., Nishi S. P., Martinez J. D., Huang M. H., Uretsky B. F., and Perez-Polo J. R.. 2006. Augmentation of myocardial production of 15-epi-lipoxin-a4 by pioglitazone and atorvastatin in the rat. Circulation. 114: 929–935. [DOI] [PubMed] [Google Scholar]
- 37.González-Herrera F., Cramer A., Pimentel P., Castillo C., Liempi A., Kemmerling U., Machado F. S., and Maya J. D.. 2017. Simvastatin attenuates endothelial activation through 15-epi-lipoxin A4 production in murine chronic Chagas cardiomyopathy. Antimicrob. Agents Chemother. 61: doi:10.1128/AAC.02137-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Planagumà A., Pfeffer M. A., Rubin G., Croze R., Uddin M., Serhan C. N., and Levy B. D.. 2010. Lovastatin decreases acute mucosal inflammation via 15-epi-lipoxin A4. Mucosal Immunol. 3: 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalli J., Chiang N., and Serhan C. N.. 2015. Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat. Med. 21: 1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serhan C. N., Clish C. B., Brannon J., Colgan S. P., Chiang N., and Gronert K.. 2000. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 192: 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serhan C. N. 2014. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 510: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weylandt K. H., Chiu C. Y., Gomolka B., Waechter S. F., and Wiedenmann B.. 2012. Omega-3 fatty acids and their lipid mediators: towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 97: 73–82. [DOI] [PubMed] [Google Scholar]
- 43.Ostermann A. I., Willenberg I., and Schebb N. H.. 2015. Comparison of sample preparation methods for the quantitative analysis of eicosanoids and other oxylipins in plasma by means of LC-MS/MS. Anal. Bioanal. Chem. 407: 1403–1414. [DOI] [PubMed] [Google Scholar]
- 44.Kawamori T., Uchiya N., Sugimura T., and Wakabayashi K.. 2003. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis. 24: 985–990. [DOI] [PubMed] [Google Scholar]
- 45.Wang D., and Dubois R. N.. 2010. Eicosanoids and cancer. Nat. Rev. Cancer. 10: 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zelenay S., van der Veen A. G., Bottcher J. P., Snelgrove K. J., Rogers N., Acton S. E., Chakravarty P., Girotti M. R., Marais R., Quezada S. A., et al. 2015. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell. 162: 1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim H. B., Kim M., Park Y. S., Park I., Kim T., Yang S. Y., Cho C. J., Hwang D., Jung J. H., Markowitz S. D., et al. 2017. Prostaglandin E2 activates YAP and a positive-signaling loop to promote colon regeneration following colitis but also carcinogenesis in mice. Gastroenterology. 152: 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iwanaga K., Nakamura T., Maeda S., Aritake K., Hori M., Urade Y., Ozaki H., and Murata T.. 2014. Mast cell-derived prostaglandin D2 inhibits colitis and colitis-associated colon cancer in mice. Cancer Res. 74: 3011–3019. [DOI] [PubMed] [Google Scholar]
- 49.Cianchi F., Cortesini C., Magnelli L., Fanti E., Papucci L., Schiavone N., Messerini L., Vannacci A., Capaccioli S., Perna F., et al. 2006. Inhibition of 5-lipoxygenase by MK886 augments the antitumor activity of celecoxib in human colon cancer cells. Mol. Cancer Ther. 5: 2716–2726. [DOI] [PubMed] [Google Scholar]
- 50.Che X. H., Chen C. L., Ye X. L., Weng G. B., Guo X. Z., Yu W. Y., Tao J., Chen Y. C., and Chen X.. 2016. Dual inhibition of COX-2/5-LOX blocks colon cancer proliferation, migration and invasion in vitro. Oncol. Rep. 35: 1680–1688. [DOI] [PubMed] [Google Scholar]
- 51.Gabbs M., Leng S., Devassy J. G., Monirujjaman M., and Aukema H. M.. 2015. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv. Nutr. 6: 513–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma N. P., Dong L., Yuan C., Noon K. R., and Smith W. L.. 2010. Asymmetric acetylation of the cyclooxygenase-2 homodimer by aspirin and its effects on the oxygenation of arachidonic, eicosapentaenoic, and docosahexaenoic acids. Mol. Pharmacol. 77: 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serhan C. N., Takano T., and Maddox J. F.. 1999. Aspirin-triggered 15-epi-lipoxin A4 and stable analogs on lipoxin A4 are potent inhibitors of acute inflammation. Receptors and pathways. Adv. Exp. Med. Biol. 447: 133–149. [DOI] [PubMed] [Google Scholar]
