Abstract
Background
Soil bacterium Sinorhizobium meliloti (S. meliloti) forms an endosymbiotic partnership with Medicago truncatula (M. truncatula) roots which results in root nodules. The bacteria live within root nodules where they function to fix atmospheric N2 and supply the host plant with reduced nitrogen. The bacterial RNA-binding protein Hfq (Hfq) is an important regulator for the effectiveness of the nitrogen fixation. RNA immunoprecipitation (RIP) method is a powerful method for detecting the association of Hfq protein with specific RNA in cultured bacteria, yet a RIP method for bacteria living in root nodules remains to be described.
Results
A modified S. meliloti gene encoding a His-tagged Hfq protein (HfqHis) was placed under the regulation of the native Hfq gene promoter (Phfqsm). The trans produced HfqHis protein was accumulated at its nature levels during all stages of the symbiosis, allowing RNAs that associated with the given protein to be immunoprecipitated with the anti-His antibody against the protein from root nodule lysates. RNAs that associated with the protein were selectively enriched in the immunoprecipitated sample. The RNAs were recovered by a simple method using heat and subsequently analyzed by RT-PCR. The nature of PCR products was determined by DNA sequencing. Hfq association with specific RNAs can be analyzed at different conditions (e. g. young or older root nodules) and/or in wild-type versus mutant strains.
Conclusions
This article describes the RIP method for determining Sinorhizobium meliloti RNA-Hfq associations in vivo. It is also applicable to other rhizobia living in planta, although some tissue-specific modification related to sample disruption and homogenization may be needed.
Keywords: RNA immunoprecipitation, S. meliloti Hfq, Nodule lysate, Rhizobia symbiosis
Background
Sinorhizobium meliloti (S. meliloti) forms an important endosymbiotic partnership with Medicago truncatula (M. truncatula) roots which results in the development of specialized organs called root nodules [1]. Bacteria live within root nodules where they function to fix atmospheric N2 and supply the host plant with reduced nitrogen. This interaction of S. meliloti and M. truncatula provides a model system to study the molecular basis of Rhizobium-legume N2-fixing symbioses [2, 3]. Forty to sixty (40 – 60) million tons of nitrogen are fixed annually by the Rhizobia-legume N2-fixing symbioses in cultivated legumes, saving about $10 billion on nitrogen fertilizer [4, 5]. S. meliloti RNA-binding protein Hfq is an important regulator that governs the effectiveness of the S. meliloti-legume interaction [6–8]. The N2-fixing efficiency is severely reduced if S. meliloti mutants carry mutations in Hfq gene (hfq) and this reduced efficiency is accompanied by reduced stress tolerance.
Hfq fulfills its function through association with specific RNA sequences [9]. Putative Hfq-binding sites i.e., free 3′-hydroxyl end of an oligo-U stretch or A/U-rich regions in S. meliloti RNA molecules are predicted in silico [10]. Two lines of experimental evidence, with a strong genetic base, have now demonstrated that Hfq binds to A/U-rich regions in mRNAs for both ExpR and for FixL proteins in the bacterium and that the bindings cause changes the stability and translation efficiency of those RNAs [11, 12]. A global regulatory role for Hfq in controlling gene regulation in the bacterium is supported by high-throughput transcriptomic studies, which demonstrated that, dependent on conditions, Hfq can regulate large number (1315) of S. meliloti RNAs including noncoding regulatory small RNAs [13].
Several RIP methods have been used for detecting the association of Hfq with specific RNA [11, 13–17] in cultured bacteria. Although very fruitful to identify and validate the Hfq-RNA association in bacteria grown in free-living state, these methods are unable to accurately detect symbiosis Hfq-RNA association because many symbiosis genes mainly express inside the host tissue [18]. Furthermore, these methods require many bacterial cells which limit their usefulness in root nodules where bacteria persist in a relative small number due to a control imposed by host plants [19]. In addition, most methods developed to recover immunoprecipitated RNA from samples involve multistep extraction using phenol-chloroform extraction and elution procedures. While generally effective, these methods are time consuming and create the potential for RNA loss during each processing step as already discussed by other investigators [20]. However, a RIP procedure for identifying Hfq associated RNA (hereafter Hfq RNA) from root nodule lysates remains to be described.
Previously, we used a RIP procedure to identify Hfq RNA from cultured bacteria [11]. For that RIP experiment, we constructed a broad host range trans-HfqHis production plasmid called p#5 [11] (Fig. 1). The trans produced His-tagged Hfq (HfqHis) is accumulated at their nature levels during different stages of culture growth, allowing Hfq RNA to be isolated in different growth conditions.
Fig. 1.
