Abstract
BACKGROUND
The Department of Health and Human Services National Blood Collection and Utilization Survey (NBCUS) has been conducted biennially since 1997. Data are used to estimate national blood collection and utilization.
STUDY DESIGN AND METHODS
The 2013 Department of Health and Human Services NBCUS is a cross-sectional survey of all US blood collection centers and hospitals as listed in the 2012 American Hospital Association Annual Survey database that perform at least 100 inpatient surgical procedures annually. The study objective was to estimate, with 95% confidence intervals (CIs), the number of blood and blood components collected and transfused in the United States.
RESULTS
In 2013, a total of 14,237,000 whole blood and apheresis red blood cell (RBC) units (95% CI, 13,639,000–14,835,000) were collected with 13,395,000 available for transfusion. Of these, 13,180,000 (95% CI, 12,389,000–13,972,000) whole blood and RBC units were transfused. This represented a 4.4% decline in the number of transfused units compared to 2011. Outdated (i.e., expired without being transfused) whole blood and RBC units declined by 17.3%. Apheresis (2,318,000; 95% CI, 2,154,000–2,482,000) and whole blood–derived platelet (PLT; 130,000; 95% CI, 23,000–237,000) distribution declined in 2013. Total PLT transfusions increased in 2013 (2,281,000) in comparison to 2011 (2,169,000). Total plasma units distributed (4,338,000) and transfused (3,624,000) declined.
CONCLUSION
Both blood collection and utilization have declined, but the gap between collection and utilization is narrowing. As collections decline further and hospitals decrease transfusions and manage products more efficiently, the decline in surplus inventory may be a concern for disaster preparedness or other unexpected utilization needs.
In the United States, blood and blood components are collected through a diverse array of both community-based nonhospital and hospital-based blood collection centers operating independently. These products are distributed to more than 4000 acute, subacute, long-term, and outpatient care facilities. Blood is collected as whole blood units and separated into components including red blood cells (RBCs), platelets (PLTs), and plasma or collected as individual components through apheresis methods. Further modifications to blood components such as leukoreduction or irradiation are performed to reduce the risk of adverse reactions. Blood transfusion practices for both medical and surgical procedures in the United States are variable across hospitals and geographic regions.1–3 In recent years, this variability has led to the establishment of patient blood management (PBM) programs. These programs include evidence-based medical and surgical approaches to manage anemia, improve hemostasis, and minimize inappropriate blood use to enhance patient safety and improve outcomes.4–6
While countries with centrally coordinated national blood services report blood collection data across facilities regularly, this is not the case in the United States.7–12 However, national estimates of collections and transfusions are necessary to understand current and projected demand for blood products to ensure a safe and adequate supply. Such estimates also inform disaster and emergency-preparedness planning and improve efforts to understand the magnitude of adverse reactions that may occur among patients receiving blood transfusions.13,14
Since 1971, national surveys have been administered intermittently in the United States to estimate blood collection and utilization.15 The biennial National Blood Collection and Utilization Survey (NBCUS) has been the primary method of gathering these data in the United States since 1997, historically (2005–2011) published as a Department of Health and Human Services survey report, with results last reported in peer-reviewed literature in 2007.16,17 Here we report the results from the 2013 NBCUS.
MATERIALS AND METHODS
The survey instrument included 83 questions on general facility information; blood collections, processing, and testing; blood and blood component transfusions; modification of components; and prices paid by hospitals for blood components. The survey was administered in a Web-based, electronic format. Participants were sent email invitations, which included a Web link that directed the respondent to the NBCUS survey portal where responses could be entered.
To estimate blood collection and utilization, all US blood collection centers and all US acute care hospitals performing at least 100 inpatient surgical procedures per year and located within the 50 states and the District of Columbia were included in the sample frame. Blood centers were identified from two sources, the FDA Blood Establishment Registration database and the America’s Blood Center’s member contact list.18 Acute care hospitals were selected from the 2012 American Hospital Association annual survey database. Military, Department of Justice, psychiatric, rehabilitation, acute long-term care, and other specialty treatment institutions, facilities outside the 50 US states, and hospitals with fewer than 100 inpatient surgical procedures were excluded. The survey was opened for participation in December 2014. Nonresponders were contacted electronically and by telephone from January to early March 2015. Data collection concluded on March 27, 2015.
The objective of data analyses was to estimate whole blood and blood components collected, including RBCs, PLTs, plasma, and cryoprecipitate units, and those rejected on testing, outdated, and transfused nationally. Estimates were rounded to the nearest 1000 units. The numbers and proportions of RBC and PLT units that were irradiated or leukoreduced were estimated. The unweighted minimum, maximum, and mean amounts paid in US dollars for RBC, PLT, and plasma units were also calculated. Extreme outliers (e.g., hospitals that reported mean prices paid <1 and >99% compared to respondents) were excluded from mean calculations. National rates of whole blood and RBC units collected per 1000 population (aged 16–64 years) and transfused per 1000 population (all ages) were calculated using US Census Bureau population estimates from 2013.19 These rates were compared with estimated national rates from 1992 to 2011.20
Respondents could include aggregate data from more than one facility on a single survey response. Of all responding facilities, 954 were for a single facility completing one response while 147 were contained on responses including data from more than one facility. When responses included more than one facility, each facility contained within the aggregated response was assigned transfusion values proportionate to the volume of annual inpatient surgical procedures performed.
