Abstract
Background
In 2012, one third of cases in a multistate outbreak of variant influenza A(H3N2) virus ([H3N2]v) infection occurred in Ohio. We conducted an investigation of (H3N2)v cases associated with agricultural Fair A in Ohio.
Methods
We surveyed Fair A swine exhibitors and their household members. Confirmed cases had influenza-like illness (ILI) and a positive laboratory test for (H3N2)v, and probable cases had ILI. We calculated attack rates. We determined risk factors for infection, using multivariable log-binomial regression.
Results
We identified 20 confirmed and 94 probable cases associated with Fair A. Among 114 cases, the median age was 10 years, there were no hospitalizations or deaths, and 82% had swine exposure. In the exhibitor household cohort of 359 persons (83 households), we identified 6 confirmed cases (2%) and 40 probable cases (11%). An age of <10 years was a significant risk factor (P < .01) for illness. One instance of likely human-to-human transmission was identified.
Conclusions
In this (H3N2)v outbreak, no evidence of sustained human-to-human (H3N2)v transmission was found. Our risk factor analysis contributed to the development of the recommendation that people at increased risk of influenza-associated complications, including children aged <5 years, avoid swine barns at fairs during the 2012 fair season.
Keywords: influenza, variant influenza virus, outbreak
Variant influenza viruses pose a public health threat because preexisting immunity in the general population may be low, and current seasonal influenza vaccination may not confer protection against them (influenza viruses that typically infect swine are referred to as “variant” when they infect humans) [1]. From January 2005 to June 2012, only 36 cases of variant influenza virus infection were identified in the United States [1–3]. However, during July–September 2012, an outbreak of variant influenza A(H3N2) virus ([H3N2]v) infection involving 10 states resulted in 306 confirmed cases [3, 4]. The (H3N2)v strain causing this outbreak had acquired the matrix (M) gene from the 2009 pandemic influenza A(H1N1) virus (A[H1N1]pdm09). This virus was first identified in humans in 2011, and there was concern that it could be efficiently transmitted among humans because the M gene has been hypothesized to contribute to efficient transmission of A(H1N1)pdm09 in animal models [5–8].
Ohio was one of the first states involved in the 2012 (H3N2)v outbreak and reported over one third of the cases of (H3N2)v infection (n = 107) and more than half of all (H3N2)v-associated hospitalizations [3, 9]. The first 2 Ohio cases were reported to the Ohio Department of Health (ODH) on 27 July 2012. Both cases were in children who presented to a local emergency department with influenza-like illness (ILI) and who had recently exhibited swine at an agricultural fair (Fair A) in County A (population, 370 000) [10]. Fair A, which took place during 22–28 July, attracted approximately 100 000 visitors and 200 swine exhibitors, who showed approximately 300 swine. Animal testing concurrent with this investigation showed that several swine exhibited at Fair A were positive for the same virus that was later identified in humans as (H3N2)v [11–13]. After reports of ILI in other Fair A attendees, ODH, the County A health department, and the Centers for Disease Control and Prevention (CDC) conducted a field investigation to define the magnitude of the (H3N2)v outbreak associated with Fair A, determine the severity of illness associated with (H3N2)v infection, identify and characterize instances of human-to-human transmission of (H3N2)v, examine possible risk factors for infection, and estimate an attack rate among persons thought to be at risk for infection.
METHODS
This investigation included case finding during 30 July–13 August 2012, and a subsequent swine exhibitor household cohort study. To identify cases, we (1) surveyed County A community members who called the local health department reporting illness or concern for illness, using questionnaires conducted via phone interviews; (2) requested that all cases of ILI identified by physician offices, health clinics, and hospitals in County A be evaluated for influenza and reported to the local public health department; (3) interviewed all patients who were tested for influenza virus in County A since the start of Fair A; and (4) performed contact tracing of confirmed and probable cases of (H3N2)v infection. For the swine exhibitor household cohort study, we surveyed all swine exhibitors at Fair A and their household members for ILI and risk factors for (H3N2) v. We tested persons reporting ILI and calculated attack rates for confirmed and probable (H3N2)v infection.
Confirmed cases were defined as meeting clinical and exposure criteria, with positive laboratory results for (H3N2)v. Clinical criteria were defined as ILI (subjective fever or temperature >38.0°C plus either cough or sore throat). Exposure criteria included any one of the following occurring ≤7 days before symptom onset: (1) direct contact with swine (touching swine), (2) indirect contact with swine (spending time in a swine barn at Fair A), (3) no direct or indirect contact with swine but attendance at an event or location where confirmed cases had been identified (such as Fair A), or (4) no direct or indirect contact with swine but contact with a confirmed or probable case. Probable cases met the clinical and exposure criteria described above but either did not have diagnostic testing performed or had inconclusive results. Noncases did not meet clinical and exposure criteria or tested negative for (H3N2)v.
