Abstract
3-(5′-Hydroxymethyl-2′-furyl)-1-benzylindazole (YC-1), has been demonstrated to inhibit platelet aggregation, vascular contraction and hypoxia-inducible factor 1 activity in vitro and in vivo. The present study investigated the neuroprotective efficacy of YC-1 in cultured neurons exposed to glutamate-induced excitotoxicity and in an animal model of stroke. In a cortical neuronal culture model, YC-1 demonstrated neurotoxicity at a concentration >100 µM, and YC-1 (10–30 µM) achieved potent cytoprotection against glutamate-induced neuronal damage. Additionally, YC-1 (30 µM) effectively attenuated the increase in intracellular Ca2+ levels. Delayed treatment of YC-1 (30 µM) also protected against glutamate-induced neuronal damage and cell swelling in cultured neurons, though only at 4 h post-treatment. In addition, immediate treatment of YC-1 (30 µM) following the exposure of cortical neurons to glutamate (300 µM) produced a marked reduction in intracellular pH. Delayed treatment of YC-1 (25 mg/kg) protected against ischemic brain damage in vivo, though only when administered at 3 h post-insult. Thus, YC-1 exhibited neuroprotection against glutamate-induced neuronal damage and in mice subjected to transient focal cerebral ischemia. This neuroprotection may be mediated via its ability to limit the glutamate-induced excitotoxicity. However, the neuroprotective therapeutic window of YC-1 is only at 3 h in vivo and 4 h in vitro, which may, at least in part, be attributed to its ability to reduce the intracellular pH in the early phase of ischemic stroke. Although YC-1 provided the potential for clinical therapy, the treatment time point must be carefully evaluated following ischemia.
Keywords: 3-(5′-Hydroxymethyl-2′-furyl)-1-benzylindazole, glutamate, intracellular pH, therapeutic window, ischemic stroke
Introduction
Ischemic stroke is an important disease worldwide and a leading cause of mortality and long-term disability (1). Ischemic stroke often results in focal motor weakness, sensory loss, visual damage, impaired speech comprehension and memory disturbances (2). Despite the high incidence of the disease, there remain only a few treatments available, namely thrombolysis and neurosurgery. These are beneficial to only a small proportion of patients with acute stage stroke and thus, more widely applicable therapies are required (1–3).
In the brain, energy is stored mainly in the form of the high energy phosphate compound, adenosine triphosphate (ATP). The normal brain requires complete oxidation of glucose to fulfill its energy requirements (4). However, under ischemic conditions, oxygen depletion forces the brain to switch to anaerobic glycolysis. As a result, the majority of ATP is produced by the glycolytic pathway, converting pyruvate to lactate and hydrogen ions, leading to a gradual decrease in overall brain pH. A stable intracellular pH [(pH)(i)] is critical for normal cellular function, and the majority of biological processes are markedly sensitive to pH. Even small changes in (pH)(i) affect the properties of various ion channels, plasma membrane excitability, and cellular metabolism (5–7). It has been proposed that this drop in pH results in acidotoxicity, which contributes to neuronal injury following cerebral ischemia (8).
Under ischemic conditions, plasma membrane depolarization leads to an increased synaptic release of glutamate. This released glutamate can cause an excessive influx of Na+ through glutamate-gated ion channels, leading to the subsequent influx of CI− and H2O, which results in plasma membrane rupture and further release of cytoplasmic glutamate into the extracellular space (9). Excessive glutamate overactivates glutamate receptors, specifically N-methyl-D-aspartate receptor (NMDAR), causing high levels of calcium ions (Ca2+) to influx into the postsynaptic cell and increase intracellular calcium [(Ca2+)(i)] (10). NMDAR overactivation disrupts antioxidant defenses and critical survival pathways, which increases the susceptibility of neurons and glia to ischemic damage, and also triggers numerous ischemic cascades, leading to further neuronal degeneration, or in some cases mortality. Therefore, improving neuronal defenses against glutamate-induced excitotoxicity and/or decreasing the (Ca2+)(i) influx into the ischemic neurons is crucial following ischemic stroke (9,11).
