Abstract
Renin-angiotensin system (RAS) inhibitors (RASi)—widely prescribed for the treatment of cardiovascular diseases—have considerable potential in oncology. The RAS plays a crucial role in cancer biology and affects tumor growth and dissemination directly and indirectly by remodeling the tumor microenvironment. We review clinical data on the benefit of RASi in primary and metastatic tumors and propose that, by activating immunostimulatory pathways, these inhibitors can enhance immunotherapy of cancer.
INTRODUCTION
The circulating renin-angiotensin system (RAS) is mainly known for its pivotal role in maintaining cardiovascular homeostasis and fluid and electrolyte balance. In addition, a local RAS is expressed in many tissues and mainly acts at the cellular level, where it mediates cell proliferation, growth, and metabolism. The local RAS works synergistically and independently of the systemic RAS. Angiotensin II (AngII) is the main effector and maintains tissue homeostasis by exerting regulatory and counterregulatory effects through its different receptors. Alternative peptide-receptor axes also assist in maintaining this balance (1–7). Figure 1 provides an overview of the main components of the RAS. Dysregulation of the RAS, for example, by overexpression of certain RAS components [such as renin, Ang-converting enzyme (ACE), or AngII type 1 receptor (AT1R)], can be involved in the pathophysiology and progression of a broad range of diseases, such as arterial hypertension, kidney disease, and other cardiovascular conditions (5, 8, 9).
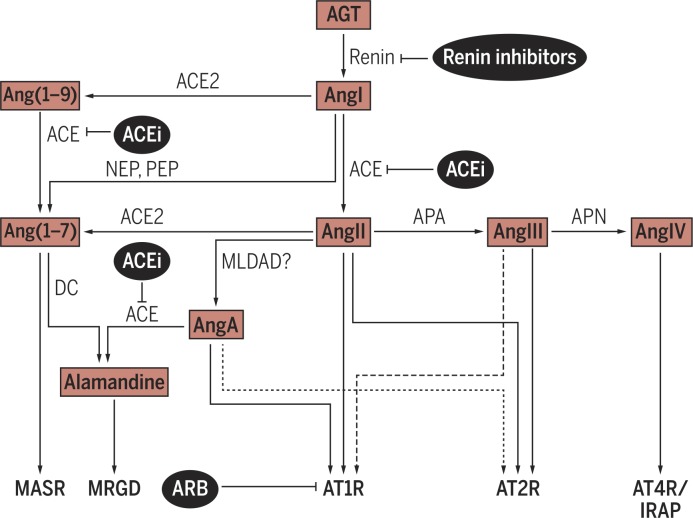
Fig. 1.
The RAS is a complex system whose bioactive peptides signal through different receptors. Angiotensinogen (AGT), generated and released into circulation by the liver, is hydrolyzed by renin, a product of the kidneys’ juxtaglomerular cells, to form AngI. AngI is then hydrolyzed by ACE, predominantly expressed by endothelial cells in the vascular territory of the lungs, to form the biologically active AngII. In addition to AngII, other truncated bioactive peptides have been identified, such as AngIII, AngIV, Ang(1–7), Ang(1–9), AngA, and alamandine. AngII interacts with two seven-transmembrane receptors, AT1R and AT2R, both of which also mediate the effects of AngA. Ang(1–7) mainly acts via the MAS receptor (MASR), and alamandine binds and signals through MRGD (MAS-related G protein– coupled receptor D). IRAP (insulin-regulated membrane aminopeptidase; also known as AT4R) is a binding site for AngIV (1–7). APA, aminopeptidase A; APN, aminopeptidase N; DC, decarboxylase; MLDAD, mononuclear leukocyte-derived aspartate DC; NEP, neutral endopeptidase; PEP, prolyendopeptidase.
The discoveries of captopril—the first orally active ACE inhibitor (ACEi)—in the mid-1970s (10) and losartan—the first orally active, selective AT1R blocker (ARB)—around a decade later (11) represent milestones in the history of the RAS. Numerous ACEis and ARBs have been developed since then. Now, ACEis and ARBs are the most common inhibitors of the RAS and are widely used in the management of several diseases, such as arterial hypertension, heart failure, myocardial infarction, and chronic kidney disease (12–15). Direct renin inhibitors (such as aliskiren) represent a third class of RAS-acting agents and have been added to the armamentarium more recently (16). A list of RAS inhibitors (RASi) approved by the U.S. Food and Drug Administration (FDA) is provided in table S1.
After being in clinical use for more than two decades in nonmalignant diseases, ACEi/ARBs have recently received considerable attention in oncology. A large-scale meta-analysis (17), published in 2010, found an increased overall occurrence of cancer in ARB users. However, two other meta-analyses published subsequently did not confirm these data (18, 19). The FDA also rebutted these findings with their own meta-analysis (20) and an integrated analysis of all 19 rodent carcinogenicity assays of ARBs (21). Thus, the data to date do not support an association between ACEi/ARB use and an increased cancer risk. However, they do not suggest a reduced occurrence of cancer either.
Of interest, an increasing number of preclinical studies support the involvement of RAS signaling in cancer development, growth, and progression (4). These data have led to investigations of the effects of RASi— both retrospectively and prospectively—in patients with different types of cancer. Interim analysis of a recent phase 2 trial—stemming from our preclinical findings (22)—showed encouraging R0 (microscopically margin-negative) resection rates in patients with locally advanced pancreatic ductal adenocarcinoma (PDAC) receiving neoadjuvant losartan plus chemoradiation (23). Moreover, our recent retrospective analysis indicated that RASi use is associated with improved survival of patients with nonmetastatic PDAC, presumably by stimulating the tumor’s immune microenvironment, normalizing its extracellular matrix (ECM), and reducing the malignant potential of cancer cells (24).
In light of these emerging data, we discuss the role of the RAS in cancer biology with a special emphasis on tumor immunity. In addition, by carefully analyzing the studies with positive versus negative outcomes, we make a case for targeting the RAS to improve treatment of certain malignancies. Moreover, RASi may not only improve the outcome of immunotherapies but also reduce or even prevent adverse effects associated with these therapies.
The AngII/AT1R axis shapes the tumor microenvironment and promotes an immunosuppressive milieu
Components of the RAS are expressed in various human cancers and cell lines (4). Overexpression of AT1R is typically associated with more aggressive tumor features (larger tumors, higher grade, and higher vascular density) and worse outcomes (25–29).
Moreover, RAS components are also expressed in many cell types of the tumor microenvironment, such as endothelial cells, fibroblasts, monocytes, macrophages, neutrophils, dendritic cells, and T cells (4, 30–34). RAS signaling in these cells can facilitate or hinder growth and dissemination and has been shown to affect cell proliferation, migration, invasion, metastasis, apoptosis, angiogenesis, cancer-associated inflammation, immunomodulation, and tumor fibrosis/desmoplasia (1, 4). Generally, the AngII/AT1R axis is considered to favor tumor growth, whereas AngII/AT2R and Ang(1–7)/MAS signaling have opposing effects (1, 4). However, there are also conflicting reports suggesting potential tumor type–specific differences (35–39).
The tumor-promoting actions of the ACE/AngII/AT1R axis, the main target of classical RASi, have been reviewed elsewhere (1, 4). In this section, we focus on its role in tumor immunity and propose RASi as an adjunct for immunotherapy. Immune checkpoint inhibitors have recently achieved compelling success in melanoma and other solid tumors (40). However, their efficacy is diminished by a major barrier—the immunosuppressive tumor microenvironment (41). Here, we review how AngII/AT1R signaling shapes the tumor immune microenvironment by modulating desmoplasia, vasculature, inflammation, and immune cells. We also discuss how RASi could alleviate immunosuppression and enhance the outcome of immunotherapy.
Tumor desmoplasia and solid stress
By regulating cancer-associated fibroblasts (CAFs) and profibrogenic pathways [such as transforming growth factor–β (TGF-β)], the RAS plays a key role in establishing a desmoplastic environment (22, 42), which affects the immune response in multiple ways (Fig. 2). CAFs can manipulate the immune system directly by inhibiting T and NK (natural killer) cell functions, promoting accumulation of suppressive cell types, and maintaining an inflammatory protumorigenic milieu (43). TGF-β can also directly induce immune suppression by inhibiting the T cell response (44). Dense tumor fibrosis represents a physical barrier to T cell infiltration (45). It also compresses blood vessels by increasing solid stress (46, 47). The reduced tumor perfusion results in a hypoxic and acidic milieu, which promotes reprogramming of macrophages into an immunosuppressive phenotype, impairs tumor killing functions of immune cells, and up-regulates the expression of inhibitory immune checkpoint molecules, such as programmed death-ligand 1 (PD-L1), by immune, stromal, and tumor cells (Fig. 3) (46–51). Normalizing the desmoplastic milieu (for example, by targeting profibrotic pathways and CAFs) can improve the efficacy of immunotherapy (52–54).
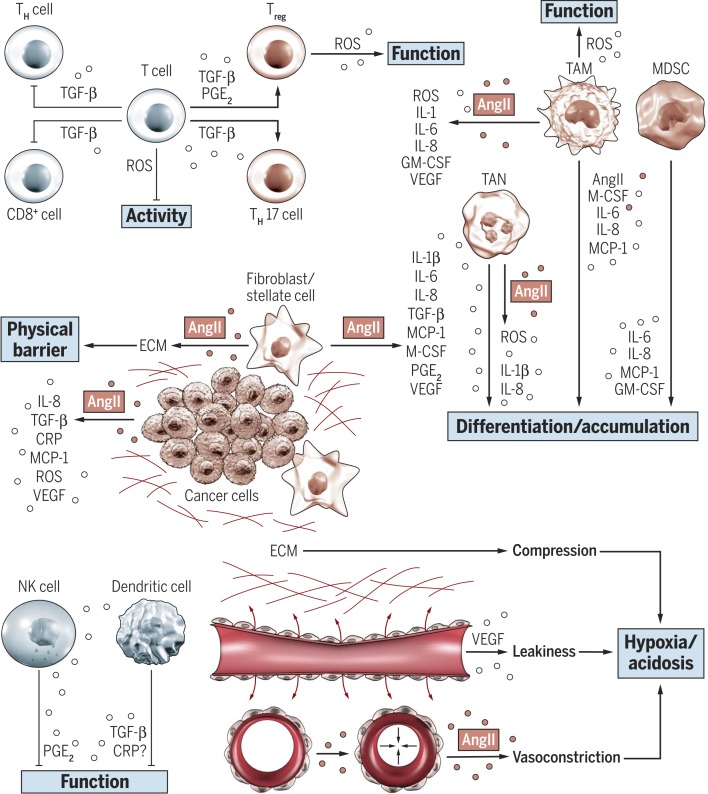
Fig. 2.
The AngII/AT1R axis regulates the tumor stroma and contributes to an immunosuppressive microenvironment. AngII/AT1R signaling can increase production and release of several proinflammatory cytokines in both tumor and stromal cells. Immunomodulatory cytokines regulate a myriad of immunosuppressive immune responses by modulating differentiation, recruitment, and function of both myeloid and lymphoid immune cell types (4, 43, 44). More precisely, these cytokines suppress the differentiation and function of immunostimulatory cell types [for example, TH (T helper) and CD8+ cells, NK cells, and dendritic cells] and activate recruitment and function of tumor-promoting cell types [such as Tregs, TH17 cells, TANs, TAMs (tumor-associated macrophages), and MDSCs (myeloid-derived suppressor cells)]. Fibroblasts are a major source of cytokines and also play a key role in establishing a desmoplastic stroma by production and deposition of ECM. The dense tumor fibrosis represents a physical barrier to immune cell infiltration (45) and compresses blood vessels by increasing tissue stiffness and solid stress. The reduced tumor perfusion results in a hypoxic and acidic milieu, which further promotes immunosuppression (46–48). Vascular endothelial growth factor (VEGF)–induced vascular leakiness (48) and AngII-mediated vasoconstriction (76, 77, 80) further impair tumor perfusion and aggravate hypoxia. GM-CSF, granulocyte-macrophage colony-stimulating factor. PGE2, prostaglandin E2.