Cases were tested for influenza virus by using reverse transcription– polymerase chain reaction (RT-PCR) analysis at ODH or CDC laboratories, according to protocols reported previously [14–16]. Respiratory specimens were obtained using nasopharyngeal swabs from patients who had symptoms at the time of survey administration and from patients who were no longer symptomatic, if symptom onset was ≤10 days from the day the survey was administered [17, 18]. Negative test results obtained for persons >4 days after symptom onset were determined to be inconclusive, given the sensitivity limits of RT-PCR; these patients were categorized as probable cases [19, 20].
We asked all confirmed and probable cases about swine exposures, symptoms, and illness in all household members. For the exhibitor household cohort, we surveyed all household members and asked additional questions about potential risk factors for (H3N2)v infection, including status as a swine exhibitor or household member of exhibitor, attendance at Fair A, presence in the swine barn at Fair A, total number of days and time per day spent in the swine barn (≤4 hours vs >4 hours), hand washing after contact with swine (none to less than half the time vs more than half the time to always), and contact with any ill person since the beginning of Fair A. We also asked about underlying medical conditions, including asthma, other chronic lung disease, heart disease (excluding hypertension), diabetes, kidney disease, immunosuppressive condition, or neurologic disorders.
We investigated the potential for human-to-human transmission among all confirmed or probable cases with illness but without swine exposure or with illness onset >4 days after last swine exposure [3, 18, 21]. Contact tracing was conducted for all residents of County A with confirmed or probable (H3N2)v infection.
We compared confirmed and probable cases to noncases by using the χ2 statistic for categorical variables and simple linear regression for continuous variables. Multivariable log-binomial regression with generalized linear models was used to identify risk factors associated with confirmed and probable case status [22]. Variables were chosen on the basis of their significance in bivariate analysis (P < .05) and biologic plausibility. The attack rate was calculated as the number of cases divided by the total number of people in the exhibitor household cohort. Given that households are important in the transmission of seasonal influenza viruses, we also performed multivariable regression, identifying households as cluster units [23, 24]. Analysis was performed in SAS 9.3. (Cary, North Carolina).
This investigation was conducted as part of a public health response and was not considered to be human subjects research in accordance with the federal human subjects protection regulations and the CDC’s Guidelines for Defining Public Health Research and Public Health Nonresearch.
RESULTS
Case Finding
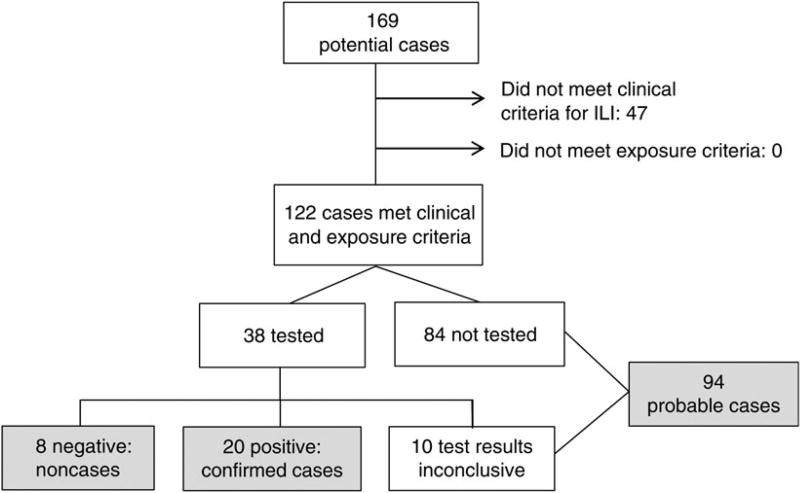
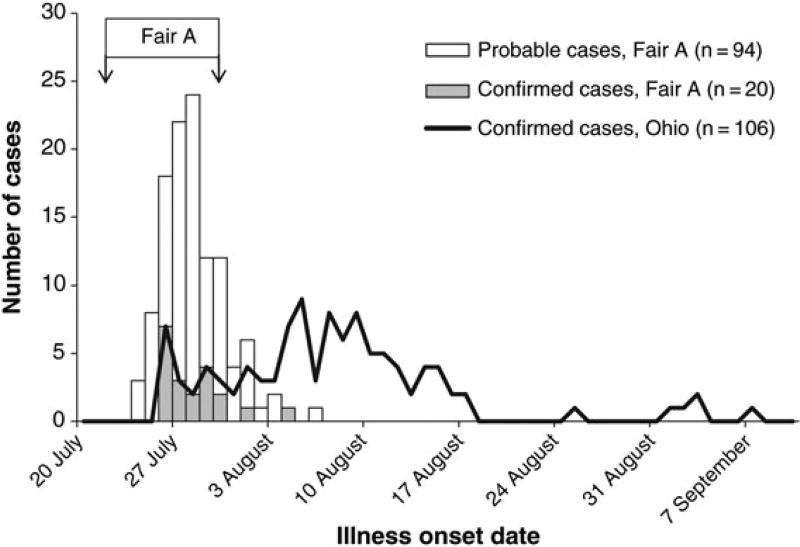
We identified 20 confirmed and 94 probable cases associated with Fair A (Figure 1); none of these cases resulted in hospitalization or deaths. Dates of illness onset for confirmed and probable cases were from 24 July through 6 August 2012 (Figure 2). Among the 114 confirmed and probable cases, 94 (82%) had either direct or indirect exposure to swine, 14 (12%) attended Fair A but denied swine exposure, 3 (3%) did not attend Fair A, and 3 (3%) had an unknown exposure. Among confirmed and probable cases, the median age was 10 years (range, <1 to 68 years), 58 (51%) were female, and the median length of illness was 3 days (range, 1–11 days). In addition to ILI, 8 confirmed cases (40%) had gastrointestinal symptoms, and 6 (30%) had eye pain or redness. Eleven (55%) confirmed cases were prescribed influenza antiviral medications.
