Abstract
Background aims
Modulation of inflammation after brain trauma is a key therapeutic goal aimed at limiting the consequences of the subsequent injury cascade. Mesenchymal stromal cells (MSCs) have been demonstrated to dynamically regulate the inflammatory environment in several tissue systems, including the central nervous system. There has been limited success, however, with the use of direct implantation of cells in the brain caused by low viability and engraftment at the injury site. To circumvent this, we encapsulated MSCs in alginate microspheres and evaluated the ability of these encapsulated MSCs to attenuate inflammation in rat organotypic hippocampal slice cultures (OHSC).
Methods
OHSC were administered lipo-polysaccharide to induce inflammation and immediately co-cultured with encapsulated or monolayer human MSCs. After 24 h, culture media was assayed for the pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-α) produced by OHSC, as well as MSC-produced trophic mediators.
Results
Encapsulated MSCs reduced TNF-α more effectively than did monolayer MSCs. Additionally, there was a strong correlation between increased prostaglandin E2 (PGE2) and reduction of TNF-α. In contrast to monolayer MSCs, inflammatory signals were not required to stimulate PGE2 production by encapsulated MSCs. Further encapsulation-stimulated changes were revealed in a multiplex panel analyzing 27 MSC-produced cytokines and growth factors, from which additional mediators with strong correlations to TNF-α levels were identified.
Conclusions
These results suggest that alginate encapsulation of MSCs may not only provide an improved delivery vehicle for transplantation but may also enhance MSC therapeutic benefit for treating neuro-inflammation.
Keywords: co-culture, inflammation mediators, mesenchymal stromal cells, organ culture, traumatic brain injury
Introduction
Neuro-inflammation is a major component of the secondary injury cascade after brain trauma, contributing significantly to tissue damage and the propagation of degenerative mechanisms [1,2] over a period of days to months after the initial trauma. Mesenchymal stromal cells (MSCs) have been reported as a promising therapeutic for such injury through the use of both in vitro and in vivo models of stroke [3] and traumatic brain injury [4,5]. Rather than serve as a direct cell replacement, in this therapy, MSCs are proposed to act as trophic mediators [6]—responding to several secondary injury mechanisms, including inflammation [7–9].
Despite promising evidence for the use of MSCs as a therapeutic for central nervous system (CNS) trauma, there has been varied success with the use of direct implantation of the cells for prolonged treatment of the secondary injury cascade because of diminished localization and survival at the injury site [10,11] as well as migration to other tissues [12,13]. To control long-term effects and localization, we have previously developed and characterized a method to encapsulate MSCs within alginate microspheres [14]. These encapsulated MSCs, but not free MSCs, significantly increased the number of anti-inflammatory macrophages in a spinal cord injury model [15] and prevented tissue degradation of organotypic hippocampal slice cultures (OHSC) [16]. However, the mechanism by which encapsulated MSCs alleviate CNS inflammation and pathology has not yet been identified. In this study, we used a lipopolysaccharide (LPS)-treated OHSC model to determine whether encapsulated MSCs, compared with monolayer MSCs, could modulate the neuro-inflammatory response. OHSC provides an in vitro model with a heterogeneous CNS cell population that maintains cellular interactions and tissue architecture; it is less complex, more tightly controlled and has higher throughput than in vivo CNS injury models. With the use of OHSC and transwell co-culture treatment, we studied MSC treatment effects on direct CNS cellular targets and identified MSC-secreted trophic mediators responsible for therapeutic benefit.
We demonstrate that encapsulated MSCs markedly reduce levels of the pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-α) produced by OHSC in response to LPS and are more effective than monolayer MSCs. Our studies corroborate previous findings that prostaglandin E2 (PGE2) is a key inflammatory mediator produced by MSCs [17,18] and further demonstrate that whereas inflammatory signals are required to induce monolayer MSC PGE2 production, alginate encapsulation of MSCs alone is enough to stimulate PGE2 production.
Similar alginate encapsulation–stimulated changes were also observed across a multiplex panel analyzing 27 MSC-produced cytokines and growth factors, from which additional mediators with strong correlations to TNF-α levels were identified. Together, these results indicate that alginate encapsulation enhances MSC therapeutic benefit for experimental inflammation and induces MSC secretome changes that may be responsible for the improved anti-inflammatory effects of encapsulated MSC treatment.
Methods
Organotypic hippocampal slice culture
All animal procedures were approved by the Rutgers University Institutional Animal Care and Use Committee (Piscataway, NJ, USA), and we carefully adhered to the animal welfare guidelines set out in the Guide for the Care and Use of Laboratory Animals, US Department of Health and Human Services, Publication No. 85–23, 1985. Outbred Sprague-Dawley dams with litters (10 pups/dam) were received and housed together, and approximately two to four rat pups were used per experiment. OHSC were prepared according to established methods [19]. Briefly, Sprague-Dawley rat pups (Taconic Biosciences Inc) at postnatal days 8–10 were decapitated; the hippocampus was rapidly dissected, sliced into 400-μm sections with the use of a McIllwain tissue chopper (Vibratome) and immersed in ice-cold Gey’s balanced salt solution (Sigma-Aldrich) supplemented with 4.5 mg/mL glucose (Sigma-Aldrich). Slices were separated and plated onto Millicell culture inserts (12 mm, hydrophilic Polytetrafluoroethylene, 0.4 μm, EMD Millipore), one slice per insert, and maintained at 37°C in 5% CO2 for 14 days. Maintenance medium consisted of 25% heat-inactivated horse serum (Life Technologies), 25% Hank’s balanced salt solution (HBSS) (Sigma-Aldrich) and 50% minimum essential medium (MEM) with added Earle’s salts (Sigma-Aldrich), supplemented with 1 mmol/L glutamine (Sigma-Aldrich) and 4.5 mg/mL glucose (Sigma-Aldrich). Medium was changed every 3 to 4 days.
Human MSC culture
Human bone marrow MSCs from a single donor (male, 28 years) were purchased from Texas A&M at passage 1 and cultured as previously described [20]. Briefly, MSCs were cultured in MEM-α medium without ribo- and deoxyribonucleosides (Life Technologies), supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals), 1 ng/mL basic fibroblast growth factor (Peprotech), 100 units/mL penicillin and 100 μg/mL streptomycin (Life Technologies). Cells were plated at 5000 cells per cm2 and allowed to proliferate to 70% confluence (approximately 4 to 5 days) before passaging. Only MSCs at passages 2 through 5 were used to initiate subsequent experiments. Monolayer cultures of MSCs, used as controls in all experiments, were seeded 1 day before use in well plates at 2.5 × 104, 5 × 104 or 1 × 105 cells/well. All cultures were incubated at 37°C in 5% CO2.