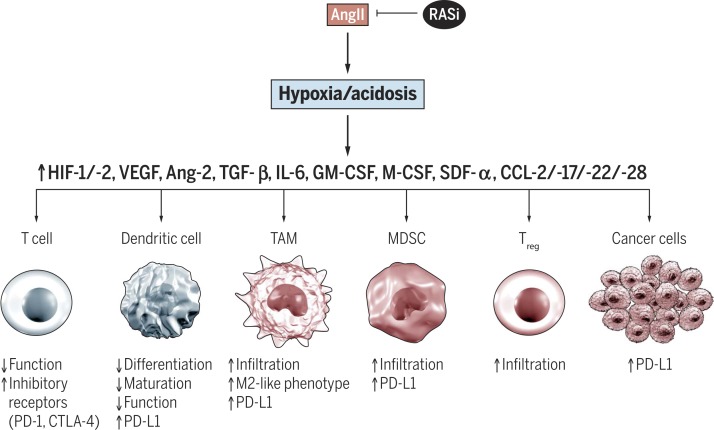
Fig. 3.
Tumor hypoxia and acidosis promote immunosuppression. AngII/AT1R-mediated effects on tumor vasculature (shown in Fig. 2) can impair tumor perfusion and oxygenation, resulting in hypoxia and acidosis within the tumor stroma. The resulting up-regulation of various cytokines, growth factors, and transcription factors [including HIF (hypoxia-inducible factor), VEGF, and TGF-b] enhances an immunosuppressive microenvironment, characterized by impaired T and dendritic cell function, accumulation of immunosuppressive cell types (M2-like macrophages, MDSCs, and Tregs), and increased expression of inhibitory immune checkpoint molecules such as PD-L1 in tumor and immune cell types (48–50, 68). Ang-2, angiopoietin-2; CCL, CC chemokine ligand; CTLA-4, cytotoxic T lymphocyte– associated protein 4; SDF, stromal cell–derived factor.
Several studies have demonstrated that RASi can successfully normalize the fibrotic stroma. Co-injection of cancer cells with stromal cells increases tumor size and fibrosis, and treatment with ARBs attenuates these effects (55, 56). Losartan inhibits collagen I production by CAFs and reduces stromal collagen and hyaluronic acid (HA) in several desmoplastic tumor models by decreasing profibrotic signaling via TGF-β, connective tissue growth factor, HA synthase 1 and 3, and endothelin-1 (22). Therefore, losartan reduces solid stress and improves vascular perfusion, resulting in decreased tumor hypoxia and improved distribution and efficacy of anticancer drugs and nanotherapeutics (22, 42). Similarly, inhalation delivery of losartan and telmisartan reduces active TGF-β and collagen I expression and increases the intratumoral distribution of nanoparticles (57, 58). Moreover, the cross-talk between tumor-associated neutrophils (TANs), adipocytes, and pancreatic stellate cells (PSCs) promotes tumor desmoplasia and pancreatic cancer growth in obesity (59). AT1R inhibition attenuates obesity-induced fibrosis and tumor progression and improves response to chemotherapy (CHT). The AT1R blockade also reduces TANs and regulatory T cells (Tregs) and increases CD8+ T cells through inhibition of PSC activation and subsequent reduction of interleukin-1β (IL-1β) expression (59). In another orthotopic model of pancreatic cancer, inhibition of aberrant TGF-β activity by losartan reduced collagen deposition and accumulation of Tregs (60).
Collectively, these data support the idea that targeting AngII/AT1R signaling with RASi can effectively reduce tumor desmoplasia and thereby decrease solid stress, increase tumor perfusion, reduce hypoxia, enhance T cell infiltration and antitumor immunity, and improve delivery and efficacy of anticancer drugs. Thus, inhibiting the AngII/AT1R axis appears to be an attractive strategy, especially for highly desmoplastic tumors, such as PDAC and some subtypes of breast and lung cancer, and RASi may represent a promising combination partner for immunotherapy.
Angiogenesis and tumor vasculature
Considerable evidence suggests that AngII/AT1R signaling promotes VEGF-mediated angiogenesis in solid tumors. AT1R expression correlates with VEGF and VEGF receptor (VEGFR) expression and microvessel density (MVD) in different human tumors (26, 27, 29). In experimental studies, AngII promoted VEGF expression in tumor (61–63) and stromal cells (64). Treatment with either ACEi or ARB reduced VEGF expression and decreased MVD and neovascularization in vivo (65, 66). VEGF also induces vascular hyperpermeability, one of the main characteristics of the abnormal tumor vasculature (46, 48). Tumor vessel leakiness promotes tumor hypoxia and acidosis by impairing tumor blood flow (Fig. 2) (48, 67). As mentioned above, hypoxia helps to create an immunosuppressive milieu (Fig. 3) and promotes tumor progression and dissemination (48, 68). Tumor vessel normalization can alleviate hypoxia, reprogram the immunosuppressive microenvironment, and improve the efficacy of immunotherapy in mice (68, 69). Glioblastoma patients who show enhanced tumor blood perfusion under anti-angiogenic therapy have markedly prolonged survival compared to subjects who experience no change or a decrease in perfusion (70–72). RASi also reduces VEGF-mediated vascular leakiness in the dermis and retina of rodents (73, 74).
In an orthotopic model of PDAC, inhibition of aberrant TGF-β signaling by losartan restored vessel diameter and permeability (60). In a retrospective study of glioblastoma patients receiving anticancer therapy, a concomitant treatment with matrix-depleting antihypertensive drugs improved vascular function as assessed by magnetic resonance imaging (75). The impaired perfusion and hypoxic condition of tumors can be further aggravated by AngII-induced vasoconstriction and increased vascular resistance (Fig. 2) (76, 77). Our laboratory has shown that AngII transiently enhanced tumor blood flow and interstitial fluid pressure by increasing the mean arterial blood pressure in different tumor types (78, 79). However, Thews and colleagues (80) found that AngII infusion decreased tumor perfusion and oxygenation in small subcutaneous sarcomas but increased both parameters in large tumors. They concluded that perfusion decreased due to vasoconstriction of preexisting functionally intact host vessels in small sarcomas, whereas the newly formed tumor vessels in large tumors did not seem to have this vasoresponsive capability, possibly due to lack of smooth muscle cells and/or angiotensin (AT) receptors (80).
Together, available data indicate that AngII/AT1R signaling impairs tumor blood supply through multiple mechanisms, such as desmoplasia-mediated vessel compression, VEGF-induced vessel leakiness and abnormal morphology, and AngIImediated vasoconstriction of host vessels. The resulting tumor hypoxia aggravates immunosuppression and evasion. Although RASi can reduce VEGF-mediated angiogenesis and desmoplasia, additional studies are needed to ascertain whether RASis have the ability to normalize the tumor vasculature, similar to anti-VEGF agents (48).
Inflammation and immune cell modulation
The RAS promotes cancer-related inflammation and infiltration of tumor-promoting immune cells (1, 4, 81), both of which enhance the immunosuppressive microenvironment (41, 82). Here, we discuss how the RAS modulates the expression of inflammatory cytokines and orchestrates the recruitment of cancer-associated immune cells to the tumor microenvironment.
Inflammatory cytokines
A number of studies have shown that AngII/AT1R signaling can increase the production and release of several proin-flammatory cytokines in both tumor and stromal cells (4). Fibroblasts represent a main target of the RAS and play a pivotal role in maintaining an inflammatory response. Cytokines released from tumor and stromal cells upon AT1R activation by AngII include TGF-β, IL-1a, IL-1β, IL-6, IL-8, MCP-1 (monocyte chemoattractant protein–1), M-CSF, COX-2 (cyclooxygenase-2), and CRP (C-reactive protein) (Fig. 2) (4, 22, 42, 56, 59, 65, 83–87). Immunomodulatory cytokines (such as TGF-β, IL-1β, MCP-1, IL-6, and IL-8) can up-regulate multiple—mostly immunosuppressive— pathways by modulating the differentiation and recruitment of both myeloid and lymphoid immune cell types (Fig. 2) (44, 82, 88–91). COX-2 suppresses antitumor immunity and contributes to resistance to immunotherapy, mainly through prostaglandin E2 synthesis (92, 93). The role of tumor-derived CRP in tumor immunity is less clear, but it may impair dendritic cell function by reducing their migration activity (94).
Oxidative stress represents another aspect of cancer-related inflammation. Although reactive oxygen species (ROS) are involved in T cell activation (95, 96), exposure to ROS can reduce T cell fitness (90, 97, 98) and enhance the function of Tregs (99) andTAMs (100). TAMs typically show a polarized M2-like phenotype and contribute to immunosuppression, whereas M1-like macrophages are known to induce antitumor immunity (101). AngII/AT1R signaling induces ROS generation in tumor cells and stromal cells (4). In prostate cancer cells, AngIImediated expression of oxidative stress–related proteins (such as inducible nitric oxide synthase) and the generation of the ROS family member O2 − radical are attenuated by the ARB candesartan (102).
Immune cells
Several studies have shown that RASi can reduce infiltration of TAMs. In human prostate cancer, high MCP-1 and macrophage infiltration are associated with more aggressive tumor features, and MCP-1 independently correlates with prostate-specific antigen recurrence (103). AngII/AT1R signaling promotes production and infiltration of TAMs in experimental tumor models; inhibition of AngII production or AT1R signaling down-regulates MCP-1, restrains tumor-induced TAM response, reduces tumor growth, and prolongs survival (34, 103–105).
AngII/AT1R signaling is also important for myeloid differentiation and functional maturation (106). ACE knockout mice show enhanced extramedullary myelopoiesis and increased numbers of cells with MDSC phenotype (32). In contrast, cultured bone marrow from ACE 10/10 mice, a mouse line overexpressing ACE in monocytic cells, demonstrates enhanced myeloid maturation and reduced MDSC production; macrophages from these mice have a more proinflammatory phenotype and more antitumor activity compared to those from wild-type mice (107). Similarly, tumor-bearing ACE 10/10 mice showed enhanced immune response, which ultimately resulted in a reduced tumor growth. Notably, ACEi reversed the beneficial effects on tumor growth, but AT1R blockade did not, suggesting that the effects of ACE overexpression were not dependent on AngII/AT1R signaling (108, 109).
Together, available data clearly demonstrate that AngII/AT1R signaling stimulates the expression of different cytokines and growth factors from tumor and stromal cells, which enhance cancer-related inflammation and promote an immuno-suppressive microenvironment (Fig. 2). Beyond the tumor immune microenvironment, the AngII/AT1R axis is also crucial for the maturation and function of immunostimulatory myeloid cells, and ACE overexpression in monocytic cells enhances antitumor immunity, although the latter effect seems to be independent of the AngII/AT1R axis. These conflicting data highlight the complexity of the RAS in cancer immunity. However, because studies supporting a stimulatory role of RAS in tumor immunosuppression considerably outweigh opposing data, we propose that RASi can effectively reprogram the tumor microenvironment toward an immunostimulatory milieu and enhance the efficacy of immunotherapy.
RASi to reduce side effects of immunotherapy
As discussed above, RASi may increase the intratumoral delivery of T cells and immunotherapeutic agents by modulating tumor vasculature and desmoplasia. This may allow for reduction in the dose of immunotherapeutic agents without decreasing the therapeutic benefit and could ultimately result in a decreased number of severe (grades 3 and 4) immunotherapy-related adverse effects. These side effects can occur in more than 50% of patients, especially if certain checkpoint blockers are combined, and some can be even lifethreatening (110, 111).
Obesity and associated chronic inflammation seem to play a critical role in inducing immunotherapy-associated toxicities (112, 113). Systemic stimulatory immunotherapy, such as αCD40/IL-2, can cause a cytokine storm, characterized by high tumor necrosis factor–α (TNF-α) and IL-6, resulting in multiorgan pathologies and lethality in obese but not in lean mice (112, 113). The TNF blockade ameliorates the observed toxicities in obese mice (113). Inhibition of the RAS can also ameliorate chronic inflammation, as shown by reduced serum concentrations of proinflammatory cytokines (TNF-α and IL-6) in patients with hypertension and diabetes (114–116). This represents another way that RASi may help to reduce or even prevent immunotherapy-induced toxicity.
RAS inhibition can improve treatment of certain tumors
The effect of RASi on the clinical outcome of patients with different tumor types has been extensively studied in recent years. Tables S2 and S3 provide an overview of the published prospective (117–126) and retrospective studies (24, 127–175), respectively. Here, we summarize the main conclusions based on the available data.