Figure 1.
Variant influenza A(H3N2) virus ([H3N2]v) infection case finding associated with Fair A, Ohio, July–August 2012. Confirmed cases met clinical and exposure criteria and had a positive laboratory test for (H3N2)v. Test results were considered inconclusive if they were negative but samples were collected >4 days after illness onset. Probable cases met clinical criteria (influenza-like illness [ILI]) and exposure criteria (direct or indirect contact with swine, attendance at an event or location where confirmed cases were identified, or contact with a confirmed or probable case) ≤7 days prior to symptom onset but had no testing or inconclusive results of testing for (H3N2)v.
Figure 2.
Number of confirmed and probable cases of variant influenza A(H3N2) virus ([H3N2]v) infection, by illness onset date, identified through case finding as associated with Fair A and the total number of confirmed cases for Ohio, July–September 2012. Probable cases met clinical criteria (influenza-like illness) and exposure criteria (direct or indirect contact with swine, attendance at an event or location where confirmed cases were identified, or contact with a confirmed or probable case) ≤7 days prior to symptom onset but had no testing or inconclusive testing for (H3N2)v. Confirmed cases met clinical and exposure criteria and had a positive laboratory test for (H3N2)v. Illness onset date for 1 confirmed case in Ohio was unknown, and this case is not included.
Exhibitor Household Cohort
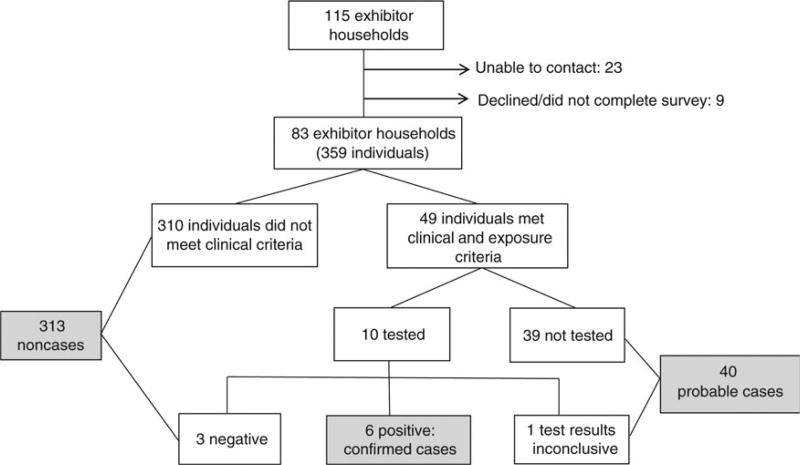
We identified 182 swine exhibitors at Fair A, comprising 115 households (Figure 3). We were unable to contact 23 households, and 9 declined participation. The final exhibitor household cohort included 83 households consisting of 359 individuals; 150 were swine exhibitors, and 209 were household members who did not exhibit swine. All members of the exhibitor household cohort lived in County A. Among exhibitors and their household members, we identified 6 confirmed cases and 40 probable cases, 24 (52%) of whom were swine exhibitors. The 6 confirmed and 40 probable cases in the exhibitor household cohort are included in the results of case finding. Using only confirmed cases (n = 6), we calculated an attack rate of 2%. Use confirmed and probable cases (n = 46) yielded an attack rate of 13%.
Figure 3.