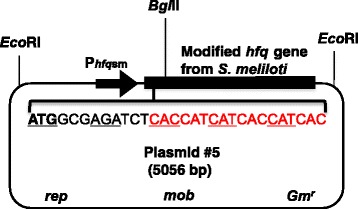
Structure of the plasmid p#5. p#5 contains the modified Hfq gene from S. meliloti with sequence (red) for an affinity His-tag to produce HfqHis. The gene is cloned under the regulation of the native Hfq gene promoter (Phfqsm) [37]. p#5, a derivative of pBBR1MCS-5 [38], also carries a gentamicin resistance gene (Gmr), the broad host range replication (rep) origin and the sequence (mob) allowing for conjugal mobilization
During that work, we realized that the use of the p#5 based RIP procedure could be further extended to assist the rhizobia research for determining S. meliloti RNA-Hfq associations in root nodules if the p#5 is stable in plants and complements Hfq mutants for symbiosis. This idea was initially tested in young root nodules, which revealed fixLJ mRNA as a Hfq RNA [12]. Here, we continue testing the idea and demonstrate that p#5 is stable in plants and complements Hfq mutants for symbiosis. Testing the procedure in matured nodules (49 days post-inoculation) revealed smelA075 as a Hfq RNA. This newly characterized regulatory RNA is conserved in rhizobia and has been proposed to play a role in stress tolerance during the symbiosis [21].
Methods
Reagents, Materials and Equipment
Nuclease-Free Water, DNA-free Kit, and Phosphate-Buffered Saline (PBS) were purchased from Ambion (TX, USA). Cell Extraction Buffer, anti-His were purchased from ThermoFisher Scientific (CA, USA). The SuperScript VILO cDNA Synthesis Kit, Bacto-tryptone Yeast extract and CaCl2 were purchased from Fisher Scientific (NJ, USA). Daynabeads Protein G was purchased from Life technologies (CA, USA). Power SYBR Green PCR Master Mix was purchased from Applied Biosystems (CA, USA). Sonic Dismembrator Model 100 was purchased from Fisher Scientific (CA, USA). ORBIT 1900 was purchased from Labnet International, Inc. (NJ, USA). 18 l Freeze Dry System (LABCONCO) was purchased from Labconco Corporation (MO, USA).
Plant Growth and Inoculation
Cultures of the S. meliloti 1021 [22], Hfq mutant [23], and HfqHis production plasmid /mutant [11] strains were grown to mid-log phase in tryptone yeast (TY) medium [5 g/l Bacto-tryptone, 3 g/l Yeast extract, 6 mM CaCl2 (added after autoclaving), pH = 7.2] [24] and centrifuged. The bacterial pellets were resuspended in an equal volume of water for inoculation onto seedlings. Seeds of M. truncatula A17 from the South Australian Research and Development Institute were surface sterilized with 95% ethanol and 6% hypochlorite, followed by extensive washing with sterile water. The seeds were kept at 4 °C overnight and then transferred to water agar plates. When seedling roots were 1.5 to 2 cm long, the seedlings were transferred to seedling growth pouches (Mega International, MN, USA), which were wetted to saturation with 9 ml of sterile, fourfold-diluted, and N-free Jensen’s medium [CaHPO4 0.1%, K2HPO4, MgSO4 and NaCl each 0.02%, FeCl2 0.01%] [25]. There were four holes, about 1 cm each, made in the punched bottom of the seed trench with sterile forceps and a seedling was carefully inserted through each hole with the root oriented toward the bottom and the cotyledon in the trench. Pouches were kept in an upright position in a box with spacers between sets of 10 to 15 pouches to prevent bending, incubated in a growth chamber (at 24 °C under a cycle of 16 h of light and 8 h of darkness), and restored back to original moisture levels each day with sterile water. When seedling main roots were 7 to 10 cm long (usually after 4 days of growth), seedlings were inoculated along the length of the root with 100 μl of bacterial suspension per seedling. The nodules on the primary root were harvested from plants on 10-, 18-, and 49- days post-inoculation (dpi).
Measuring Shoot Dry Mass
Remove shoots from roots by cutting. Dry shoots in a freeze dryer Labconco 96 h. Let the shoots warm in a dry environment (a Ziploc bag will keep moisture out). Once the shoots have warmed weigh them on a scale (APX-60, d = 0.1 mg, Denver Instrument, Bohemia, NY). Average value of shoot dry mass for bacteria infected plants that differ significantly from the corresponding value for plants inoculated with the rhizobia-free water according to Student’s t test are indicated as follows: *, P < 0.001; **, P < 0.005.
Reverse Transcription (RT)
RNA obtained by heat release were used in RT-PCR mixtures as described [11] with modifications. Thin-walled RT-PCR tubes were used, the reaction volume was scaled down to 10 μl, and reactions were run in a PTC-1148 thermal cycler with a hot bonnet (Bio-Rad Lab., Inc., Hercules, California). Following 10 min of incubation at 25 °C, the cDNA was synthesized at 50 °C for 90 min and heat denaturation of the enzyme at 85 °C for 5 min and hold at 4 °C.
Quantitative PCR (qPCR)
cDNA samples were analysis by qPCR as previously described [12] with primers listed in Table 1. Briefly, 16S rRNA gene was used as internal control because cycle threshold (Ct) values of this gene is similar under several conditions [26]. A qPCR mix contained: 5 μl of SYBR Green Master mix, 1 μl of 5 μM stock solution for each primer, 20 ng of cDNA, and a proper amount of water to bring the total volume to 10 μl. qPCR reactions were run on a StepOnePlus real-time PCR system (Applied Biosystem, Fisher Scientific, NJ, USA). Reaction conditions were: 40 cycles at 95 °C for 15 s, 60 °C for 60 s, and 72 °C for 60 s, followed by a melt curve. RNA samples containing no RT were run as controls to ensure that samples were free from DNA contamination. Melt curve tests did not exhibit a second melting temperature for primer pairs used. qPCR data were processed using StepOne software version 2.2.2.