RASi usage in conjunction with CHT
Available clinical data suggest that RASi may potentiate the effect of certain systemic antitumor therapies. The use of RASi was associated with better outcomes in patients with different solid tumors who received platinum-based CHT (142, 143, 149, 165, 172). The gain in overall survival (OS; the length of time from either the date of diagnosis or the start of treatment that patients are still alive) ranged from ~3 months in advanced non–small cell lung cancer (NSCLC) to 5.7 months in advanced gastric cancer and even 11 months in metastatic colorectal cancer (CRC) (142, 149, 165, 172). In line with the clinical data, experimental studies showed that platinum-based CHT can increase VEGF production through up-regulation of AT1R expression. This seems to represent a mechanism for platinum resistance that can be successfully targeted by RASi (176, 177).
In addition, concomitant RASi treatment was associated with better survival in patients with metastatic renal cell carcinoma (RCC; gain in OS, 7 to 26 months) (137–140), metastatic CRC (gain in OS, ~11 months) (172), glioblastoma (175), and advanced hepatocellular carcinoma (HCC; gain in OS, ~5 months) (173) who received VEGF-targeted therapies. Because AngII/AT1R signaling promotes VEGF-mediated angiogenesis (4), RASi may potentiate the effect of anti-VEGF therapy. In a mouse model of Ehrlichs’s ascites carcinoma, the ARB olmesartan augmented the antiangiogenic effect of the tyrosine kinase inhibitor (TKI) sorafenib (178). RASi may also represent a strategy to inhibit rapid revascularization (179, 180) and regrowth of tumors (181, 182) after cessation of anti-VEGF therapy, which is often necessary due to treatment-related side effects, especially with VEGFR TKIs (183, 184). Notably, arterial hypertension is a common side effect of anti-VEGF therapy and can be associated with better survival outcomes (185). VEGF-targeted therapy-induced hypertension is often treated with RASi, which could represent a potential confounder for the reported beneficial survival results associated with RASi use in patients who received anti-VEGF therapies. However, two points suggest otherwise: First, some studies reported the number of patients who received RASi either at baseline or after initiation of anti-VEGF therapy and showed that most of the patients were taking RASi already at baseline (137, 139). Second, McKay and colleagues (140) demonstrated that even in the subgroup of patients who developed anti-VEGF therapy-induced hypertension, RASi users had improved survival compared to nonusers.
Finally, two studies suggested a putative clinical benefit of RASi use in patients who received epidermal growth factor receptor (EGFR) TKIs (128, 143). This could be explained by the preclinical finding that AT1R signaling can regulate proliferation and migration of cancer cells through transactivation of the EGFR by metalloproteinase-dependent shedding of EGF ligands (4).
Tumor characteristics as determinants of RASi efficacy
RASi use was associated with better outcomes in multiple studies, whereas no association was found in others. This suggests that response to RASi treatment may also vary by tumor type and depend on certain tumor characteristics, as discussed below.
In breast cancer, only 2 of 13 studies shown in tables S2 and S3 reported beneficial effects of RASi use, whereas 3 studies found worse outcomes. A meta-analysis found no association of ACEi/ARB use with disease-free survival (DFS; the length of time after primary treatment for a cancer ends that the patient survives without any signs or symptoms of that cancer) or OS in breast cancer (186). The heterogeneity in terms of tumor stage, hormone receptor status, human epidermal growth factor receptor 2 overexpression, and (neo)adjuvant treatment regimen could have masked a potential benefit of RASi in certain subgroups and highlights the need for careful patient selection to obtain homogenous and comparable study cohorts.
The use of RASi was associated with better outcomes in patients with RCC, CRC, and HCC (tables S2 and S3). These tumors are well known to respond to anti-VEGF therapy (187–189). As discussed earlier, RASi may enhance the efficacy of VEGF-targeted therapies and thereby improve clinical outcome. However, in HCC (125, 126, 159, 164) and some CRC (167) and RCC (144) studies listed in tables S2 and S3, most of the patients were not treated with anti-VEGF treatment, suggesting that anti-VEGF–responsive tumors generally seem to be more sensitive to RASi.
RASi therapy had a clinical benefit in both slowly progressing cancers, such as prostate cancer, and highly aggressive tumor types, such as glioblastoma and pancreatic cancer (tables S2 and S3). A phase 2 study at the Massachusetts General Hospital (MGH) is currently investigating whether adding losartan to CHT (FOLFIRINOX), followed by chemoradiation, can convert locally advanced PDAC to resectable tumors (23). Preliminary results from this trial showed that R0 resection was achieved in 13 of 25 patients (52%), which is a major improvement compared to previously reported R0 resection rates obtained with neoadjuvant FOLFIRINOX and radiation in locally advanced PDAC (23 to 24%) (190, 191). The median OS was 33 months, with a 2-year survival rate of 65% for all patients and 83% for resected patients (23).
In addition, RASi use was effective in both early and advanced tumor stages. In some tumor types, the effect of RASi was investigated primarily in either early tumors (such as resected urinary tract cancer) (130, 147, 150, 151) or advanced stages (such as metastatic NSCLC) (142, 149). In RCC and CRC, positive outcomes were reported for both early (144, 167) and metastatic diseases (137–140, 172). Notably, in PDAC, a survival benefit in RASi users was only shown for locally advanced/metastatic diseases treated with CHT (168–170) but not for resected early/locally advanced tumors (174).
In contrast, in our own retrospective analysis, RASi use was associated with longer OS in pancreatic cancer patients with resected primary tumors (median OS, 36.3 versus 19.3 months) and locally advanced tumors (median OS, 11.3 versus 9.3 months) but not in meta-static patients. To obtain mechanistic insights, we performed RNA sequencing expression profiling of prospectively collected cancer treatment–naïve pancreatic cancer samples (four lisinopril-treated patients versus four controls). Our data suggest that lisinopril, which was the most commonly used ACEi in our cohort, normalized the ECM, down-regulated genes involved in cancer progression (such as Wnt and Notch signaling), and up-regulated genes associated with the activity of T cells and antigen-presenting cells. In addition, we identified a predictive gene signature for RASi-mediated survival, which was validated in two publicly available cohorts (24). A recently published meta-analysis pooling data on different solid tumor types (192) showed that the use of ACEi or ARB was associated with improved DFS and OS. After pooling studies that were classified as early (I/II) or advanced (III/IV) stage-dominant, the association with DFS remained significant in both stages (P = 0.04 and P = 0.03, respectively); a positive association with OS was only observed in advanced tumor stage (192).
Finally, HCC usually develops in patients with underlying liver fibrosis/cirrhosis (193). The peritumoral liver tissue and the severity of liver dysfunction determine prognosis of HCC, and complications of cirrhosis (portal hypertension and variceal bleeding) are a common cause of death in patients with HCC (193). The AngII/AT1R axis plays a crucial role in the pathophysiology of liver cirrhosis (194), and RASi can improve both liver fibrosis (195) and portal hypertension (196). These effects, in addition to the direct antitumor effects of RASi, may also contribute to the improved outcome observed in HCC patients treated with RASi (125, 126, 159, 164, 173).
CONCLUSIONS
Preclinical studies have provided compelling evidence that the AngII/ AT1R axis regulates almost all hallmarks of cancer. RASi can directly attenuate tumor growth and dissemination and improve the efficacy of systemic therapies by increasing drug delivery to the tumor tissue. The latter should help to reduce the dose of CHT and immunotherapy without decreasing the benefit and consequently decrease the anticancer therapy–induced side effects.
It is also clear that AngII/AT1R signaling contributes to the immuno-suppressive tumor microenvironment in multiple ways. The immuno-suppressive milieu is a major barrier for immunotherapy and may explain why immune checkpoint inhibitors have failed in some tumor types, such as PDAC, and have benefited only a fraction of patients in other indications where these agents are approved. Studies have shown that AT1R inhibition can decrease infiltration of immunosuppressive cell types and increase the number of effector T cells. This could also help to reduce the dose of immunotherapy without lowering drug efficacy, eventually resulting in a decreased number of severe immunotherapy-induced side effects. Although not yet studied in the context of tumor immunity, the AngII/AT1R axis is also important for the maturation of immune effector cells.
Multiple clinical studies have also revealed that RASi may have beneficial effects in a broad range of malignancies. The gain in survival is tumor type– and stage-dependent and ranged from 3 months (advanced NSCLC) to more than 25 months (metastatic RCC) in retrospective studies. However, response to RASi treatment may not only vary with tumor types but also depend on certain tumor characteristics, cancer treatment, and RASi type and dosing. More precisely, RCC, HCC, PDAC, glioblastoma, urinary tract cancer, and NSCLC seem to belong to the responsive tumor types, whereas breast cancer is rather unresponsive to RASi. With respect to cancer treatment, RASi use was associated with better outcomes in patients with NSCLC, gastric cancer, and CRC who received platinum-based CHT and in those with RCC, HCC, and CRC treated with anti-VEGF therapy (for example, sunitinib). More data are needed for other tumor types, such as melanoma, thyroid cancer, head and neck cancer, and hematologic malignancies.
Because the clinical evidence largely came from retrospective studies and small prospective pilot trials, these findings should be considered as hypothesis-generating. However, given the large amount of preclinical and clinical data suggesting a beneficial effect of RASi in different cancer types, we propose that RASis have a great potential to become an adjunct within the oncological armamentarium. Ongoing trials testing whether RASi can improve the antitumor effect of certain anticancer treatments are listed in table S4.
Future perspectives and translational challenges
Advancing the promising strategy to reprogram the tumor microenvironment with RASi to enhance anticancer treatment will require a close interplay between basic and clinical research and addressing a number of outstanding questions. Preclinical research should combine immune checkpoint inhibitors or other immunotherapy approaches with RASi to confirm whether RASis have the potential to reprogram the immunosuppressive microenvironment and eventually render tumors more sensitive to immunotherapies. In addition, mechanistic studies should not only focus on effects of RASi on the tumor stroma but also investigate treatment-related changes within immune cell populations in the bone marrow and lymphoid organs. This will help to better understand the role of the RAS in cancer immunity.
Moreover, clinical pilot studies focusing on biological readouts— such as intratumoral ECM deposition, immune cell infiltration, and drug distribution—should be designed to confirm the available preclinical data and to pave the way for large randomized controlled efficacy trials. These studies should seek to identify those patients who may benefit most from concomitant RASi use. Such personalized approaches require a tight integration between measurements of various biomarkers —circulating (profibrotic molecules, immune cells, and chemokines), tissue (profibrotic molecules, collagen, and HA), and imaging (perfusion, oxygenation, and drug distribution)—and the treatment outcome (197). Assessing the intratumoral expression of the components of the RAS may also have the potential to predict response to RASi treatment.
Finally, the beneficial response of tumors to RASi is dose-dependent. For example, the collagen content of desmoplastic tumors decreases with an increasing dose of ARBs (42). However, increasing the dose can cause hypotension and other adverse effects. One potential solution to this challenge is to develop nanoformulations of RASi that will preferentially deliver RASi to the tumor microenvironment. Addressing these issues and challenges will unravel the complexity of RAS signaling and its role in different malignancies and enable development of new strategies to deliver RASi to tumors in safe doses with an even better outcome.
Supplementary Material
Acknowledgments
We thank Y. Boucher, I. Chen, A. Crane, M. Datta, M. Khandekar, H. Liu, M. Pittet, and K. Naxerova for helpful comments. We also apologize to all authors whose papers are not cited because of the limitations in number of references.
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/9/410/eaan5616/DC1
Table S1. RASi approved by the FDA.
Table S2. Published prospective studies using RASi in different types of cancer.
Table S3. Published retrospective studies using RASi in different types of cancer.
Table S4. Ongoing prospective studies investigating the effect of RASi in solid malignant tumors.
Funding
M.P. is supported by an Erwin-Schroedinger Fellowship by the Austrian Science Fund (project no. J 3747-B28). R.K.J. is supported by the National Cancer Institute (NCI; grants P01-CA080124, P50-CA165962, R01-CA129371, R01-CA208205, and U01-CA 224348), NCI Outstanding Investigator Award (R35-CA197743), the Lustgarten Foundation, the Ludwig Center at Harvard, the National Foundation for Cancer Research, and the Gates Foundation.
