Abstract
Glutamate is a major excitatory neurotransmitter that is stored in vesicles located in the presynaptic terminal. Glutamate is transported into vesicles via the vesicular glutamate transporter (VGLUT). In the present study, the age-associated changes of the major VGLUTs, VGLUT1 and VGLUT2, in the hippocampus were investigated, based on immunohistochemistry and western blot analysis at postnatal month 1 (PM1; adolescent), PM6, PM12 (adult group), PM18 and PM24 (the aged groups). VGLUT1 immunoreactivity was primarily detected in the mossy fibers, Schaffer collaterals and stratum lacunosum-moleculare. By contrast, VGLUT2 immunoreactivity was observed in the granule cell layer and the outer molecular layer of the dentate gyrus, stratum pyramidale, Schaffer collaterals and stratum lacunosum-moleculare in the hippocampal CA1-3 regions. VGLUT1 immunoreactivity and protein levels remained constant across all age groups. However, VGLUT2 immunoreactivity and protein levels decreased in the PM3 group when compared with the PM1 group. VGLUT2 immunoreactivity and protein levels were not altered in the PM12 group; however, they increased in the PM18 group. In addition, in the PM18 group, highly immunoreactive VGLUT2 cells were also identified in the stratum radiatum and oriens of the hippocampal CA1 region. In the PM24 group, VGLUT2 immunoreactivity and protein levels were significantly decreased and were the lowest levels observed amongst the different groups. These results suggested that VGLUT1 may be less susceptible to the aging process; however, the increase of VGLUT2 in the non-pyramidal cells in the PM18 group, and the consequent decrease in VGLUT2, may be closely linked to age-associated memory impairment in the hippocampus.
Keywords: aging, gerbil, hippocampus, vesicular glutamate transporter
Introduction
Glutamate, the principal excitatory neurotransmitter in the brain, is stored in the vesicles of the presynaptic terminal via the membrane-bound vesicular glutamate transporter (VGLUT) (1). Glutamate is released into the synaptic cleft following the fusion of a vesicle with the cell membrane (2); once vesicles are bound to cell membrane VGLUTs, they are emptied of glutamate, which is released into the cell (3). Therefore, the expression level of VGLUTs reflects the amount of glutamate in vesicles (4,5). Three isoforms of VGLUTs have been isolated to date, with VGLUT1 and VGLUT2 being expressed primarily in the hippocampus and cerebral cortex (6). Knockdown of VGLUT1 in mice has resulted in depression-like behavior and impaired long-term recognition memory; however, short-term memory was retained in the novel object recognition test, as well as spatial memory as observed in the Morris water maze test (7). Knockout of VGLUT2 attenuated spatial learning and memory as well as synaptic plasticity in the mouse hippocampus (8).
Glutamate levels, as well as VGLUT1 and VGLUT2 expression, are known to be altered during the aging process (9–13), as well as in Alzheimer's disease (14,15). However, there have been conflicting results regarding the alterations in VGLUT1 and VGLUT2 in the aged hippocampus. For example, VGLUT1 levels are relatively resistant to aging processes, whereas VGLUT2 are more susceptible to the aging process. In an aging model using senescence-accelerated mice (SAMP8), VGLUT1 expression was maintained at several ages compared with age-matched control groups (13). However, in the normal aging model using Wistar rats, VGLUT1 expression was decreased in the hippocampus from PM18 (9). By contrast, VGLUT2 decreased significantly at PM12 in Wistar rats (9) and SAMP8 (13).
Mongolian gerbils are ideal experimental models for studies on aging and epilepsy as they have relatively short lifespans, a homogenous genetic background and are easy to perform behavioral tests on (16,17). The average lifespan of half the population was 110 and 139 weeks in male and female gerbils, respectively (18). Therefore, in the present study, the changes in hippocampal VGLUT1 and VGLUT2 immunoreactivity and protein levels were investigated in Mongolian gerbils of different ages to estimate the changes of glutamate transporting systems in the aged gerbils and to elucidate the association between VGLUT and aging processes in the hippocampus.
Materials and methods
Experimental animals
Male Mongolian gerbils (n=5-; 3-month-old; 50–60 g) were purchased from Japan SLC, Inc. (Shizuoka, Japan). They were housed under standard conditions at a constant temperature (22°C) and humidity (60%), with a 12-h light/dark cycle, and free access to food and water. The handling and care of the animals conformed to guidelines compliant with the current international laws and policies [National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals; NIH Publication no. 85-23, 1985, revised 1996] (19). Ethical approval was obtained from the Institutional Animal Care and Use Committee of Kangwon National University (Chuncheon, Gangwon, Republic of Korea) for all animal procedures in the present study (no. KW-160802-2). Animals were firstly divided into 3 groups, and were then further divided into 5 subgroups (n=10 in each subgroup): Adolescent [postnatal month (PM) 1], adult (PM6 and PM12) and aged (PM18 and PM24) groups. All experiments were conducted with an effort to minimize the number of animals required and the suffering caused by the procedures employed in the present study.