Exhibitor household cohort variant influenza A(H3N2) virus ([H3N2]v) infection investigation, Fair A Ohio, July–August 2012. Confirmed cases met clinical and exposure criteria and had a positive laboratory test for (H3N2)v. Test results were considered inconclusive if they were negative but samples were collected >4 days after illness onset. Probable cases met clinical criteria (influenza-like illness) and exposure criteria (direct or indirect contact with swine, attendance at an event or location where confirmed cases were identified, or contact with a confirmed or probable case) ≤7 days prior to symptom onset but had no testing or inconclusive results of testing for (H3N2)v.
Confirmed and probable cases in the exhibitor household cohort were significantly younger than noncases, with a median age of 11 years vs 20 years (P < .01; Table 1). More cases than noncases were <10 years old (41% vs 11%; P < .01). There was no significant difference in sex, race, or median household size between cases and noncases.
Table 1.
Demographic, Clinical, and Exposure Characteristics of Exhibitor Household Cohort, Fair A Ohio July–August 2012
| Variable | Confirmed and Probable Cases (n = 46) |
Noncases (n = 313) | Relative Riska (95% CI) | P Value |
|---|---|---|---|---|
| Demographic characteristic | ||||
|
| ||||
| Age, y | ||||
| Median (range) | 10.5 (0.8–55) | 20.0 (0.3–78) | 1.0 (.9–1.0)b | <.01 |
| <10 | 19 (41) | 34 (11) | 4.0 (2.4–6.6) | <.01 |
| ≥10 | 27 (59) | 271 (89) | Reference | |
| Sex | ||||
| Male | 27 (59) | 158 (50) | Reference | |
| Female | 19 (41) | 155 (50) | 0.7 (.4–1.3) | .3 |
| Race | ||||
| White | 46 (100) | 312 (99) | Reference | |
| Black | 0 (0) | 1 (1) | 0 (.0–7.8) | 1.0 |
| Household size, no., median (range) | 5 (3–8) | 4 (2–8) | 1.1 (.9–1.3)c | .3 |
|
| ||||
| Risk factor | ||||
|
| ||||
| At least 1 medical conditiond | 9 (20) | 38 (12) | 1.6 (.8–3.1) | .2 |
| Asthma or chronic lung disease | 7 (15) | 24 (8) | 1.9 (.9–3.9) | .1 |
| Current smoker | 1/45 (2) | 12/305 (4) | 0.6 (.0–4.0) | 1.0 |
| Attended Fair A | 46 (100) | 308 (98) | 0 (0–3.5) | 1.0 |
| Time at fair, d, median (IQR) | 8 (7–8) | 8 (8–8) | 1.0 (.8–1.1) | .5 |
| Household status | ||||
| Swine exhibitor | 24 (52) | 126 (40) | 1.5 (.9–2.6) | .1 |
| Household member of swine exhibitor | 22 (48) | 187 (60) | Reference | |
| Contact with swine at Fair Ae | ||||
| Direct | 46 (100) | 286 (91) | Reference | |
| Indirect | 0 (0) | 18 (6) | .03 | |
| Attended Fair A | 0 (0) | 4 (1) | 0 (0–.8) | |
| Household member | 0 (0) | 5 (2) | ||
| Went into swine barn | 46 (100) | 300/312 (96) | 0 (0–1.7) | .4 |
| Time in swine barn, h | ||||
| <4/day | 18 (39) | 178 (57) | Reference | |
| ≥4/day | 28 (61) | 131 (42) | 1.9 (1.1–3.3) | .02 |
| Total hours in barn,f median (range) | 22 (0.5–40) | 20 (0–36) | 1.0 (.9–1.0) | .1 |
| Hand washing | ||||
| None to <50% of time | 26/42 (62) | 131/274 (48) | Reference | |
| 50% of time to always | 16/42 (38) | 143/274 (52) | 0.6 (.3–1.1) | .1 |
| Recalled contact with ill pig at fair | 18/43 (42) | 87/259 (34) | 1.4 (.8–2.4) | .3 |
| Close contact with swine at home or work | 18/45 (40) | 124/309 (40) | 1.0 (.6–1.7) | 1.0 |
| Contact with any ill person (inside or outside household) | 21 (46) | 88 (28) | 1.9 (1.1–3.3) | .02 |
| Contact with ill household member | 13 (28) | 56 (18) | 1.7 (.9–3.0) | .1 |
|
| ||||
| Clinical characteristic | ||||
|
| ||||
| lllness duration, d, median (range) | 3 (1–38) | NA | NA | |
| Sought medical care | 12/44 (27) | 8/36 (22) | 1.1 (.7–1.7) | .6 |
| Hospitalized | 0 (0) | 0 (0) | NA | |
| Death | 0 (0) | 0 (0) | NA | |
Data are no. (%) of subjects or no. of subjects with the characteristic/no. evaluated (%), unless otherwise indicated. Not all numbers sum to the total, because some individuals did not complete all questions in the survey.