Table 1.
RT-PCR Primers
| Target | Primer name | Left primer sequence (5′-3′) | Primer name | Right primer sequence (5′-3′) | Reference | |
|---|---|---|---|---|---|---|
| 1 | Smrc7 | MG3462 | GCACTCATACAATGCCGTGA | MG3463 | CTCTTTGAAAGCGGGACAAA | This work |
| 2 | SMrc15 | MG2290 | GGTGCATCTAGCGGCTTTCT | MG2291 | GGGCCCTTTCAGTTGTGAAG | [11] |
| 3 | SMrc16 | MG2292 | CCACCGCAGCAGCTGTT | MG2293 | GGCCCTTGTAGTTGTGAAGGTA | [11] |
| 4 | SMrc45 | MG2294 | TCGATTAGGTGAGGTTATCG | MG2295 | CGGTTGGCCGGAATAGC | [11] |
| 5 | rRNA-16S | AB2777 | GATAAGCCGAGAGGAAGGTG | AB2778 | GTGTAGCCCAGCCCGTAAG | [40] |
| 6 | smelA075 | RJ11 | GTCGAAGCGCTGTACCT | RJ16 | GGGAGGAGGTGGCTCGGGG | This work |
| 7 | Intergenic region between Smc00849-Smc00850 | In850F | ATTTCTTCAATGACGTTCTCGTCA | In849R | ATACGTTCAAATTTTATCAT | [31] |
Results
HfqHis production Complements Hfq Gene Mutations for the Symbiosis
As previously reported [2, 27], and as shown in Fig. 2, inoculation of M. truncatula roots with wild-type S. meliloti induced cylinder-shaped N2 fixing root nodules, which maintained their morphology and function for a period of 49 days following inoculation (Fig. 2d). The nitrogen fixation rescued nitrogen starvation of M. truncatula plants as evidenced by their green-colored leaves (Fig. 2a) and normal shoot dry mass (42.2 ± 0.9 mg per plant, n = 56) (Fig. 2g). By comparison, inoculation of M. truncatula roots with Hfq mutant bacteria carrying p#5, either S. meliloti 8530∆hfq (n = 34, data not shown) or S. meliloti 1021∆hfq, also induced cylinder-shaped nitrogen fixing root nodules (Fig. 2e). The plants had green-colored leaves (Fig. 2b) and their shoot dry mass was normal (40.3 ± 0.6 mg per plant, n = 56) (Fig. 2h). The green leaves and normal shoot mass reflected the normal nitrogen fixation carried out by wild-type S. meliloti. Inoculation of M. truncatula roots with the Hfq mutant bacteria induced small white nodules (Fig. 2f). The plants had yellowish leaves (Fig. 2c) and reduced shoot mass (20.1 ± 0.6 mg per plant, n = 56) which were an indication of nitrogen starvation (Fig. 2i). There were no visible changes on M. truncatula roots that were inoculated with rhizobia-free water (data not shown). The similarities between functions of M. truncatula nodules formed by the Hfq mutant strain carrying p#5 plasmid and by wild-type strain were further compared for p#5 restored nodulin LegHb (leghemoglobin, defined as pink-colored nodules, is required for nitrogen-fixation [28]). In normal M. truncatula nodules, LegHb production is accumulated in the nodules containing nitrogen fixing bacteria as indicated by arrowheads in Fig. 2d and e. The production of LegHb was observed both in nodules formed by wild-type S. meliloti (Fig. 2d) and by Hfq mutants carrying p#5 plasmid (Fig. 2e), but much less in nodules formed by the Hfq mutant (Fig. 2f). Our results demonstrated that S. meliloti Hfq mutant strains carrying the modified Hfq gene complemented Hfq gene mutations for nodule morphology, plant nodulin LegHb production, and the nitrogen fixation.
Fig. 2.
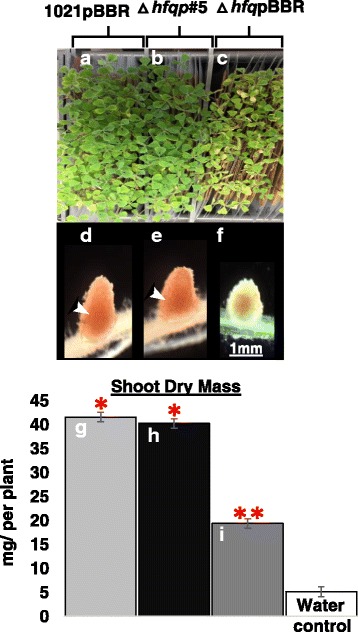
Symbiotic phenotypes of S. meliloti 1021Δhfqp#5. Leaves of plants inoculated with S. meliloti strains a, Rm1021pBBR1MCS-5; b, 1021∆hfqp#5; and c, 1021∆hfq pBBR1MCS-5. d, e and f, Corresponding representative nodules. Arrowheads indicate legHb protein produced in nitrogen fixation zone. g, h, and i, Shoot dry mass mean values of 2 independent cultures are given in mg/plant ± SEM. At least 56 plants were used per culture for each independent culture (n = 56). Average values of shoot dry mass for bacteria infected plants that differ significantly from the corresponding value for plants inoculated with water were determined using Student’s t test and indicated as follows: *, P < 0.001; **, P < 0.005
The HfqHis Production Plasmid P#5 Is Stably Maintained in S. meliloti
The gentamicin is the antibiotic used to select for maintenance of the broad-host-range HfqHis production plasmid p#5, but it is toxic to plants. We had to inoculate plants with bacteria in the absence of the selection for plasmid maintenance. To assess plasmid stability in planta, we crushed nodules and plated nodule bacteria on nonselective media and subsequently screened for the plasmid marker gentamicin (Gmr) by replica plating on the agar containing the antibiotic at the concentration of 50 μg/ml. At the end of the experiment (49-dpi), about 85% of the Hfq mutant bacterial cells retained the p#5 plasmid and only 65% of them retained the parental plasmid pBBR, presumably because of Hfq regulated stress resistance functions [6, 7, 13] which are essential for survival of Hfq mutant bacteria in root nodules.