Blood centers (both community-based, nonhospital and hospital-based centers) were stratified into seven groups, and hospitals were stratified into six groups for imputation and development of estimates. Nonhospital blood centers were stratified into four categories based on expected volume of whole blood and RBC collections: fewer than 50,000, 50,000 or more to fewer than 200,000, 200,000 or more to 400,000 or fewer units, and more than 400,000 units. Hospital-based blood centers were stratified into three categories based on volume of annual inpatient surgical procedures: fewer than 1000, 1000 or more to fewer than 8000, and 8000 or more procedures. For utilization estimates, hospitals were stratified into six categories based on volume of annual inpatient surgical procedures: 100 to 999, 1000 to 1399, 1400 to 2399, 2400 to 4999, 5000 to 7999, and 8000 or more procedures.
Multiple imputation was performed for missing data on variables in the collection and utilization sections.21 These included 1) collection or distribution—whole blood, apheresis RBCs, rejected for unacceptable disease markers and other reasons, apheresis PLTs, plasma, and cryoprecipitate units; 2) utilization—allogeneic (nondirected), autologous, and directed whole blood and RBCs, PLTs, plasma, and cryoprecipitate units; and 3) all outdated units. Because all analyzed variables were continuous and non-normally distributed, logarithm transformation and predictive mean matching methods were utilized to ensure plausibility of imputed values.22,23 A two-stage imputation procedure was performed for variables with data skewed toward zero.24 The strata defined above for blood centers and hospitals were used as the covariates to impute allogeneic whole blood collections and allogeneic RBC transfusions, respectively. The imputed values for these variables were included with strata classes as covariates to impute variables in the collection and utilization sections defined above.
Blood center (both nonhospital and hospital) and hospital responses were weighted to adjust for nonresponse. Sampling weights were calculated for each stratum by dividing the total number of eligible respondents by the actual number of respondents in that stratum. Blood centers with the largest expected collection volume (>400,000 units) were each assigned a weight of 1.0. All other blood center and hospital responses, to estimate collection or utilization, were weighted using the earlier-described strata. National estimates were calculated based on the sampling weights and Taylor series method was used to calculate the variance of the national estimates to develop confidence intervals (CIs).25,26 The results of the multiple imputed data sets were combined to account for imputation error.21 All analyses were performed using computer software (SAS, Version 9.3, SAS Institute, Inc.).
RESULTS
Survey response rates
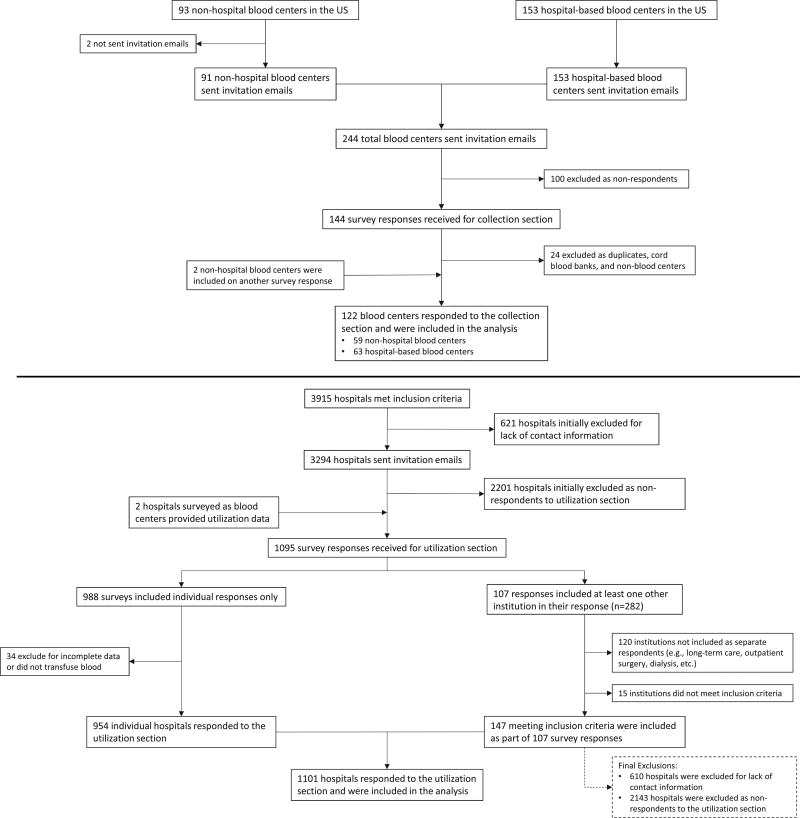
Ninety-three nonhospital blood centers were identified in the United States. Invitation e-mails were sent to 91 nonhospital blood centers. Two centers were not sent invitations due to lack of contact information. Of these 91, a total of 59 (64%) nonhospital blood centers responded to the survey. A total of 153 hospital-based blood centers were identified and invited to participate. Of these, 63 (41%) responded to the survey (Fig. 1). Nonhospital blood center collections represent an estimated 94% of the RBC, PLT, and plasma supply.
Fig. 1.