Tissue processing
For histology experiments, the animals (n=5/group) at PM1, 6, 12, 18 and 24 were anesthetized with 1 g/kg urethane by intraperitoneal injection (Sigma-Aldrich; Merck KGaA), then perfused transcardially with 0.1 M of phosphate-buffered saline (PBS; pH 7.4) followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.4). Brains were then removed and postfixed in the same fixative for 12 h at 25°C prior to cryopreservation via overnight storage in 30% sucrose at 4°C. Serial coronal brain sections (30 µm) were generated using a cryostat (Leica Microsystems GmbH, Wetzlar, Germany) at −25°C and maintained in 6-well plates containing PBS until further processing.
Immunohistochemistry
To ensure that the immunohistochemical data were comparable between groups, sections were carefully processed under parallel conditions. Tissue sections located 90 µm apart from each other were selected from within an area between 1.4–2.0 mm posterior to the bregma, as defined by a gerbil atlas (20). Five sections, (30 µm) from each of the tissue sections collected, located 90 µm apart from each other were sequentially incubated with 0.3% hydrogen peroxide (H2O2) in PBS for 30 min and 10% normal goat serum (cat no. S1000; Vector Laboratories, Inc., Burlingame, CA, USA) in 0.05 M PBS for 30 min at 25°C. Sections were then incubated with the guinea pig anti-VGLUT1 antibody (dilution 1:5,000; cat no. AB5905; Chemicon, Temecula, CA, USA) and guinea pig anti-VGLUT2 (dilution 1:10,000; cat no. AB2251; Chemicon) overnight at room temperature. Sections were then incubated with a biotinylated goat anti-guinea pig IgG (1:200; cat no. BA-7000; Vector Laboratories, Inc.) for 2 h at room temperature, followed by a streptavidin-peroxidase complex (ABC kit; cat no. PK-6100; Vector Laboratories, Inc.) for 1 h at room temperature. Immunostaining was visualized via detection with DAB in 0.1 M Tris-HCl buffer (pH 7.2). Sections were then dehydrated in graded ethanol (70, 80, 90, 95, 100, 100 or 70–100%) and mounted on gelatin-coated slides in Canada balsam (Kanto Chemical Co., Inc., Tokyo, Japan).
In order to establish the specificity of the VGLUT1 and VGLUT2 antibodies, the procedure included the omission of the VGLUT1 and VGLUT2 antibodies, goat anti-guinea pig IgG, and the substitution of normal goat serum for the primary antibody. In addition, for positive control test, immunohistochemistry was conducted with VGLUT1 and VGLUT2 antibodies in the cerebellum of gerbils as VGLUT1 and VGLUT2 are specifically detected in cerebellum (21).
Analysis of the hippocampal CA1 and CA2/3 regions, and dentate gyrus was performed using an ImageJ software v.1.5 (NIH, Bethesda, MD, USA). Digital images of the mid-point of each region were captured using a BX51 light microscope (Olympus Corporation, Tokyo, Japan) equipped with a digital camera (DP72; Olympus Corporation) connected to a computer monitor. Images were calibrated into an array of 512×512 pixels corresponding to a tissue area of 1,200×900 µm (magnification, ×100). Each pixel resolution was 256 gray levels, and the area was divided into the strata oriens, pyramidale and radiatum. The intensity of VGLUT1 and VGLUT2 immunoreactivity was evaluated by relative optical density (ROD), which was obtained following transformation of the mean gray level using the formula: ROD=log (256/mean gray level). The ROD of background staining was determined in unlabeled portions of the sections using Photoshop CC 2015 software (Adobe Systems, Inc., San Jose, CA, USA); this value was subtracted to correct for nonspecific staining, using ImageJ v.1.50 software (NIH). Data are expressed as a percentage of the PM1 group values (set to 100%).