The P#5 RNA Immunoprecipitation (RIP) Method
We tested the p#5 RIP method by targeting SmrC15 and SmrC16, two previously identified Hfq RNAs of moderate abundance in nodule-associated bacteria [29, 30]. In this test, nodules formed by S. meliloti Hfq mutant bacteria carrying p#5 (named Sm1021∆hfqp#5, [12]) were harvested on the 49-dpi and ground to very thin powder in liquid nitrogen in a mortar with a pestle, waited for liquid nitrogen to evaporate. Then we immediately transferred an aliquot of nodule powder (100 mg) into 1 ml of TRIZOL for total RNA isolation and another aliquot (2 g) for RIP. The latter (2 g of nodule powder) was processed as follows: The sample was suspended in pre-chilled Cell Extraction Buffer (50% [wt/vol] suspension), incubated for 3 min on ice, sonicated with Sonic Dismembrator Model 100 five times (15 s per cycle at 28-30 w [11]). Resulting lysates were incubated at 4 °C for 10 min for homogenization. Cell debris in the lysates were pelleted by centrifugation for 20 min at 4000 x g at 4 °C and then the pellet was discarded. Six-hundred-microliters (600 μl) of cleared lysates were processed by the p#5 RIP method shown in Fig. 3a. To obtain efficient binding anti-His antibody (see Step 1), the cleared lysates containing HfqHis-RNA molecules were first diluted (4:1 [wt/vol]) in PBS (pH = 7.0) to reduce viscosity and then mixed with 10 μg of anti-His antibody. The mixture was incubated on a rotator at 4 °C for 30 min to allow the binding to occur [11, 14], and the resulting material was immunoprecipitated on 1.5 mg IgG beads (see Step 2) by consecutively incubating aliquots of the material with the beads for 10 min at room temperature. Then those beads were washed five times with PBS. Fifty percent (50%) of the bead slurry was frozen for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis after the second wash. The remaining beads were suspended in 20 μl of Ambion nuclease-free water, then diluted (5:1, 1:1 and 1:5 [vol/vol]) for a thorough suspension of beads and for reducing potential inhibitors presented in processed samples therefore to reduce and prevent false-negative results. Samples were heated to 90 °C for 30 s to release RNA [11, 20] (see Step 3), chilled on ice for 2 min, and assayed immediately for RNA by RT-PCR. The amount of total RNA in RT-PCR mix was determined by Bioanalyzer data. A control experiment using lysates of nodules formed by wild-type bacterium (S. meliloti 1021 in which Hfq was not tagged with hexahistidine) was processed in parallel as described before [12, 15]. The oligonucleotide primers used for the RT-PCR were in Table 1. Genomic DNA specific primers In850 and In849 [31] (Table 1) failed to amplify genomic DNA in RNA samples which indicated that genomic DNA in RNA samples was below the level detectable by PCR (Fig. 3b, lane 8). RNA specific primers amplified SmrC15 cDNA (85 nt) (Fig. 3b, lanes 4, 5, and 6) and SmrC16 cDNA (60 nt) (Fig. 3b, lanes 9, 10, and 11) in all the diluted, heat release RNA content of p#5 nodule lysate. Because the amplification worked best for the smallest dilutions [5:1] in both cases of SmrC15 (lane 6) and SmrC16 (lane 11), there were no obvious inhibitions to be reported in those samples. DNA sequencing confirmed the PCR amplified bands (Fig. 3b, lanes 4, 5, and 6) as SmrC15 cDNA and the segment of confirmed sequence was as follow: CCTCCCCAGCCGCTGCAGCAGCTGTT. Also, DNA sequencing confirmed the PCR amplified bands (Fig. 3b, lanes 9, 10, and 11) as Smrc16 cDNA and the segment of the confirmed sequence was as follow: CCTCCCCAGCCGCTGCAGCAGCTGTT. Primers failed to amplify either SmrC15 or SmrC16 cDNA from control sample nodule lysates (Fig. 3b, lanes 1 and 2) which indicated that the His-tag worked effectively. Furthermore, our results were consistent with GUS gene fusions experiment data which showed the accumulation of SmrC15 is stronger than SmrC16 in planta [32]. Taken together, we concluded the followings: First, HfqHis protein bound RNA specifically in M. truncatula nodules. Second, the p#5 RIP method worked properly. We routinely recovered sufficient amounts of HfqHis─anti-His antibody complex from 100 μl of frozen bead slurry to detect HfqHis band by Coomassie staining (Fig. 3c). The clean protein band of HfqHis with size about ~ 11 kDa was further verified by LC-MS (Liquid Chromatography – Mass Spectrometry) analysis, confirming the His-tag at the N-terminus of HfqHis (Fig. 3d).