Author contributions
M.P. and R.K.J. performed the literature search, wrote the manuscript, and approved the final version of the manuscript.
Competing interests
M.P. received travel support from Bayer and speaking fees and consultant fees from Bayer and Bristol-Myers Squibb. R.K.J. received consultant fees from Ophthotech, Sun Pharma Advanced Research Corporation (SPARC), SynDevRx, and XTuit; owns equity in Enlight, Ophthotech, SynDevRx, and XTuit; and serves on the Board of Directors of XTuit and the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, and Tekla World Healthcare Fund. R.K.J. is an inventor on patent application (USSN 61/43 8,240 and USSN 61/643,487) submitted by MGH that covers the use of antihypertensive agents for cancer therapy.
REFERENCES AND NOTES
- 1.Ager E. I., Neo J., Christophi C., The renin-angiotensin system and malignancy. Carcinogenesis 29, 1675–1684 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Bader M., Tissue renin-angiotensin-aldosterone systems: Targets for pharmacological therapy. Annu. Rev. Pharmacol. Toxicol. 50, 439–465 (2010). [DOI] [PubMed] [Google Scholar]
- 3.de Gasparo M., Catt K. J., Inagami T., Wright J. W., Unger T., International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 52, 415–472 (2000). [PubMed] [Google Scholar]
- 4.George A. J., Thomas W. G., Hannan R. D., The renin-angiotensin system and cancer: Old dog, new tricks. Nat. Rev. Cancer 10, 745–759 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Paul M., Poyan Mehr A., Kreutz R., Physiology of local renin-angiotensin systems. Physiol. Rev. 86, 747–803 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Qaradakhi T., Apostolopoulos V., Zulli A., Angiotensin (1–7) and alamandine: Similarities and differences. Pharmacol. Res. 111, 820–826 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues-Ferreira S., Nahmias C., G-protein coupled receptors of the renin-angiotensin system: New targets against breast cancer? Front. Pharmacol. 6, 24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobori H., Nangaku M., Navar L. G., Nishiyama A., The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol. Rev. 59, 251–287 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Ferrario C. M, Role of angiotensin II in cardiovascular disease—Therapeutic implications of more than a century of research. J. Renin Angiotensin Aldosterone Syst. 7, 3–14 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Cushman D. W., Ondetti M. A, History of the design of captopril and related inhibitors of angiotensin converting enzyme. Hypertension 17, 589–592 (1991). [DOI] [PubMed] [Google Scholar]
- 11.Bhardwaj G., How the antihypertensive losartan was discovered. Expert Opin. Drug Discov. 1, 609–618 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Ponikowski P., Voors A. A., Anker S. D., Bueno H., Cleland J. G. F., Coats A. J. S., Falk V., González-Juanatey J. R., Harjola V.-P., Jankowska E. A., Jessup M., Linde C., Nihoyannopoulos P., Parissis J. T., Pieske B., Riley J. P., Rosano G. M. C., Ruilope L. M., Ruschitzka F., Rutten F. H., van der Meer P.; Authors/Task Force Members , 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 37, 2129–2200 (2016). [DOI] [PubMed] [Google Scholar]
- 13.James P. A., Oparil S., Carter B. L., Cushman W. C., Dennison-Himmelfarb C., Handler J., Lackland D. T., LeFevre M. L., MacKenzie T. D., Ogedegbe O., Smith S. C. Jr., Svetkey L. P., Taler S. J., Townsend R. R., Wright J. T. Jr., Narva A. S., Ortiz E., 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311, 507–520 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Levin A., Stevens P. E., Summary of KDIGO 2012 CKD Guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 85, 49–61 (2014). [DOI] [PubMed] [Google Scholar]
- 15.O’Gara P. T., Kushner F. G., Ascheim D. D., Casey D. E. Jr., Chung M. K., de Lemos J. A., Ettinger S. M., Fang J. C., Fesmire F. M., Franklin B. A., Granger C. B., Krumholz H. M., Linderbaum J. A., Morrow D. A., Newby L. K., Ornato J. P., Ou N., Radford M. J., Tamis-Holland J. E., Tommaso C. L., Tracy C. M., Woo Y. J., Zhao D. X., Anderson J. L., Jacobs A. K., Halperin J. L., Albert N. M., Brindis R. G., Creager M. A., DeMets D., Guyton R. A., Hochman J. S., Kovacs R. J., Ohman E. M., Stevenson W. G., Yancy C. W.; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines , 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 127, e362–e425 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Shafiq M. M., Menon D. V., Victor R. G., Oral direct renin inhibition: Premise, promise, and potential limitations of a new antihypertensive drug. Am. J. Med. 121, 265–271 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sipahi I., Debanne S. M., Rowland D. Y., Simon D. I., Fang J. C., Angiotensin-receptor blockade and risk of cancer: Meta-analysis of randomised controlled trials. Lancet Oncol. 11, 627–636 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ARB Trialists Collaboration , Effects of telmisartan, irbesartan, valsartan, candesartan, and losartan on cancers in 15 trials enrolling 138,769 individuals. J. Hypertens. 29, 623–635 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Bangalore S., Kumar S., Kjeldsen S. E., Makani H., Grossman E., Wetterslev J., Gupta A. K., Sever P. S., Gluud C., Messerli F. H., Antihypertensive drugs and risk of cancer: Network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol. 12, 65–82 (2011). [DOI] [PubMed] [Google Scholar]
- 20.FDA Drug Safety Communication: No increase in risk of cancer with certain blood pressure drugs—Angiotensin Receptor Blockers (ARBs) , 15 July 2010; www.fda.gov/Drugs/DrugSafety/ucm257516.htm.
- 21.Link W. T., De Felice A., An FDA overview of rodent carcinogenicity studies of angiotensin II AT-1 receptor blockers: Pulmonary adenomas and carcinomas. Regul. Toxicol. Pharmacol. 70, 555–563 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Chauhan V. P., Martin J. D., Liu H., Lacorre D. A., Jain S. R., Kozin S. V., Stylianopoulos T., Mousa A. S., Han X., Adstamongkonkul P., Popović Z., Huang P., Bawendi M. G., Boucher Y., Jain R. K., Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 4, 2516 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy J. E., Wo J. Y.-L., Ferrone C., Jiang W., Yeap B. Y., Blaszkowsky L. S., Kwak E. L., Allen J. N., Clark J. W., Faris J. E., Zhu A. X., Goyal L., Mamon H. J., Lillemoe K. D., Ryan D. P., DeLaney T. F., Fernandez-del Castillo C., Boucher Y., Hong T. S., TGF-B1 inhibition with losartan in combination with FOLFIRINOX (F-NOX) in locally advanced pancreatic cancer (LAPC): Preliminary feasibility and R0 resection rates from a prospective phase II study. J. Clin. Oncol. 35 (suppl. 4S), 386 (2017). [Google Scholar]
- 24.Liu H., Naxerova K., Pinter M., Incio J., Lee H., Shigeta K., Ho W. W., Crain J. A., Jacobson A., Michelakos T., Dias-Santos D., Zanconato A., Hong T. S., Clark J. W., Murphy J. E., Ryan D. P., Deshpande V., Lillemoe K. D., Fernandez-del Castillo C., Downes M., Evans R. M., Michaelson J., Ferrone C. R., Boucher Y., Jain R. K., Use of angiotensin system inhibitors is associated with immune activation and longer survival in non-metastatic pancreatic ductal adenocarcinoma. Clin. Cancer Res. (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arrieta O., Pineda-Olvera B., Guevara-Salazar P., Hernández-Pedro N., Morales-Espinosa D., Cerón-Lizarraga T. L., González-De la Rosa C. H., Rembao D., Segura-Pacheco B., Sotelo J., Expression of AT1 and AT2 angiotensin receptors in astrocytomas is associated with poor prognosis. Br. J. Cancer 99, 160–166 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arrieta O., Villarreal-Garza C., Vizcaíno G., Pineda B., Hernández-Pedro N., Guevara-Salazar P., Wegman-Ostrosky T., Villanueva-Rodríguez G., Gamboa-Domínguez A., Association between AT1 and AT2 angiotensin II receptor expression with cell proliferation and angiogenesis in operable breast cancer. Tumour Biol. 36, 5627–5634 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Ino K., Shibata K., Kajiyama H., Yamamoto E., Nagasaka T., Nawa A., Nomura S., Kikkawa F., Angiotensin II type 1 receptor expression in ovarian cancer and its correlation with tumour angiogenesis and patient survival. Br. J. Cancer 94, 552–560 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rocken C., Rohl F. W., Diebler E., Lendeckel U., Pross M., Carl-McGrath S., Ebert M. P., The angiotensin II/angiotensin II receptor system correlates with nodal spread in intestinal type gastric cancer. Cancer Epidemiol. Biomarkers Prev. 16, 1206–1212 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Shirotake S., Miyajima A., Kosaka T., Tanaka N., Maeda T., Kikuchi E., Oya M., Angiotensin II type 1 receptor expression and microvessel density in human bladder cancer. Urology 77, 1009.e19–1009.e25 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Balyasnikova I. V., Danilov S. M., Muzykantov V. R., Fisher A. B., Modulation of angiotensin-converting enzyme in cultured human vascular endothelial cells. In Vitro Cell. Dev. Biol. Anim. 34, 545–554 (1998). [DOI] [PubMed] [Google Scholar]
- 31.Danilov S. M., Sadovnikova E., Scharenborg N., Balyasnikova I. V., Svinareva D. A., Semikina E. L., Parovichnikova E. N., Savchenko V. G., Adema G. J., Angiotensin-converting enzyme (CD143) is abundantly expressed by dendritic cells and discriminates human monocyte-derived dendritic cells from acute myeloid leukemia-derived dendritic cells. Exp. Hematol. 31, 1301–1309 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Lin C., Datta V., Okwan-Duodu D., Chen X., Fuchs S., Alsabeh R., Billet S., Bernstein K. E., Shen X. Z., Angiotensin-converting enzyme is required for normal myelopoiesis. FASEB J. 25, 1145–1155 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen X. Z., Billet S., Lin C., Okwan-Duodu D., Chen X., Lukacher A. E., Bernstein K. E., The carboxypeptidase ACE shapes the MHC class I peptide repertoire. Nat. Immunol. 12, 1078–1085 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cortez-Retamozo V., Etzrodt M., Newton A., Ryan R., Pucci F., Sio S. W., Kuswanto W., Rauch P. J., Chudnovskiy A., Iwamoto Y., Kohler R., Marinelli B., Gorbatov R., Wojtkiewicz G., Panizzi P., Mino-Kenudson M., Forghani R., Figueiredo J.-L., Chen J. W., Xavier R., Swirski F. K., Nahrendorf M., Weissleder R., Pittet M. J., Angiotensin II drives the production of tumor-promoting macrophages. Immunity 38, 296–308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azevedo H., Fujita A., Bando S. Y., Iamashita P., Moreira-Filho C. A., Transcriptional network analysis reveals that AT1 and AT2 angiotensin II receptors are both involved in the regulation of genes essential for glioma progression. PLOS ONE 9, e110934 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X., Zhang H., Soledad-Conrad V., Zhuang J., Uhal B. D., Bleomycin-induced apoptosis of alveolar epithelial cells requires angiotensin synthesis de novo. Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L501–L507 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Nguyen L., Ager E. I., Neo J., Christophi C., Regulation of colorectal cancer cell epithelial to mesenchymal transition by the renin angiotensin system. J. Gastroenterol. Hepatol. 31, 1773–1782 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Papp M., Li X., Zhuang J., Wang R., Uhal B. D., Angiotensin receptor subtype AT1 mediates alveolar epithelial cell apoptosis in response to ANG II. Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L713–L718 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Zheng S., Yang Y., Song R., Yang X., Liu H., Ma Q., Yang L., Meng R., Tao T., Wang S., He J., Ang-(1–7) promotes the migration and invasion of human renal cell carcinoma cells via Mas-mediated AKT signaling pathway. Biochem. Biophys. Res. Commun. 460, 333–340 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Smyth M. J., Ngiow S. F., Ribas A., Teng M. W. L., Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 13, 143–158 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Munn D. H., Bronte V., Immune suppressive mechanisms in the tumor microenvironment. Curr. Opin. Immunol. 39, 1–6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diop-Frimpong B., Chauhan V. P., Krane S., Boucher Y., Jain R. K., Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl. Acad. Sci. U.S.A. 108, 2909–2914 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Öhlund D., Elyada E., Tuveson D., Fibroblast heterogeneity in the cancer wound. J. Exp. Med. 211, 1503–1523 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li M. O., Flavell R. A., TGF-b: A master of all T cell trades. Cell 134, 392–404 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watt J., Kocher H. M., The desmoplastic stroma of pancreatic cancer is a barrier to immune cell infiltration. OncoImmunology 2, e26788 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jain R. K., Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. J. Clin. Oncol. 31, 2205–2218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain R. K., Martin J. D., Stylianopoulos T., The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 16, 321–346 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jain R. K., Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 26, 605–622 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noman M. Z., Hasmim M., Messai Y., Terry S., Kieda C., Janji B., Chouaib S., Hypoxia: A key player in antitumor immune response. A review in the theme: Cellular responses to hypoxia. Am. J. Physiol. Cell Physiol. 309, C569–C579 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palazón A., Aragonés J., Morales-Kastresana A., de Landázuri M. O., Melero I., Molecular pathways: Hypoxia response in immune cells fighting or promoting cancer. Clin. Cancer Res. 18, 1207–1213 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Voron T., Colussi O., Marcheteau E., Pernot S., Nizard M., Pointet A.-L., Latreche S., Bergaya S., Benhamouda N., Tanchot C., Stockmann C., Combe P., Berger A., Zinzindohoue F., Yagita H., Tartour E., Taieb J., Terme M., VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 212, 139–148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y., Ramjiawan R. R., Reiberger T., Ng M. R., Hato T., Huang Y., Ochiai H., Kitahara S., Unan E. C., Reddy T. P., Fan C., Huang P., Bardeesy N., Zhu A. X., Jain R. K., Duda D. G., CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology 61, 1591–1602 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feig C., Jones J. O., Kraman M., Wells R. J. B., Deonarine A., Chan D. S., Connell C. M., Roberts E. W., Zhao Q., Caballero O. L., Teichmann S. A., Janowitz T., Jodrell D. I., Tuveson D. A., Fearon D. T., Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti–PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. U.S.A. 110, 20212–20217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang H., Hegde S., Knolhoff B. L., Zhu Y., Herndon J. M., Meyer M. A., Nywening T. M., Hawkins W. G., Shapiro I. M., Weaver D. T., Pachter J. A., Wang-Gillam A., DeNardo D. G., Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 22, 851–860 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masamune A., Hamada S., Kikuta K., Takikawa T., Miura S., Nakano E., Shimosegawa T., The angiotensin II type I receptor blocker olmesartan inhibits the growth of pancreatic cancer by targeting stellate cell activities in mice. Scand. J. Gastroenterol. 48, 602–609 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Okazaki M., Fushida S., Harada S., Tsukada T., Kinoshita J., Oyama K., Tajima H., Ninomiya I., Fujimura T., Ohta T., The angiotensin II type 1 receptor blocker candesartan suppresses proliferation and fibrosis in gastric cancer. Cancer Lett. 355, 46–53 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Godugu C., Patel A. R., Doddapaneni R., Marepally S., Jackson T., Singh M., Inhalation delivery of Telmisartan enhances intratumoral distribution of nanoparticles in lung cancer models. J. Control. Release 172, 86–95 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel K., Doddapaneni R., Chowdhury N., Boakye C. H. A., Behl G., Singh M., Tumor stromal disrupting agent enhances the anticancer efficacy of docetaxel loaded PEGylated liposomes in lung cancer. Nanomedicine 11, 1377–1392 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Incio J., Liu H., Suboj P., Chin S. M., Chen I. X., Pinter M., Ng M. R., Nia H. T., Grahovac J., Kao S., Babykutty S., Huang Y., Jung K., Rahbari N. N., Han X., Chauhan V. P., Martin J. D., Kahn J., Huang P., Desphande V., Michaelson J., Michelakos T. P., Ferrone C. R., Soares R., Boucher Y., Fukumura D., Jain R. K., Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 6, 852–869 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arnold S. A., Rivera L. B., Carbon J. G., Toombs J. E., Chang C.-L., Bradshaw A. D., Brekken R. A., Losartan slows pancreatic tumor progression and extends survival of SPARC-null mice by abrogating aberrant TGFb activation. PLOS ONE 7, e31384 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anandanadesan R., Gong Q., Chipitsyna G., Witkiewicz A., Yeo C. J., Arafat H. A., Angiotensin II induces vascular endothelial growth factor in pancreatic cancer cells through an angiotensin II type 1 receptor and ERK1/2 signaling. J. Gastrointest. Surg. 12, 57–66 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Ji Y., Wang Z., Li Z., Li K., Le X., Zhang T., Angiotensin II induces angiogenic factors production partly via AT1/JAK2/STAT3/SOCS3 signaling pathway in MHCC97H cells. Cell. Physiol. Biochem. 29, 863–874 (2012). [DOI] [PubMed] [Google Scholar]
- 63.Kosaka T., Miyajima A., Shirotake S., Kikuchi E., Hasegawa M., Mikami S., Oya M., Ets-1 and hypoxia inducible factor-1alpha inhibition by angiotensin II type-1 receptor blockade in hormone-refractory prostate cancer. Prostate 70, 162–169 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Fujita M., Hayashi I., Yamashina S., Fukamizu A., Itoman M., Majima M., Angiotensin type 1a receptor signaling-dependent induction of vascular endothelial growth factor in stroma is relevant to tumor-associated angiogenesis and tumor growth. Carcinogenesis 26, 271–279 (2005). [DOI] [PubMed] [Google Scholar]
- 65.Kosugi M., Miyajima A., Kikuchi E., Horiguchi Y., Murai M., Angiotensin II type 1 receptor antagonist candesartan as an angiogenic inhibitor in a xenograft model of bladder cancer. Clin. Cancer Res. 12, 2888–2893 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Yoshiji H., Kuriyama S., Kawata M., Yoshii J., Ikenaka Y., Noguchi R., Nakatani T., Tsujinoue H., Fukui H., The angiotensin-I–converting enzyme inhibitor perindopril suppresses tumor growth and angiogenesis: Possible role of the vascular endothelial growth factor. Clin. Cancer Res. 7, 1073–1078 (2001). [PubMed] [Google Scholar]
- 67.Jain R. K., Determinants of tumor blood flow: A review. Cancer Res. 48, 2641–2658 (1988). [PubMed] [Google Scholar]
- 68.Huang Y., Goel S., Duda D. G., Fukumura D., Jain R. K., Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 73, 2943–2948 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang Y., Yuan J., Righi E., Kamoun W. S., Ancukiewicz M., Nezivar J., Santosuosso M., Martin J. D., Martin M. R., Vianello F., Leblanc P., Munn L. L., Huang P., Duda D. G., Fukumura D., Jain R. K., Poznansky M. C., Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl. Acad. Sci. U.S.A. 109, 17561–17566 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Batchelor T. T., Gerstner E. R., Emblem K. E., Duda D. G., Kalpathy-Cramer J., Snuderl M., Ancukiewicz M., Polaskova P., Pinho M. C., Jennings D., Plotkin S. R., Chi A. S., Eichler A. F., Dietrich J., Hochberg F. H., Lu-Emerson C., Iafrate A. J., Ivy S. P., Rosen B. R., Loeffler J. S., Wen P. Y., Sorensen A. G., Jain R. K., Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc. Natl. Acad. Sci. U.S.A. 110, 19059–19064 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Emblem K. E., Mouridsen K., Bjornerud A., Farrar C. T., Jennings D., Borra R. J. H., Wen P. Y., Ivy P., Batchelor T. T., Rosen B. R., Jain R. K., Sorensen A. G., Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat. Med. 19, 1178–1183 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sorensen A. G., Emblem K. E., Polaskova P., Jennings D., Kim H., Ancukiewicz M., Wang M., Wen P. Y., Ivy P., Batchelor T. T., Jain R. K., Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 72, 402–407 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gilbert R. E., Kelly D. J., Cox A. J., Wilkinson-Berka J. L., Rumble J. R., Osicka T., Panagiotopoulos S., Lee V., Hendrich E. C., Jerums G., Cooper M. E., Angiotensin converting enzyme inhibition reduces retinal overexpression of vascular endothelial growth factor and hyperpermeability in experimental diabetes. Diabetologia 43, 1360–1367 (2000). [DOI] [PubMed] [Google Scholar]
- 74.Sano H., Hosokawa K., Kidoya H., Takakura N., Negative regulation of VEGF-induced vascular leakage by blockade of angiotensin II type 1 receptor. Arterioscler. Thromb. Vasc. Biol. 26, 2673–2680 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Emblem K. E., Gerstner E. R., Sorensen G., Rosen B. R., Wen P. Y., Batchelor T. T., Jain R. K., Abstract 3975: Matrix-depleting anti-hypertensives decompress tumor blood vessels and improve perfusion in patients with glioblastoma receiving anti-angiogenic therapy. Cancer Res. 76 (suppl. 14), 3975 (2016). [Google Scholar]
- 76.Bell K. M., Prise V. E., Shaffi K. M., Chaplin D. J., Tozer G. M., A comparative study of tumour-blood-flow modification in two rat-tumour systems using endothelin-1 and angiotensin II: Influence of tumour size on angiotensin-II response. Int. J. Cancer 67, 730–738 (1996). [DOI] [PubMed] [Google Scholar]
- 77.Tozer G. M., Shaffi K. M, The response of tumour vasculature to angiotensin II revealed by its systemic and local administration to ‘tissue-isolated’ tumours. Br. J. Cancer 72, 595–600 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zlotecki R. A., Baxter L. T., Boucher Y., Jain R. K., Pharmacologic modification of tumor blood flow and interstitial fluid pressure in a human tumor xenograft: Network analysis and mechanistic interpretation. Microvasc. Res. 50, 429–443 (1995). [DOI] [PubMed] [Google Scholar]
- 79.Zlotecki R. A., Boucher Y., Lee I., Baxter L. T., Jain R. K., Effect of angiotensin II induced hypertension on tumor blood flow and interstitial fluid pressure. Cancer Res. 53, 2466–2468 (1993). [PubMed] [Google Scholar]
- 80.Thews O., Kelleher D. K., Vaupel P., Disparate responses of tumour vessels to angiotensin II: Tumour volume-dependent effects on perfusion and oxygenation. Br. J. Cancer 83, 225–231 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okwan-Duodu D., Landry J., Shen X. Z., Diaz R., Angiotensin-converting enzyme and the tumor microenvironment: Mechanisms beyond angiogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R205–R215 (2013). [DOI] [PubMed] [Google Scholar]