3-(5′-Hydroxymethyl-2′-furyl)-1-benzylindazole (YC-1) is a nitric oxide (NO)-independent and direct activator of soluble guanylyl cyclase, and a hypoxia-inducible factor-1 inhibitor. YC-1 has been demonstrated to downregulate vascular endothelial growth factor, erythropoietin, endothelin-1 and inducible NO synthase signaling and protein levels (12,13). YC-1 can downregulate matrix metalloproteinase-9 protein levels in the human retina and protect blood-brain-barrier permeability following ischemia/reperfusion-induced injury in rats (14).
The present study assessed YC-1-mediated neuroprotection and its therapeutic window in an in vitro model of a cultured neurons exposed to glutamate-induced excitotoxicity, and further determined the crucial therapeutic window using intraperitoneal administration of YC-1 in a mouse model of transient focal cerebral ischemia.
Materials and methods
Animal preparation
A total of 74 2-day-old Sprague-Dawley rats and 43 mice (20–26 g) (all female) were supplied by the National Cheng Kung University Laboratory Animal Center and were allowed free access to food and water. Unless otherwise indicated, mice were housed individually in static microisolation caging (45.5×23×20.5 cm). Cages were maintained in temperature-controlled chambers (29±1°C) on a 12-h light/dark cycle. For in vitro experiments of cell viability, cell swelling, (Ca2+)(i) and (pH)(i), 74 rats underwent cortical isolation for primary cultured neurons. For in vivo experiments of brain infraction, 43 mice were used for transient middle cerebral artery (MCA) occlusion.
The present study received affidavits of ethical approval for the use of animals in the present study; ethical approval was provided by National Cheng Kung University (Tainan, Taiwan, R.O.C.). An affidavit of approval for animal use protocols was obtained for in vivo experiments (approval no. 97175) and in vitro primary cortical neuron culture experiments (approval no. 103177).
Primary cortical neuronal culture
According the method described previously (11,15), cultured neurons were obtained from the cerebral cortices of 1-day-old Sprague-Dawley rats. Following animal sacrifice, the cortices were excised in ice-cold Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Tissue samples were minced and then incubated with Hank's Balanced Salt Solution (HBSS; Gibco; Thermo Fisher Scientific, Inc.) containing papain (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and DNase I (Bionovas Biotechnology Co., Ltd., Toronto, Canada) at 37°C to dissociate the cells. Following 30 min incubation, heat-inactivated fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) was added to the cell suspensions to terminate the reaction and the suspensions were then centrifuged at 800 × g for 10 min at 4°C. The pellets were collected and resuspended in DMEM with 10% FBS and then filtrated with a 70 µm cell strainer (BD Biosciences, Franklin Lakes, NJ, USA). Dissociated cells were plated onto poly-D-lysine-coated petri dishes and incubated at 37°C in a humidified incubator with 5% CO2. Then, 4 h following plating, the culture medium was replaced by neurobasal medium containing 25 µM glutamate, 0.5 mM L-glutamine and 2% B27 supplement (Invitrogen; Thermo Fisher Scientific, Inc.). Culture medium was changed every 3 days and the cultured neurons were allowed to grow for ~7–14 days.
Neurotoxicity of YC-1
Neurons were incubated with YC-1 (3–300 µM) or vehicle (0.1% DMSO) at 37°C overnight. Neuronal damage was assessed by densitometric measurements of the cellular propidium iodide (PI; Thermo Fisher Scientific, Inc.) uptake following 24 h. PI was solubilized in water to a final concentration of 50 µg/ml and stored in aliquots at −20°C for a maximum of 3 months. Cells were washed 3 times in PBS and then stained by the addition of 1 ml PI (50 µg/ml) to each dish for 10 min in the dark at room temperature. PI positive cell and total cell numbers were measured on an Olympus IX71 inverted phase/fluorescence microscope (Olympus Corporation, Tokyo, Japan), equipped with a cooled charge-couple device camera (300T-RC; Dage-MTI, Inc., Michigan City, IN, USA). PI %=[(PI positive cell numbers)/(total cell numbers)] × 100.
Glutamate-induced cell cytotoxicity
Cells were administered the following experimental treatments: i) Pre-treatment group were administered YC-1 (1–100 µM) or vehicle (0.1% DMSO) for 30 min and then exposed to glutamate (300 mM) for 24 h; ii) post-treatment group in which YC-1 (30 µM) or vehicle (0.1% DMSO) was added to the culture medium at 0, 2, 4 or 6 h following glutamate exposure. The neuronal cytotoxicity was determined at 24 h following treatment using a PI assay kit (Thermo Fisher Scientific, Inc.). PI positive cell and total cell numbers were measured on an Olympus IX71 inverted phase/fluorescence microscope, equipped with a cooled charge-couple device camera as above. PI %=[(PI positive cell numbers)/(total cell numbers)] × 100.