Alginate micro-encapsulation
Alginate poly-L-lysine micro-encapsulation of MSCs was performed as previously described [14]. A 2.2% (wt/vol) alginate solution (molecular weight [MW]: 100,000–200,000 g/mol, G-content: 65% to 70%, Sigma-Aldrich) was generated with Ca2+-free Dulbecco’s modified Eagle’s medium (DMEM) (Life Technologies). Cultured MSCs were dissociated and re-suspended in 2.2% alginate to yield a final solution of 4 × 106 cells/mL in 2% (wt/vol) alginate (resulting in approximately 150 cells/capsule), which has been previously determined to maintain MSC viability and an undifferentiated state [15]. The cell solution was transferred to a syringe pump (KD Scientific) set at a flow rate of 10 mL/h. Alginate beads were generated with the use of an electrostatic bead generator (Nisco), at an applied voltage of 6.4 kV. The resulting bead diameter was 500 × 50 μm. The beads were extruded into a bath of CaCl2 (100 mmol/L) (Sigma-Aldrich) containing 145 mmol/L NaCl (Sigma-Aldrich) and 10 mmol/L 3-(N-morpholino)propanesulfonic acid (MOPS) (Sigma-Aldrich). Micro-encapsulated cells were washed once with phosphate-buffered saline (PBS) (Sigma-Aldrich) and then were treated for 2 min with poly-L-lysine (Sigma-Aldrich, MW: 68,600 g/mol) (0.05% wt/vol), followed by an additional PBS wash. The micro-encapsulated cells were re-suspended in 5 mL of MEM-α (Life Technologies) and transferred to a 25-cm2 tissue culture flask, maintained in an upright position. Encapsulated cells were incubated at 37°C in 5% CO2 and used for experiments 1 day after encapsulation. To determine the average number of cells per capsule for dosing purposes, 15 μL of capsules was added to 200 μL of 1% ethylene diamine tetra-acetic acid (EDTA). Capsules were immediately counted in this volume (n = 3), and the average number of capsules/mL was calculated accordingly. The capsule+EDTA solutions were incubated at room temperature for 5 min to allow lysis of the alginate and release of MSC from capsules. A 10-μL volume of these cell suspensions was counted on a hemacytometer to determine average number of cells/mL (n = 3). The average number of cells/capsule was calculated as (cells/mL)/(capsules/mL) and used to determine the number of capsules necessary for experimental treatment. On the basis of the number of capsules necessary to achieve the desired MSC dose, an equivalent number of capsules was chosen for empty-capsule controls.
LPS injury and co-culture
Organotypic slices cultured on membrane inserts were added to 24-well plates containing monolayer or encapsulated MSCs, and maintenance medium was exchanged for serum-free medium (75% MEM with added Earle’s salts, 25% HBSS, 1 mmol/L glutamine and 4.5 mg/mL glucose). Slice cultures were randomly placed into treatment and control groups, with each group comprising cultures prepared from at least two different animals. For MSC treatment, cultures were stimulated with 1 μg/mL LPS (Escherichia coli 055:B5, Sigma-Aldrich) [21,22] and immediately co-cultured with monolayer or encapsulated MSCs at 2.5 × 104, 5 × 104 or 1 × 105 cells/well. Non-stimulated and stimulated host cultures without MSC co-culture were used as controls. Cultures were returned to incubators at 37°C in 5% CO2 for 24 h, after which media supernatants were collected.
PGE2 treatment
Organotypic slices cultured on membrane inserts were added to 24-well plates containing serum-free medium supplemented with 1 μg/mL LPS ± human PGE2 (Cayman Chemical) at 2, 4, 6, 8, 10 or 12 ng/mL. Cultures were returned to incubators at 37°C in 5% CO2 for 24 h, after which media supernatants were collected.
Cytokine measurement
At the end of each treatment, cell culture media supernatants were collected and stored at −20°C. Media supernatants were assayed for TNF-α produced by the organotypic slice culture through the use of a rat-specific TNF-α enzyme-linked immunosorbent assay (ELISA) (Biolegend) according to the manufacturer’s instructions. Total PGE2 secretion (rat + human) was evaluated through the use of PGE2 enzyme immunoassay (EIA) (Cayman Chemical), and secretion by MSCs was evaluated with the use of a Bioplex multiplex bead analysis (Bio-Rad Inc) for 27 human-specific growth factors and cytokines, both according to the manufacturer’s instructions.
Hierarchical cluster analysis
Bioplex secretome data were normalized to the monolayer MSC condition and analyzed by use of an unsupervised agglomerative clustering algorithm in Matlab (MathWorks).
Statistical analysis
All results are expressed as a mean ± standard error (SE). All data presented, with the exception of Bioplex data, are averaged from ≥3 separate experiments, each with n = 2–3 individual slice cultures per condition (≥6–9 total cultures per condition). Bio-plex data are averaged from one experiment, with n = 3 individual slice cultures per condition, assayed in duplicate. KaleidaGraph (Synergy Software) was used for statistical evaluation. Data obtained from individual samples for each condition were pooled, and comparisons between different conditions were performed with the use of one-way analysis of variance followed by post hoc Tukey honestly significant differences test, with statistical significance determined at P ≤ 0.05. For correlation analyses, data from each sample set were standardized to a normal distribution by calculating the z-score for each sample:
where x is the sample, μ is the mean and σ is the standard deviation. Pearson’s correlation of coefficient, r, was calculated from standard scores and considered significant for values of P ≤ 0.05.
Results
Treatment with MSCs inhibits production of pro-inflammatory TNF-α in LPS-stimulated OHSC
The bacterial endotoxin LPS is known to induce experimental inflammation through activation of the immune response and stimulation of cytokine production and has been commonly used to model the neuro-inflammatory component of secondary CNS injury both in vitro [23,24] and in vivo [25,26]. To evaluate the ability of MSC treatment to mitigate the inflammatory response, we stimulated OHSC with 1 μg/mL LPS and concurrently treated with monolayer or encapsulated MSCs. After 24 h, cell culture media was assayed for TNF-α, a pro-inflammatory cytokine known to be rapidly elevated after brain injury in animal models [27] and in the clinic [28]. With the use of ELISA specific for rat TNF-α, we were able to measure OHSC-produced TNF-α.
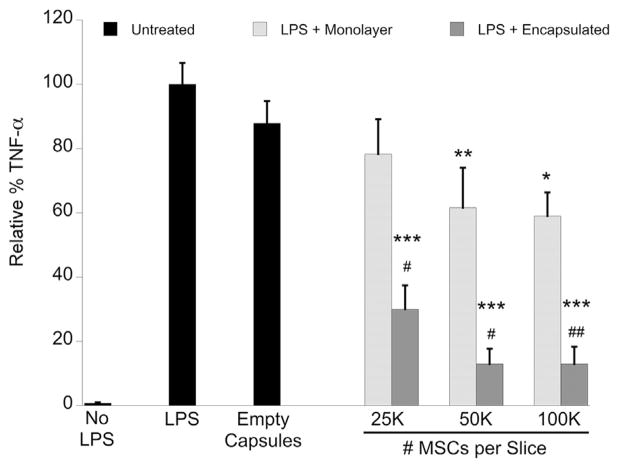
In untreated OHSC, LPS caused a significant increase in TNF-α production (13.21 ± 1.44 ng/mL) by the OHSC, and, for the purpose of analysis, we set this condition as maximum TNF-α production (100%). All other conditions are expressed as a relative percentage of this maximum value. Both monolayer and encapsulated MSCs reduced TNF-α production in a dose-dependent manner, which was significant for encapsulated MSCs at all doses (2.5 × 104, 5 × 104, 1 × 105 cells/well) but was only significant for monolayer MSCs at 5 × 104 and 1 × 105 cells/well (Figure 1). Additionally, encapsulated MSCs at all doses had a significantly greater effect on reducing TNF-α as compared with an equivalent dose of monolayer MSCs. Empty-capsule treatment had no significant effect on TNF-α reduction.