Flow diagram describing blood center and transfusing hospital respondents, 2013.
From the 2012 American Hospital Association annual survey database, 3915 hospitals were identified that met inclusion criteria. Of these, contact information was unobtainable for 610 hospitals. Therefore, 3305 hospitals were sent invitations to participate. In total, 1101 of 3305 (33.3%) hospitals provided responses to the survey. Two additional institutions were sent surveys as blood centers, but provided utilization data only and were therefore included in the utilization estimate analyses. (Fig. 1).
Whole blood and RBC collections and transfusions
In 2013, a total of 14,237,000 whole blood and apheresis RBC units (95% CI, 13,639,000–14,835,000) were collected in the United States (Table 1), representing a 9.4% decline compared to 2011 (15,721,000; 95% CI, 15,521,000–15,921,000). Of these, 13,563,000 units (95.3%) were collected by nonhospital blood centers with an additional 674,000 units (4.7%) collected by hospitals. Allogeneic, nondirected whole blood units accounted for 12,109,000 (95% CI, 11,439,000–12,779,000) of total whole blood and RBC collections representing a 10.9% decline compared to 2011. Apheresis RBC units accounted for 2,043,000 (95% CI, 1,659,000–2,427,000) collections. After units that were rejected for unacceptable disease markers or other reasons were removed, 13,395,000 RBC units (95% CI, 12,823,000–13,966,000) were available for transfusion. This number represents an 8.2% decline compared to 2011.
TABLE 1.
Estimated numbers of whole blood and RBC units collected, transfused, and outdated in 2013 (expressed in thousands)
| Activity | Blood centers |
Hospitals | Combined totals |
95% CI | 2011 Totals* | % Change 2011–2013 |
|---|---|---|---|---|---|---|
| Collections | ||||||
| Whole blood units | ||||||
| Allogeneic, nondirected | 11,511 | 598 | 12,109 | 11,439–12,779 | 13,586 | −10.9 |
| Autologous | 40 | 21 | 61 | 48–74 | 113 | −46.0 |
| Directed | 15 | 9 | 24 | 15–32 | 45 | −46.7 |
| Apheresis RBC units† | 1,997 | 46 | 2,043 | 1,659–2,427 | 1,978 | 3.3 |
| Total supply | 13,563 | 674 | 14,237 | 13,639–14,835 | 15,721 | −9.4 |
| Rejected on testing | 87 | 10 | 98 | 87–109 | 102 | −3.9 |
| Rejected for other reasons‡ | 712 | 32 | 744 | 663–825 | 1,030 | −27.8 |
| Total available supply | 12,764 | 631 | 13,395 | 12,823–13,966 | 14,589 | −8.2 |
| Transfusions | ||||||
| Allogeneic, nondirected | 13,093 | 12,296–13,890 | 13,684 | −4.3 | ||
| Autologous | 44 | 25–64 | 65 | −32.3 | ||
| Directed | 43 | 12–73 | 37 | 16.2 | ||
| Total transfusions | 13,180 | 12,389–13,972 | 13,785 | −4.4 | ||
| Outdated whole blood or RBCs | 306 | 269–343 | 370 | −17.3 |
2011 totals were obtained from the 2011 NBCUS Report.20
Apheresis RBC units include allogeneic, autologous, directed, and concurrent collections.
Units rejected for other reasons does not include outdated units.
In 2013, a total of 13,180,000 whole blood and RBC units (95% CI, 12,389,000–13,972,000) were transfused in the United States. This was a 4.4% decline compared with 2011. Of these, 13,093,000 units (99.3%; 95% CI, 12,296,000–13,890,000) were allogeneic, nondirected whole blood and RBC units transfused. Nonhospital and hospital-based blood centers reported 306,000 units (95% CI, 269,000–343,000) outdated, representing a 17.3% decline compared to 2011.
PLT, plasma, and cryoprecipitate distribution and transfusion
In 2013, a total of 2,448,000 whole blood–derived and apheresis PLTs units (95% CI, 2,237,000–2,659,000) were distributed by blood centers, representing a 10.6% decline compared to 2011 (2,738,000; 95% CI, 2,652,000–2,824,000; Table 2). Of these, 2,318,000 units (94.7%; 95% CI, 2,154,000–2,482,000) were apheresis PLTs and 130,000 units (5.3%; 95% CI, 23,000–237,000) were whole blood–derived PLTs, representing declines of 7.9% (2011, 2,516,000) and 41.4% (2011, 222,000) compared to 2011, respectively. A total of 4,338,000 (95% CI, 3,432,000–5,244,000) plasma units were distributed in 2013, a 26.8% decline compared to 2011 (5,926,000; 95% CI, 5,758,000–6,094,000). These include fresh-frozen plasma (FFP), plasma frozen within 24 hours of collection, cryoprecipitate-reduced plasma, and liquid plasma. A total of 978,000 units (95% CI, 798,000–1,157,000) of cryoprecipitate were distributed in 2013, a 42.1% decline compared to 2011.
TABLE 2.