- 54.Titos E., Chiang N., Serhan C. N., Romano M., Gaya J., Pueyo G., and Claria J.. 1999. Hepatocytes are a rich source of novel aspirin-triggered 15-epi-lipoxin A(4). Am. J. Physiol. 277: C870–C877. [DOI] [PubMed] [Google Scholar]
- 55.Clària J., Lee M. H., and Serhan C. N.. 1996. Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)-neutrophil interactions and are potent inhibitors of cell proliferation. Mol. Med. 2: 583–596. [PMC free article] [PubMed] [Google Scholar]
- 56.Mangino M. J., Brounts L., Harms B., and Heise C.. 2006. Lipoxin biosynthesis in inflammatory bowel disease. Prostaglandins Other Lipid Mediat. 79: 84–92. [DOI] [PubMed] [Google Scholar]
- 57.Kowalski M. L., Ptasinska A., Bienkiewicz B., Pawliczak R., and DuBuske L.. 2003. Differential effects of aspirin and misoprostol on 15-hydroxyeicosatetraenoic acid generation by leukocytes from aspirin-sensitive asthmatic patients. J. Allergy Clin. Immunol. 112: 505–512. [DOI] [PubMed] [Google Scholar]
- 58.Korosec P., Tisler U., Bajrovic N., Silar M., Mrhar A., and Kosnik M.. 2011. Acetylsalicylic acid-triggered 15-HETE generation by peripheral leukocytes for identifying ASA sensitivity. Respir. Med. 105(Suppl 1): S81–S83. [DOI] [PubMed] [Google Scholar]
- 59.Wiese F. W., Thompson P. A., Warneke J., Einspahr J., Alberts D. S., and Kadlubar F. F.. 2003. Variation in cyclooxygenase expression levels within the colorectum. Mol. Carcinog. 37: 25–31. [DOI] [PubMed] [Google Scholar]
- 60.Chang W. L., Jackson C., Riel S., Cooper H. S., Devarajan K., Hensley H. H., Zhou Y., Vanderveer L. A., Nguyen M. T., and Clapper M. L.. Differential preventive activity of sulindac and atorvastatin in Apc+/Min-FCCCmice with or without colorectal adenomas. Gut. Epub ahead of print. November 9, 2017; doi:10.1136/gutjni-2017-313942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teraoka N., Mutoh M., Takasu S., Ueno T., Yamamoto M., Sugimura T., and Wakabayashi K.. 2011. Inhibition of intestinal polyp formation by pitavastatin, a HMG-CoA reductase inhibitor. Cancer Prev. Res. (Phila.). 4: 445–453. [DOI] [PubMed] [Google Scholar]
- 62.Ye Y., Nishi S. P., Manickavasagam S., Lin Y., Huang M. H., Perez-Polo J. R., Uretsky B. F., and Birnbaum Y.. 2007. Activation of peroxisome proliferator-activated receptor-gamma (PPAR-gamma) by atorvastatin is mediated by 15-deoxy-delta-12,14-PGJ2. Prostaglandins Other Lipid Mediat. 84: 43–53. [DOI] [PubMed] [Google Scholar]
- 63.Suh N., Reddy B. S., DeCastro A., Paul S., Lee H. J., Smolarek A. K., So J. Y., Simi B., Wang C. X., Janakiram N. B., et al. 2011. Combination of atorvastatin with sulindac or naproxen profoundly inhibits colonic adenocarcinomas by suppressing the p65/beta-catenin/cyclin D1 signaling pathway in rats. Cancer Prev. Res. (Phila.). 4: 1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao H., Zhang Q., Lin Y., Reddy B. S., and Yang C. S.. 2008. Combination of atorvastatin and celecoxib synergistically induces cell cycle arrest and apoptosis in colon cancer cells. Int. J. Cancer. 122: 2115–2124. [DOI] [PubMed] [Google Scholar]
- 65.Agarwal B., Rao C. V., Bhendwal S., Ramey W. R., Shirin H., Reddy B. S., and Holt P. R.. 1999. Lovastatin augments sulindac-induced apoptosis in colon cancer cells and potentiates chemopreventive effects of sulindac. Gastroenterology. 117: 838–847. [DOI] [PubMed] [Google Scholar]
- 66.Reddy B. S., Wang C. X., Kong A. N., Khor T. O., Zheng X., Steele V. E., Kopelovich L., and Rao C. V.. 2006. Prevention of azoxymethane-induced colon cancer by combination of low doses of atorvastatin, aspirin, and celecoxib in F 344 rats. Cancer Res. 66: 4542–4546. [DOI] [PubMed] [Google Scholar]
- 67.Hoffmeister M., Chang-Claude J., and Brenner H.. 2007. Individual and joint use of statins and low-dose aspirin and risk of colorectal cancer: a population-based case-control study. Int. J. Cancer. 121: 1325–1330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.