Abbreviations: CI, confidence interval; d, days; h, hours; IQR, interquartile range; NA, not applicable; y, years.
To compare confirmed and probable vs noncases, χ2 analysis was used for categorical variables, and log-binomial regression was used for continuous variables.
Risk per year of age.
Risk per household member.
Medical conditions include asthma, other chronic lung disease, heart disease, diabetes, kidney disease, immunosuppressive condition, or neurologic disorders.
Direct contact defined as touching swine. Indirect contact defined as spending time in swine barn without touching swine. Attended Fair A was defined as no direct or indirect contact with swine but attendance at Fair A. Household member was defined as no direct or indirect contact with swine and no attendance at Fair A.
Total barn time is calculated from the total days in the swine barn multiplied by the time per day in the swine barn. Time per day in the swine barn was defined as <10 minutes, 10 minutes to 1 hour, 1–4 hours, and >4 hours. For total barn time calculations, the mean duration of each period was used (ie, 0.2 hours, 0.5 hours, 2.5 hours, and 4 hours, respectively).
Among confirmed and probable cases in the exhibitor household cohort, the median illness duration was 3 days (range, 1–38 days; Table 1). There was no difference in the proportion of cases and noncases who reported having at least 1 underlying medical condition (P = .2). Although not statistically significant, the risk of illness was almost twice as high among those with asthma or other chronic lung conditions (relative risk [RR], 1.9; 95% confidence interval [CI], .9–3.9; P = .1).
Ninety-nine percent of the exhibitor household cohort either visited Fair A or had direct or indirect contact with swine; all confirmed and probable cases in the household cohort had direct swine contact. Among 346 of 358 cohort members (97%) who went into the swine barn at Fair A, more confirmed and probable cases than noncases spent ≥4 hours per day in the barn (61% vs 42%; P = .02). However, the total median time spent in the barn over the course of Fair A was not significantly different between the 2 groups (22 hours in cases vs 20 hours in noncases; P = .1). Risk factors not significantly associated with confirmed or probable case status in bivariate analysis included number of days spent at Fair A, exhibitor versus household status, hand-washing behavior, recalling contact with ill swine at Fair A, and close contact with swine at home or work outside Fair A (Table 1). Forty-six percent of confirmed and probable cases recalled an ill human contact, either inside or outside the household, compared with 28% of noncases (P = .02). However, this difference was not significant when taking into account only ill contacts within the household (P = .1).
In a multivariable model that included age (<10 vs ≥10 years), hours per day in the swine barn (<4 vs ≥4 hours), report of ill human contact (yes vs no), and presence of an underlying medical condition (yes vs no), only age of <10 years was a significant risk factor for illness in a confirmed or probable case (adjusted RR, 3.4; 95% CI, 2.0–6.0; P < .01). Results of the multivariable analysis were similar when household clustering was accounted for, and the correlation coefficient (0.2), which indicates the importance of household clustering, was low.
Human-to-Human Transmission
We investigated 8 cases of potential human-to-human transmission associated with Fair A. There were 3 contacts of confirmed cases who were ill and had no known swine exposure; 2 tested negative, and 1 was not tested. There were 4 contacts of probable cases who were ill and had no known swine exposure; 2 tested negative, 1 had inconclusive test results (the individual tested negative >4 days after symptom onset), and 1 was not tested. In one case, a 1-year-old child without swine contact was exposed to an ill sibling who was not tested for influenza virus and did report swine contact. The 1-year-old child tested positive and was the only case likely due to human-to-human transmission associated with Fair A. Five additional community members with ILI but no association with Fair A were tested and were negative.
DISCUSSION
This investigation provides a detailed epidemiological description of (H3N2)v infections associated with an agricultural fair in Ohio during the largest known outbreak of variant influenza virus infection in the United States. From July–September 2012, 306 confirmed cases from 10 states were reported to the CDC, and 107 (35%) were from Ohio (Figure 2) [3]. This investigation includes 20 confirmed cases identified in Ohio associated with Fair A and describes one of the earliest and largest clusters of confirmed cases in the state. At the time of this investigation, the magnitude of the nationwide outbreak, including its pandemic potential, was unknown. Understanding that younger age was a risk factor, identification of a relatively low attack rate, and a lack of evidence of sustained human-to-human transmission associated with Fair A provided essential information that shaped the local and national public health response to this outbreak.
