Abstract
Identifying periods of increased vulnerability to air pollution during pregnancy with respect to the development of adverse birth outcomes can improve understanding of possible mechanisms of disease development and provide guidelines for protection of the child. Exposure to air pollution during pregnancy is typically based on the mother’s residence at delivery, potentially resulting in exposure misclassification and biasing the estimation of critical windows of pregnancy. In this study, we determined the impact of maternal residential mobility during pregnancy on defining weekly exposure to particulate matter less than or equal to 10 μm in aerodynamic diameter (PM10) and estimating windows of susceptibility to term low birth weight. We utilized data sets from 4 Connecticut birth cohorts (1988–2008) that included information on all residential addresses between conception and delivery for each woman. We designed a simulation study to investigate the impact of increasing levels of mobility on identification of critical windows. Increased PM10 exposure during pregnancy weeks 16–18 was associated with an increased probability of term low birth weight. Ignoring residential mobility when defining weekly exposure had only a minor impact on the identification of critical windows for PM10 and term low birth weight in the data application and simulation study. Identification of critical pregnancy windows was robust to exposure misclassification caused by ignoring residential mobility in these Connecticut birth cohorts.
Keywords: Bayesian statistics, critical pregnancy windows, exposure misclassification, term low birth weight
Exposure to ambient air pollution during pregnancy is associated with a number of adverse birth outcomes, including but not limited to preterm birth, low birth weight, and development of congenital anomalies (1, 2). The majority of past statistical models investigating these associations have incorporated exposure to ambient pollution concentrations into a regression framework using averages based on prespecified periods of pregnancy, such as specific weeks, months, and trimesters of pregnancy and the entire pregnancy. Typically, separate models are fitted for each exposure definition and multiple comparisons are made to test specific hypotheses regarding timing of exposure.
More recently, there has been increasing interest in identifying more specific periods of increased vulnerability to environmental exposures, known as critical windows of pregnancy, within a single modeling framework. The National Institute of Environmental Health Sciences recently included the identification of critical windows of susceptibility as part of its set of strategic goals (3). A better understanding of the specific timing of exposure and outcome development could lead to improved mechanistic explanations for disease development, as well as focused guidelines for protection of the fetus. A number of statistical methods have been developed for estimating these critical windows of development and have been successfully applied to adverse birth outcomes, including preterm birth (4, 5), low birth weight (6, 7), and cardiac congenital anomalies (8, 9).
In the majority of these past studies, exposures incurred during pregnancy have been defined on the basis of the mother’s residence at delivery, under the assumption(s) that only a small proportion of women move between conception and delivery and/or that these women typically move only a short distance. Such approaches to exposure ascertainment are typically necessitated by data sets that have residence information available only for the time of birth. A recent review suggested that 9%–32% of women move at least once during pregnancy, though the majority move a relatively small distance (<10 km), and that residential mobility can differ by individual characteristics, including age, parity, socioeconomic status, and marital status (10). Ignoring maternal residential mobility during pregnancy can lead to exposure misclassification for these women. In related work, Pereira et al. (11) investigated the impact of this misclassification error when standard epidemiologic analyses based on prespecified pollution averaging periods (e.g., trimester, entire pregnancy) are used in separately fitted statistical models. Their findings suggested that results from these models are robust to the introduced error. However, when estimating critical windows of exposure, smaller pollution-exposure averaging periods (e.g., daily or weekly exposures) are often considered jointly in a model. The impact that this misclassification has on finer-scale averaging periods and the resulting critical window estimation is currently unknown and is difficult to address given the limited availability of full residential histories.
In this work, we aimed to determine what impact maternal residential mobility has on identification of critical windows of susceptibility during pregnancy. Working with multiple birth cohort studies from Connecticut (1988–2008) that included full residential histories, we defined exposure 1) on the basis of residence at delivery and 2) accounting for all residential movement during pregnancy. These exposure definitions were compared and misclassification error was quantified across each week of pregnancy. We then applied the critical window identification method of Warren et al. (5) to investigate the timing of development of term low birth weight with respect to average weekly exposures to particulate matter less than or equal to 10 μm in aerodynamic diameter (PM10). We applied the method to each exposure definition and compared the results. Finally, in a simulation study, we investigated the potential impact on critical window identification as the proportion of women who moved during pregnancy increased.
METHODS
Data description
We utilized data on 4 Connecticut birth cohorts (1988–2008), consisting of information from the Environmental Tobacco Smoke Study (12) (1988–1991; n = 2,781), the Nutrition in Pregnancy Study (13) (1996–1999; n = 2,344), the Asthma in Pregnancy Study (14) (1996–2000; n = 2,255), and the Pink and Blue Study, a study of depression during pregnancy (15) (2005–2008; n = 2,645). Full geocoded residential history information was available for each woman throughout the entire pregnancy. These data have been previously described (11). The Yale University institutional review board approved the study protocol, and participation of human subjects did not occur until after informed consent had been obtained.