Cell swelling measurements
The experimental treatments were divided into four groups: i) Vehicle (0.1% DMSO); ii) glutamate-treated group; iii) delayed treatment, YC-1 at 2 h following glutamate exposure; and, iv) delayed treatment, YC-1 at 4 h following glutamate exposure. The glutamate (300 mM)-induced neuronal morphological changes were measured by time-lapse imaging techniques on an Olympus IX71 inverted microscope equipped with a thermo-controllable heating stage, differential interference contrast (DIC) and an image analyzer (MCID Elite 6.0 Rev. 1.4, Imaging Research Inc., St. Catharines, ON, Canada) by the method described previously (11,16). In each microscopic field ~6 to 12 neurons per culture were measured and compared over time. Data are expressed as a percentage relative to the baseline values.
(Ca2+)(i) measurement
The level of (Ca2+)(i) was measured on a single cell fluorimeter, as previously described (11). Briefly, neuronal cultures were incubated with 3 µM Fura 2-Acetoxymethylester (Fura-2 AM; Invitrogen; Thermo Fisher Scientific, Inc.) and 10 µM ionomycin in a standard buffer (composition in mM: NaCl, 140; KCl, 3.5; KH2PO4, 0.4; Na2HPO4, 1.25; CaCl2, 2.2; MgSO4, 2; glucose, 10; HEPES, 10, pH 7.3) for 30 min, followed by incubation in dye-free standard buffer for 30 min and then the addition of vehicle or YC-1 (30 µM) for 20 min, and exposure to glutamate (300 µM) all at 37°C. The glass coverslip was placed into the stage chamber of an Olympus IX71 inverted microscope, equipped with a 75 W xenon illumination system, a cooled charge-couple device (CCD) camera (300T-RC; Dage-MTI, Inc., Michigan City, IN, USA) coupled to an image intensifier (Gen II S-25 image intensifier; Dage-MTI, Inc.), a Lambda 10-2 filter-wheel and shutter (Sutter Instruments, Novato, CA, USA) and a computerized image analyzer (MCID Elite 6.0 Rev. 1.4; Imaging Research Inc.). The cells were alternatively illuminated with light at 340 and 380 nm wavelengths, and the emitted light was passed through a 510-nm barrier filter. The 340 and 380 nm images were captured at 6 sec intervals, and the ratio signals (340 nm excited image/380 nm excited image) were processed and examined for changes in (Ca2+)(i). A total of ~10 neurons in the microscope field were individually measured.
(pH)(i) measurement
According to the method described previously (17), the fluorescent pH indicator dye, 2′,7′-Bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF; Invitrogen; Thermo Fisher Scientific, Inc.) was loaded into cells. Following 30 min incubation in 5 µM BCECF-AM (acetoxymethyl), the glass coverslip was placed into the stage chamber of an Olympus IX71 inverted microscope, equipped with a 75 W xenon illumination system, a cooled CCD camera (300T-RC; Dage-MTI, Inc.) coupled to an image intensifier (Gen II S-25 image intensifier; Dage-MTI, Inc.), a Lambda 10-2 filter-wheel and shutter (Sutter Instruments) and a computerized image analyzer (MCID Elite 6.0 Rev. 1.4, Imaging Research Inc.). The cells were alternatively illuminated with light at 440 and 490 nm wavelengths, and the emitted light was passed through a 535-nm barrier filter. The 440 and 490 nm images were captured at 6 sec intervals and the ratio signals (490 nm excited image/440 nm excited image) were processed and examined for changes in (pH)(i). A total of ~10 neurons in the microscopic field were individually measured. pH calibrations using BCECF measurement were performed by incubating cells with nigericin, a K+/H+ ionophore. Cells were washed with HBSS buffer (nigericin 1 µM, KCl 140 mM and MgCl2 1 mM) 3 times and cells were then incubated with HBSS buffer at pH 6, 6.5, 7, 7.5 and 8. The (pH)(i) level was calculated using the following equation: (pH)(i)=pKa + log [(R-Rmin)/(Rmax-R)] × [F440 min/F440 max)], where R is the ratio of fluorescence intensity recorded at 490 and 440 nm, and Rmin and Rmax are the ratios of 490/440 nm fluorescence intensity recorded at pH 6 and pH 8. pKa was converted by known pH concentrations of HBSS buffer.