After extensive case finding and contact tracing, we identified only 1 instance of likely human-to-human transmission; however, 2 of 8 persons evaluated for the potential of human-to-human transmission were not tested for (H3N2)v infection and may have been unidentified cases. Despite this, we found no evidence of sustained human-to-human transmission. Our results are consistent with findings from the national (H3N2)v outbreak, in which 14 instances of possible human-to-human transmission were identified out of 306 confirmed cases (excluding the case associated with Fair A) [3]. Therefore, most transmission events associated with Fair A likely occurred between swine and humans. Human infection with variant influenza viruses has been associated with human-swine interactions in various settings, including agricultural fairs, farms, and other events where livestock and humans interact [25–29]. Other outbreaks of variant influenza viruses have resulted in limited or no human-to-human transmission [1, 25, 27, 28, 30, 31].
We found that younger age was an independent predictor of illness in multivariable analysis. An investigation of a 2011 outbreak of (H3N2)v infection associated with an agricultural fair in Pennsylvania also showed that younger age, especially <10 years, was a potential risk factor for infection [32]. Several serologic studies have shown lower levels of cross-reactive antibodies to (H3N2)v among children <10 years, compared with older children and adults [33–35]. Lower levels of cross-protective antibody and the high level of swine exposure experienced by many children at agricultural fairs may place them at higher risk of (H3N2)v infection. Thus, prevention strategies to limit (H3N2)v infection should target children who may have exposure to swine. This is consistent with CDC guidelines for seasonal influenza, which identify children aged <5 years as being at higher risk for influenza-associated complications. Our data were not sufficient to stratify by age <10 years [36].
By establishing the exhibitor household cohort early in the outbreak, we were able to estimate an attack rate for confirmed (H3N2)v infection (2%) and probable cases (13%). These findings are consistent with the Pennsylvania (H3N2)v infection outbreak in 2011, in which 11% of members of a children’s agricultural club who attended the fair were suspected cases with symptoms consistent with influenza [32]. But attack rates, even in similar outbreaks or epidemics, can vary depending on the population, setting (eg, school, community, or nursing home), and virus characteristics.
Approximately 150 million people attend fairs in North America every year, and several national public health recommendations were shaped in part by results from this investigation (Marla Calico, International Association of Fairs and Expositions, personal communication, December 2012). Children aged <5 years, adults aged ≥65 years, pregnant women, and people whose underlying medical conditions place them at high risk for influenza-associated complications were recommended to avoid swine and swine barns during the summer and fall of 2012 [37]. People in these categories are known to be at higher risk of developing complications from seasonal influenza and were advised to take care to prevent (H3N2)v infection [38]. This investigation did not find older age or the presence of underlying medical conditions to be significant risk factors for case status, but this may have been due to the small number of older individuals and those with medical problems in our cohort. Recommendations to fair organizers included posting signs encouraging hand washing after touching swine and monitoring the health of swine at fairs [36]. In addition, healthcare providers were encouraged to have a low threshold to test for influenza virus, especially in instances of ILI outside of the influenza season and when close contact with swine was reported [38].
There are several limitations to this investigation. First, swine exposure was widespread among the exhibitor household cohort, with 92% of all individuals reporting direct exposure to swine. This made it difficult to assess varying degrees of swine exposure as a risk factor for illness and might explain why total swine exposure time and exposure intensity (direct vs indirect) were not significant risk factors in this analysis. Second, timely testing among cases meeting exposure and clinical criteria was limited, resulting in a large number of probable cases whose illness etiology could not be confirmed or refuted. Third, testing of swine was performed at Fair A separately from this investigation, and additional details regarding clinical signs in swine, the timing of illness among swine as compared to humans, and interactions between ill swine and fair attendees was unknown. Additional details would be helpful in understanding the animal-to-human transmission dynamics of (H3N2)v during this outbreak. Fourth, for most of this analysis, we combined confirmed and probable cases, and thus misclassification of viral infection is possible. While it is unlikely that every probable case was due to (H3N2)v infection, it is likely that confirmed cases underestimate true disease incidence, since not all those who are infected are symptomatic, seek healthcare, or are tested for influenza virus. During seasonal influenza epidemics, laboratory-confirmed cases of influenza often represent a fraction of all infections [39]. Similarly, it is estimated that there are approximately 200 cases of (H3N2)v infection for every reported case [40]. With active case finding and active surveillance, we identified >4 probable cases for each confirmed case.
During August–September 2012, this investigation in Ohio provided data for public health decision making in response to the larger outbreak of (H3N2)v infection in 10 states. The low severity, relatively low attack rate, and lack of sustained human-to-human transmission helped determine that this virus had low pandemic potential, an insight that helped shape a focused public health response to this outbreak. Results from this study also helped reinforce specific recommendations to fair attendees; for example, finding that younger age was an independent predictor of illness supported the recommendation that children aged <5 years avoid swine throughout the fair season. Vigilant surveillance during future agricultural fair seasons will be essential to determine whether ongoing transmission of (H3N2)v between swine and humans continues or whether efficient human-to-human transmission of variant viruses emerges.