All analyses were limited to singletons born alive at or after term (gestational age ≥37 weeks). We removed 785 women from the data set because their pregnancies did not meet these conditions. Weekly ambient PM10 concentrations (inverse distance–weighted value of all monitors within 100 km of the residence) were calculated for each woman in the study based on her specific calendar dates of pregnancy and spatial location. Gestational age was obtained from birth certificate records and represented the best available clinical estimate for each woman. When available, ultrasound estimates were used; otherwise, the date of the last menstrual period was used. PM10 was explored rather than particulate matter less than or equal to 2.5 μm in aerodynamic diameter (PM2.5) because PM2.5 was not routinely measured during the study period. The spatial linking of exposures was done in 2 ways: 1) ignoring residential mobility by linking exposures based on residence at delivery and 2) accounting for full residential history during the pregnancy. In the study, we focused on term low birth weight, which occurs when the birth weight of an infant at 37 or more completed weeks of gestation is less than 2,500 g. Term low birth weight is associated with a number of immediate health concerns in newborns, as well as the development of adverse health outcomes later in life. Table 1 displays the available covariates in the final analysis data set. We removed 445 women from the data set because of missing outcome or covariate information, leaving 8,795 births for analysis.
Table 1.
Characteristics of 4 Connecticut Birth Cohortsa Included in a Study of Residential Mobility and Identification of Critical Windows of Susceptibility to Air Pollution Exposure, 1988–2008
| Characteristic | Movers (n = 965) | Nonmovers (n = 7,830) | ||
|---|---|---|---|---|
| Mean (SD) | % | Mean (SD) | % | |
| Gravidityb | 2.29 (1.40) | 2.49 (1.46) | ||
| Parityb | 0.64 (0.90) | 0.85 (0.92) | ||
| Maternal body mass indexc | 24.62 (5.52) | 24.56 (5.52) | ||
| Gestational age, weeksb | 39.65 (1.33) | 39.50 (1.31) | ||
| Previous preterm birth | 0.03 | 0.03 | ||
| Low-birth-weight outcome | 0.02 | 0.02 | ||
| Previous low-birth-weight birth | 0.05 | 0.05 | ||
| Sex of child (female) | 0.50 | 0.50 | ||
| Marital status (single)b | 0.43 | 0.19 | ||
| Maternal race/ethnicityb | ||||
| White | 0.66 | 0.79 | ||
| Black | 0.11 | 0.07 | ||
| Other | 0.23 | 0.15 | ||
| Maternal educational levelb | ||||
| Did not complete high school | 0.15 | 0.07 | ||
| Completed high school | 0.21 | 0.16 | ||
| Postsecondary education | 0.47 | 0.51 | ||
| Graduate school or above | 0.17 | 0.26 | ||
| Maternal age, yearsb | ||||
| <25.0 | 0.33 | 0.15 | ||
| 25.0–29.9 | 0.30 | 0.27 | ||
| 30.0–34.9 | 0.27 | 0.37 | ||
| ≥35.0 | 0.10 | 0.21 | ||
| Season of birth | ||||
| Winter (December–February) | 0.26 | 0.25 | ||
| Spring (March–May) | 0.23 | 0.26 | ||
| Summer (June–August) | 0.24 | 0.24 | ||
| Fall (September–November) | 0.28 | 0.25 | ||
| PM10 exposure, μg/m3 | ||||
| Entire pregnancyb | 22.18 (9.58) | 21.99 (9.55) | ||
| Trimester 1 | 22.32 (9.68) | 22.15 (9.68) | ||
| Trimester 2 | 22.11 (9.66) | 22.05 (9.66) | ||
| Trimester 3b | 22.11 (9.40) | 21.77 (9.32) | ||
Abbreviations: PM10, particulate matter less than or equal to 10 μm in aerodynamic diameter; SD, standard deviation.
a The Environmental Tobacco Smoke Study (12) (1988–1991; n = 2,781), the Nutrition in Pregnancy Study (13) (1996–1999; n = 2,344), the Asthma in Pregnancy Study (14) (1996–2000; n = 2,255), and the Pink and Blue Study (15) (2005–2008; n = 2,645).
bP < 0.05 for difference between the 2 groups of women.
c Weight (kg)/height (m)2.