Fig. 3.
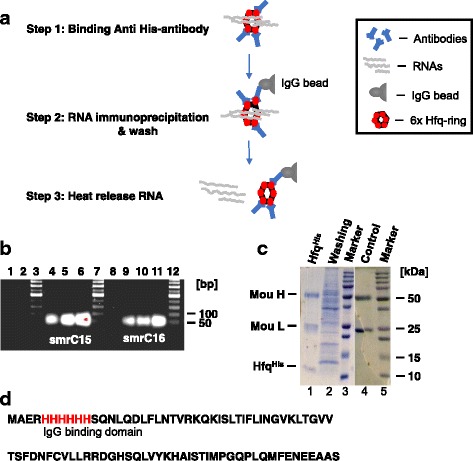
Testing the p#5 method. a An overview of the method. Hfq forms a hexametric ring for action [37] b smrC15 (lanes, 4, 5, and 6) and smrC16 (lanes 9, 10, and 11) RNAs were detected by RT-PCR in the samples heat-released from HfqHis, but not in the negative control samples (lanes 1 and 2). PCR failed to amplify genomic DNA in RNA samples (lane 8) with primers In850F and In849R (Table 1, [31]). c An image of 12% SDS-PAGE gel stained with 1% Coomassie blue R-250. Lane 1: The immunoprecipitated HfqHis from p#5 nodule samples. H and L are heavy and light chains of the mouse anti-His antibody, respectively. Lane 2: Protein contents in the ‘first wash’ of the p#5 nodule sample. Lane 3 and 5: Protein molecular markers to calculate sample molecular weights. Lane 4: Precipitated antibody from the negative control. d The sequence and the position of the His-tag
Using the P#5 RIP Method Discovers New Hfq RNA smelA075
To test the generality of the p#5 RIP method, we targeted the S. meliloti smelA075 [13]. The smelA075 is a stress-induced small regulatory RNA with an uncharacterized relationship with Hfq, although it was seen in 5-week-old M. truncatula nodules [33] and exhibited Hfq responsive accumulation in cells grown in TY medium [23].
The smelA075 RNA was immunoprecipitated specifically with the p#5 procedure from 300 mg of nodule lysate at different nodule stages (10-, 18- and 49- dpi). RT-PCR data showed (Fig. 4a, lanes 2, 3, 4) the existence of smelA075 in RNA-Hfq complexes. Two primers used in PCR experiment, were RJ11 and RJ16 (Table 1). DNA sequencing confirmed the PCR amplified band (Fig. 4a, lane 2) as smelA075 cDNA and the segment of the confirmed sequence was as follow: CCTCCCACGGCGCCCGGCATTCGGT. The primers failed to amplify smelA075 from the control experiment as shown (Fig. 4a, lane 5). Quantitative PCR analysis (Fig. 4b) further indicated that the relative enrichment of smelA075 by the protein was nearly tripled in matured nodules (49-dpi compared to 18-dpi, Fig. 4b) which indicated a tightened regulation of smelA075 by Hfq at the later stages of the symbiosis.
Fig. 4.
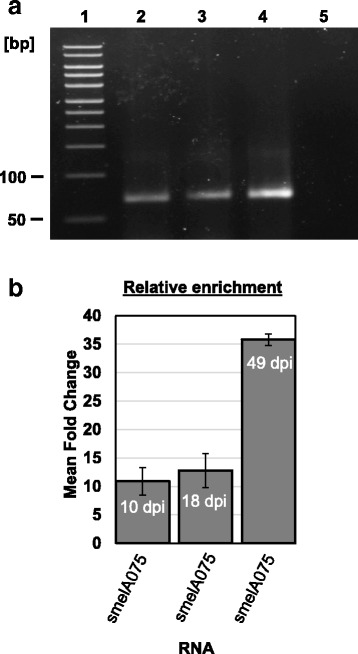
Hfq complexes smelA075 in M. truncatula nodules. a RT-PCR detected smelA075 from Hfq complexes immunoprecipitated from lysates of M. truncatula nodules 10-dpi (lane 2), 18-dpi (lane 3) and 49-dpi (lanes 4), but failed to detect smelA075 from control samples (lane 5). Markers (lane 1). This experiment was repeated twice and similar results were obtained (data not shown). b smelA075 immunoprecipitated with the HfqHis was analyzed by qPCR. Relative enrichments were calculated with the 2-∆∆Ct method [39]. The data are presented as mean fold change ± SEM and normalized to S. meliloti 16S rRNA gene
The fact that small RNA smelA075 shows a broad distribution pattern within the Rhizobiales [21] and carries three anti-Shine Dalgarno (anti-SD) sequences makes smelA075 likely to involve in targeting multiple downstream mRNAs.