Estimated number of PLT, plasma, and cryoprecipitate units distributed by blood centers, transfused by hospitals, and outdated in blood centers and hospitals in 2013 (expressed in thousands)
| Activity | Blood centers |
Hospitals | Combined totals |
95% CI | 2011 totals* |
% Change 2011-2013 |
|---|---|---|---|---|---|---|
| Distributed | ||||||
| Apheresis PLTs | 2133 | 185 | 2318 | 2154–2482 | 2516 | −7.9 |
| Whole blood–derived PLTs† | 75 | 55 | 130 | 23–237 | 222 | −41.4 |
| Total PLTs | 2209 | 240 | 2448 | 2237–2659 | 2738 | −10.6 |
| Total plasma | 4005 | 333 | 4338 | 3432–5244 | 5926 | −26.8 |
| Cryoprecipitate‡ | 857 | 120 | 978 | 798–1157 | 1690 | −42.1 |
| Blood center outdates§ | 205 | 34 | 239 | 200–278 | 301 | −31.9 |
| Transfused | ||||||
| Apheresis PLTs | 2137 | 1773–2500 | 1970 | 8.5 | ||
| Whole blood–derived PLTs† | 128 | 83–172 | 199 | −35.7 | ||
| Total PLTs (includes directed units) | 2281 | 1915–2646 | 2169 | 5.2 | ||
| Total plasma | 3624 | 3304–3943 | 3882 | −6.6 | ||
| Cryoprecipitate‡ | 1095 | 916–1274 | 1094 | 0 | ||
| Hospital outdates¶ | 395 | 345–446 | 521 | −24.2 |
2011 totals were obtained from the 2011 NBCUS Report.20
Whole blood–derived PLTs are expressed as apheresis equivalents.
Cryoprecipitates are expressed as individual unit equivalents.
Blood center outdates are units that were outdated at nonhospital and hospital-based blood centers.
Hospital outdates are units that were outdated at transfusing hospitals.
In 2013, 2,281,000 total PLTs units (95% CI, 1,915,000–2,646,000) were transfused in the United States, constituting a 5.2% increase from 2011 (2,169,000; 95% CI, 2,001,000–2,337,000). Larger numbers of apheresis PLT units were transfused (2013, 2,137,000; 95% CI, 1,773,000–2,500,000) in comparison to 2011 (1,970,000; 95% CI, 1,809,000–2,131,000). The number of whole blood–derived PLT units transfused (2013, 128,000; 95% CI, 83,000–172,000), however, declined in comparison to 2011 (199,000; 95% CI, 139,000–259,000). Plasma units transfused in 2013 (3,624,000; 95% CI, 3,304,000–3,943,000) declined by 6.6% in comparison to 2011 (3,882,000; 95% CI, 3,665,000–4,101,000). Cryoprecipitate transfusion estimates were unchanged from 2011. Nonhospital and hospital-based blood centers reported 239,000 units (95% CI, 200,000–278,000) outdated and transfusing hospitals reported 395,000 units (95% CI, 345,000–446,000) outdated representing a 31.9 and 24.2% decline, respectively.
Leukoreduction and irradiation of components
The proportion of whole blood and RBC units that were leukoreduced at any stage (before, after storage, or at the bedside) remained unchanged from 2011 (70.5%) to 2013 (71.7%). The proportion of whole blood–derived PLT units that were subjected to leukoreduction (35.0% in 2013 and 37.0% in 2011) remained similar. In 2013, 16.8% (2,216,000; 95% CI: 1,735,000–2,697,000) of whole blood and RBC units were irradiated, compared to 12.0% in 2011 (1,648,000; 95% CI not reported). In 2011, 46.4% of transfused apheresis PLT units (915,000; 95% CI not reported) were irradiated. In 2013, that proportion increased to 57.6% (1,231,000 units; 95% CI, 970,000–1,492,000). The proportion of whole blood–derived PLTs subjected to irradiation was similar in both years (2011, 34.2%; 2013, 31.7%).
Component prices paid by hospitals
The mean price paid by hospitals for a single leukoreduced RBC unit in 2013 ($225.74; range, $166–$440) was unchanged from 2011 ($225.42). Prices for single FFP units were similar in 2011 (mean, $57.91) and 2013 (mean, $60.54; range, $35–$174). Prices for single plasma frozen within 24 hours of collection were also similar in 2011 (mean, $56.08) and 2013 (mean, $58.55; range, $31–$175). Leukoreduced apheresis PLT unit prices may have modestly increased in 2013 (mean, $547.29; range, $300–$950), compared with 2011 (mean, $535.17).
Donor deferrals
In 2013, an estimated 15,236,895 persons presented to donate blood (95% CI, 14,437,000–16,037,000; Table 3). In 2011, a total of 17,984,000 persons presented to donate blood. Approximately 15% (2,368,211 persons; 95% CI, 2,119,000–2,617,000) of persons presenting to donate were deferred. Almost half (1,187,910; 95% CI, 1,119,000–1,257,000) of deferrals were due to low hemoglobin (Hb). Almost 20% (472,236; 95% CI, 432,000–513,000) of deferrals were due to other medical reasons (e.g., use of medications on the medication deferral list, hepatitis B immune globulin, unlicensed vaccines, etc.). An estimated 7.3% were deferred for travel and 3.0% due to prescription drug use. Men who have sex with men behavior resulted in 7751 (95% CI, 6936–8566) persons being deferred from donation. Other high-risk behaviors (14,486; 95% CI, 10,393–18,579) accounted for less than 1% of all deferrals.