Statistical model
We applied the previously established statistical model of Warren et al. (5) to identify critical windows of susceptibility with respect to weekly exposure to PM10 and development of term low birth weight. This method represents a probit regression model, fitted in the Bayesian setting, that includes weekly PM10 exposure across the entire pregnancy for each woman within a single modeling framework while accounting for the correlation between exposures through the use of a temporally smoothed Gaussian process prior distribution for the risk parameters. Risk parameters associated with pregnancy weeks that are closer together in time are assumed to be more similar, allowing for smoothing of the estimation over the pregnancy weeks. This model helps overcome issues related to multicollinearity when dealing with highly correlated daily and weekly exposures. The form of the model is similar to that of a multivariable probit regression that accounts for exposure and covariates, but the introduced prior structure helps to stabilize parameter estimation and reduce uncertainty in the estimated parameters. Additional details on the statistical model, prior distribution specifications, and model fitting are presented in the Web Appendix (available at https://academic.oup.com/aje).
Data application
We begin by creating 2 different PM10 exposure data sets for our Connecticut birth cohorts. The first defines weekly exposure throughout the pregnancy based only on residence at delivery, ignoring the possibility that a portion of the women moved at some point between conception and delivery. This exposure definition represents the most common metric used in practice and mimics a data set for which only residential address at birth is available. The second defines exposure on the basis of the full set of residential addresses for each woman in the study and thereby accounts for changes in exposure that occur due to the woman’s moving during pregnancy. These data are often unavailable because of data collection limitations, such as the frequent use of birth registries. Using the Connecticut birth cohort data and both exposure data sets, we quantify the number of women who moved between conception and delivery and how this movement changed the average exposure level during each week of pregnancy.
Next, we apply the critical window statistical model to both exposure data sets in separate models and compare the estimated critical windows of susceptibility with respect to development of term low birth weight.
Simulation study
We design a simulation study to investigate the impact of increasing levels of residential mobility on critical window estimation. We explore the impact on this estimation with simulations assuming that 25%, 50%, and 75% of the population moves at least once during pregnancy.
In order to create a data set with the required proportion of population mobility, we begin by using our actual Connecticut birth cohort data to establish the overall sample size and characteristics of the population. Next, we randomly sample the women whom we designate as “movers” during the pregnancy. The number of women selected depends on the proportion of mobility we are currently working with (25%, 50%, or 75% of the entire sample). We ensure that the selected group of movers is similar to our observed data set by assigning higher sampling weights to women who are younger, less educated, nonwhite, and single (see Table 1). Once the movers are identified, we displace their true PM10 exposure in each pregnancy week to create misclassified exposures. To do this, we randomly sample from the true distribution of exposure differences in each pregnancy week that we observe in our actual data (see Figure 1). Displaced exposures less than zero and greater than the largest observed exposure are redisplaced in order to create realistic exposures. Those women designated as nonmovers do not have their exposures altered from the original estimates. The women designated as movers and nonmovers are reselected for each simulated data set.
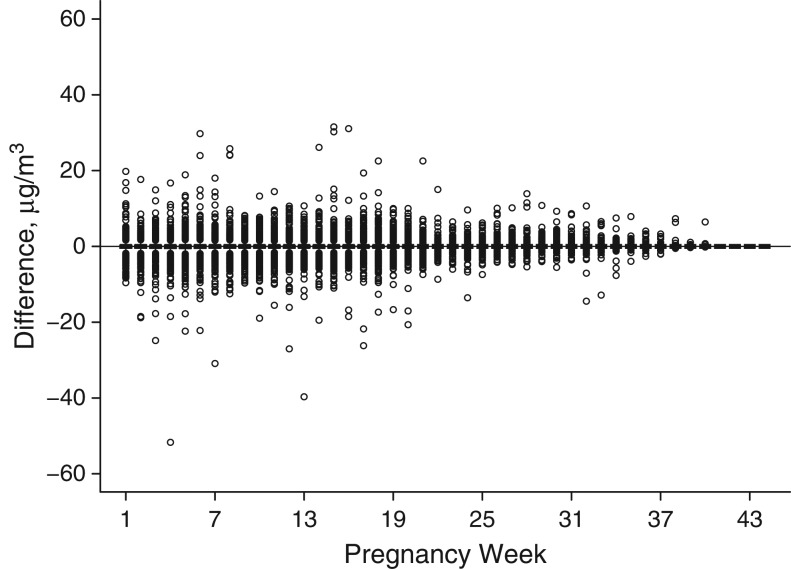
Figure 1.
Differences in air pollution exposure metrics according to week of pregnancy for 4 Connecticut birth cohorts, 1988–2008. Exposure metric 1: exposures based on full maternal residential address histories; exposure metric 2: exposures based on residence at delivery. Differences are shown as exposure metric 2 minus exposure metric 1.