Figure 1.
Rat TNF-α ELISA of cell culture media supernatant collected after 24 h of LPS stimulation ± MSC treatment in OHSC. Data are normalized to untreated LPS-stimulated OHSC and represented as mean ± SE from five experiments, each with n = 2–3 cultures per condition. Encapsulated MSC treatment significantly reduced TNF-α levels in a dose-dependent manner that was more effective than monolayer MSC treatment. Empty capsule treatment had no significant effect on TNF-α reduction. *P < 0.02, **P < 0.002, ***P < 0.0001 compared with LPS + no treatment; #P < 0.01, ##P < 0.002 compared with treatment with equivalent number of free MSCs.
Encapsulated MSCs increase total PGE2 concentration when co-cultured with LPS-stimulated OHSC
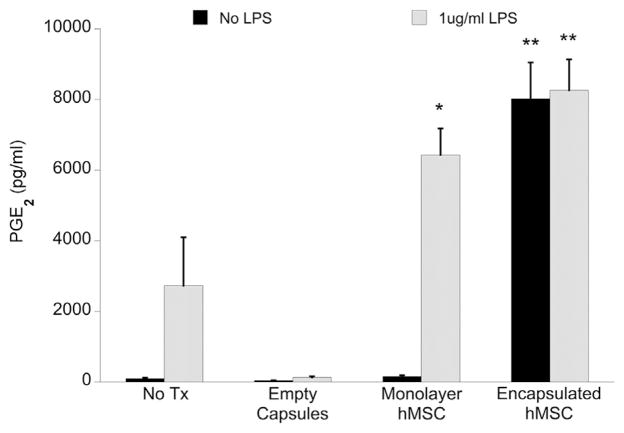
PGE2 is a potent inflammatory mediator and has been reported to participate in MSC-mediated modulation of the inflammatory and immune responses [17,18,29]. We evaluated total (rat + human) PGE2 concentration in OHSC media after 24 h with/without stimulation with 1 μg/mL LPS and treatment with monolayer or encapsulated MSC (1 × 105 cells/well) (Figure 2). Both monolayer and encapsulated MSCs increased total PGE2 production in LPS-stimulated cultures, either through MSC secretion in response to inflammatory factors or by stimulating endogenous PGE2 production by OHSC. Although the presence of LPS was necessary to induce PGE2 production in the monolayer MSC condition, encapsulated MSCs produced PGE2 regardless of LPS stimulation, suggesting that the alginate capsule micro-environment may regulate MSC PGE2 secretion.
Figure 2.
Total PGE2 concentration in cell culture media supernatant collected after 24 h of culture ± MSC treatment (1 × 105 MSCs/well, with or without LPS), as measured by EIA. Data are represented as mean ± SE from three experiments, each with n = 2–3 cultures per condition. Encapsulated MSC conditions resulted in a significant increase of total PGE2 concentration as compared with untreated LPS-stimulated OHSC, regardless of LPS presence. Only monolayer MSC treatment with LPS stimulation resulted in a significant increase of PGE2 as compared with untreated LPS-stimulated OHSC. Monolayer MSC treatment without LPS stimulation, as well as treatment with empty capsules, produced negligible amounts of PGE2. *P < 0.05, **P < 0.0005 compared with OHSC + LPS + no MSC treatment.
Increasing PGE2 concentration is responsible for TNF-α reduction in OHSC
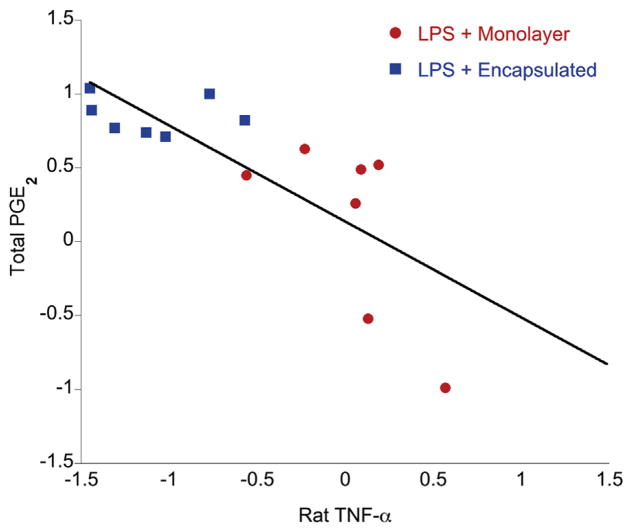
Given our findings that MSCs (i) reduce TNF-α production and (ii) increase PGE2 levels in LPS-stimulated slice cultures, we used paired data to determine if a correlation exists between levels of total PGE2 and rat TNF-α. Data for MSC treatment conditions were first standardized using z-score scaling, and Pearson’s coefficient of correlation (for linear correlation) was determined from standard scores. We found a strong, significant negative correlation (r = −0.7614, P = 0.0008) between levels of total PGE2 and rat TNF-α (Figure 3). Additionally, we observed that treatment conditions cluster together: encapsulated MSC treatment clusters at high PGE2/low TNF-α; and monolayer MSC treatment clusters at lower levels of PGE2 and higher TNF-α.
Figure 3.
Correlation between total PGE2 and rat TNF-α measured in OHSC culture supernatants for MSC treatment conditions (1 × 105 MSCs/well). Linear regression and Pearson’s co-efficient (r) were derived from z-scores of standardized data. There is a significant (P = 0.0008) negative correlation—increasing PGE2 correlates with decreasing TNF-α—and a clear grouping of treatment conditions: encapsulated MSC treatment clusters at high levels of PGE2 and low levels of TNF-α, and monolayer MSC treatment clusters at low levels of PGE2 and higher TNF-α.