Footnotes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Ohio Department of Health.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Update: influenza A (H3N2)v transmission and guidelines - five states, 2011. MMWR Morb Mortal Wkly Rep. 2012;60:1741–4. [PubMed] [Google Scholar]
- 2.Shinde V, Bridges CB, Uyeki TM, et al. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009. N Engl J Med. 2009;360:2616–25. doi: 10.1056/NEJMoa0903812. [DOI] [PubMed] [Google Scholar]
- 3.Jhung MA, Epperson S, Biggerstaff M, et al. Outbreak of variant influenza A(H3N2) virus in the United States. Clin Infect Dis. 2013;57:1703–12. doi: 10.1093/cid/cit649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (US) Update: influenza activity - United States and worldwide, May 20–September 22, 2012. MMWR Morb Mortal Wkly Rep. 2012;61:785–9. [PubMed] [Google Scholar]
- 5.Chou YY, Albrecht RA, Pica N, et al. The M segment of the 2009 new pandemic H1N1 influenza virus is critical for its high transmission efficiency in the guinea pig model. J Virol. 2011;85:11235–41. doi: 10.1128/JVI.05794-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakdawala SS, Lamirande EW, Suguitan AL, Jr, et al. Eurasian-origin gene segments contribute to the transmissibility, aerosol release, and morphology of the 2009 pandemic H1N1 influenza virus. PLoS Pathog. 2011;7:e1002443. doi: 10.1371/journal.ppat.1002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma W, Liu Q, Bawa B, et al. The neuraminidase and matrix genes of the 2009 pandemic influenza H1N1 virus cooperate functionally to facilitate efficient replication and transmissibility in pigs. J Gen Virol. 2012;93:1261–8. doi: 10.1099/vir.0.040535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (US) Swine-origin influenza A (H3N2) virus infection in two children–Indiana and Pennsylvania, July–August 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1213–5. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (US) Influenza A (H3N2) variant virus-related hospitalizations: Ohio, 2012. MMWR Morb Mortal Wkly Rep. 2012;61:764–7. [PubMed] [Google Scholar]
- 10.US Census Bureau. [Accessed March 2013];State and county quick facts. http://quickfacts.census.gov/qfd/index.html.
- 11.Bowman AS, Sreevatsan S, Killian ML, et al. Molecular evidence for interspecies transmission of H3N2pM/H3N2v influenza A viruses at an Ohio agricultural fair, July 2012. Emerg Microbes Infect. 2012;1:e33. doi: 10.1038/emi.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowman AS, Nelson SW, Page SL, et al. Swine-to-human transmission of influenza A(H3N2) virus at agricultural fairs, Ohio, USA, 2012. Emerg Infect Dis. 2014;20:1472–80. doi: 10.3201/eid2009.131082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Z, Gomez J, Bowman AS, et al. Antigenic characterization of H3N2 influenza A viruses from Ohio agricultural fairs. J Virol. 2013;87:7655–67. doi: 10.1128/JVI.00804-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (US) Evaluation of rapid influenza diagnostic tests for influenza A (H3N2)v virus and updated case count–United States, 2012. MMWR Morb Mortal Wkly Rep. 2012;61:619–21. [PubMed] [Google Scholar]
- 15.Lindstrom S, Garten R, Balish A, et al. Human infections with novel reassortant influenza A(H3N2)v viruses, United States, 2011. Emerg Infect Dis. 2012;18:834–7. doi: 10.3201/eid1805.111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (US) [Accessed March 2013];Data interpretation update to CDC flu rRT-PCR Dx panel. http://www.cdc.gov/flu/pdf/swineflu/data-interpretation-update.pdf.