We repeat this process 100 times for each of the 3 mobility pattern proportions, creating 100 data sets for analysis in each setting. For each data set, we apply the critical window statistical model, ignoring the residential mobility of the population, and compare the estimated critical windows with the critical windows that would be estimated if we accounted for full residential mobility (full mobility results).
We estimate the bias, mean absolute error, and mean squared error for each weekly risk parameter estimator (posterior mean), lower limit of the 95% credible interval (0.025 posterior quantile), and upper limit of the 95% credible interval (0.975 posterior quantile) with respect to the full mobility results. We then average these metrics over all pregnancy weeks to obtain an average bias, average mean absolute error, and average mean squared error for each estimator. This process is repeated for each setting of the proportion of movers in the population. We also monitor the proportion of times (out of 100 simulated data sets) that each pregnancy week was identified as a critical window, meaning that its 95% credible interval failed to include zero, regardless of the sign of the estimate. In addition, the mean value of the 100 posterior mean estimates of risk during each pregnancy week and the mean of the 100 lower and upper 95% credible interval limits in each pregnancy week are collected and compared graphically with the actual posterior means and quantiles in the full mobility results.
RESULTS
Data description
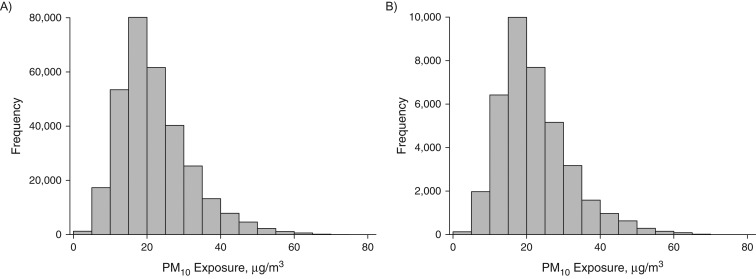
Table 1 describes the Connecticut birth cohorts and available covariates by mobility status and gives statistical testing results for comparisons of attributes between the 2 groups (t tests for continuous variables and χ2 tests for categorical variables). Overall, approximately 1.6% of the sample pregnancies resulted in term low birth weight, lower than the most recent reported US prevalence of 2.8% (16). Women who moved during pregnancy were more likely to be younger, single, less educated, and nonwhite, to have lower gravidity and parity, to have a longer period of gestation, and to have higher third-trimester average exposure to PM10. In Figure 2, we display the histograms of weekly PM10 exposures for all women across all pregnancy weeks by mobility status.
Figure 2.
Weekly exposure to particulate matter less than or equal to 10 μm in aerodynamic diameter (PM10) according to pregnancy mobility status for 4 Connecticut birth cohorts, 1988–2008. A) Nonmovers; B) movers.
Data application
For the full set of women who moved during pregnancy (before removing women with missing covariates), the median distance traveled was 5 km (interquartile range, 2–13 km), with women moving to areas with lower levels of PM10, on average (11). In the final analysis data set, 965 of the 8,795 women (10.97%) moved at least once during pregnancy. For these 965 women, 45.05% of their weekly PM10 exposures based on full residential mobility did not differ from exposures based on residence at delivery alone. This indicates that the distance traveled may have been short for these women overall, in agreement with the review of Bell and Belanger (10). We compared the 2 exposure definitions for these women who moved at least once and for whom some change in a weekly exposure occurred, by calculating the absolute value of the difference in PM10 exposures during those particular pregnancy weeks. The average value, standard deviation, and range of these absolute differences were 1.05, 1.84, and 0–51.7 μg/m3, respectively. A histogram of these absolute differences in the exposure metrics is displayed in Web Figure 1.
Next, we investigate how these exposure differences are distributed over each pregnancy week. In Figure 1, we display the differences in exposure definitions (residence at delivery minus full residential mobility) for the women who moved at least once during the pregnancy, in each pregnancy week, while in Web Figure 2 we show the number of these women who have not given birth by each week of pregnancy. It is clear from Figure 1 that as gestational age increases, the exposure misclassification decreases. This may be a result of women moving earlier in pregnancy and remaining stationary later in the pregnancy. Web Figure 2 suggests that after week 37 of pregnancy, the number of women who are still pregnant begins to decrease, as expected. This would also lead to a smaller amount of misclassification error during these later pregnancy weeks.