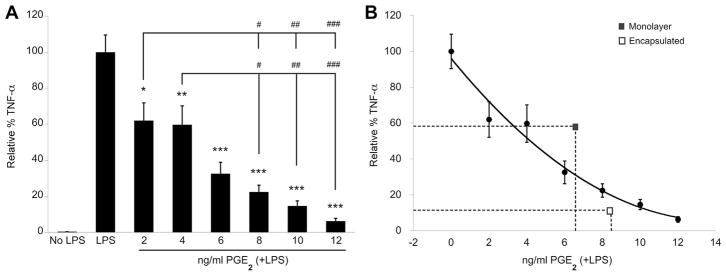
To determine if PGE2 is a direct mediator of TNF-α reduction in our culture model, we added exogenous human PGE2 to LPS-stimulated slice cultures and evaluated culture media for rat TNF-α secretion after 24 h. There is a clear dose-response effect of increasing human PGE2 on reducing TNF-α produced by OHSC (Figure 4A). We then compared the effects of exogenous versus MSC-produced PGE2 on TNF-α reduction. As seen in Figure 2, monolayer and encapsulated MSCs produced 6.43 ± 0.74 ng/mL and 8.30 ± 0.87 ng/mL PGE2, respectively. On the basis of a polynomial curve fit to the exogenous PGE2 data in Figure 4A, at these levels of PGE2 we would expect TNF-α reductions to 32% and 20% of maximum, respectively, if PGE2 was the primary mediator responsible for TNF-α modulation. Encapsulated MSCs achieved a lower level of TNF-α relative to maximum (13% ± 5.3%) than suggested by this model, but monolayer MSCs did not (59% ± 7.4%) (Figure 4B, Table I). These deviations suggest that inflammatory mediation is regulated differently by encapsulated MSCs compared with monolayer MSCs and may be explained by differences in encapsulated versus monolayer MSC secretion of other factors that enhance or limit the effect of MSC-produced PGE2.
Figure 4.
(A) Rat TNF-α ELISA of cell culture media supernatant collected from organotypic cultures after 24 h of LPS stimulation ± human PGE2. Data are normalized to untreated LPS-stimulated OHSC and represented as mean ± SE from three experiments, each with n = 3 cultures per condition. Addition of exogenous human PGE2 significantly reduced TNF-α levels in a dose-dependent manner. *P < 0.01, **P < 0.005, ***P < 0.0001 compared with LPS + no treatment. #P < 0.01, ##P < 0.001, ###P < 0.0001 between dose groups. (B) Polynomial curve fit of data presented in A, overlaid with corresponding mean levels of PGE2 production and TNF-α reduction by monolayer and encapsulated MSCs.
Table I.
Experimental versus PGE2-estimated TNF-α reduction by hMSCs.
| Monolayer | Encapsulated | |
|---|---|---|
| PGE2 (ng/mL) | 6.43 ± 0.74 | 8.30 ± 0.87 |
| TNF-α (% maximum, experimental) | 59 ± 7.4 | 13 ± 5.3 |
| TNF-α (% maximum, estimated) | 32 | 20 |
Experimental versus estimated values of rat TNF-α (percentage of maximum production) after treatment with monolayer or encapsulated hMSCs (1 × 105 cells/well). Estimated TNF-α values were calculated from a polynomial curve fit of TNF-α levels after exogenous PGE2 administration (Figure 4B), with use of the concentration of PGE2 detected in media collected from LPS-stimulated OHSC treated with hMSCs (Figure 2).
Alginate-encapsulated MSCs exhibit secretome changes
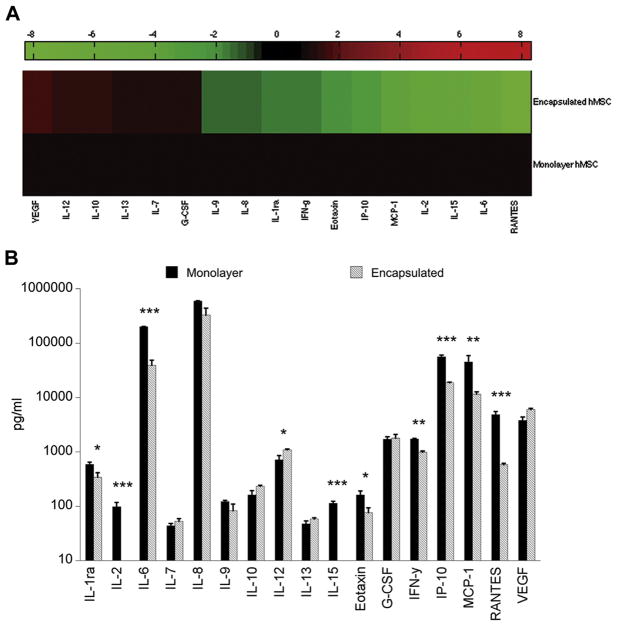
To further understand the mechanisms by which encapsulated MSCs differentially modulate the inflammatory response, we used a multiplex assay to screen for 27 human growth factors and cytokines that may be differentially regulated in monolayer versus encapsulated MSCs. Using cell culture media from monolayer and encapsulated MSCs (1 × 105 cells/well ± 1 μg/mL LPS ± OHSC), we first examined secretion by encapsulated MSCs compared with monolayer MSC secretion, for conditions in which MSCs were co-cultured with LPS-stimulated OHSC. With the use of heat-map representation of the 17 analytes detectable by multiplex assay, encapsulated MSC secretion was normalized relative to monolayer MSC secretion, and we identified distinct panels of cytokines either upregulated or downregulated by encapsulated MSCs (Figure 5A). We then compared quantitative levels of secretion and determined that 10 of the 17 analytes detected in the multiplex assay exhibited significantly different levels of secretion by encapsulated MSCs as compared with monolayer MSCs, after co-culture with LPS-stimulated OHSC (Figure 5B).
Figure 5.
(A) Heat map representation of multiplex (human) secretome data. Secretion by encapsulated MSCs (1 × 105 cells/well) co-cultured with LPS-stimulated OHSC is normalized to secretion by monolayer MSCs (1 × 105 cells/well) co-cultured with LPS-stimulated OHSC. Increased levels of secretion are represented in shades of red and decreased levels in shades of green. (B) Multiplex analysis of cell culture media collected after 24 h of MSC co-culture with LPS-stimulated hippocampal slices. Data are represented as mean ± SE from one experiment, with n = 3 cultures per condition. Of 17 detectable analytes, 10 were identified as exhibiting significantly different levels of secretion by encapsulated MSCs as compared with monolayer MSCs. *P < 0.05, **P < 0.005, ***P < 0.0001.
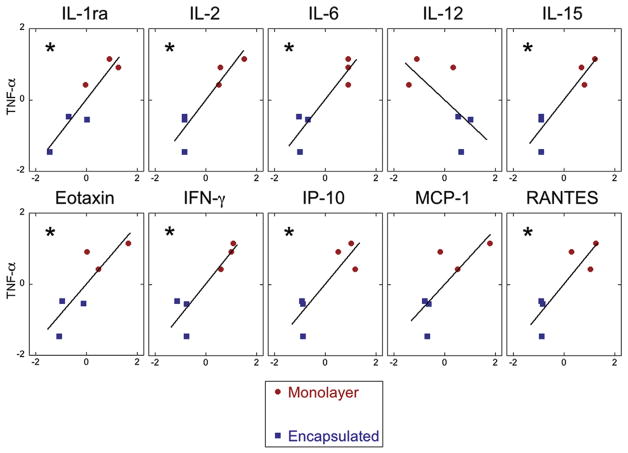
From this subset of 10 analytes, we sought to identify potential candidates responsible for the improved benefit of encapsulated MSCs by using paired data to correlate levels of MSC-secreted factors to rat TNF-α. Data for MSC treatment conditions was first standardized through z-score scaling, and Pearson’s coefficient of correlation (for linear correlation) was determined from standard scores. We found a strong, significant correlation for several MSC-secreted mediators (Figure 6). These correlations, along with the correlation for PGE2, are summarized in Table II in order of decreasing correlation. Nine of the 11 analytes demonstrate a significant correlation with TNF-α.