- 17.Bhattarai A, Villanueva J, Palekar RS, et al. Viral shedding duration of pandemic influenza A H1N1 virus during an elementary school outbreak–Pennsylvania, May–June 2009. Clin Infect Dis. 2011;52(suppl 1):S102–8. doi: 10.1093/cid/ciq026. [DOI] [PubMed] [Google Scholar]
- 18.Carrat F, Vergu E, Ferguson NM, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167:775–85. doi: 10.1093/aje/kwm375. [DOI] [PubMed] [Google Scholar]
- 19.Lau LL, Cowling BJ, Fang VJ, et al. Viral shedding and clinical illness in naturally acquired influenza virus infections. J Infect Dis. 2010;201:1509–16. doi: 10.1086/652241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loeb M, Singh PK, Fox J, et al. Longitudinal study of influenza molecular viral shedding in Hutterite communities. J Infect Dis. 2012;206:1078–84. doi: 10.1093/infdis/jis450. [DOI] [PubMed] [Google Scholar]
- 21.Lessler J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, Cummings DA. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9:291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2011;22:661–70. doi: 10.1177/0962280211427759. [DOI] [PubMed] [Google Scholar]
- 23.Ohmit SE, Petrie JG, Malosh RE, et al. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis. 2013;56:1363–9. doi: 10.1093/cid/cit060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–52. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawood FS, Dong L, Liu F, et al. A pre-pandemic outbreak of triple-reassortant swine influenza virus infection among university students, South Dakota, 2008. J Infect Dis. 2011;204:1165–71. doi: 10.1093/infdis/jir502. [DOI] [PubMed] [Google Scholar]
- 26.Vincent AL, Swenson SL, Lager KM, Gauger PC, Loiacono C, Zhang Y. Characterization of an influenza A virus isolated from pigs during an outbreak of respiratory disease in swine and people during a county fair in the United States. Vet Microbiol. 2009;137:51–9. doi: 10.1016/j.vetmic.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Wells DL, Hopfensperger DJ, Arden NH, et al. Swine influenza virus infections. Transmission from ill pigs to humans at a Wisconsin agricultural fair and subsequent probable person-to-person transmission. JAMA. 1991;265:478–81. doi: 10.1001/jama.265.4.478. [DOI] [PubMed] [Google Scholar]
- 28.Wentworth DE, Thompson BL, Xu X, et al. An influenza A (H1N1) virus, closely related to swine influenza virus, responsible for a fatal case of human influenza. J Virol. 1994;68:2051–8. doi: 10.1128/jvi.68.4.2051-2058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terebuh P, Olsen CW, Wright J, et al. Transmission of influenza A viruses between pigs and people, Iowa, 2002–2004. Influenza Other Respir Viruses. 2010;4:387–96. doi: 10.1111/j.1750-2659.2010.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaydos JC, Top FH, Jr, Hodder RA, Russell PK. Swine influenza a outbreak, Fort Dix, New Jersey, 1976. Emerg Infect Dis. 2006;12:23–8. doi: 10.3201/eid1201.050965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers KP, Olsen CW, Gray GC. Cases of swine influenza in humans: a review of the literature. Clin Infect Dis. 2007;44:1084–8. doi: 10.1086/512813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong KK, Greenbaum A, Moll ME, et al. Outbreak of influenza A(H3N2) variant virus infection among attendees of an agricultural fair, Pennsylvania, USA, 2011. Emerg Infect Dis. 2012;18:1937–44. doi: 10.3201/eid1812.121097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention (US) Antibodies cross-reactive to influenza A (H3N2) variant virus and impact of 2010–11 seasonal influenza vaccine on cross-reactive antibodies - United States. MMWR Morb Mortal Wkly Rep. 2012;61:237–41. [PubMed] [Google Scholar]
- 34.Waalen K, Kilander A, Dudman SG, Ramos-Ocao R, Hungnes O. Age-dependent prevalence of antibodies cross-reactive to the influenza A (H3N2) variant virus in sera collected in Norway in 2011. Euro Surveill. 2012;17:pii:20170. [PubMed] [Google Scholar]
- 35.Skowronski DM, De Serres G, Janjua NZ, et al. Cross-reactive antibody to swine influenza A(H3N2) subtype virus in children and adults before and after immunisation with 2010/11 trivalent inactivated influenza vaccine in Canada, August to November 2010. Euro Surveill. 2012;17:pii:20066. doi: 10.2807/ese.17.04.20066-en. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention (US) [Accessed March 2013];Influenza A (H3N2) variant virus: other guidance documents. http://www.cdc.gov/flu/swineflu/h3n2v-other-guidance.htm.
- 37.Centers for Disease Control and Prevention (US) [Accessed March 2013];Influenza H3N2 variant virus: H3N2v and you. http://www.cdc.gov/flu/swineflu/h3n2v-basics.htm.
- 38.Centers for Disease Control and Prevention (US) [Accessed March 2013];Interim information for clinicians about human infections with H3N2v virus. http://www.cdc.gov/flu/swineflu/h3n2v-clinician.htm.
- 39.Reed C, Angulo FJ, Swerdlow DL, et al. Estimates of the prevalence of pandemic (H1N1) 2009, United States, April–July 2009. Emerg Infect Dis. 2009;15:2004–7. doi: 10.3201/eid1512.091413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biggerstaff M, Reed C, Epperson S, et al. Estimates of the number of human infections with influenza A(H3N2) variant virus, United States, August 2011–April 2012. Clin Infect Dis. 2013;57(suppl 1):S12–5. doi: 10.1093/cid/cit273. [DOI] [PMC free article] [PubMed] [Google Scholar]