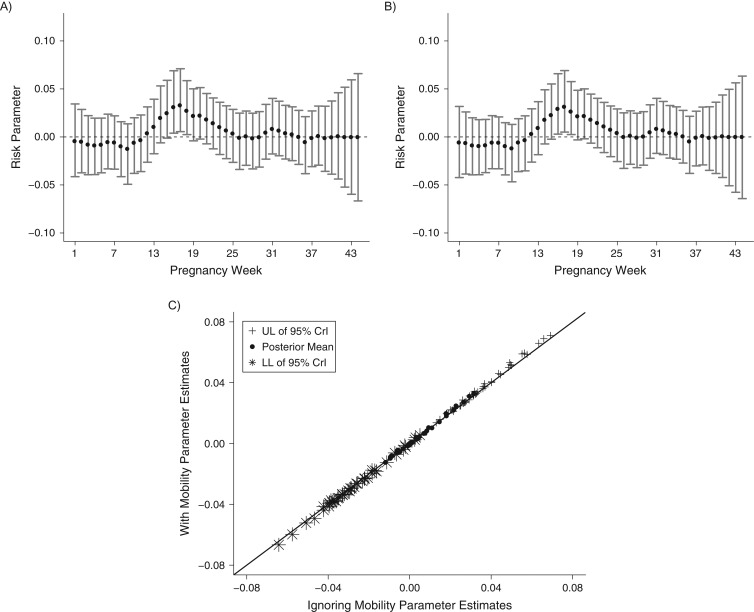
Finally, we analyze results from the critical window identification statistical model fitted to each exposure data set. Figure 3 shows the estimated critical window results (posterior means and 95% credible intervals) for the model fit to both data sets, as well as a scatterplot for comparing estimates between the 2 models. Individual week risk parameters with 95% credible intervals that are completely above zero are referred to as critical windows. Increased exposure to PM10 during these weeks is associated with an increased probability of term low birth weight. The results in Figure 3 from the different exposure data sets are nearly identical, with the same critical windows being identified: pregnancy weeks 16–18. Web Tables 1 and 2 display inference for the included covariates and remaining model parameters, respectively.
Figure 3.
Estimated critical windows for development of low birth weight with respect to weekly PM10 exposure during pregnancy for 4 Connecticut birth cohorts, 1988–2008. A) Exposure metric 1: exposures based on full maternal residential address histories; B) exposure metric 2: exposures based on residence at delivery; C) scatterplot of parameter estimates from parts A and B. CrI, credible interval; LL, lower limit; PM10, particulate matter less than or equal to 10 μm in aerodynamic diameter; UL, upper limit.
Simulation study
In Table 2, we present the average bias, mean absolute error, and mean squared error for all estimators and for each setting of the proportion of movers in the population. There is a steady increase in average mean absolute error for each of 3 estimators as the proportion of movers increases from 25% to 75%. We also present the proportion of times (out of the 100 simulated data sets) that a pregnancy week was identified as a critical window. If estimation if not affected by the increased exposure misclassification, we would expect these proportions to be large during pregnancy weeks 16–18 (as seen in our data application) and low otherwise. This appears to be the case across each of the 3 proportions of movers, though the probability of incorrectly identifying a critical window increases as the proportion of movers increases, while remaining low overall.
Table 2.
Identification of Critical Windows of Susceptibility to Air Pollution Exposure According to Residential Mobility (Simulation Study) in 4 Connecticut Birth Cohorts, 1988–2008
| Estimator | Maternal Residential Mobilitya | ||
|---|---|---|---|
| 25% Mobility | 50% Mobility | 75% Mobility | |
| Posterior meanb | |||
| Average bias | −0.03 (0.03)c | −0.03 (0.03) | −0.01 (0.03) |
| Average MAE | 0.77 (0.03) | 0.94 (0.04) | 1.07 (0.04) |
| Average MSE | 0.001 (0.0000) | 0.002 (0.0001) | 0.002 (0.0002) |
| Posterior 0.975 quantileb | |||
| Average bias | −0.42 (0.05) | −0.49 (0.05) | −0.39 (0.05) |
| Average MAE | 1.38 (0.06) | 1.66 (0.08) | 1.68 (0.07) |
| Average MSE | 0.004 (0.0003) | 0.005 (0.0005) | 0.005 (0.0004) |
| Posterior 0.025 quantileb | |||
| Average bias | 0.28 (0.04) | 0.35 (0.04) | 0.33 (0.04) |
| Average MAE | 1.38 (0.05) | 1.61 (0.06) | 1.69 (0.05) |
| Average MSE | 0.007 (0.0002) | 0.005 (0.0003) | 0.005 (0.0003) |
| Estimated critical window (proportion of times significant) | |||
| Week 16 | 1.00 | 1.00 | 1.00 |
| Week 17 | 1.00 | 1.00 | 1.00 |
| Week 18 | 1.00 | 1.00 | 0.97 |
| Maximum in any other week (proportion) | 0.05 | 0.08 | 0.20 |
| Average across any other weeks (proportion) | 0.001 | 0.002 | 0.005 |
Abbreviations: MAE, mean absolute error; MSE, mean squared error.
a Percentage of women who moved during pregnancy.
b Estimates were multiplied by 1,000 for display purposes.
c Standard errors are presented in parentheses where applicable.