Discussion
Specialized RIP methods are required to analyze RNA-protein association during the N2-fixing symbiosis for two main reasons: First, symbiosis RNAs are often nodule-specific. Second, the number of bacteria in root nodules are relatively small compared to the bacteria grown in free-living state due to the host plant control [19]. Those make it difficult to use an in vitro RIP method for an in vivo study. There are very few protocols that work in N2-fixing symbiosis to produce rapid, direct information on RNA-Hfq association, except one [12] that was only tested in early stages of N2-fixation. We now describe a simple RIP method for determining S. meliloti RNA-Hfq association in all stages of N2-fixing symbiosis. The method has the necessary combination of simple procedure, sensitivity and consistent results to be a useful tool for determining S. meliloti RNA-Hfq associations in vivo.
In this in vivo RIP method, 2 g of nodule material can be processed in a short period of time without using phenol extraction. This method permits to reduce potential Hfq RNA lose during the extraction steps. The trans- produced HfqHis protein has a sensitivity for recovering Hfq RNA of moderate abundance (Fig. 3b) and low abundance transcripts under optimal conditions [12]. Heat release was first used in recovery viral RNA from complex stool sample as described by Schwab et al. [20]. We modified Schwab’s method by shortening the time of heat from 5 min to 30 s (at 90 °C) to avoid RNA degradation. We used genome specific primers to detect trace DNA contamination for reducing the risk of false-positive results. We used dilution to reduce potential inhibitors presented in processed samples therefore to reduce false-negative results. DNase1 can be used to remove DNA contamination if necessary. The SuperScript VILO enzyme has optimal reaction temperature of 50-55 °C and can be used in reverse transcription for difficult templates such as small RNAs with secondary structures.
Although this procedure is designed for small scale analysis of the immunoprecipitated RNA from plant and the identity and relative amount of RNA sequence in control and immunoprecipitated samples are determined individually by RT-PCR and by quantitative RT-PCR, the amount of starting material can be scaled up and the RIP method can theoretically be combined with microarray technology or RNA sequencing to identify immunoprecipitated RNAs on a “genome”-wide basis. In fact, high-throughput RIP-chip and RIP-Seq methods have already been reported [34, 35].
Conclusion
This article describes the RIP method for cells of the model symbiotic bacterium, S. meliloti. Hfq is conserved among the nodule forming symbiotic bacteria [36]. Therefore, the p#5 RIP method will have broader applications in study RNA-Hfq associations of Rhizobia-legume symbioses (e. g., Azorhizobium, R. leguminosarum). Some tissue-specific modifications related to sample disruption and homogenization may be needed.
Acknowledgments
This research was supported by a United States Department of Agriculture National Institute of Food and Agriculture grant 2015-67013-22837 to M. Gao. We would also like to thank Tai-Jung Wu and Shanna Xia for their valuable contributions as reviewers and technical assistance with figure/table editing and manuscript formatting.
Funding
This research was supported by a United States Department of Agriculture National Institute of Food and Agriculture grant 2015-67013-22837 to M. Gao and by University of Florida’s Institute of Food and Agricultural Science summer internships to A. Benge and J. Mesa.
Abbreviations
- dpi
Days of Post-Inoculation
- Hfq RNA
RNAs that Associate with Hfq
- HfqHis
His-tagged Hfq Protein
- LC-MS
Liquid Chromatography – Mass Spectrometry
- LegHb
Leghemoglobin
- PCR
Polymerase Chain Reaction
- qPCR
Quantitative PCR
- RIP
RNA Immunoprecipitation
- RT-PCR
Reverse Transcription-PCR
- SDS-PAGE
Sodium Dodecyl Sulfate-polyacrylamide Gel Electrophoresis
- SEM
Standard Error of the Mean
- TY
Tryptone Yeast Medium
Authors’ Contributions
MG designed this project, analyzed data and wrote the manuscript. MG, AB, JM, RJ, FL, conducted the experiments. All authors reviewed and approved the final manuscript.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mengsheng Gao, Email: msgao@ufl.edu.
Anne Benge, Email: abengie88@gmail.com.
Julia M. Mesa, Email: julia.mesa1@gmail.com
Regina Javier, Email: rmjavier12@gmail.com.
Feng-Xia Liu, Email: liufx@cau.edu.cn.