TABLE 3.
Estimated number of individuals deferred from donation in the United States, 2013
| Number of individuals | % of total deferrals |
|||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Deferrals | Blood centers | Hospitals | Combined totals | 95% CI‡ | 2013 | 2011* |
| Low Hb | 1,120,223 | 67,687 | 1,187,910 | 1,119–1,257 | 50.2 | 48.8 |
| Prescription drug use | 66,602 | 3,801 | 70,403 | 65–76 | 3.0 | 3.6 |
| Other medical reasons | 446,877 | 25,360 | 472,236 | 432–513 | 19.9 | 19.2 |
| High-risk behavior (MSM only)† | 7,291 | 460 | 7,751 | 7–9 | 0.3 | 0.4 |
| High-risk behavior (all other behaviors) | 13,558 | 928 | 14,486 | 10–19 | 0.6 | 1.0 |
| Travel | 160,392 | 12,429 | 172,821 | 158–187 | 7.3 | 7.4 |
| Tattoo/piercing | 71,797 | 3,980 | 75,777 | 70–82 | 3.2 | 4.0 |
| Other | 349,204 | 17,623 | 366,827 | 166–567 | 15.5 | 15.7 |
| Total | 2,235,944 | 132,268 | 2,368,211 | 2,119–2,617 | ||
| Total presenting to donate | 14,452,441 | 784,454 | 15,236,895 | 14,437–16,037 | ||
Percentage of total deferrals in 2011 were obtained from the 2011 NBCUS Report.20
MSM = men who have sex with men.
95% CI Expressed in thousands.
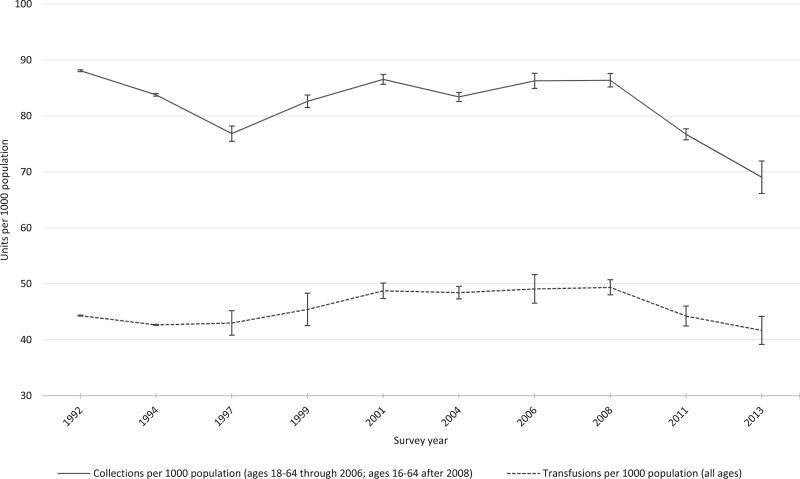
Rate of blood collection and utilization
Figure 2 illustrates the estimated rate per 1000 population of whole blood and RBC units collected and transfused in the United States from1992 to 2013. Whole blood and RBC collections per 1000 population declined in 2013 (69.0 units per 1000 population aged 16–64 years) in comparison to 2011 (76.2 units per 1000 population aged 16–64 years). The rate of whole blood and RBC transfusions per 1000 population also declined (2013, 41.7 per 1000 population, all ages; 2011, 44.0 per 1000 population, all ages).
Fig. 2.
Trends in estimated rates of whole blood and RBC collections and transfusions in the United States, 1992–2013.US population estimates from 1992 to 2013 were obtained from the US Census Bureau population estimates.19 Estimated whole blood and RBC collections and transfusions were obtained from previous surveys.20
DISCUSSION
In 2013, our data suggest that blood collection and utilization in the United States continued to decline following a trend that was initially described in 2011 (Fig. 2). The decline in collections included whole blood, PLTs, plasma, and cryoprecipitate overall. With the exception of apheresis PLT and cryoprecipitate units, blood and blood component utilization declined, although the magnitude was not as steep as with collections. In 2011, a total of 804,000 more whole blood and RBC units were collected than were transfused in the United States, and that margin closed considerably in 2013, to 215,000 units. As blood centers are collecting less blood, units are managed more efficiently, with less wastage (e.g., fewer rejections and outdates) than in previous years.