- 82.Balkwill F. R., Mantovani A., Cancer-related inflammation: Common themes and therapeutic opportunities. Semin. Cancer Biol. 22, 33–40 (2012). [DOI] [PubMed] [Google Scholar]
- 83.Ji Y., Wang Z., Li Z., Zhang A., Jin Y., Chen H., Le X., Angiotensin II enhances proliferation and inflammation through AT1/PKC/NF-kB signaling pathway in hepatocellular carcinoma cells. Cell. Physiol. Biochem. 39, 13–32 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Uemura H., Ishiguro H., Nagashima Y., Sasaki T., Nakaigawa N., Hasumi H., Kato S., Kubota Y., Antiproliferative activity of angiotensin II receptor blocker through cross-talk between stromal and epithelial prostate cancer cells. Mol. Cancer Ther. 4, 1699–1709 (2005). [DOI] [PubMed] [Google Scholar]
- 85.Matsuzuka T., Miller K., Pickel L., Doi C., Ayuzawa R., Tamura M., The synergistic induction of cyclooxygenase-2 in lung fibroblasts by angiotensin II and pro-inflammatory cytokines. Mol. Cell. Biochem. 320, 163–171 (2009). [DOI] [PubMed] [Google Scholar]
- 86.Pham H., Chong B., Vincenti R., Slice L. W., Ang II and EGF synergistically induce COX-2 expression via CREB in intestinal epithelial cells. J. Cell. Physiol. 214, 96–109 (2008). [DOI] [PubMed] [Google Scholar]
- 87.Slice L. W., Chiu T., Rozengurt E., Angiotensin II and epidermal growth factor induce cyclooxygenase-2 expression in intestinal epithelial cells through small GTPases using distinct signaling pathways. J. Biol. Chem. 280, 1582–1593 (2005). [DOI] [PubMed] [Google Scholar]
- 88.David J. M., Dominguez C., Hamilton D. H., Palena C., The IL-8/IL-8R axis: A double agent in tumor immune resistance. Vaccines 4, E22 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martin F., Apetoh L., Ghiringhelli F., Controversies on the role of Th17 in cancer: A TGF-b–dependent immunosuppressive activity? Trends Mol. Med. 18, 742–749 (2012). [DOI] [PubMed] [Google Scholar]
- 90.Ugel S., De Sanctis F., Mandruzzato S., Bronte V., Tumor-induced myeloid deviation: When myeloid-derived suppressor cells meet tumor-associated macrophages. J. Clin. Invest. 125, 3365–3376 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vinay D. S., Ryan E. P., Pawelec G., Talib W. H., Stagg J., Elkord E., Lichtor T., Decker W. K., Whelan R. L., Kumara H. M. C. S., Signori E., Honoki K., Georgakilas A. G., Amin A., Helferich W. G., Boosani C. S., Guha G., Ciriolo M. R., Chen S., Mohammed S. I., Azmi A. S., Keith W. N., Bilsland A., Bhakta D., Halicka D., Fujii H., Aquilano K., Ashraf S. S., Nowsheen S., Yang X., Choi B. K., Kwon B. S., Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 35 (Suppl.), S185–S198 (2015). [DOI] [PubMed] [Google Scholar]
- 92.Brown J. R., DuBois R. N., COX-2: A molecular target for colorectal cancer prevention. J. Clin. Oncol. 23, 2840–2855 (2005). [DOI] [PubMed] [Google Scholar]
- 93.Zelenay S., van der Veen A. G., Böttcher J. P., Snelgrove K. J., Rogers N., Acton S. E., Chakravarty P., Girotti M. R., Marais R., Quezada S. A., Sahai E., Reis e Sousa C., Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell 162, 1257–1270 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frenzel H., Pries R., Brocks C. P., Jabs W. J., Wittkopf N., Wollenberg B., Decreased migration of myeloid dendritic cells through increased levels of C-reactive protein. Anticancer Res. 27, 4111–4115 (2007). [PubMed] [Google Scholar]
- 95.Jackson S. H., Devadas S., Kwon J., Pinto L. A., Williams M. S., T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat. Immunol. 5, 818–827 (2004). [DOI] [PubMed] [Google Scholar]
- 96.Sena L. A., Li S., Jairaman A., Prakriya M., Ezponda T., Hildeman D. A., Wang C.-R., Schumacker P. T., Licht J. D., Perlman H., Bryce P. J., Chandel N. S., Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38, 225–236 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gringhuis S. I., Leow A., Papendrecht-van der Voort E. A. M., Remans P. H. J., Breedveld F. C., Verweij C. L., Displacement of linker for activation of T cells from the plasma membrane due to redox balance alterations results in hyporesponsiveness of synovial fluid T lymphocytes in rheumatoid arthritis. J. Immunol. 164, 2170–2179 (2000). [DOI] [PubMed] [Google Scholar]
- 98.Lahdenpohja N., Savinainen K., Hurme M., Pre-exposure to oxidative stress decreases the nuclear factor-kB–dependent transcription in T lymphocytes. J. Immunol. 160, 1354–1358 (1998). [PubMed] [Google Scholar]
- 99.Kim H.-R., Lee A., Choi E.-J., Hong M.-P., Kie J.-H., Lim W., Lee H. K., Moon B.-I., Seoh J.-Y., Reactive oxygen species prevent imiquimod-induced psoriatic dermatitis through enhancing regulatory T cell function. PLOS ONE 9, e91146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin X., Zheng W., Liu J., Zhang Y., Qin H., Wu H., Xue B., Lu Y., Shen P., Oxidative stress in malignant melanoma enhances tumor necrosis factor-a secretion of tumor-associated macrophages that promote cancer cell invasion. Antioxid. Redox Signal. 19, 1337–1355 (2013). [DOI] [PubMed] [Google Scholar]
- 101.Sica A., Schioppa T., Mantovani A., Allavena P., Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti-cancer therapy. Eur. J. Cancer 42, 717–727 (2006). [DOI] [PubMed] [Google Scholar]
- 102.Uemura H., Ishiguro H., Ishiguro Y., Hoshino K., Takahashi S., Kubota Y., Angiotensin II induces oxidative stress in prostate cancer. Mol. Cancer Res. 6, 250–258 (2008). [DOI] [PubMed] [Google Scholar]
- 103.Shirotake S., Miyajima A., Kosaka T., Tanaka N., Kikuchi E., Mikami S., Okada Y., Oya M., Regulation of monocyte chemoattractant protein-1 through angiotensin II type 1 receptor in prostate cancer. Am. J. Pathol. 180, 1008–1016 (2012). [DOI] [PubMed] [Google Scholar]
- 104.Egami K., Murohara T., Shimada T., Sasaki K.-i, Shintani S., Sugaya T., Ishii M., Akagi T., Ikeda H., Matsuishi T., Imaizumi T., Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth. J. Clin. Invest. 112, 67–75 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chehl N., Gong Q., Chipitsyna G., Aziz T., Yeo C. J., Arafat H. A., Angiotensin II regulates the expression of monocyte chemoattractant protein-1 in pancreatic cancer cells. J. Gastrointest. Surg. 13, 2189–2200 (2009). [DOI] [PubMed] [Google Scholar]
- 106.Shen X. Z., Bernstein K. E., The peptide network regulated by angiotensin converting enzyme (ACE) in hematopoiesis. Cell Cycle 10, 1363–1369 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shen X. Z., Okwan-Duodu D., Blackwell W.-L., Ong F. S., Janjulia T., Bernstein E. A., Fuchs S., Alkan S., Bernstein K. E., Myeloid expression of angiotensin-converting enzyme facilitates myeloid maturation and inhibits the development of myeloid-derived suppressor cells. Lab. Invest. 94, 536–544 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shen X. Z., Li P., Weiss D., Fuchs S., Xiao H. D., Adams J. A., Williams I. R., Capecchi M. R., Taylor W. R., Bernstein K. E., Mice with enhanced macrophage angiotensin-converting enzyme are resistant to melanoma. Am. J. Pathol. 170, 2122–2134 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shen X. Z., Lukacher A. E., Billet S., Williams I. R., Bernstein K. E., Expression of angiotensin-converting enzyme changes major histocompatibility complex class I peptide presentation by modifying C termini of peptide precursors. J. Biol. Chem. 283, 9957–9965 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hofmann L., Forschner A., Loquai C., Goldinger S. M., Zimmer L., Ugurel S., Schmidgen M. I., Gutzmer R., Utikal J. S., Göppner D., Hassel J. C., Meier F., Tietze J. K., Thomas I., Weishaupt C., Leverkus M., Wahl R., Dietrich U., Garbe C., Kirchberger M. C., Eigentler T., Berking C., Gesierich A., Krackhardt A. M., Schadendorf D., Schuler G., Dummer R., Heinzerling L. M., Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti–PD-1 therapy. Eur. J. Cancer 60, 190–209 (2016). [DOI] [PubMed] [Google Scholar]
- 111.Postow M. A., Chesney J., Pavlick A. C., Robert C., Grossmann K., McDermott D., Linette G. P., Meyer N., Giguere J. K., Agarwala S. S., Shaheen M., Ernstoff M. S., Minor D., Salama A. K., Taylor M., Ott P. A., Rollin L. M., Horak C., Gagnier P., Wolchok J. D., Hodi F. S., Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372, 2006–2017 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bouchlaka M. N., Sckisel G. D., Chen M., Mirsoian A., Zamora A. E., Maverakis E., Wilkins D. E. C., Alderson K. L., Hsiao H.-H., Weiss J. M., Monjazeb A. M., Hesdorffer C., Ferrucci L., Longo D. L., Blazar B. R., Wiltrout R. H., Redelman D., Taub D. D., Murphy W. J., Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. J. Exp. Med. 210, 2223–2237 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mirsoian A., Bouchlaka M. N., Sckisel G. D., Chen M., Pai C.-C. S., Maverakis E., Spencer R. G., Fishbein K. W., Siddiqui S., Monjazeb A. M., Martin B., Maudsley S., Hesdorffer C., Ferrucci L., Longo D. L., Blazar B. R., Wiltrout R. H., Taub D. D., Murphy W. J., Adiposity induces lethal cytokine storm after systemic administration of stimulatory immunotherapy regimens in aged mice. J. Exp. Med. 211, 2373–2383 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fliser D., Buchholz K., Haller H.; EUropean Trial on Olmesartan and Pravastatin in Inflammation and Atherosclerosis (EUTOPIA) Investigators , Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation 110, 1103–1107 (2004). [DOI] [PubMed] [Google Scholar]
- 115.Manabe S., Okura T., Watanabe S., Fukuoka T., Higaki J., Effects of angiotensin II receptor blockade with valsartan on pro-inflammatory cytokines in patients with essential hypertension. J. Cardiovasc. Pharmacol. 46, 735–739 (2005). [DOI] [PubMed] [Google Scholar]
- 116.Pavlatou M. G., Mastorakos G., Margeli A., Kouskouni E., Tentolouris N., Katsilambros N., Chrousos G. P., Papassotiriou I., Angiotensin blockade in diabetic patients decreases insulin resistance-associated low-grade inflammation. Eur. J. Clin. Invest. 41, 652–658 (2011). [DOI] [PubMed] [Google Scholar]
- 117.Holmes M. D., Hankinson S. E., Feskanich D., Chen W. Y., Beta blockers and angiotensin-converting enzyme inhibitors’ purported benefit on breast cancer survival may be explained by aspirin use. Breast Cancer Res. Treat. 139, 507–513 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jones P. H., Christodoulos K., Dobbs N., Thavasu P., Balkwill F., Blann A. D., Caine G. J., Kumar S., Kakkar A. J., Gompertz N., Talbot D. C., Ganesan T. S., Harris A. L., Combination antiangiogenesis therapy with marimastat, captopril and fragmin in patients with advanced cancer. Br. J. Cancer 91, 30–36 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nakai Y., Isayama H., Ijichi H., Sasaki T., Kogure H., Yagioka H., Miyabayashi K., Mizuno S., Yamamoto K., Mouri D., Kawakubo K., Yamamoto N., Hirano K., Sasahira N., Tateishi K., Tada M., Koike K., Phase I trial of gemcitabine and candesartan combination therapy in normotensive patients with advanced pancreatic cancer: GECA1. Cancer Sci. 103, 1489–1492 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nakai Y., Isayama H., Ijichi H., Sasaki T., Takahara N., Ito Y., Matsubara S., Uchino R., Yagioka H., Arizumi T., Hamada T., Miyabayashi K., Mizuno S., Yamamoto K., Kogure H., Yamamoto N., Hirano K., Sasahira N., Tateishi K., Tada M., Koike K., A multicenter phase II trial of gemcitabine and candesartan combination therapy in patients with advanced pancreatic cancer: GECA2. Invest. New Drugs 31, 1294–1299 (2013). [DOI] [PubMed] [Google Scholar]