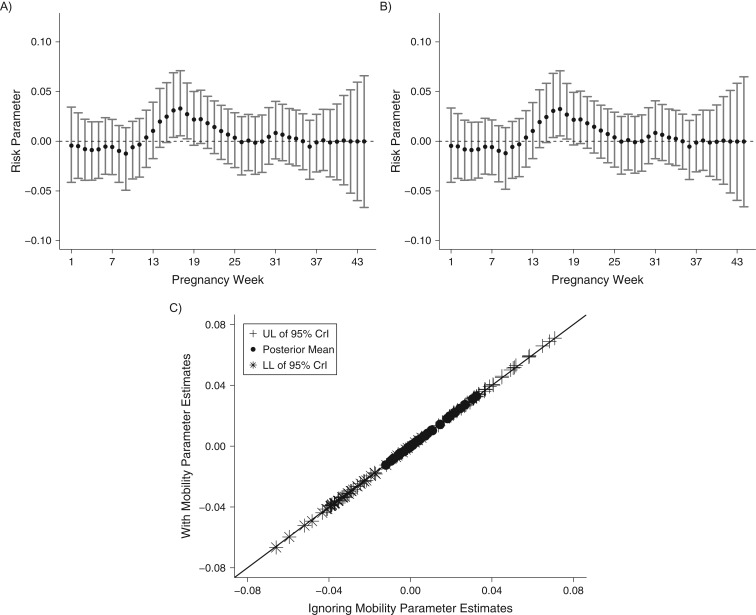
In Figure 4, we display the results assuming 75% mobility of the population. Web Figures 3 and 4 display the results for 25% and 50% mobility, respectively (results consistent across mobility level). These figures are comparable to Figure 3 in their content. Overall, these findings suggest that even though estimation of the critical windows changes as the proportion of movers increases, the changes are minimal and do not greatly affect estimation and identification of statistically significant critical windows of susceptibility.
Figure 4.
Results from a simulation study of 2 air pollution (PM10) exposure metrics, assuming that 75% of the pregnant population moves between conception and delivery, in 4 Connecticut birth cohorts, 1988–2008. A) Exposure metric 1: exposures based on full maternal residential address histories; B) exposure metric 2: exposures based on residence at delivery; C) scatterplot of parameter estimates from parts A and B. CrI, credible interval; LL, lower limit; PM10, particulate matter less than or equal to 10 μm in aerodynamic diameter; UL, upper limit.
DISCUSSION
The results from our data application (i.e., analysis of the cohort data) indicate an association between term low birth weight and exposure to PM10 during weeks 16–18 of pregnancy. In a Texas-based study of critical window estimation carried out during 2001–2004, similar weeks of increased vulnerability to PM2.5 were identified in the second trimester (weeks 19–21) using the same statistical model as that implemented in this analysis (6). In that study, the authors did not have access to the full material residential histories needed to more accurately link weekly exposures to the pregnant women. In an unadjusted analysis, Hao et al. (17) observed elevated risk of term low birth weight with increased exposure to PM2.5 during the second trimester for women across the United States. General agreement in the estimated critical windows between these different populations, with a focus on different pollutants, strengthens the evidence suggesting that there is an association between particulate matter exposure and term low birth weight sometime in the second trimester. Exposures to PM10 and PM2.5 throughout pregnancy have been linked to decreases in birth weight in a number of past epidemiologic analyses using different populations, pollutant averaging periods, and statistical methods (1, 18–22).
The biological pathway by which prenatal air pollution exposure may affect birth weight is not yet fully understood. A recent study suggested that placental mitochondrial DNA may act as a mediator of the association between air pollution exposure during pregnancy and reduced birth weight (23). Prenatal air pollution exposure may deplete the placenta’s mitochondria content through increased oxidative stress (23, 24). These mitochondria are important in ensuring that the placenta can support proper growth of the fetus, and damage to them could result in a reduction in birth weight for a fetus (23–26).
Using the same cohorts of pregnant women as those included in this study, Pereira et al. (11) investigated the association between exposure to PM10 during pregnancy and a number of adverse birth outcomes using separately fitted standard epidemiologic statistical models with prespecified pollution averaging periods. They also considered different exposure definitions 1) based on first recorded residential address, 2) based on last recorded residential address, and 3) accounting for full maternal residential mobility. Increased exposures were associated with reduced birth weight during the second trimester and across the entire pregnancy. Similarly, the authors found no substantial changes in risk effect estimation when the different exposure definitions were used. Their work established the adequacy of using residence at delivery to define exposures for larger aggregated periods of pregnancy in the cohorts. Our work extends this by focusing on the joint estimation of critical windows of increased vulnerability using more advanced statistical methods.