References
- 1.Cooper JB, Long SR. Morphogenetic Rescue of Rhizobium meliloti nodulation mutants by trans-Zeatin secretion. Plant Cell. 1994;6(2):215–225. doi: 10.1105/tpc.6.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long SR. Rhizobium symbiosis: nod factors in perspective. Plant Cell. 1996;8(10):1885–1898. doi: 10.1105/tpc.8.10.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Downie JA. The roles of extracellular proteins, polysaccharides and signals in the interactions of rhizobia with legume roots. FEMS Microbiol Rev. 2010;34(2):150–170. doi: 10.1111/j.1574-6976.2009.00205.x. [DOI] [PubMed] [Google Scholar]
- 4.Graham PH, Vance CP. Legumes: importance and constraints to greater use. Plant Physiol. 2003;131(3):872–877. doi: 10.1104/pp.017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benedito VA, Torres-Jerez I, Murray JD, Andriankaja A, Allen S, Kakar K, Wandrey M, Verdier J, Zuber H, Ott T, et al. A gene expression atlas of the model legume Medicago truncatula. Plant J. 2008;55(3):504–513. doi: 10.1111/j.1365-313X.2008.03519.x. [DOI] [PubMed] [Google Scholar]
- 6.Barra-Bily L, Pandey SP, Trautwetter A, Blanco C, Walker GC. The Sinorhizobium meliloti RNA chaperone Hfq mediates symbiosis of S. meliloti and alfalfa. J Bacteriol. 2010;192(6):1710–1718. doi: 10.1128/JB.01427-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao M, Barnett MJ, Long SR, Teplitski M. Role of the Sinorhizobium meliloti global regulator Hfq in gene regulation and symbiosis. Mol Plant-Microbe Interact. 2010;23(4):355–365. doi: 10.1094/MPMI-23-4-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres-Quesada O, Oruezabal RI, Peregrina A, Jofre E, Lloret J, Rivilla R, Toro N, Jimenez-Zurdo JI. The Sinorhizobium meliloti RNA chaperone Hfq influences central carbon metabolism and the symbiotic interaction with alfalfa. BMC Microbiol. 2010;10:71. doi: 10.1186/1471-2180-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennan RG, Link TM. Hfq structure, function and ligand binding. Curr Opin Microbiol. 2007;10(2):125–133. doi: 10.1016/j.mib.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 10.del Val C, Romero-Zaliz R, Torres-Quesada O, Peregrina A, Toro N, Jimenez-Zurdo JI. A survey of sRNA families in alpha-proteobacteria. RNA Biol. 2012;9(2):119–129. doi: 10.4161/rna.18643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao M, Tang M, Guerich L, Salas-Gonzalez I, Teplitski M. Modulation of Sinorhizobium meliloti quorum sensing by Hfq-mediated post-transcriptional regulation of ExpR. Environ Microbiol Rep. 2015;7(1):148–154. doi: 10.1111/1758-2229.12235. [DOI] [PubMed] [Google Scholar]
- 12.Gao M, Nguyen H, Salas Gonzalez I, Teplitski M. Regulation of fixLJ by Hfq controls symbiotically important genes in Sinorhizobium meliloti. Mol Plant-Microbe Interact. 2016;29(11):844–853. doi: 10.1094/MPMI-09-16-0182-R. [DOI] [PubMed] [Google Scholar]
- 13.Torres-Quesada O, Reinkensmeier J, Schluter JP, Robledo M, Peregrina A, Giegerich R, Toro N, Becker A, Jimenez-Zurdo JI. Genome-wide profiling of Hfq-binding RNAs uncovers extensive post-transcriptional rewiring of major stress response and symbiotic regulons in Sinorhizobium meliloti. RNA Biol. 2014;11(5):563–579. doi: 10.4161/rna.28239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfeiffer V, Sittka A, Tomer R, Tedin K, Brinkmann V, Vogel J. A small non-coding RNA of the invasion gene island (SPI-1) represses outer membrane protein synthesis from the Salmonella core genome. Mol Microbiol. 2007;66(5):1174–1191. doi: 10.1111/j.1365-2958.2007.05991.x. [DOI] [PubMed] [Google Scholar]
- 15.Sittka A, Lucchini S, Papenfort K, Sharma CM, Rolle K, Binnewies TT, Hinton JC, Vogel J. Deep sequencing analysis of small noncoding RNA and mRNA targets of the global post-transcriptional regulator, Hfq. PLoS Genet. 2008;4(8):e1000163. doi: 10.1371/journal.pgen.1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schluter JP, Reinkensmeier J, Daschkey S, Evguenieva-Hackenberg E, Janssen S, Janicke S, Becker JD, Giegerich R, Becker A. A genome-wide survey of sRNAs in the symbiotic nitrogen-fixing alpha-proteobacterium Sinorhizobium meliloti. BMC Genomics. 2010;11:245. doi: 10.1186/1471-2164-11-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dambach M, Irnov I, Winkler WC. Association of RNAs with Bacillus subtilis Hfq. PLoS One. 2013;8(2):e55156. doi: 10.1371/journal.pone.0055156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnett MJ, Fisher RF. Review article: global gene expression in the rhizobial-legume symbiosis. Symbiosis. 2006;42:1–24. [Google Scholar]