While overall collections have decreased, increasing proportions of whole blood, RBCs, and apheresis PLT units were irradiated in 2013, continuing a trend which has been observed since 2004. The proportion of leukoreduced whole blood, RBCs, and apheresis PLT units transfused from 2008 to 2013 remained similar and are still not comparable to other developed countries where universal leukoreduction has been adopted (e.g., Austria, France, Germany, United Kingdom).11
This survey, consistent with previous NBCUS surveys conducted in 2008 and 2011, suggests declining transfusion utilization in the United States. While recent analyses using the Nationwide Inpatient Sample have suggested an increase in transfusion use, estimating blood utilization using the Nationwide Inpatient Sample, which relies on diagnoses and procedure coding by clinicians, is likely limited because inpatient transfusion reimbursement is included within diagnoses-related group payments by the Centers for Medicare and Medicaid Services in the United States and may therefore not be separately recorded as part of hospital discharge documentation.27 Therefore, a direct survey is more likely to yield accurate estimates of transfusion use. Declining blood utilization in the United States more likely represents the impact of PBM initiatives and other improvements in clinical practices, such as decrease in myeloablative transplantation, increase in minimally invasive surgery, success with cytokine-based therapies, and immunosuppression for aplastic anemia.28–31 Previous studies have described wide variations in transfusion practice with a substantial proportion of transfusions identified as inappropriate.32–34 Additionally, risks related to transfusions, including adverse transfusion-related reactions, associations with healthcare-associated infections, increased cancer recurrence, and poorer surgical outcomes have been described.2,13,35–37 Therefore, PBM interventions have focused on the reduction of the need for transfusion and thereby improve patient outcomes. Alternatives to transfusion include pharmacologic treatment of anemia, closer management of anticoagulation, surgical blood conservation techniques, and restrictive transfusion practices for surgical procedures and medical conditions.38 Similar reductions in blood utilization attributable to PBM initiatives have been noted in other developed countries.39,40 As these evidence-based practices gain wider implementation across the United States, the trend of reduction in blood transfusions is likely to continue. In addition to PBM interventions, the findings of this survey suggest that hospitals are improving blood product inventory management as reflected by the decline in outdated units. The reduction in wastage is likely to also decrease the demand for blood units by hospitals.
The impact of reductions in blood collection and utilization raises important concerns related to sustainability of blood collection centers and public health-preparedness. In the United States, these reductions along with consolidation of institutions into hospital systems may result in declining revenue for nonhospital collection centers.41,42 Our findings suggest that the prices paid per RBC unit have not changed since 2011 which, along with fewer collections, has resulted in declining revenue for collection centers. The present data also suggest that collection centers appear to have shifted production toward apheresis PLT collections and distribution of higher numbers of irradiated blood products. Reasons for these changes may be multifactorial. Hospitals could prefer apheresis PLT units, which obviate the need for pooling before transfusion. The increased proportion of irradiated products could be due to growing transfusion use among patients being treated for malignancies and other immunosuppressed conditions. However, the growth of these products may reflect higher reimbursement than whole blood–derived PLTs and nonirradiated alternatives. Consolidation of blood services has also occurred in response to declining revenue.42 Collectively, these consolidations have resulted in reductions in capacity and may impact the provisions of necessary blood components available for transfusion as well as public health emergency preparedness. 41 Additional blood safety measures have recently been introduced or proposed in the United States, which could result in additional cost to blood centers and hospitals. These include pathogen reduction technology, mandatory PLT testing for bacterial contamination, and geographic-based blood donor screening for babesiosis.43–45 Efforts are currently under way to study the impact of declining revenues and additional safety measures on the sustainability of the US blood supply.41
These findings are subject to the following limitations. The survey instrument was disseminated electronically. For facilities that did not participate, receipt of the survey was difficult to confirm. All facilities that did not receive the survey link due to information technology–related challenges (e.g., e-mail filtering and firewall blockades) could not be identified, but could have been as high as one-third of facilities initially included in the sample. The impact of respondent bias on collection or utilization estimates cannot be quantified. Although decreasing response rates from hospitals, where blood products are utilized, could lead to reporting bias around transfusion trends, the uniformity in response rate across strata (Table S1, available as supporting information in the online version of this paper) and consistency of trends in both collection and utilization estimates across product types suggest that the impact is likely to be minimal.
Next, respondents could aggregate data from more than one facility on a single survey response. These responses were difficult to disaggregate and resulted in an assumption that distribution of transfusions were proportionate to the volume of annual inpatient surgical procedures. This assumption is consistent with procedures used in previous US national collection and utilization surveys since 1989 and unlikely to have a significant impact on the survey findings.46 Finally, the survey was not distributed to outpatient facilities, and some military and specialty hospitals, which may transfuse substantial numbers of blood and blood components (e.g., large cancer treatment centers). The survey was also not distributed to military collection facilities. This may result in underestimation of collection and utilization.
In summary, blood collection is declining in the United States, most likely in response to decreased utilization in healthcare facilities that have implemented PBM initiatives. Given calls for new donor testing and product modification strategies, requiring continued investment, and need for resilience of the blood supply for disaster preparedness, these trends could create challenges for both blood safety and availability.
Supplementary Material
Table S1. Hospital sample frame and sample weights by number of inpatient surgical procedures and WB/RBC collections in 2013.