Figure 6.
Correlation between rat TNF-α and MSC-secreted factors measured by means of multiplex bead assay for monolayer and encapsulated MSC treatment conditions. Only analytes that exhibited significant differences between treatment groups (monolayer versus encapsulated MSC) are depicted. Linear regression and Pearson’s coefficient (r) were derived from z-scores of standardized data. Co-efficients of correlation (r) and significance for each analyte can be found in Table II. *P < 0.05.
Table II.
Correlations between levels of hMSC-secreted factors and OHSC-produced TNF-α detected in culture media.
| Analyte | r (Pearson) | P value | TNF-α ↓ |
|---|---|---|---|
| PGE2a | −0.7614 | 0.0008 | ↑ |
| IL-1ra | 0.9230 | 0.0087 | ↓ |
| IL-15 | 0.9170 | 0.0100 | ↓ |
| IL-6 | 0.9158 | 0.0103 | ↓ |
| IL-2 | 0.9138 | 0.0108 | ↓ |
| IFN-g | 0.8994 | 0.0147 | ↓ |
| RANTES | 0.8622 | 0.0257 | ↓ |
| IP-10 | 0.8590 | 0.0284 | ↓ |
| Eotaxin | 0.8402 | 0.0363 | ↓ |
| MCP-1 | 0.7687 | 0.0741 | ↓ |
| IL-12 | −0.6570 | 0.1563 | ↑ |
Correlation between hMSC-secreted factors found to be significantly different between treatment groups (monolayer versus encapsulation hMSC) and rat TNF-α measured in OHSC culture supernatants. Pearson’s coefficient of correlation (r) and significance of correlation, calculated from z-scores of standardized data, are listed for each detectable analyte and ranked in order of increasing significance. Of 11 hMSC-secreted factors significantly different between treatment groups, 9 demonstrate a significantly (P < 0.05) strong correlation (r > 0.75) with rat TNF-α. Whether the factor is detected as increased or decreased when TNF-α is reduced is denoted. n = 14 for PGE2, n = 6 for all others.
Total PGE2 in supernatant measured, all other analytes measured are human-specific.
Alginate is an effector of MSC secretion
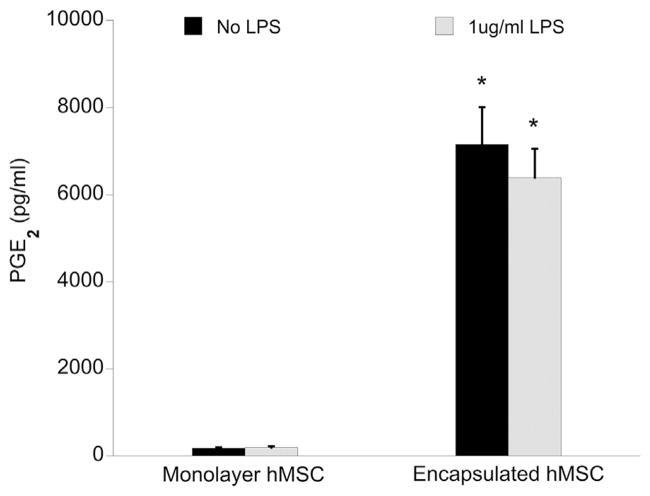
It is clear that alginate encapsulation enhances MSC modulation of the inflammatory response through TNF-α reduction and induces changes in the MSC secretome. To better understand the influence of alginate encapsulation on MSC behavior in isolation, we compared the effects of inflammatory stimuli (LPS and/or co-culture) on the secretion profile of monolayer and encapsulated MSCs. We first evaluated PGE2 concentration in media collected from MSCs cultured alone (1 × 105 cells/well) after 24 h ± stimulation with 1 μg/mL LPS. We have already reported in Figure 2 that, when co-cultured with OHSC, encapsulated MSCs are stimulated to produce PGE2 even in the absence of LPS-stimulation. Correspondingly, encapsulated MSCs cultured alone produced PGE2 regardless of LPS presence, whereas neither condition induced PGE2 production by monolayer MSCs (Figure 7). These data suggest that alginate encapsulation alone is capable of inducing MSC PGE2 production, regardless of inflammatory stimuli, and that monolayer MSC culture requires the presence of both slice co-culture and LPS to stimulate PGE2 production.
Figure 7.
Total PGE2 concentration in MSC cell culture media supernatant (1 × 105 cells/well) collected after 24 h ± 1 μg/mL LPS, as measured by EIA. Data are represented as mean ± SE from three experiments, each with n = 2–3 cultures per condition. Encapsulated MSCs produced a significantly greater amount of PGE2 compared with monolayer MSC, regardless of LPS presence. *P < 0.0001 compared with monolayer MSC counterpart.
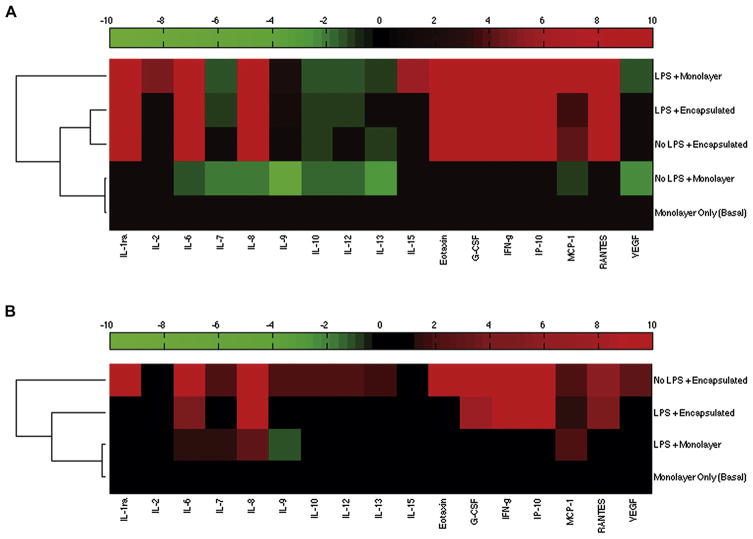
Through the use of hierarchical cluster analysis (HCA) of our multiplex data, we identified similar patterns governing changes in the MSC secretome when examining the effects of (i) OHSC ± LPS or (ii) ± LPS (MSCs cultured alone). In the first instance, we normalized all MSC “treatment” conditions (co-culture with OHSC ± 1 μg/mL LPS) to “baseline” secretion: monolayer MSC cultured alone (no LPS) (Figure 8A). The first node in the dendrogram clusters “No LPS + Monolayer” nearest to the “Monolayer Only (Basal)” condition, indicating similar secretion patterns and little-to-no change above baseline secretion. Farthest away from the baseline node is the secretion pattern of the “LPS + Monolayer” condition, demonstrating a larger change relative to baseline secretion. The “No LPS + Encapsulated” and “LPS + Encapsulated” conditions cluster together and branch further away from the baseline condition. These patterns mirror those found in our PGE2 data: the presence of both LPS and slice co-culture were necessary to stimulate secretome changes by monolayer MSCs, and the presence of LPS had little direct effect on encapsulated MSC secretion.