- 19.Mergaert P, Uchiumi T, Alunni B, Evanno G, Cheron A, Catrice O, Mausset AE, Barloy-Hubler F, Galibert F, Kondorosi A, et al. Eukaryotic control on bacterial cell cycle and differentiation in the rhizobium-legume symbiosis. Proc Natl Acad Sci U S A. 2006;103(13):5230–5235. doi: 10.1073/pnas.0600912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwab KJ, Estes MK, Neill FH, Atmar RL. Use of heat release and an internal RNA standard control in reverse transcription-PCR detection of Norwalk virus from stool samples. J Clin Microbiol. 1997;35(2):511–514. doi: 10.1128/jcm.35.2.511-514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinkensmeier J, Schluter J-P, Giegerich R, Becker A. Conservation and occurrence of trans-encoded sRNAs in the Rhizobiales. Genes (Basel) 2011;2(4):925–956. doi: 10.3390/genes2040925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galibert F, Finan TM, Long SR, Puhler A, Abola P, Ampe F, Barloy-Hubler F, Barnett MJ, Becker A, Boistard P, et al. The composite genome of the legume symbiont Sinorhizobium meliloti. Science. 2001;293(5530):668–672. doi: 10.1126/science.1060966. [DOI] [PubMed] [Google Scholar]
- 23.Poole P, Ramachandran V, Terpolilli J. Rhizobia: from saprophytes to endosymbionts. Nat Rev Microbiol. 10.1038/nrmicro.2017.171. [DOI] [PubMed]
- 24.Beringer JE. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- 25.Jensen HL. Nitrogen fixation in leguminous plants. I. General characters of root nodule bacteria isolated from species of Medicago and Trifolium in Australia. Proc Linn Soc N S W. 1942;66:98–108. [Google Scholar]
- 26.Becker A, Berges H, Krol E, Bruand C, Ruberg S, Capela D, Lauber E, Meilhoc E, Ampe F, de Bruijn FJ, et al. Global changes in gene expression in Sinorhizobium meliloti 1021 under microoxic and symbiotic conditions. Mol Plant-Microbe Interact. 2004;17(3):292–303. doi: 10.1094/MPMI.2004.17.3.292. [DOI] [PubMed] [Google Scholar]
- 27.Van de Velde W, Guerra JC, De Keyser A, De Rycke R, Rombauts S, Maunoury N, Mergaert P, Kondorosi E, Holsters M, Goormachtig S. Aging in legume symbiosis. A molecular view on nodule senescence in Medicago truncatula. Plant Physiol. 2006;141(2):711–720. doi: 10.1104/pp.106.078691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Downie JA. Legume haemoglobins: symbiotic nitrogen fixation needs bloody nodules. Curr Biol. 2005;15(6):R196–R198. doi: 10.1016/j.cub.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 29.del Val C, Rivas E, Torres-Quesada O, Toro N, Jimenez-Zurdo JI. Identification of differentially expressed small non-coding RNAs in the legume endosymbiont Sinorhizobium meliloti by comparative genomics. Mol Microbiol. 2007;66(5):1080–1091. doi: 10.1111/j.1365-2958.2007.05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voss B, Holscher M, Baumgarth B, Kalbfleisch A, Kaya C, Hess WR, Becker A, Evguenieva-Hackenberg E. Expression of small RNAs in Rhizobiales and protection of a small RNA and its degradation products by Hfq in Sinorhizobium meliloti. Biochem Biophys Res Commun. 2009;390(2):331–336. doi: 10.1016/j.bbrc.2009.09.125. [DOI] [PubMed] [Google Scholar]
- 31.Gao M, Teplitski M. RIVET-a tool for in vivo analysis of symbiotically relevant gene expression in Sinorhizobium meliloti. Mol Plant-Microbe Interact. 2008;21(2):162–170. doi: 10.1094/MPMI-21-2-0162. [DOI] [PubMed] [Google Scholar]
- 32.Torres-Quesada O, Millan V, Nisa-Martinez R, Bardou F, Crespi M, Toro N, Jimenez-Zurdo JI. Independent activity of the homologous small regulatory RNAs AbcR1 and AbcR2 in the legume symbiont Sinorhizobium meliloti. PLoS One. 2013;8(7):e68147. doi: 10.1371/journal.pone.0068147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnett MJ, Toman CJ, Fisher RF, Long SR. A dual-genome Symbiosis Chip for coordinate study of signal exchange and development in a prokaryote-host interaction. Proc Natl Acad Sci U S A. 2004;101(47):16636–16641. doi: 10.1073/pnas.0407269101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erhard F, Dolken L, Zimmer R. RIP-chip enrichment analysis. Bioinformatics. 2013;29(1):77–83. doi: 10.1093/bioinformatics/bts631. [DOI] [PubMed] [Google Scholar]
- 35.Jayaseelan S, Doyle F, Tenenbaum SA. Profiling post-transcriptionally networked mRNA subsets using RIP-Chip and RIP-Seq. Methods. 2014;67(1):13–19. doi: 10.1016/j.ymeth.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaminski PA, Desnoues N, Elmerich C. The expression of nifA in Azorhizobium caulinodans requires a gene product homologous to Escherichia coli HF-I, an RNA-binding protein involved in the replication of phage Q beta RNA. Proc Natl Acad Sci U S A. 1994;91(11):4663–4667. doi: 10.1073/pnas.91.11.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sobrero P, Valverde C. Evidences of autoregulation of hfq expression in Sinorhizobium meliloti strain 2011. Arch Microbiol. 2011;193(9):629–639. doi: 10.1007/s00203-011-0701-1. [DOI] [PubMed] [Google Scholar]
- 38.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, 2nd, Peterson KM. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166(1):175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Marino D, Damiani I, Gucciardo S, Mijangos I, Pauly N, Puppo A. Inhibition of nitrogen fixation in symbiotic Medicago truncatula upon cd exposure is a local process involving leghaemoglobin. J Exp Bot. 2013;64(18):5651–5660. doi: 10.1093/jxb/ert334. [DOI] [PMC free article] [PubMed] [Google Scholar]