ABBREVIATIONS
- NBCUS
National Blood Collection and Utilization Survey
- PBM
patient blood management
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. The use of trade names is for identification purposes only and does not constitute endorsement by the US Centers for Disease Control and Prevention or the Department of Health and Human Services.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Additional Supporting Information may be found in the online version of this article at the publisher’s website:
References
- 1.Bennett-Guerrero E, Zhao Y, O’Brien SM, et al. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA. 2010;304:1568–75. doi: 10.1001/jama.2010.1406. [DOI] [PubMed] [Google Scholar]
- 2.Sherwood MW, Wang Y, Curtis JP, et al. Patterns and outcomes of red blood cell transfusion in patients undergoing percutaneous coronary intervention. JAMA. 2014;311:836–43. doi: 10.1001/jama.2014.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloch EM, Cohn C, Bruhn R, et al. A cross-sectional pilot study of blood utilization in 27 hospitals in Northern California. Am J Clin Pathol. 2014;142:498–505. doi: 10.1309/AJCP8WFIQ0JRCSIR. [DOI] [PubMed] [Google Scholar]
- 4.Goodnough LT. Blood management: transfusion medicine comes of age. Lancet. 2013;381:1791–2. doi: 10.1016/S0140-6736(13)60673-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodnough LT, Shander A. Patient blood management. Anesthesiology. 2012;116:1367–76. doi: 10.1097/ALN.0b013e318254d1a3. [DOI] [PubMed] [Google Scholar]
- 6.Williamson LM, Devine DV. Challenges in the management of the blood supply. Lancet. 2013;381:1866–75. doi: 10.1016/S0140-6736(13)60631-5. [DOI] [PubMed] [Google Scholar]
- 7.Drackley A, Newbold KB, Paez A, et al. Forecasting Ontario’s blood supply and demand. Transfusion. 2012;52:366–74. doi: 10.1111/j.1537-2995.2011.03280.x. [DOI] [PubMed] [Google Scholar]
- 8.The facts about whole blood: the national inventory trend [Internet] Ottawa: Canadian Blood Services; 2015. [cited 2016 Jan 13]. Available from: https://www.blood.ca/en/bloodtype. [Google Scholar]
- 9.Borkent-Raven BA, Janssen MP, Van Der Poel CL. Demographic changes and predicting blood supply and demand in the Netherlands. Transfusion. 2010;50:2455–60. doi: 10.1111/j.1537-2995.2010.02716.x. [DOI] [PubMed] [Google Scholar]
- 10.2013 extended version [Internet] Leiden: Transfusion and Transplantation Reactions in Patients (TRIP) National Hemovigilance Office; 2015. [cited 2016 Jan 13]. TRIP annual report. Hemovigilance. Available from: http://www.tripnet.nl/pages/en/documents/TRIP2013Hemovigilance-English.pdf. [Google Scholar]
- 11.Janssen MP, van Hoeven LR, Rautmann G. Trends and observations on the collection, testing and use of blood and blood components in Europe 2001–2011 report. Strasbourg: Council of Europe. 2015 [Google Scholar]
- 12.Latest stocks and statistics [Internet] London: National Health Service Blood and Transplant (NHSBT); 2015. [cited 2016 Jan 13]. Available from: http://www.blood.co.uk/about-blood/stock-levels-statistics/ [Google Scholar]
- 13.Harvey AR, Basavaraju SV, Chung KW, et al. Transfusion-related adverse reactions reported to the National Healthcare Safety Network Hemovigilance Module, United States, 2010 to 2012. Transfusion. 2015;55:709–18. doi: 10.1111/trf.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamp C, Heiden M, Henseler O, et al. Management of blood supplies during an influenza pandemic. Transfusion. 2010;50:231–9. doi: 10.1111/j.1537-2995.2009.02498.x. [DOI] [PubMed] [Google Scholar]
- 15.Wallace EL, Churchill WH, Surgenor DM, et al. Collection and transfusion of blood and blood components in the United States, 1994. Transfusion. 1998;38:625–36. doi: 10.1046/j.1537-2995.1998.38798346630.x. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan MT, Cotten R, Read EJ, et al. Blood collection and transfusion in the United States in 2001. Transfusion. 2007;47:385–94. doi: 10.1111/j.1537-2995.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- 17.National blood collection & utilization survey [Internet] Washington (DC): US Department of Health and Human Services; 2015. [cited 2015 Aug 21]. Available from: http://.www.hhs.gov/ash/bloodsafety/nbcus/ [Google Scholar]
- 18.FDA. Blood establishment registration database [Internet] Silver Spring (MD): US Food and Drug Administration; 2015. [cited 2016 Jan 13]. Available from: https://www.accessdata.fda.gov/scripts/cber/CFAppsPub/ [Google Scholar]
- 19.US Census Bureau. Population and housing unit estimates [Internet] Washington (DC): US Department of Commerce; 2015. [cited 2016 Jan 13]. Available from: http://www.census.gov/popest/index.html. [Google Scholar]
- 20.The 2011 National Blood Collection and Utilization Survey report. Washington (DC): US Department of Health and Human Services; 2013. [Google Scholar]
- 21.Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken (NJ): Wiley; 2004. [Google Scholar]
- 22.Heitjan DF, Roderick JA. Multiple imputation for the fatal accident reporting system. J R Stat Soc Ser C Appl Stat. 1991;40:13–29. [Google Scholar]
- 23.Schenker N, Taylor JM. Partially parametric techniques for multiple imputation. Comput Stat Data Anal. 1996;22:425–46. [Google Scholar]
- 24.He Y, Raghunathan TE. Tukey’s gh distribution for multiple imputation. Am Stat. 2006;60:251–6. [Google Scholar]