Western blot analysis
To confirm the age-associated changes of VGLUT1 and VGLUT2 in the hippocampus, animals at PM1, 6, 12, 18, and 24 (n=5/subgroup) were sacrificed and used for western blot analysis. Following euthanasia and the removal of brains, tissues were cut to 500 µm thick sections using a vibratome (Leica Microsystems GmbH) and the hippocampus was dissected out the using a surgical blade. Hippocampal tissues were homogenized in 50 mM PBS (pH 7.4) at 4°C for 30 sec containing 0.1 mM ethylene glycol bis (2-aminoethyl ether)-N,N,N,N tetraacetic acid (pH 8.0), 0.2% nonidet P-40, 10 mM ethylenediamine tetraacetic acid (pH 8.0), 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF, 150 mM NaCl, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride and 1 mM dithiothreitol (DTT). Following centrifugation for 5 min at 16,000 × g at 4°C, the protein level was determined in the supernatants using a Micro Bicinchoninic Acid protein assay kit with bovine serum albumin as the standard according to the manufacturer's instructions (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Aliquots containing 20 µg of total protein were boiled in loading buffer containing 150 mM Tris (pH 6.8), 3 mM DTT, 6% SDS, 0.3% bromophenol blue and 30% glycerol. Then, each aliquot was loaded onto a 12% polyacrylamide gel. Following electrophoresis, proteins were transferred to nitrocellulose membranes (Pall Corporation, East Hills, NY, USA). The membranes were then incubated with 5% non-fat dry milk in PBS containing 0.1% Tween-20 for 45 min at 25°C, followed by incubation with guinea pig anti-VGLUT1 (dilution 1:10,000; cat no. AB5905; EMD Millipore, Billerica, MA, USA) and guinea pig anti-VGUT2 (dilution 1:20,000; cat no. AB2251; EMD Millipore) for 12 h at 4°C. Then, the membrane was incubated with peroxidase-conjugated anti-guinea pig antibody (1:400; cat no. BA-7000; Vector Laboratories, Inc.). Visualization was performed using an enhanced luminol-based chemiluminescent kit (Pierce; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The blots were scanned and densitometry was performed for the quantification of ROD of each band using Scion Image software (version 4.0.3; Scion Corp., Frederick, MD, USA). These data were normalized against β-actin (dilution 1:500; cat no. ab8229; Abcam, Cambridge, UK).
Statistical analysis
The data are presented as the mean ± standard error mean of triplicate measurement. Differences among the groups were statistically analyzed by one-way analysis of variance followed by a Bonferroni's post hoc test, using GraphPad Prism v5.01 software (GraphPad Software, Inc., La Jolla, CA). P<0.05 was considered to indicate a statistically significant difference.
Results
Antibody specificity
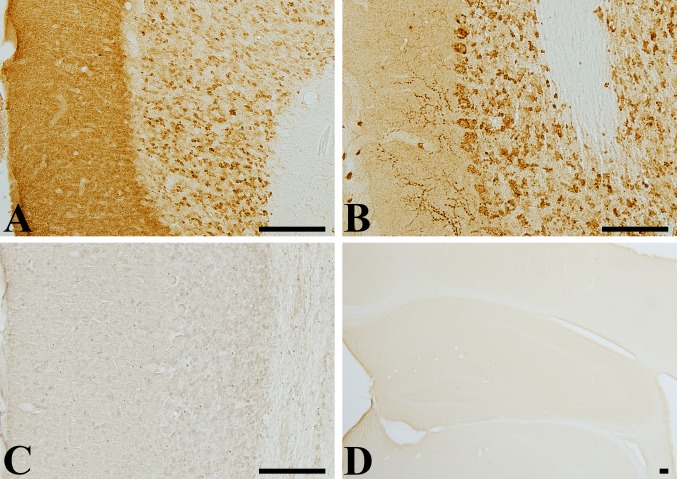
VGLUT1 immunoreactivity was identified in the molecular and granular layer of cerebellum, while VGLUT2 immunoreactivity was mainly detected in the Purkinje cell and granular layer of cerebellum. In the molecular layer, VGLUT1 immunoreactivity was diffusely observed, while VGLUT2 immunoreactivity was found in some coarse fibers (Fig. 1A and B). Negative control test with pre-immune serum did not show any marked staining of VGLUT1 and VGLUT2 in the cerebellum (Fig. 1C and D).
Figure 1.
Immunohistochemistry for (A) VGLUT1 and (B) VGLUT2 in the cerebellum and with pre-immune serum in (C) cerebellum and (D) hippocampus of adult PM6 gerbils. VGLUT1 and VGLUT2 immunoreactivity is found in the molecular and granular layer of cerebellum. Immunostaining with pre-immune serum does not show any immunoreactivities in cerebellum and hippocampus. Scale bar=100 µm.