Figure 8.
Heat-map representation of hierarchical cluster analysis on secretome data of (A) MSC treatment conditions (+OHSC, ±LPS) normalized to baseline monolayer MSC-only condition (no LPS, no co-culture) and (B) monolayer (+LPS) and encapsulated MSC (±LPS) culture alone normalized to baseline monolayer MSC only condition (no LPS). For all conditions, 1 × 105 MSCs/well were used. Increased levels of secretion are represented in shades of red and decreased levels in shades of green.
HCA conducted for secretion by MSCs cultured alone reveals similar trends (Figure 8B). The addition of LPS to monolayer MSC culture induced very little change above baseline secretion. Alginate encapsulation stimulated greater secretome changes, seemingly regardless of LPS stimulation. These data combined suggest that the presence of alginate and the capsule micro-environment exert dominant effects on MSC secretion.
Discussion
There is a clear need to develop a delivery platform to facilitate clinical translation of MSC therapeutics. Although MSCs have been shown to provide neuro-protection and promote regeneration after brain trauma [30], several studies have reported low efficiency of engraftment at the injury site and a decrease in cell number at the site over time [10,11]. Additionally, several studies have reported that a percentage of intravenously administered MSCs have been detected in the liver, spleen, kidney, lungs and other tissues even up to 1 year after treatment [13,31]. Our previous efforts have aimed to use alginate micro-encapsulation of MSCs to deliver cells after spinal cord injury (SCI). The MSC encapsulation process has been optimized to maintain cell viability up to 2 months, support proliferation up to 3 weeks within the alginate capsule environment [15] and maintain MSCs in an undifferentiated state. With the use of these encapsulated MSCs, we demonstrated, using both in vitro macrophage culture and an in vivo model of SCI, that encapsulated MSCs promote the anti-inflammatory M2 macrophage phenotype, even in the absence of direct cell contact. Furthermore, encapsulated MSCs co-cultured with LPS-stimulated macrophages reduced levels of pro-inflammatory TNF-α and the activation marker inducible nitric oxide synthase [15].
In this study, we sought to evaluate the ability of encapsulated MSCs to specifically attenuate the neuro-inflammatory component of CNS injury with the use of LPS-stimulated OHSCs, in which astrocytes and glial cells are the primary mediators of the inflammatory response [32,33]. Our results demonstrate that MSCs are capable of attenuating TNF-α produced by OHSC, in a dose-dependent manner. The findings in monolayer MSC treatment are consistent with previous studies [17]. However, our studies demonstrated that encapsulated MSC treatment results in a significantly greater reduction of TNF-α compared with equivalent doses of monolayer MSC treatment.
To determine a mechanism for the improved action of encapsulated MSCs, we evaluated PGE2 as a potential inflammatory mediator. PGE2 is a critical component of the early inflammatory response, and, although it has been previously recognized for its pro-inflammatory actions [34,35], recent studies provide evidence that PGE2 acts as an anti-inflammatory mediator dependent on receptor subtype binding and affinity, as well as local PGE2 concentration [36]. In experimental models of cerebral ischemia, PGE2 signaling through the EP2 receptor was found to have neuro-protective effects [37,38], and induction of PGE2 synthesis was demonstrated to reduce inflammation in experimental pleuritis [39,40]. Moreover, MSC PGE2 production has been identified as a primary mediator responsible for the anti-inflammatory and immunomodulatory effects of MSC treatment in several in vitro models [17,29,41], and our previous studies evaluating MSC treatment in LPS-stimulated macrophage culture have demonstrated that MSC-secreted PGE2 facilitates macrophage reprogramming by attenuating the pro-inflammatory M1 phenotype and promoting the anti-inflammatory M2 phenotype [18]. Our results, consistent with such previous reports, demonstrate the role of PGE2 as an important mediator of LPS-induced inflammation and that increased levels of MSC-secreted PGE2 are correlated with decreased production of TNF-α by OHSC. Interestingly, although not statistically significant, our observations suggest that encapsulated MSCs may be capable of producing, or stimulating host production of, more PGE2 in response to inflammatory stimuli than their monolayer MSC counterpart.
Despite the strong evidence indicating a direct effect of PGE2 on reduced TNF-α production, our data comparing the effects of exogenous versus MSC-produced PGE2 suggests that other MSC-secreted factors might play a role in enhancing or limiting their therapeutic benefit. As such, we identified several additional MSC-secreted mediators with strong correlations to TNF-α levels. Several pro-inflammatory factors demonstrated strong positive correlations with TNF-α and were produced in higher quantities by monolayer MSCs compared with encapsulated MSCs. The increased production of known inflammatory mediators by monolayer MSCs may explain the limited effect of monolayer MSC-produced PGE2 on TNF-α reduction. Although administration of the equivalent amount of exogenous PGE2 predicts greater inflammatory modulation, monolayer MSC-produced PGE2 may not be sufficient to overcome the concurrent effects of MSC-produced pro-inflammatory mediators.
Of particular interest is our observation that, although production of inflammatory mediators and/or changes in secretion patterns by monolayer MSCs are dependent on inflammatory stimuli (co-culture with LPS-stimulated OHSC), encapsulated MSCs exhibit these changes regardless of LPS stimulation or the presence of stimuli produced by OHSC co-culture. These results indicate that the alginate material or the 3D culture environment within the micro-capsule effects changes in the MSC secretome. The data are corroborated by our previously reported findings that the alginate capsule micro-environment enhances MSC secretion patterns as compared with monolayer MSCs, both in the presence and absence of inflammatory cues [15]. Though alginate has been widely used and studied as a biomaterial for immobilization and delivery of cell therapies [42,43] and is generally accepted as a biocompatible material for implantation and long-term efficacy [44–46], some studies have reported activation of host immune and inflammatory responses to alginate [47] that may be dependent on the purity and composition of alginate [48–50]. It is possible that encapsulated MSC secretion is changing in response to such cues, and, consequently, MSCs are becoming primed to modulate the host inflammatory response. Alternatively, alginate-encapsulated MSCs may be responding to the capsule micro-environment. It has been well documented that cells respond to the mechanical properties of the substrate on which they are cultured [51–53]. MSCs encapsulated in a gellan gum hydrogel modified with extracellular matrix–like peptides demonstrated enhanced proliferation and secretion of neurotrophic factors when compared with MSCs in unmodified capsules [54], and, in cross-linked methacrylated hyaluronic acid hydrogels, MSC secretion of cytokines and angiogenic factors was found to be dependent on hydrogel stiffness [55]. In addition, MSCs encapsulated in alginate hydrogels were reported to upregulate secreted growth factor expression when subject to compression forces, suggesting the ability of MSCs to modulate gene expression in response to their mechanical environment [56]. Finally, there is recent evidence that spheroid aggregate culture of MSCs enhances anti-inflammatory properties [57,58], suggesting that the 3-D conformation of MSC culture plays a role in improving therapeutic benefit.