The simulation study results suggest that the distance traveled may be a more important factor in terms of exposure misclassification than the proportion of the population who move during pregnancy. Moving larger distances would more greatly incorporate the geographical variability of ambient air pollution and therefore lead to larger exposure classification. Among the women who moved in our data set, exposure misclassification was relatively small and decreased as pregnancy progressed. Other subpopulations may have different patterns of mobility during pregnancy. In this study, this minimal amount of error apparently had only a minor impact on critical window estimation, even as a larger proportion of women moved. It is possible that with different pollutants of interest, critical window estimation could be affected more severely, particularly if there are abrupt changes in the composition or magnitude of that pollutant at shorter distances (e.g., greater spatial heterogeneity due to point sources). This would result in increased exposure misclassification and would tend to pull the estimation of critical windows towards the null (9). Our cohorts of women had lower rates of term low birth weight and pregnancy mobility than the general pregnant population. Future studies are needed to determine the robustness of our findings in populations with higher rates of both of these factors.
Changing the level of exposure aggregation (e.g., from weekly to daily) could also result in different levels of exposure misclassification. In this study, we focused on weekly averages of air pollution exposure across the entire pregnancy based on similar critical window analyses of term low birth weight and preterm birth. However, for some health outcomes, the window of development is shorter than the entire pregnancy span, and therefore a finer scale of exposure timing may be needed. For example, Warren et al. (9) considered the association between daily exposures to PM2.5 during postconception weeks 2–8 and the risk of developing a number of cardiac congenital anomalies based on the developmental period of the heart. In future work, researchers should consider how critical window estimation is affected in different populations, using different adverse birth outcomes, pollutants, and exposure averaging periods, while carefully considering the biological plausibility of using such finer-scaled exposures.
In conclusion, to our knowledge, our study is the first to have quantified the impact of maternal residential mobility on estimation of critical pregnancy windows. Using Connecticut birth cohorts with full maternal residential address histories, we were able to investigate this impact with respect to estimating the association between term low birth weight and weekly PM10 pregnancy exposures and to characterize the periods of pregnancy with increased exposure misclassification. In line with past work, we observed an increased risk of low birth weight associated with PM exposure in the second trimester, but we were able to more specifically identify pregnancy weeks 16–18 as a particularly vulnerable period. Simulation study results suggested that even when a larger proportion of the pregnant population moves between conception and delivery, there is relatively little impact on critical window identification for PM10 and term low birth weight for this study population, probably because of the small distances being traveled.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Biostatistics, School of Public Health, Yale University, New Haven, Connecticut (Joshua L. Warren); School of Forestry and Environmental Studies, Yale University, New Haven, Connecticut (Ji-Young Son, Brian P. Leaderer, Michelle L. Bell); School of Public Health, Curtin University, Perth, Western Australia, Australia (Gavin Pereira); and Department of Environmental Health Sciences, School of Public Health, Yale University, New Haven, Connecticut (Brian P. Leaderer, Michelle L. Bell).
This work was supported by the National Center for Advancing Translational Science, the National Institutes of Health, and the National Institutes of Health Roadmap for Medical Research (Clinical and Translational Science Awards UL1 TR001863 and KL2 TR001862 to J. L. W.); the Environmental Protection Agency (Assistance Agreement RD835871 with M. L. B); the National Institute of Allergy and Infectious Diseases (grant 5R01AI041040); the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant 5R01HD045735); the National Institute of Environmental Health Sciences (grant R01ES019587); and the National Institute on Drug Abuse (grant 5R01DA005484).
This work has not been formally reviewed by the Environmental Protection Agency (EPA). The views expressed in this document are solely those of the authors and do not necessarily reflect those of the EPA. The EPA does not endorse any products or commercial services mentioned in this publication. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health.
Conflict of interest: none declared.