Age-associated changes in VGLUT1 immunoreactivity
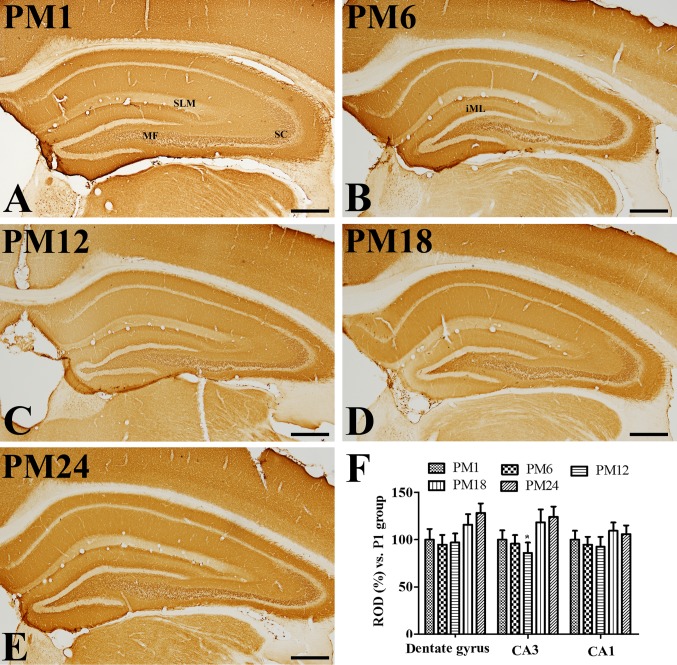
In all groups, VGLUT1 immunoreactivity was observed in the mossy fibers of the dentate gyrus, as well as the Schaffer collaterals and stratum lacunosum-moleculare in the hippocampal CA1 region (Fig. 2A-E). There were some variations in the VGLUT1 expression pattern in the inner molecular layer of the dentate gyrus; VGLUT1 immunoreactivity was clearly visible in the PM6 and PM24 groups (Fig. 2B and E). VGLUT1 immunoreactivity was altered in the dentate gyrus, however, not in the hippocampal CA1-3 regions, with increasing age. VGLUT1 immunoreactivity was markedly increased in the dentate gyrus of the PM18 and PM24 groups when compared with the PM1 group (Fig. 2F).
Figure 2.
Immunohistochemistry for VGLUT1 in the hippocampus of the (A) adolescent PM1, adult (B) PM6 and (C) PM12, and aged (D) PM18 and (E) PM24 groups. VGLUT1 immunoreactivity was observed in the mossy fibers (indicated by MF) of the dentate gyrus as well as the Schaffer collaterals (indicated by SC) and stratum lacunosum-moleculare (indicated by SLM). VGLUT1 immunoreactivity was detected in the inner molecular layer (indicated by iML) in the PM6 and PM24 groups. Scale bar=500 µm. (F) ROD are expressed as a percentage of the value of the VGLUT1 immunoreactivity in the PM1 group in the dentate gyrus, hippocampal CA1 and CA2/3 regions per section of the PM1, PM6, PM12, PM18 and PM24 groups (n=5/group). Data are presented as the mean ± standard error mean. *P<0.05 vs. PM1. GLUT, vesicular glutamate transporter; PM, postnatal month; ROD, relative optical densities.
Age-associated changes in VGLUT2 immunoreactivity
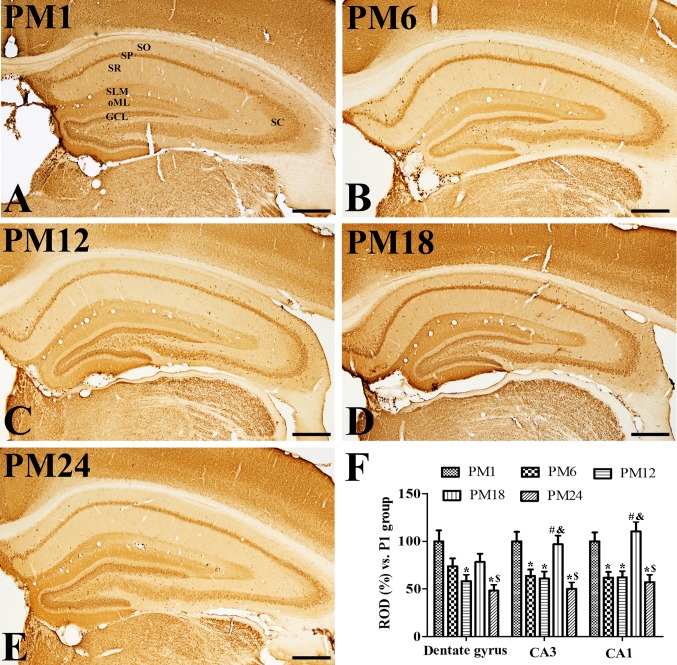
In all groups, VGLUT2 immunoreactivity was observed in the granule cell layer and outer molecular layer of the dentate gyrus. In addition, VGLUT2 immunoreactivity was also detected in the stratum pyramidale, Schaffer collaterals and stratum lacunosum-moleculare in the hippocampal CA1-3 (Fig. 3A-E). VGLUT2 immunoreactivity gradually decreased in the dentate gyrus with age; however, VGLUT2 immunoreactivity in the PM18 group increased significantly in the CA1-3 regions when compared with the PM1 and PM12 groups (Fig. 3F). In addition, VGLUT2 immunoreactivity was also markedly detected in the non-pyramidal cells of the stratum oriens and radiatum of the CA1-3 regions (Fig. 3D). However, VGLUT2 immunoreactivity in the PM24 group was significantly decreased in the hippocampal CA1-3 regions when compared with the PM1 group (Fig. 3E and F).