- 121.Ronquist G., Frithz G., Wang Y.-H., Lindeborg T., Captopril may reduce biochemical (prostate-specific antigen) failure following radical prostatectomy for clinically localized prostate cancer. Scand. J. Urol. Nephrol. 43, 32–36 (2009). [DOI] [PubMed] [Google Scholar]
- 122.Sørensen G. V., Ganz P. A., Cole S. W., Pedersen L. A., Sørensen H. T., Cronin-Fenton D. P., Garne J. P., Christiansen P. M., Lash T. L., Ahern T. P., Use of β-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and risk of breast cancer recurrence: A Danish nationwide prospective cohort study. J. Clin. Oncol. 31, 2265–2272 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tatokoro M., Fujii Y., Kawakami S., Saito K., Koga F., Matsuoka Y., Iimura Y., Masuda H., Kihara K., Phase-II trial of combination treatment of interferon-a, cimetidine, cyclooxygenase-2 inhibitor and renin-angiotensin-system inhibitor (I-CCA therapy) for advanced renal cell carcinoma. Cancer Sci. 102, 137–143 (2011). [DOI] [PubMed] [Google Scholar]
- 124.Uemura H., Hasumi H., Kawahara T., Sugiura S., Miyoshi Y., Nakaigawa N., Teranishi J.-i, Noguchi K., Ishiguro H., Kubota Y., Pilot study of angiotensin II receptor blocker in advanced hormone-refractory prostate cancer. Int. J. Clin. Oncol. 10, 405–410 (2005). [DOI] [PubMed] [Google Scholar]
- 125.Yoshiji H., Noguchi R., Ikenaka Y., Kaji K., Aihara Y., Yamazaki M., Yamao J., Toyohara M., Mitoro A., Sawai M., Yoshida M., Morioka C., Fujimoto M., Uemura M., Fukui H., Combination of branched-chain amino acids and angiotensin-converting enzyme inhibitor suppresses the cumulative recurrence of hepatocellular carcinoma: A randomized control trial. Oncol. Rep. 26, 1547–1553 (2011). [DOI] [PubMed] [Google Scholar]
- 126.Yoshiji H., Noguchi R., Toyohara M., Ikenaka Y., Kitade M., Kaji K., Yamazaki M., Yamao J., Mitoro A., Sawai M., Yoshida M., Fujimoto M., Tsujimoto T., Kawaratani H., Uemura M., Fukui H., Combination of vitamin K2 and angiotensin-converting enzyme inhibitor ameliorates cumulative recurrence of hepatocellular carcinoma. J. Hepatol. 51, 315–321 (2009). [DOI] [PubMed] [Google Scholar]
- 127.Alashkham A., Paterson C., Windsor P., Struthers A., Rauchhaus P., Nabi G., The incidence and risk of biochemical recurrence following radical radiotherapy for prostate cancer in men on angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs). Clin. Genitourin. Cancer 14, 398–405 (2016). [DOI] [PubMed] [Google Scholar]
- 128.Aydiner A., Ciftci R., Sen F., Renin-Angiotensin system blockers may prolong survival of metastatic non–small cell lung cancer patients receiving erlotinib. Medicine 94, e887 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Babacan T., Balakan O., Kuzan T. Y., Sarici F., Koca E., Kertmen N., Petekkaya I., Altundag K., The effect of renin-angiotensin-system inhibition on survival and recurrence of N3+ breast cancer patients. J. BUON 20, 50–56 (2015). [PubMed] [Google Scholar]
- 130.Blute M. L. Jr., Rushmer T. J., Shi F., Fuller B. J., Abel E. J., Jarrard D. F., Downs T. M., Renin-angiotensin inhibitors decrease recurrence after transurethral resection of bladder tumor in patients with nonmuscle invasive bladder cancer. J. Urol. 194, 1214–1219 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Botteri E., Munzone E., Rotmensz N., Cipolla C., De Giorgi V., Santillo B., Zanelotti A., Adamoli L., Colleoni M., Viale G., Goldhirsch A., Gandini S., Therapeutic effect of β-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res. Treat. 140, 567–575 (2013). [DOI] [PubMed] [Google Scholar]
- 132.Boudreau D. M., Yu O., Chubak J., Wirtz H. S., Bowles E. J. A., Fujii M., Buist D. S. M., Comparative safety of cardiovascular medication use and breast cancer outcomes among women with early stage breast cancer. Breast Cancer Res. Treat. 144, 405–416 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chae Y. K., Brown E. N., Lei X., Melhem-Bertrandt A., Giordano S. H., Litton J. K., Hortobagyi G. N., Gonzalez-Angulo A. M., Chavez-MacGregor M., Use of ACE inhibitors and angiotensin receptor blockers and primary breast cancer outcomes. J. Cancer 4, 549–556 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chae Y. K., Valsecchi M. E., Kim J., Bianchi A. L., Khemasuwan D., Desai A., Tester W., Reduced risk of breast cancer recurrence in patients using ACE inhibitors, ARBs, and/or statins. Cancer Invest. 29, 585–593 (2011). [DOI] [PubMed] [Google Scholar]
- 135.Ganz P. A., Habel L. A., Weltzien E. K., Caan B. J., Cole S. W., Examining the influence of beta blockers and ACE inhibitors on the risk for breast cancer recurrence: Results from the LACE cohort. Breast Cancer Res. Treat. 129, 549–556 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Goldvaser H., Rizel S., Hendler D., Neiman V., Shepshelovich D., Shochat T., Sulkes A., Brenner B., Yerushalmi R., The association between angiotensin receptor blocker usage and breast cancer characteristics. Oncology 91, 217–223 (2016). [DOI] [PubMed] [Google Scholar]
- 137.Izzedine H., Derosa L., Le Teuff G., Albiges L., Escudier B., Hypertension and angiotensin system inhibitors: Impact on outcome in sunitinib-treated patients for metastatic renal cell carcinoma. Ann. Oncol. 26, 1128–1133 (2015). [DOI] [PubMed] [Google Scholar]
- 138.Keizman D., Gottfried M., Ish-Shalom M., Maimon N., Peer A., Neumann A., Hammers H., Eisenberger M. A., Sinibaldi V., Pili R., Hayat H., Kovel S., Sella A., Boursi B., Weitzen R., Mermershtain W., Rouvinov K., Berger R., Carducci M. A., Active smoking may negatively affect response rate, progression-free survival, and overall survival of patients with metastatic renal cell carcinoma treated with sunitinib. Oncologist 19, 51–60 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Keizman D., Huang P., Eisenberger M. A., Pili R., Kim J. J., Antonarakis E. S., Hammers H., Carducci M. A., Angiotensin system inhibitors and outcome of sunitinib treatment in patients with metastatic renal cell carcinoma: A retrospective examination. Eur. J. Cancer 47, 1955–1961 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McKay R. R., Rodriguez G. E., Lin X., Kaymakcalan M. D., Hamnvik O.-P. R., Sabbisetti V. S., Bhatt R. S., Simantov R., Choueiri T. K., Angiotensin system inhibitors and survival outcomes in patients with metastatic renal cell carcinoma. Clin. Cancer Res. 21, 2471–2479 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Melhem-Bertrandt A., Chavez-MacGregor M., Lei X., Brown E. N., Lee R. T., Meric-Bernstam F., Sood A. K., Conzen S. D., Hortobagyi G. N., Gonzalez-Angulo A. M., Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J. Clin. Oncol. 29, 2645–2652 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Menter A. R., Carroll N. M., Sakoda L. C., Delate T., Hornbrook M. C., Jain R. K., Kushi L. H., Quinn V. P., Ritzwoller D. P., Effect of angiotensin system inhibitors on survival in patients receiving chemotherapy for advanced non–small-cell lung cancer. Clin. Lung Cancer 18, 189–197 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Miao L., Chen W., Zhou L., Wan H., Gao B., Feng Y., Impact of angiotensin I-converting enzyme inhibitors and angiotensin II type-1 receptor blockers on survival of patients with NSCLC. Sci. Rep. 6, 21359 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Miyajima A., Yazawa S., Kosaka T., Tanaka N., Shirotake S., Mizuno R., Kikuchi E., Oya M., Prognostic impact of renin–angiotensin system blockade on renal cell carcinoma after surgery. Ann. Surg. Oncol. 22, 3751–3759 (2015). [DOI] [PubMed] [Google Scholar]
- 145.Sendur M. A. N., Aksoy S., Yaman S., Ozdemir N. Y., Zengin N., Altundag K., Efficacy of angiotensin-receptor blockers on demographic and clinico-pathological characteristics of breast cancer. Breast 21, 419–420 (2012). [DOI] [PubMed] [Google Scholar]
- 146.Sorich M. J., Kichenadasse G., Rowland A., Woodman R. J., Mangoni A. A., Angiotensin system inhibitors and survival in patients with metastatic renal cell carcinoma treated with VEGF-targeted therapy: A pooled secondary analysis of clinical trials. Int. J. Cancer 138, 2293–2299 (2016). [DOI] [PubMed] [Google Scholar]
- 147.Tanaka N., Miyajima A., Kikuchi E., Matsumoto K., Hagiwara M., Ide H., Kosaka T., Masuda T., Nakamura S., Oya M., Prognostic impact of renin-angiotensin system blockade in localised upper-tract urothelial carcinoma. Br. J. Cancer 106, 290–296 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang H., Liao Z., Zhuang Y., Liu Y., Levy L. B., Xu T., Yusuf S. W., Gomez D. R., Incidental receipt of cardiac medications and survival outcomes among patients with stage III non–small-cell lung cancer after definitive radiotherapy. Clin. Lung Cancer 16, 128–136 (2015). [DOI] [PubMed] [Google Scholar]
- 149.Wilop S., von Hobe S., Crysandt M., Esser A., Osieka R., Jost E., Impact of angiotensin I converting enzyme inhibitors and angiotensin II type 1 receptor blockers on survival in patients with advanced non–small-cell lung cancer undergoing first-line platinum-based chemotherapy. J. Cancer Res. Clin. Oncol. 135, 1429–1435 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yoshida T., Kinoshita H., Fukui K., Matsuzaki T., Yoshida K., Mishima T., Yanishi M., Komai Y., Sugi M., Inoue T., Murota T., Matsuda T., Prognostic impact of renin-angiotensin inhibitors in patients with bladder cancer undergoing radical cystectomy. Ann. Surg. Oncol. 24, 823–831 (2017). [DOI] [PubMed] [Google Scholar]
- 151.Yuge K., Miyajima A., Tanaka N., Shirotake S., Kosaka T., Kikuchi E., Oya M., Prognostic value of renin-angiotensin system blockade in non–muscle-invasive bladder cancer. Ann. Surg. Oncol. 19, 3987–3993 (2012). [DOI] [PubMed] [Google Scholar]
- 152.Buchler T., Krejci M., Svobodnik A., Adam Z., Minarik J., Bacovsky J., Scudla V., Mayer J., Vorlicek J., Hajek R., Outcome of patients with multiple myeloma and hypertension treated with angiotensin-I–converting enzyme inhibitors during high-dose chemotherapy. Hematol. J. 5, 559–564 (2005). [DOI] [PubMed] [Google Scholar]
- 153.Cardwell C. R., Mc Menamin U. C., Hicks B. M., Hughes C., Cantwell M. M., Murray L. J., Drugs affecting the renin-angiotensin system and survival from cancer: A population based study of breast, colorectal and prostate cancer patient cohorts. BMC Med. 12, 28 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Carpentier A. F., Ferrari D., Bailon O., Ursu R., Banissi C., Dubessy A.-L., Belin C., Levy C., Steroid-sparing effects of angiotensin-II inhibitors in glioblastoma patients. Eur. J. Neurol. 19, 1337–1342 (2012). [DOI] [PubMed] [Google Scholar]
- 155.Chae Y. K., Dimou A., Pierce S., Kantarjian H., Andreeff M., The effect of calcium channel blockers on the outcome of acute myeloid leukemia. Leuk. Lymphoma 55, 2822–2829 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen Y.-H., Huang C.-H., Lu H.-I., Chen C.-H., Huang W.-T., Hsieh M.-J., Rau K.-M., Chang A. Y. W., Lin W.-C., Li S.-H., Prognostic impact of renin-angiotensin system blockade in esophageal squamous cell carcinoma. J. Renin Angiotensin Aldosterone Syst. 16, 1185–1192 (2015). [DOI] [PubMed] [Google Scholar]
- 157.De Giorgi V., Gandini S., Grazzini M., Benemei S., Marchionni N., Geppetti P., Effect of β-blockers and other antihypertensive drugs on the risk of melanoma recurrence and death. Mayo Clin. Proc. 88, 1196–1203 (2013). [DOI] [PubMed] [Google Scholar]