In summary, our results demonstrate that alginate encapsulation of MSCs enhances their ability to modulate experimental inflammation, through reduction of the pro-inflammatory cytokine TNF-α. Our results suggest that the enhanced benefit conferred by alginate encapsulation is due to changes in encapsulated MSC secretion patterns relative to monolayer MSC. Alginate encapsulation appears to be an effector of changes in MSC secretion regardless of external stimuli, indicating that the capsule material or environment may induce functional changes in MSCs that enhance their therapeutic properties, perhaps through priming MSCs to elevate beneficial factors. Overall, our results suggest that alginate encapsulation of MSCs may not only provide an improved delivery vehicle for transplantation and extended treatment but may also provide for enhanced MSC therapeutic benefit for CNS trauma. Future studies aim to investigate delivery modes, feasibility and long-term effects in vivo.
Acknowledgments
We would like to acknowledge Serom Lee, PhD, and Andrea Gray for their assistance and contribution to the statistical analyses, and Mehdi Ghodbane, PhD, for assistance with multiplex assay and analysis. This research was supported by the New Jersey Commission on Brain Injury Research (SNJ-DHSS-CBIR-CBIE12IPG019 and 10–3215-BIR-E-0), National Institute of Health Grant P41EB002503, Rutgers-UMDNJ Biotechnology Training Fellowship T32GM00008339–21 and NSF Stem Cell IGERT Fellowship 0801620. These funding agencies were not involved in the conduct of the research or preparation of the article.
Footnotes
Disclosure of interests: The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
References
- 1.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. British Journal of Anaesthesia. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 2.Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–36. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–11. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 4.Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Long-term recovery after bone marrow stromal cell treatment of traumatic brain injury in rats. J Neurosurg. 2006;104:272–7. doi: 10.3171/jns.2006.104.2.272. [DOI] [PubMed] [Google Scholar]
- 5.Heile AMB, Wallrapp C, Klinge PM, Samii A, Kassem M, Silverberg G, et al. Cerebral transplantation of encapsulated mesenchymal stem cells improves cellular pathology after experimental traumatic brain injury. Neurosci Lett. 2009;463:176–81. doi: 10.1016/j.neulet.2009.07.071. [DOI] [PubMed] [Google Scholar]
- 6.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 7.Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40:609–19. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- 8.Schäfer S, Calas A-G, Vergouts M, Hermans E. Immunomodulatory influence of bone marrow-derived mesenchymal stem cells on neuroinflammation in astrocyte cultures. J Neuroimmunol. 2012;249:40–8. doi: 10.1016/j.jneuroim.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Zhang C, Chi S, Xu Y, Teng J, Wang H, et al. Effects of human marrow stromal cells on activation of microglial cells and production of inflammatory factors induced by lipopolysaccharide. Brain Res. 2009;1269:23–30. doi: 10.1016/j.brainres.2009.02.049. [DOI] [PubMed] [Google Scholar]
- 10.Harting MT, Jimenez F, Xue H, Fischer UM, Baumgartner J, Dash PK, et al. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg. 2009;110:1189–97. doi: 10.3171/2008.9.JNS08158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q, Long Y, Yuan X, Zou L, Sun J, Chen S, et al. Protective effects of bone marrow stromal cell transplantation in injured rodent brain: synthesis of neurotrophic factors. J Neurosci Res. 2005;80:611–9. doi: 10.1002/jnr.20494. [DOI] [PubMed] [Google Scholar]
- 12.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–82. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 13.Detante O, Moisan A, Dimastromatteo J, Richard M-J, Riou L, Grillon E, et al. Intravenous administration of 99mTc-HMPAO-labeled human mesenchymal stem cells after stroke: in vivo imaging and biodistribution. Cell Transplant. 2009;18:1369–79. doi: 10.3727/096368909X474230. [DOI] [PubMed] [Google Scholar]
- 14.Maguire T, Novik E, Schloss R, Yarmush M. Alginate-PLL microencapsulation: effect on the differentiation of embryonic stem cells into hepatocytes. Biotechnol Bioeng. 2006;93:581–91. doi: 10.1002/bit.20748. [DOI] [PubMed] [Google Scholar]
- 15.Barminko J, Kim JH, Otsuka S, Gray A, Schloss R, Grumet M, et al. Encapsulated mesenchymal stromal cells for in vivo transplantation. Biotechnol Bioeng. 2011;108:2747–58. doi: 10.1002/bit.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dollé J-P, Barminko J, Veruva S, Moure C, Schloss R, Yarmush ML. Reversal of fibronectin-induced hippocampal degeneration with encapsulated mesenchymal stromal cells. Nano LIFE. 2013;03:1350004. doi: 10.1142/S1793984413500049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foraker JE, Oh JY, Ylostalo JH, Lee RH, Watanabe J, Prockop DJ. Cross-talk between human mesenchymal stem/progenitor cells (MSCs) and rat hippocampal slices in LPS-stimulated cocultures: the MSCs are activated to secrete prostaglandin E2. Journal of Neurochemistry. 2011;119:1052–63. doi: 10.1111/j.1471-4159.2011.07511.x. [DOI] [PubMed] [Google Scholar]
- 18.Barminko JA, Nativ NI, Schloss R, Yarmush ML. Fractional factorial design to investigate stromal cell regulation of macrophage plasticity. Biotechnol Bioeng. 2014;111:2239–51. doi: 10.1002/bit.25282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoppini L, Buchs P, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–82. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 20.Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS ONE. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci USA. 1989;86:6348–52. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung IY, Benveniste EN. Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. J Immunol. 1990;144:2999–3007. [PubMed] [Google Scholar]
- 23.Bernardino L, Balosso S, Ravizza T, Marchi N, Ku G, Randle JC, et al. Inflammatory events in hippocampal slice cultures prime neuronal susceptibility to excitotoxic injury: a crucial role of P2X7 receptor-mediated IL-1beta release. Journal of Neurochemistry. 2008;106:271–80. doi: 10.1111/j.1471-4159.2008.05387.x. [DOI] [PubMed] [Google Scholar]
- 24.Fenn AM, Skendelas JP, Moussa DN, Muccigrosso MM, Popovich PG, Lifshitz J, et al. Methylene blue attenuates traumatic brain injury-associated neuroinflammation and acute depressive-like behavior in mice. J Neurotrauma. 2015;32:127–38. doi: 10.1089/neu.2014.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–55. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szczepanik AM, Fishkin RJ, Rush DK, Wilmot CA. Effects of chronic intrahippocampal infusion of lipopolysaccharide in the rat. Neuroscience. 1996;70:57–65. doi: 10.1016/0306-4522(95)00296-u. [DOI] [PubMed] [Google Scholar]