Figure 3.
Immunohistochemistry for VGLUT2 in the hippocampus of the (A) adolescent PM1, adult (B) PM6 and (C) PM12, and aged (D) PM18 and (E) PM24 groups. VGLUT2 immunoreactivity was observed in the granule cell layer (indicated by GCL) and the outer molecular layer (indicated by oML), stratum pyramidale (indicated by SP), Schaffer collaterals (indicated by SC) and stratum lacunosum-moleculare (indicated by SLM) of the hippocampus. VGLUT2 immunoreactivity as markedly increased in the stratum radiatum (indicated by SR) and oriens (indicated by SO) of the CA1 region in the PM18 group. VGLUT2 immunoreactivity decreased in all layers of the hippocampus in the PM24 group. Scale bar=500 µm. (F) ROD are expressed as a percentage of the value of the VGLUT2 immunoreactivity in the PM1 group in the dentate gyrus, hippocampal CA1 and CA2/3 regions per section of the PM1, PM6, PM12, PM18 and PM24 groups (n=5/group). Data are presented as the mean ± standard error mean. *P<0.05 vs. the PM1 group; #P<0.05, vs. the PM6 group; $P<0.05 vs. the PM12 group; &P<0.05 vs. the PM18 group. VGLUT, vesicular glutamate transporter; PM, postnatal month; ROD, relative optical densities.
Age-associated changes in VGLUT protein levels
VGLUT1 protein levels were higher in the PM6 and PM24 groups when compared with the PM1 group; however, statistically significant differences were not identified between the different groups (Fig. 4). VGLUT2 protein levels increased in the PM18 group when compared with the PM12 group; however, no statistically significant difference was detected. VGLUT2 protein levels were significantly decreased in the PM24 group when compared with the PM1 and PM18 groups (Fig. 4).
Figure 4.
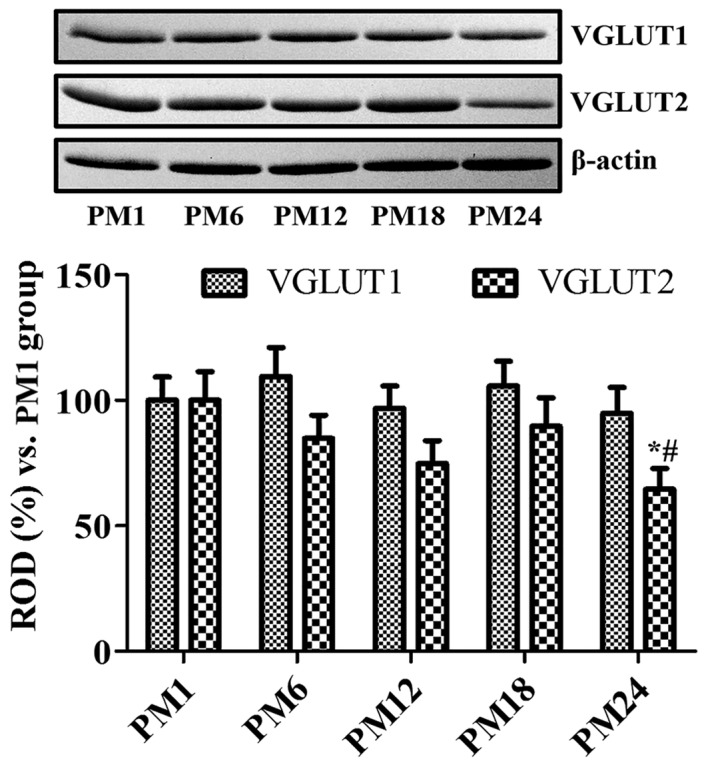
Western blot analysis. Protein levels are expressed as a percentage of VGLUT1 and VGLUT2 protein levels normalized by their respective β-actin protein level in the hippocampal homogenates of the PM1, PM6, PM12, PM18 and PM24 groups (n=5/group). Data are presented as the mean ± standard error mean. *P<0.05 vs. PM1 group; #P<0.05 vs. PM18 group.