MCA model and drug administration
Adult male C57 black (C57BL/B6) mice, (n=43, weight 20–26 g, 6–8 weeks old) were housed at 24±1°C, 60% humidity with a 12-h light/dark cycle and free access to food and water prior to and following surgery. For the surgical procedures performed on mice, 1–2% halothane in N2O:O2 (70:30%) was used for anesthesia (3–4% used for induction and 1–2% for maintenance). A precision vaporizer, adequate ventilation and scavenging were employed.
Focal cerebral ischemia was employed by intra-arterial suture occlusion of the proximal right MCA according to the method described previously (18). Briefly, the external carotid artery (ECA), internal carotid artery (ICA) and pterygopalatine artery of the ICA were exposed under an operating microscope. A silicone rubber coated nylon suture was inserted into the ICA via a slit in the ECA. The suture was advanced 7.5–8.5 mm along the ICA until the tip occluded the origin of the MCA. Reperfusion was produced by gently removed the suture and the incision closed following 1 h of MCA occlusion. Following the surgical procedures, the animals were kept in a cage with a heating lamp, monitored for 2 h and then transferred back into their original cages (ambient temperature, ~24±1°C).
YC-1 was dissolved in a mixture of polyethylene glycol (PEG) 400 (Sigma-Aldrich; Merck KGaA) and saline (PEG 400:saline=3:7). Animals were administered an intraperitoneal injection of either YC-1 (25 mg/kg) or vehicle (PEG 400:saline=3:7) at 1, 2, 3, or 4 h following MCA occlusion to test the window of opportunity. Brain infarctions were examined 48 h following ischemia reperfusion.
Animal sacrifice and quantification of ischemic brain damage
All assessments were based on the method described previously (18). At 48 h, sacrifice was performed on the mice under anesthesia via transcardial perfusion with 4% formaldehyde in 0.1 M PBS following the ischemia-reperfusion insult. Following fixation via transcardial perfusion with ice-cold 4% formaldehyde in 0.1 M PBS and dehydration, the brains were embedded in Optimal Cutting Temperature compound (OCT; Miles Inc., Elkhart, IN, USA) and frozen in liquid nitrogen. The brains were sectioned (40 µm) at 8 preselected coronal levels on a cryostat (HM-500O; Microm International GmbH, Walldorf, Germany). The sections were mounted on poly-l-lysine-coated slides (Sigma-Aldrich; Merck KGaA), dried at 37°C overnight, and were then stored at −20°C.
The 40-µm brain sections were stained with 0.5% Cresyl violet for 4 h at room temperature. Under light microscopy, the areas with neuronal perikarya displaying typical morphological features of ischemic damage were delineated using a computerized image analyzer (MCID Elite 6.0 Rev. 1.4; Imaging Research Inc.). The number of fields of view analyzed were: Control group, 10; YC-1 treated after 1 h of ischemia, 10; YC-1 treated after 2 h of ischemia, 7; YC-1 treated after 3 h of ischemia, 9; and, YC-1 treated after 4 h of ischemia 7. Infarct volumes were measured and expressed as a percentage of the contralateral hemisphere volume using the following equation: (Contralateral hemisphere area-ipsilateral non-ischemic hemisphere area)/contralateral hemisphere area. The ipsilateral brain edema was expressed as a percentage index relative to the volume of the left hemisphere [(ischemic hemisphere area/contralateral hemisphere area)-infarct volume].
Statistical analysis
All data were expressed as the mean ± standard deviation of 3 repeated experiments. Unpaired Student's t-test or one-way analysis of variance with a least significant difference post hoc test were used to evaluate differences between groups. P<0.05 was considered to indicate a statistically significant difference.