- 27.Shohami E, Novikov M, Bass R, Yamin A, Gallily R. Closed head injury triggers early production of TNF alpha and IL-6 by brain tissue. J Cereb Blood Flow Metab. 1994;14:615–9. doi: 10.1038/jcbfm.1994.76. [DOI] [PubMed] [Google Scholar]
- 28.Ross SA, Halliday MI, Campbell GC, Byrnes DP, Rowlands BJ. The presence of tumour necrosis factor in CSF and plasma after severe head injury. British Journal of Neurosurgery. 1994;8:419–25. doi: 10.3109/02688699408995109. [DOI] [PubMed] [Google Scholar]
- 29.Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS ONE. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang R, Liu Y, Yan K, Chen L, Chen X-R, Li P, et al. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. 2013;10:106. doi: 10.1186/1742-2094-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thanos CG, Bintz BE, Bell WJ, Qian H, Schneider PA, MacArthur DH, et al. Intraperitoneal stability of alginate-polyornithine microcapsules in rats: an FTIR and SEM analysis. Biomaterials. 2006;27:3570–9. doi: 10.1016/j.biomaterials.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 32.Markiewicz I, Lukomska B. The role of astrocytes in the physiology and pathology of the central nervous system. Acta Neurobiol Exp. 2006;66:343–58. doi: 10.55782/ane-2006-1623. [DOI] [PubMed] [Google Scholar]
- 33.Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. Journal of Neurochemistry. 2009;108:1343–59. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakata D, Yao C, Narumiya S. Prostaglandin E2, an immunoactivator. J Pharmacol Sci. 2010;112:1–5. doi: 10.1254/jphs.09r03cp. [DOI] [PubMed] [Google Scholar]
- 35.Sheibanie AF, Yen J-H, Khayrullina T, Emig F, Zhang M, Tuma R, et al. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23–>IL-17 axis. J Immunol. 2007;178:8138–47. doi: 10.4049/jimmunol.178.12.8138. [DOI] [PubMed] [Google Scholar]
- 36.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–8. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu D, Wu L, Breyer R, Mattson MP, Andreasson K. Neuroprotection by the PGE2 EP2 receptor in permanent focal cerebral ischemia. Ann Neurol. 2005;57:758–61. doi: 10.1002/ana.20461. [DOI] [PubMed] [Google Scholar]
- 38.McCullough L, Wu L, Haughey N, Liang X, Hand T, Wang Q, et al. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci. 2004;24:257–68. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 40.Gilroy DW, Colville-Nash PR, McMaster S, Sawatzky DA, Willoughby DA, Lawrence T. Inducible cyclooxygenase-derived 15-deoxy(Delta)12–14PGJ2 brings about acute inflammatory resolution in rat pleurisy by inducing neutrophil and macrophage apoptosis. Faseb J. 2003;17:2269–71. doi: 10.1096/fj.02-1162fje. [DOI] [PubMed] [Google Scholar]
- 41.Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS ONE. 2010;5:e9016. doi: 10.1371/journal.pone.0009016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murua A, Portero A, Orive G, Hernández RM, de Castro M, Pedraz JL. Cell microencapsulation technology: towards clinical application. J Control Release. 2008;132:76–83. doi: 10.1016/j.jconrel.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Orive G, Anitua E, Pedraz JL, Emerich DF. Biomaterials for promoting brain protection, repair and regeneration. Nature Reviews Neuroscience. 2009;10:682–92. doi: 10.1038/nrn2685. [DOI] [PubMed] [Google Scholar]
- 44.Buchser E, Goddard M, Heyd B, Joseph JM, Favre J, de Tribolet N, et al. Immunoisolated xenogenic chromaffin cell therapy for chronic pain. Initial clinical experience Anesthesiology. 1996;85:1005–12. doi: 10.1097/00000542-199611000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Ross CJ, Ralph M, Chang PL. Delivery of recombinant gene products to the central nervous system with nonautologous cells in alginate microcapsules. Hum Gene Ther. 1999;10:49–59. doi: 10.1089/10430349950019183. [DOI] [PubMed] [Google Scholar]
- 46.Zimmermann U, Thürmer F, Jork A, Weber M, Mimietz S, Hillgärtner M, et al. A novel class of amitogenic alginate microcapsules for long-term immunoisolated transplantation. Ann N Y Acad Sci. 2001;944:199–215. doi: 10.1111/j.1749-6632.2001.tb03833.x. [DOI] [PubMed] [Google Scholar]
- 47.Read TA, Stensvaag V, Vindenes H, Ulvestad E, Bjerkvig R, Thorsen F. Cells encapsulated in alginate: a potential system for delivery of recombinant proteins to malignant brain tumours. Int J Dev Neurosci. 1999;17:653–63. doi: 10.1016/s0736-5748(99)00052-0. [DOI] [PubMed] [Google Scholar]
- 48.Thanos CG, Calafiore R, Basta G, Bintz BE, Bell WJ, Hudak J, et al. Formulating the alginate-polyornithine biocapsule for prolonged stability: evaluation of composition and manufacturing technique. J Biomed Mater Res A. 2007;83:216–24. doi: 10.1002/jbm.a.31472. [DOI] [PubMed] [Google Scholar]
- 49.Orive G, Tam SK, Pedraz JL, Hallé J-P. Biocompatibility of alginate-poly-L-lysine microcapsules for cell therapy. Bio-materials. 2006;27:3691–700. doi: 10.1016/j.biomaterials.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 50.Paredes-Juarez GA, de Haan BJ, Faas MM, de Vos P. The role of pathogen-associated molecular patterns in inflammatory responses against alginate based microcapsules. J Control Release. 2013;172:983–92. doi: 10.1016/j.jconrel.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 51.Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate Compliance versus Ligand Density in Cell on Gel Responses. Biophysical Journal. 2004;86:617–28. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 53.Discher DE, Janmey P, Wang Y-L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 54.Silva NA, Moreira J, Ribeiro-Samy S, Gomes ED, Tam RY, Shoichet MS, et al. Modulation of bone marrow mesenchymal stem cell secretome by ECM-like hydrogels. Biochimie. 2013;95(12):2314–9. doi: 10.1016/j.biochi.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 55.Marklein RA, Soranno DE, Burdick JA. Magnitude and presentation of mechanical signals influence adult stem cell behavior in 3-dimensional macroporous hydrogels. Soft Matter. 2012;8:8113–20. [Google Scholar]
- 56.Haudenschild AK, Hsieh AH, Kapila S, Lotz JC. Pressure and distortion regulate human mesenchymal stem cell gene expression. Annals of Biomedical Engineering. 2009;37:492–502. doi: 10.1007/s10439-008-9629-2. [DOI] [PubMed] [Google Scholar]
- 57.Bartosh TJ, Ylostalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci USA. 2010;107:13724–9. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ylostalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells. 2012;30:2283–96. doi: 10.1002/stem.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]