Discussion
Glutamate is concentrated in synaptic vesicles by VGLUTs (6), which are specifically required for exocytic release (22,23). In the present study, the specificity of VGLUT1 and VGLUT2 antibodies was tested in the cerebellum of gerbils. Localization of VGLUT1 and VGLUT2 in gerbils was similar to that in the cerebellum of rats (21,24,25). The treatment with pre-immune serum completely blocked the immunohistochemical staining for VGLUT1 and VGLUT2 in the cerebellum and the hippocampus of gerbils.
VGLUT1 was expressed primarily in the mossy fibers of the dentate gyrus, as well as the Schaffer collaterals and stratum lacunosum-moleculare of the hippocampal CA2/3 regions. VGLUT2 was observed in the granule cell layer and outer molecular layer of the dentate gyrus, as well as the stratum pyramidale, Schaffer collaterals and stratum lacunosum-moleculare of the hippocampal CA1-3 regions. These results are consistent with previous studies, in regard to the expression of VGLUT1 and 2 glutamatergic terminals (24,26–29). In the present study, VGLUT1 was relatively resistant to the aging process, except in the dentate gyrus; VGLUT1 immunoreactivity was markedly increased in the outer molecular layer of the dentate gyrus of the PM6 and PM24 groups compared with the PM1 group; however, no significant differences in VGLUT1 protein levels were observed in the hippocampal homogenates. This result is consistent with a previous study using senescence-accelerated mice (SAMP8), in that no significant changes were observed in VGLUT1 in the hippocampus of 2-, 6- and 12-month-old control mice and SAMP8 (10). However, in Wistar rats VGLUT1 expression was decreased in the hippocampus with aging at PM18 (9). The slight increase in VGLUT1 may be associated with mild cognitive impairment as VGLUT1 is upregulated under these conditions (14), and working and reference memory in the gerbil starts to decrease at PM18 (30). In SAMP8 mice, memory impairments, based on a T-maze foot-shock avoidance task, have been reported at 8 to 12 months of age (31).
In the present study, VGLUT2 tended to decrease with age in the hippocampus. This result is consistent with a previous study, which observed that VGLUT2 was seen to constantly decrease with age from 12 months onwards in the hippocampus of Wistar rats (9). In addition, a significant decline in γ-aminobutyric acid (GABA) and glutamate levels have been observed in the hippocampal homogenates of aged (12-month-old) SAMP8 compared with those of the adult (2-month-old) SAMP8 (13). In the present study, morphological evidence has been presented of significant increases in VGLUT2 immunoreactivity in the hippocampal CA1-3 regions of the PM18 group, particularly in the non-pyramidal cells, which then markedly decreased in the hippocampus at PM24. Glutamic acid decarboxylase, a rate-limiting enzyme for GABA synthesis, immunoreactive interneurons in the CA1 region have been demonstrated to be significantly decreased in the hippocampal CA1 region of middle-aged (15–17 months) and old-aged (25–29 months) rats (32). Collectively, these results suggested that the increase in glutamate and decrease in GABA levels may be the cause of cell damage in the non-pyramidal cells of middle-aged animals. In animal models of Alzheimer's disease, glutamatergic and GABAergic presynaptic boutons are increased during the early stages of the amyloid pathology (33,34). Similarly, in humans with mild cognitive impairment, glutamatergic presynaptic bouton density has been shown to increase in the mid-frontal gyrus, while the brains of patients with moderate and severe Alzheimer's disease have exhibited a significant depletion in presynaptic bouton density (14).
In conclusion, VGLUT1 and VGLUT2 are expressed differentially in the hippocampus, and VGLUT1 is relatively resistant to changes induced by the aging process; however, VGLUT2 appears to decrease in the hippocampus with age. The increase in VGLUT2 in the non-pyramidal cells of the PM18 group may be closely associated with the reduction in memory function during the aging process of Mongolian gerbils. This result may be applicable to the development of anti-aging drugs to modulate glutamate and the GABA ratio in the hippocampus in the aging process, and the efficient transport system of glutamate may targeted overcome the decreases in hippocampal functions in the aging process.
Acknowledgements
The authors would like to thank Mr. Seung Uk Lee and Mrs. Hyun Sook Kim for technical help in the present study.