- 158.Engineer D. R., Burney B. O., Hayes T. G., Garcia J. M., Exposure to ACEI/ARB and β-blockers is associated with improved survival and decreased tumor progression and hospitalizations in patients with advanced colon cancer. Transl. Oncol. 6, 539–545 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Facciorusso A., Del Prete V., Crucinio N., Muscatiello N., Carr B. I., Di Leo A., Barone M., Angiotensin receptor blockers improve survival outcomes after radiofrequency ablation in hepatocarcinoma patients. J. Gastroenterol. Hepatol. 30, 1643–1650 (2015). [DOI] [PubMed] [Google Scholar]
- 160.He L.-R., Qiao W., Liao Z.-X., Komaki R., Ho L., Hofstetter W. L., Lin S. H., Impact of comorbidities and use of common medications on cancer and non-cancer specific survival in esophageal carcinoma. BMC Cancer 15, 1095 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Heinzerling J. H., Anthony T., Livingston E. H., Huerta S., Predictors of distant metastasis and mortality in patients with stage II colorectal cancer. Am. Surg. 73, 230–238 (2007). [PubMed] [Google Scholar]
- 162.Holmes S., Griffith E. J., Musto G., Minuk G. Y., Antihypertensive medications and survival in patients with cancer: A population-based retrospective cohort study. Cancer Epidemiol. 37, 881–885 (2013). [DOI] [PubMed] [Google Scholar]
- 163.Januel E., Ursu R., Alkhafaji A., Marantidou A., Doridam J., Belin C., Levy-Piedbois C., Carpentier F., Impact of renin-angiotensin system blockade on clinical outcome in glioblastoma. Eur. J. Neurol. 22, 1304–1309 (2015). [DOI] [PubMed] [Google Scholar]
- 164.Kaibori M., Ishizaki M., Matsui K., Kitade H., Matsui Y., Kwon A.-H., Evaluation of metabolic factors on the prognosis of patients undergoing resection of hepatocellular carcinoma. J. Gastroenterol. Hepatol. 26, 536–543 (2011). [DOI] [PubMed] [Google Scholar]
- 165.Kim S. T., Park K. H., Oh S. C., Seo J. H., Kim J. S., Shin S. W., Kim Y. H., How does inhibition of the renin-angiotensin system affect the prognosis of advanced gastric cancer patients receiving platinum-based chemotherapy? Oncology 83, 354–360 (2012). [DOI] [PubMed] [Google Scholar]
- 166.Kourilsky A., Bertrand G., Ursu R., Doridam J., Barlog C., Faillot T., Mandonnet E., Belin C., Levy C., Carpentier A. F., Impact of angiotensin-II receptor blockers on vasogenic edema in glioblastoma patients. J. Neurol. 263, 524–530 (2016). [DOI] [PubMed] [Google Scholar]
- 167.Morris Z. S., Saha S., Magnuson W. J., Morris B. A., Borkenhagen J. F., Ching A., Hirose G., McMurry V., Francis D. M., Harari P. M., Chappell R., Tsuji S., Ritter M. A., Increased tumor response to neoadjuvant therapy among rectal cancer patients taking angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Cancer 122, 2487–2495 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nakai Y., Isayama H., Ijichi H., Sasaki T., Sasahira N., Hirano K., Kogure H., Kawakubo K., Yagioka H., Yashima Y., Mizuno S., Yamamoto K., Arizumi T., Togawa O., Matsubara S., Tsujino T., Tateishi K., Tada M., Omata M., Koike K., Inhibition of renin-angiotensin system affects prognosis of advanced pancreatic cancer receiving gemcitabine. Br. J. Cancer 103, 1644–1648 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nakai Y., Isayama H., Sasaki T., Mizuno S., Sasahira N., Kogure H., Kawakubo K., Yamamoto N., Hirano K., Ijichi H., Tateishi K., Tada M., Koike K., Clinical outcomes of chemotherapy for diabetic and nondiabetic patients with pancreatic cancer: Better prognosis with statin use in diabetic patients. Pancreas 42, 202–208 (2013). [DOI] [PubMed] [Google Scholar]
- 170.Nakai Y., Isayama H., Sasaki T., Takahara N., Saito K., Ishigaki K., Hamada T., Mizuno S., Miyabayashi K., Yamamoto K., Mohri D., Kogure H., Yamamoto N., Ijichi H., Tateishi K., Tada M., Koike K., The inhibition of renin-angiotensin system in advanced pancreatic cancer: An exploratory analysis in 349 patients. J. Cancer Res. Clin. Oncol. 141, 933–939 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nakai Y., Isayama H., Sasaki T., Takahara N., Saito K., Takeda T., Umefune G., Saito T., Takagi K., Watanabe T., Hamada T., Uchino R., Mizuno S., Yamamoto K., Kogure H., Matsubara S., Yamamoto N., Ijichi H., Tateishi K., Tada M., Koike K., No survival benefit from the inhibition of renin-angiotensin system in biliary tract cancer. Anticancer Res. 36, 4965–4970 (2016). [DOI] [PubMed] [Google Scholar]
- 172.Osumi H., Matsusaka S., Wakatsuki T., Suenaga M., Shinozaki E., Mizunuma N., Angiotensin II type-1 receptor blockers enhance the effects of bevacizumab-based chemotherapy in metastatic colorectal cancer patients. Mol. Clin. Oncol. 3, 1295–1300 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pinter M., Weinmann A., Wörns M.-A., Hucke F., Bota S., Marquardt J. U., Duda D. G., Jain R. K., Galle P. R., Trauner M., Peck-Radosavljevic M., Sieghart W., Use of inhibitors of the renin–angiotensin system is associated with longer survival in patients with hepatocellular carcinoma. United European Gastroenterol. J. 10.1177/2050640617695698 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tingle S. J., Moir J. A., White S. A., Role of anti-stromal polypharmacy in increasing survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. World J Gastrointest Pathophysiol 6, 235–242 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Levin V. A., Chan J., Datta M., Yee J. L., Jain R. K., Effect of angiotensin system inhibitors on survival in newly diagnosed glioma patients and recurrent glioblastoma patients receiving chemotherapy and/or bevacizumab. J. Neurooncol. 134, 325–330 (2017). [DOI] [PubMed] [Google Scholar]
- 176.Tanaka N., Miyajima A., Kosaka T., Shirotake S., Hasegawa M., Kikuchi E., Oya M., Cis-dichlorodiammineplatinum upregulates angiotensin II type 1 receptors through reactive oxygen species generation and enhances VEGF production in bladder cancer. Mol. Cancer Ther. 9, 2982–2992 (2010). [DOI] [PubMed] [Google Scholar]
- 177.Tanaka N., Miyajima A., Kosaka T., Miyazaki Y., Shirotake S., Shirakawa H., Kikuchi E., Oya M., Acquired platinum resistance enhances tumour angiogenesis through angiotensin II type 1 receptor in bladder cancer. Br. J. Cancer 105, 1331–1337 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Abd-Alhaseeb M. M., Zaitone S. A., Abou-El-Ela S. H., Moustafa Y. M., Olmesartan potentiates the anti-angiogenic effect of sorafenib in mice bearing Ehrlich’s ascites carcinoma: Role of angiotensin (1–7). PLOS ONE 9, e85891 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mancuso M. R., Davis R., Norberg S. M., O’Brien S., Sennino B., Nakahara T., Yao V. J., Inai T., Brooks P., Freimark B., Shalinsky D. R., Hu-Lowe D. D., McDonald D. M., Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J. Clin. Invest. 116, 2610–2621 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Griffioen A. W., Mans L. A., de Graaf A. M. A., Nowak-Sliwinska P., de Hoog C. L. M. M., de Jong T. A. M., Vyth-Dreese F. A., van Beijnum J. R., Bex A., Jonasch E., Rapid angiogenesis onset after discontinuation of sunitinib treatment of renal cell carcinoma patients. Clin. Cancer Res. 18, 3961–3971 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fox W. D., Higgins B., Maiese K. M., Drobnjak M., Cordon-Cardo C., Scher H. I., Agus D. B., Antibody to vascular endothelial growth factor slows growth of an androgen-independent xenograft model of prostate cancer. Clin. Cancer Res. 8, 3226–3231 (2002). [PubMed] [Google Scholar]
- 182.Powles T., Blank C., Chowdhury S., Horenblas S., Peters J., Shamash J., Sarwar N., Boleti E., Sahdev A., O’Brien T., Berney D., Beltran L., Nathan P., Haanen J., Bex A., The outcome of patients treated with sunitinib prior to planned nephrectomy in metastatic clear cell renal cancer. Eur. Urol. 60, 448–454 (2011). [DOI] [PubMed] [Google Scholar]
- 183.Rini B. I., Bellmunt J., Clancy J., Wang K., Niethammer A. G., Hariharan S., Escudier B., Randomized phase III trial of temsirolimus and bevacizumab versus interferon alfa and bevacizumab in metastatic renal cell carcinoma: INTORACT trial. J. Clin. Oncol. 32, 752–759 (2014). [DOI] [PubMed] [Google Scholar]
- 184.Haas N. B., Manola J., Uzzo R. G., Flaherty K. T., Wood C. G., Kane C., Jewett M., Dutcher J. P., Atkins M. B., Pins M., Wilding G., Cella D., Wagner L., Matin S., Kuzel T. M., Sexton W. J., Wong Y.-N., Choueiri T. K., Pili R., Puzanov I., Kohli M., Stadler W., Carducci M., Coomes R., DiPaola R. S., Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 387, 2008–2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ravaud A., Schmidinger M., Clinical biomarkers of response in advanced renal cell carcinoma. Ann. Oncol. 24, 2935–2942 (2013). [DOI] [PubMed] [Google Scholar]
- 186.Raimondi S., Botteri E., Munzone E., Cipolla C., Rotmensz N., DeCensi A., Gandini S., Use of beta-blockers, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers and breast cancer survival: Systematic review and meta-analysis. Int. J. Cancer 139, 212–219 (2016). [DOI] [PubMed] [Google Scholar]
- 187.European Association For The Study Of The Liver ; European Organisation For Research And Treatment Of Cancer, EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 56, 908–943 (2012). [DOI] [PubMed] [Google Scholar]
- 188.Fakih M. G., Metastatic colorectal cancer: Current state and future directions. J. Clin. Oncol. 33, 1809–1824 (2015). [DOI] [PubMed] [Google Scholar]
- 189.Escudier B., Porta C., Schmidinger M., Rioux-Leclercq N., Bex A., Khoo V., Gruenvald V., Horwich A.; ESMO Guidelines Committee , Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 27 (suppl. 5), v58–v68 (2016). [DOI] [PubMed] [Google Scholar]
- 190.Faris J. E., Blaszkowsky L. S., McDermott S., Guimaraes A. R., Szymonifka J., Huynh M. A., Ferrone C. R., Wargo J. A., Allen J. N., Dias L. E., Kwak E. L., Lillemoe K. D., Thayer S. P., Murphy J. E., Zhu A. X., Sahani D. V., Wo J. Y., Clark J. W., Fernandez-del Castillo C., Ryan D. P., Hong T. S, FOLFIRINOX in locally advanced pancreatic cancer: The Massachusetts General Hospital Cancer Center experience. Oncologist 18, 543–548 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mellon E. A., Hoffe S. E., Springett G. M., Frakes J. M., Strom T. J., Hodul P. J., Malafa M. P., Chuong M. D., Shridhar R., Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol. 54, 979–985 (2015). [DOI] [PubMed] [Google Scholar]
- 192.Song T., Choi C. H., Kim M. K., Kim M.-L., Yun B. S., Seong S. J., The effect of angiotensin system inhibitors (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) on cancer recurrence and survival: A meta-analysis. Eur. J. Cancer Prev. 26, 78–85 (2017). [DOI] [PubMed] [Google Scholar]
- 193.Pinter M., Trauner M., Peck-Radosavljevic M., Sieghart W., Cancer and liver cirrhosis: Implications on prognosis and management. ESMO Open 1, e000042 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yoshiji H., Kuriyama S., Yoshii J., Ikenaka Y., Noguchi R., Nakatani T., Tsujinoue H., Fukui H., Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology 34, 745–750 (2001). [DOI] [PubMed] [Google Scholar]
- 195.Jonsson J. R., Clouston A. D., Ando Y., Kelemen L. I., Horn M. J., Adamson M. D., Purdie D. M., Powell E. E., Angiotensin-converting enzyme inhibition attenuates the progression of rat hepatic fibrosis. Gastroenterology 121, 148–155 (2001). [DOI] [PubMed] [Google Scholar]
- 196.Tandon P., Abraldes J. G., Berzigotti A., Garcia-Pagan J. C., Bosch J., Renin-angiotensin-aldosterone inhibitors in the reduction of portal pressure: A systematic review and meta-analysis. J. Hepatol. 53, 273–282 (2010). [DOI] [PubMed] [Google Scholar]
- 197.Jain R. K., Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat. Rev. Cancer 8, 309–316 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.