- 25.Woodruff RS. A simple method for approximating the variance of a complicated estimate. J Am Stat Assoc. 1971;66:411–4. [Google Scholar]
- 26.Fuller WA. Regression analysis for sample survey. Sankhya Ser C. 1975;37:117–32. [Google Scholar]
- 27.Pathak R, Bhatt VR, Karmacharya P, et al. Trends in blood-product transfusion among inpatients in the United States from 2002 to 2011: data from the Nationwide Inpatient Sample. J Hosp Med. 2014;9:800–1. doi: 10.1002/jhm.2248. [DOI] [PubMed] [Google Scholar]
- 28.Goodnough LT, Maggio P, Hadhazy E, et al. Restrictive blood transfusion practices are associated with improved patient outcomes. Transfusion. 2014;54:2753–9. doi: 10.1111/trf.12723. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz-Argüelles GJ, López-Martinez B, Gómez-Rangel D, et al. Decreased transfusion requirements in patients given stem cell allografts using a non-myeloablative conditioning regimen: a single institution experience. Hematology. 2003;8:151–4. doi: 10.1080/1024533031000084196. [DOI] [PubMed] [Google Scholar]
- 30.Scheinberg P, Nunez O, Wu C, et al. Treatment of severe aplastic anaemia with combined immunosuppression: antithymocyte globulin, ciclosporin and mycophenolate mofetil. Br J Haematol. 2006;133:606–11. doi: 10.1111/j.1365-2141.2006.06085.x. [DOI] [PubMed] [Google Scholar]
- 31.Woo YJ, Nacke EA. Robotic minimally invasive mitral valve reconstruction yields less blood product transfusion and shorter length of stay. Surgery. 2006;140:263–7. doi: 10.1016/j.surg.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Hasley PB, Lave JR, Kapoor WN. The necessary and the unnecessary transfusion: a critical review of reported appropriateness rates and criteria for red cell transfusions. Transfusion. 1994;34:110–5. doi: 10.1046/j.1537-2995.1994.34294143936.x. [DOI] [PubMed] [Google Scholar]
- 33.Triulzi D, Gottschall J, Murphy E, et al. A multicenter study of plasma use in the United States. Transfusion. 2015;55:1313–9. doi: 10.1111/trf.12970. quiz 1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Likosky DS, Al-Attar PM, Malenka DJ, et al. Geographic variability in potentially discretionary red blood cell transfusions after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2014;148:3084–9. doi: 10.1016/j.jtcvs.2014.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiergens TS, Rentsch M, Kasparek MS, et al. Impact of perioperative allogeneic red blood cell transfusion on recurrence and overall survival after resection of colorectal liver metastases. Dis Colon Rectum. 2015;58:74–82. doi: 10.1097/DCR.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 36.Rohde JM, Dimcheff DE, Blumberg N, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA. 2014;311:1317–26. doi: 10.1001/jama.2014.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012;52(Suppl 1):65S–79S. doi: 10.1111/j.1537-2995.2012.03663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shander A, Javidroozi M. Strategies to reduce the use of blood products: a US perspective. Curr Opin Anaesthesiol. 2012;25:50–8. doi: 10.1097/ACO.0b013e32834dd282. [DOI] [PubMed] [Google Scholar]
- 39.Tinegate H, Chattree S, Iqbal A, et al. Ten-year pattern of red blood cell use in the North of England. Transfusion. 2013;53:483–9. doi: 10.1111/j.1537-2995.2012.03782.x. [DOI] [PubMed] [Google Scholar]
- 40.van Hoeven L, Janssen MP, Rautmann G. The collection, testing and use of blood and blood components in Europe. Strasbourg: Concil of Europe; 2012. [Google Scholar]
- 41.Recommendations of the 47th meeting of the ACBTSA, November 2015 [Internet] Washington, DC: US Department of Health & Human Services; 2015. [cited 2016 Feb 16]. Advisory Committee on Blood and Tissue Safety Availability. Available from: http://www.hhs.gov/ash/bloodsafety/advisorycommittee/recommendations/nov2015-recommendations.html.html. [Google Scholar]
- 42.Snyder EL, Stramer SL, Benjamin RJ. The safety of the blood supply—time to raise the bar. N Engl J Med. 2015;373:882. doi: 10.1056/NEJMc1507761. [DOI] [PubMed] [Google Scholar]
- 43.FDA approves first pathogen reduction system to treat plasma [Internet] Silver Spring (MD): US Food and Drug Administration; 2014. [cited 2016 Jan 13]. [Google Scholar]
- 44.Bacterial detection testing by blood and blood collection establishments and transfusion services to enhance the safety and availability of platelets for transfusion [Internet] Silver Spring (MD): US Food and Drug Administration; 2014. [cited 2016 Apr 18]. Available from: https://www.federalregister.gov/articles/2014/12/09/2014-28809/bacterial-detection-testing-by-blood-collection-establishments-and-transfusion-services-to-enhance. [Google Scholar]
- 45.Strategies for implementation of antibody and nucleic acid-based testing for Babesia microti in blood donors [Internet] Silver Spring (MD): US Food and Drug Administration; 2015. [cited 2016 Apr 18]. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/BloodProductsAdvisoryCommittee/UCM446274.pdf. [Google Scholar]
- 46.Wallace EL, Surgenor DM, Hao HS, et al. Collection and transfusion of blood and blood components in the United States, 1989. Transfusion. 1993;33:139–44. doi: 10.1046/j.1537-2995.1993.33293158046.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Hospital sample frame and sample weights by number of inpatient surgical procedures and WB/RBC collections in 2013.