Funding
The present study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant no. NRF-2013R1A1A2059364). The present study was also partially supported by the Research Institute for Veterinary Science, Seoul National University.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Authors contributions
HJ, DY and IH designed the experiments and the study. HJ, DY, JP and JK looked after the animals and performed the morphological experiments. DK conducted western blot analysis. JC, MW and YY participated in designing and discussing the study. HJ and IH wrote this manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The handling and care of the animals conformed to guidelines compliant with the current international laws and policies [National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals; NIH Publication no. 85-23, 1985, revised 1996]. Ethical approval was obtained from the Institutional Animal Care and Use Committee of Kangwon National University (Chuncheon, Gangwon, Republic of Korea) for all animal procedures in the present study (no. KW-160802-2).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Omote H, Moriyama Y. Vesicular neurotransmitter transporters: An approach for studying transporters with purified proteins. Physiology (Bethesda) 2013;28:39–50. doi: 10.1152/physiol.00033.2012. [DOI] [PubMed] [Google Scholar]
- 2.Südhof TC. The synaptic vesicle cycle: A cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 3.Daniels RW, Collins CA, Chen K, Gelfand MV, Featherstone DE, DiAntonio A. A single vesicular glutamate transporter is sufficient to fill a synaptic vesicle. Neuron. 2006;49:11–16. doi: 10.1016/j.neuron.2005.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishikawa T, Sahara Y, Takahashi T. A single packet of transmitter does not saturate postsynaptic glutamate receptors. Neuron. 2002;34:613–621. doi: 10.1016/S0896-6273(02)00692-X. [DOI] [PubMed] [Google Scholar]
- 5.Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size; Proc Natl Acad Sci USA; 2004; pp. 7158–7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Mestikawy S, Wallén-Mackenzie A, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: Vesicular glutamate transporters. Nat Rev Neurosci. 2011;12:204–216. doi: 10.1038/nrn3054. [DOI] [PubMed] [Google Scholar]
- 7.Tordera RM, Totterdell S, Wojcik SM, Brose N, Elizalde N, Lasheras B, Del Rio J. Enhanced anxiety, depressive-like behaviour and impaired recognition memory in mice with reduced expression of the vesicular glutamate transporter 1 (VGLUT1) Eur J Neurosci. 2007;25:281–290. doi: 10.1111/j.1460-9568.2006.05259.x. [DOI] [PubMed] [Google Scholar]
- 8.He H, Mahnke AH, Doyle S, Fan N, Wang CC, Hall BJ, Tang YP, Inglis FM, Chen C, Erickson JD. Neurodevelopmental role for VGLUT2 in pyramidal neuron plasticity, dendritic refinement, and in spatial learning. J Neurosci. 2012;32:15886–15901. doi: 10.1523/JNEUROSCI.4505-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canas PM, Duarte JM, Rodrigues RJ, Köfalvi A, Cunha RA. Modification upon aging of the density of presynaptic modulation systems in the hippocampus. Neurobiol Aging. 2009;30:1877–1884. doi: 10.1016/j.neurobiolaging.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Cheng XR, Yang Y, Zhou WX, Zhang YX. Expression of VGLUTs contributes to degeneration and acquisition of learning and memory. Neurobiol Learn Mem. 2011;95:361–375. doi: 10.1016/j.nlm.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Lin L, Cao B, Xu Z, Sui Y, Chen J, Luan Q, Yang R, Li S, Li KF. In vivo HMRS and lipidomic profiling reveals comprehensive changes of hippocampal metabolism during aging in mice. Biochem Biophys Res Commun. 2016;470:9–14. doi: 10.1016/j.bbrc.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Ménard C, Quirion R, Vigneault E, Bouchard S, Ferland G, El Mestikawy S, Gaudreau P. Glutamate presynaptic vesicular transporter and postsynaptic receptor levels correlate with spatial memory status in aging rat models. Neurobiol Aging. 2015;36:1471–1482. doi: 10.1016/j.neurobiolaging.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Lian K, Han B, Wang Y, Kuo SH, Geng Y, Qiang J, Sun M, Wang M. Age-related alterations in the metabolic profile in the hippocampus of the senescence-accelerated mouse prone 8: A spontaneous Alzheimer's disease mouse model. J Alzheimers Dis. 2014;39:841–848. doi: 10.3233/JAD-131463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell KF, Bennett DA, Cuello AC. Paradoxical upregulation of glutamatergic presynaptic boutons during mild cognitive impairment. J Neurosci. 