Results
YC-1 reduces glutamate-induced cell death
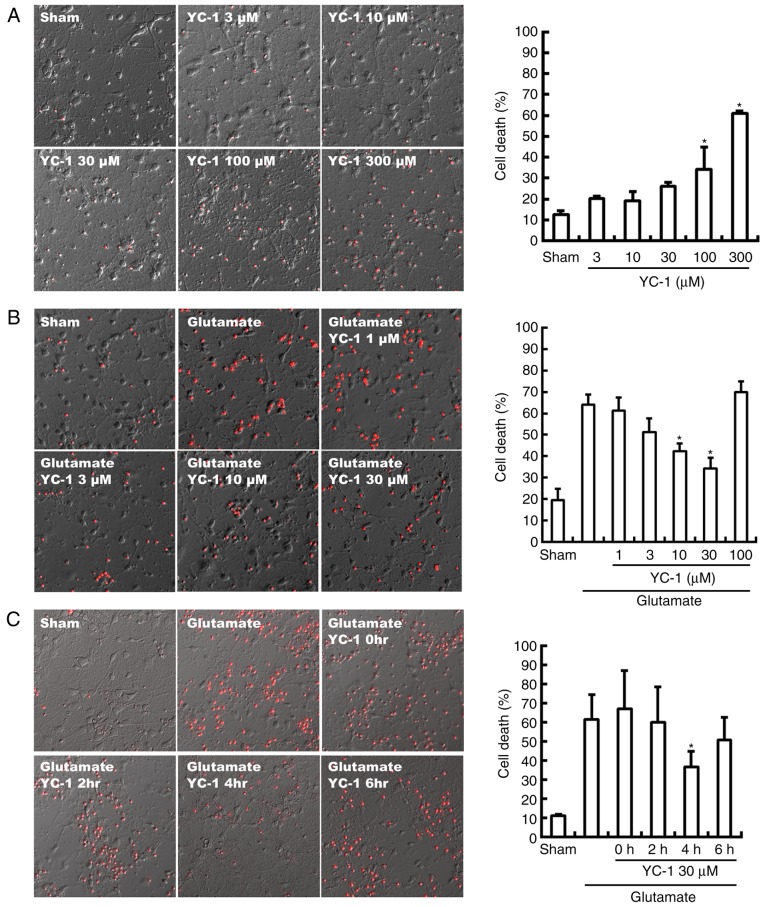
Neurotoxicity of YC-1 was observed with a concentration beyond 100 µM, compared with sham group (Fig. 1A; P<0.05). Conversely, the glutamate-induced neurotoxicity was significantly attenuated by YC-1 at 10–30 µM, compared with the glutamate only group (Fig. 1B; P<0.05). Delayed treatment with YC-1 (30 µM) at 4 h, though not at 2 or 6 h, significantly reduced glutamate-induced cell death, compared with the glutamate group (Fig. 1C; P<0.05).
Figure 1.
YC-1 reduced glutamate induced cell death. (A) Differential interference contrast photomicrographs demonstrated significant YC-1 neurotoxicity at a concentration >100 mM (n=4/group; magnification, ×200). (B) YC-1 (10–30 µM) achieved potent cytoprotection against glutamate-induced neuronal damage (n=4/group; magnification, ×200). (C) Delayed treatment of YC-1 (30 µM) at 4 h, though not 2 or 6 h, significantly reduced glutamate induced cell deaths (n=4/group; magnification, ×200), compared with controls. *P<0.05 vs. controls. YC-1, 3-(5′-Hydroxymethyl-2′-furyl)-1-benzylindazole; n, number of rats.
Delayed YC-1 treatment at 4 h attenuates glutamate-induced cell swelling in cultured neurons
The cultured neurons were exposed to 300 µM glutamate and then delayed treatment with 30 µM YC-1 at 2 or 4 h (Fig. 2A). Time-course DIC photomicrographs of cultured neurons demonstrated that only delayed treatment with 30 µM YC-1 at 4 h significantly attenuated the glutamate-induced cell swelling over time (Fig. 2B; P<0.05).
Figure 2.