Abbreviations
- PM2.5
particulate matter less than or equal to 2.5 μm in aerodynamic diameter
- PM10
particulate matter less than or equal to 10 μm in aerodynamic diameter
REFERENCES
- 1. Stieb DM, Chen L, Eshoul M, et al. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012;117:100–111. [DOI] [PubMed] [Google Scholar]
- 2. Vrijheid M, Martinez D, Manzanares S, et al. Ambient air pollution and risk of congenital anomalies: a systematic review and meta-analysis. Environ Health Perspect. 2011;119(5):598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Institute of Environmental Health Sciences 2012–2017 Strategic Plan—Advancing Science, Improving Health: A Plan for Environmental Health Research Bethesda, MD: National Institute of Environmental Health Sciences; 2012. (NIH publication no. 12-7935). [Google Scholar]
- 4. Chang HH, Warren JL, Darrow LA, et al. Assessment of critical exposure and outcome windows in time-to-event analysis with application to air pollution and preterm birth study. Biostatistics. 2015;16(3):509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Warren J, Fuentes M, Herring A, et al. Spatial-temporal modeling of the association between air pollution exposure and preterm birth: identifying critical windows of exposure. Biometrics. 2012;68(4):1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Warren JL, Fuentes M, Herring AH, et al. Air pollution metric analysis while determining susceptible periods of pregnancy for low birth weight. ISRN Obstet Gynecol. 2013;2013:387452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilson A, Chiu YM, Hsu HL, et al. Bayesian distributed lag interaction models to identify perinatal windows of vulnerability in children’s health. Biostatistics. 2017;18(3):537–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Warren J, Fuentes M, Herring A, et al. Bayesian spatial-temporal model for cardiac congenital anomalies and ambient air pollution risk assessment. Environmetrics. 2012;23(8):673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Warren JL, Stingone JA, Herring AH, et al. Bayesian multinomial probit modeling of daily windows of susceptibility for maternal PM2.5 exposure and congenital heart defects. Stat Med. 2016;35(16):2786–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bell ML, Belanger K. Review of research on residential mobility during pregnancy: consequences for assessment of prenatal environmental exposures. J Expo Sci Anal Environ Epidemiol. 2012;22(5):429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pereira G, Bracken MB, Bell ML. Particulate air pollution, fetal growth, and gestational length: the influence of residential mobility in pregnancy. Environ Res. 2016;147:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sadler L, Belanger K, Saftlas A, et al. Environmental tobacco smoke exposure and small-for-gestational-age birth. Am J Epidemiol. 1999;150(7):695–705. [DOI] [PubMed] [Google Scholar]
- 13. Bracken MB, Triche EW, Belanger K, et al. Association of maternal caffeine consumption with decrements in fetal growth. Am J Epidemiol. 2003;157(5):456–466. [DOI] [PubMed] [Google Scholar]
- 14. Triche EW, Saftlas AF, Belanger K, et al. Association of asthma diagnosis, severity, symptoms, and treatment with risk of preeclampsia. Obstet Gynecol. 2004;104(3):585–593. [DOI] [PubMed] [Google Scholar]
- 15. Spoozak L, Gotman N, Smith MV, et al. Evaluation of a social support measure that may indicate risk of depression during pregnancy. J Affect Disord. 2009;114(1–3):216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2015. Natl vital Stat Rep. 2017;66(1):1. [PubMed] [Google Scholar]
- 17. Hao Y, Strosnider H, Balluz L, et al. Geographic variation in the association between ambient fine particulate matter (PM2.5) and term low birth weight in the United States. Environ Health Perspect. 2016;124(2):250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Backes CH, Nelin T, Gorr MW, et al. Early life exposure to air pollution: how bad is it? Toxicol Lett. 2013;216(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dadvand P, Parker J, Bell ML, et al. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect. 2013;121(3):367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fleischer NL, Merialdi M, van Donkelaar A, et al. Outdoor air pollution, preterm birth, and low birth weight: analysis of the World Health Organization Global Survey on Maternal and Perinatal Health. Environ Health Perspect. 2014;122(4):425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pedersen M, Giorgis-Allemand L, Bernard C, et al. Ambient air pollution and low birthweight: a European cohort study (ESCAPE). Lancet Respir Med. 2013;1(9):695–704. [DOI] [PubMed] [Google Scholar]
- 22. Sapkota A, Chelikowsky AP, Nachman KE, et al. Exposure to particulate matter and adverse birth outcomes: a comprehensive review and meta-analysis. Air Qual Atmos Health. 2012;5(4):369–381. [Google Scholar]
- 23. Clemente DB, Casas M, Vilahur N, et al. Prenatal ambient air pollution, placental mitochondrial content, and birth weight in the INMA (Spain) and ENVIRONAGE (Belgium) birth cohorts. Environ Health Perspect. 2016;124(5):659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van den Hooven EH, Pierik FH, de Kluizenaar Y, et al. Air pollution exposure and markers of placental growth and function: the Generation R Study. Environ Health Perspect. 2012;120(12):1753–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Janssen BG, Munters E, Pieters N, et al. Placental mitochondrial DNA content and particulate air pollution during in utero life. Environ Health Perspect. 2012;120(9):1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barrett JR. Prenatal air pollution and reduced birth weight: decline in placental mitochondria as a potential mechanism. Environ Health Perspect. 2016;124(5):A98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.