2007;27:10810–10817. doi: 10.1523/JNEUROSCI.3269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashani A, Lepicard E, Poirel O, Videau C, David JP, Fallet-Bianco C, Simon A, Delacourte A, Giros B, Epelbaum J, et al. Loss of VGLUT1 and VGLUT2 in the prefrontal cortex is correlated with cognitive decline in Alzheimer disease. Neurobiol Aging. 2008;29:1619–1630. doi: 10.1016/j.neurobiolaging.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Buchhalter JR. Animal models of inherited epilepsy. Epilepsia. 1993;34(Suppl 3):S31–S41. doi: 10.1111/j.1528-1167.1993.tb06257.x. [DOI] [PubMed] [Google Scholar]
- 17.Paul LA, Fried I, Watanabe K, Forsythe AB, Scheibel AB. Structural correlates of seizure behavior in the Mongolian gerbil. Science. 1981;213:924–926. doi: 10.1126/science.7256289. [DOI] [PubMed] [Google Scholar]
- 18.Troup GM, Smith GS, Walford RL. Life span, chronologic disease patterns, and age-related changes in relative spleen weights for the mongolian gerbil (Meriones unguiculatus) Exp Gerontol. 1969;4:139–143. doi: 10.1016/0531-5565(69)90001-1. [DOI] [PubMed] [Google Scholar]
- 19.[NRC] National Research Council: Guide for the Care and Use of Laboratory Animals. 7th. National Academy Press; Washington DC: 1996. [Google Scholar]
- 20.Loskota WA, Lomax P, Verity MA. Ann Arbor Science Publishers Inc.; Ann Arbor, MI: 1974. A stereotaxic atlas of the Mongolian Gerbil Brain (Meriones unguiculatus) pp. 70–79. [Google Scholar]
- 21.Hioki H, Fujiyama F, Taki K, Tomioka R, Furuta T, Tamamaki N, Kaneko T. Differential distribution of vesicular glutamate transporters in the rat cerebellar cortex. Neuroscience. 2003;117:1–6. doi: 10.1016/S0306-4522(02)00943-0. [DOI] [PubMed] [Google Scholar]
- 22.Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- 23.Takamori S. VGLUTs: ‘Exciting’ times for glutamatergic research? Neurosci Res. 2006;55:343–351. doi: 10.1016/j.neures.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/S0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- 25.Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004;304:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- 27.Fujiyama F, Furuta T, Kaneko T. Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. J Comp Neurol. 2001;435:379–387. doi: 10.1002/cne.1037. [DOI] [PubMed] [Google Scholar]
- 28.Halasy K, Hajszan T, Kovács EG, Lam TT, Leranth C. Distribution and origin of vesicular glutamate transporter 2-immunoreactive fibers in the rat hippocampus. Hippocampus. 2004;14:908–918. doi: 10.1002/hipo.20006. [DOI] [PubMed] [Google Scholar]
- 29.Herzog E, Takamori S, Jahn R, Brose N, Wojcik SM. Synaptic and vesicular co-localization of the glutamate transporters VGLUT1 and VGLUT2 in the mouse hippocampus. J Neurochem. 2006;99:1011–1018. doi: 10.1111/j.1471-4159.2006.04144.x. [DOI] [PubMed] [Google Scholar]
- 30.Hwang IK, Yoo KY, Jung BK, Cho JH, Kim DH, Kang TC, Kwon YG, Kim YS, Won MH. Correlations between neuronal loss, decrease of memory, and decrease expression of brain-derived neurotrophic factor in the gerbil hippocampus during normal aging. Exp Neurol. 2006;201:75–83. doi: 10.1016/j.expneurol.2006.02.129. [DOI] [PubMed] [Google Scholar]
- 31.Flood JF, Morley JE. Age-related changes in footshock avoidance acquisition and retention in senescence accelerated mouse (SAM) Neurobiol Aging. 1993;14:153–157. doi: 10.1016/0197-4580(93)90091-O. [DOI] [PubMed] [Google Scholar]
- 32.Shi L, Argenta AE, Winseck AK, Brunso-Bechtold JK. Stereological quantification of GAD-67-immunoreactive neurons and boutons in the hippocampus of middle-aged and old Fischer 344 × Brown Norway rats. J Comp Neurol. 2004;478:282–291. doi: 10.1002/cne.20303. [DOI] [PubMed] [Google Scholar]
- 33.Bell KF, de Kort GJ, Steggerda S, Shigemoto R, Ribeiro-da-Silva A, Cuello AC. Structural involvement of the glutamatergic presynaptic boutons in a transgenic mouse model expressing early onset amyloid pathology. Neurosci Lett. 2003;353:143–147. doi: 10.1016/j.neulet.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 34.Hu L, Wong TP, Côté SL, Bell KF, Cuello AC. The impact of Aβ-plaques on cortical cholinergic and non-cholinergic presynaptic boutons in Alzheimer's disease-like transgenic mice. Neuroscience. 2003;121:421–432. doi: 10.1016/S0306-4522(03)00394-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.