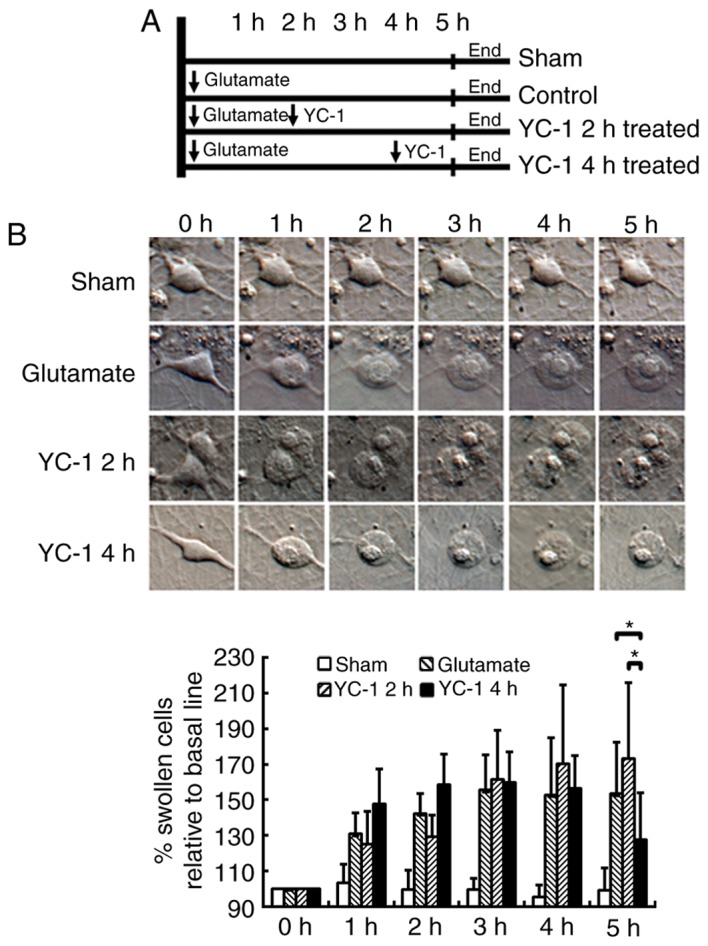
YC-1 attenuated glutamate-induced cell swelling in cultured neurons. (A) Schematic diagram of grouping and the different treatments applied. (B) Time-course differential interference contrast photomicrographs of cultured neurons demonstrated that delayed treatment with YC-1 (30 µM) at 4 h only significantly attenuated the glutamate-induced cell swelling over time when compared with controls, and delayed treatment of YC-1 at 2 h (n=9/group; magnification, ×400). *P<0.05, as indicated. YC-1, 3-(5′-Hydroxymethyl-2′-furyl)-1-benzylindazole; n, number of rats.
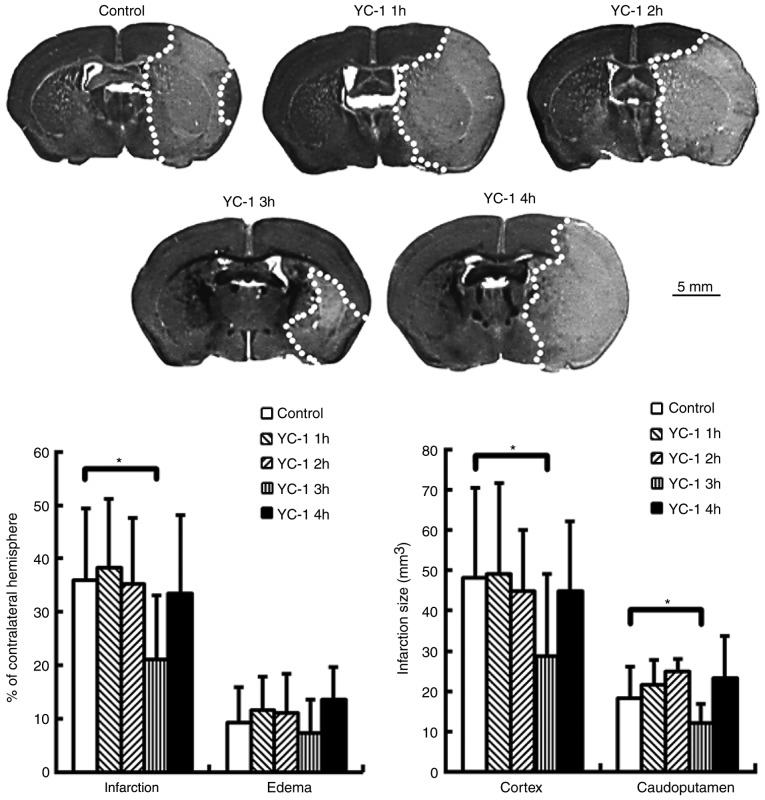
YC-1 reduces brain infarction in mice subjected to transient MCA occlusion
Adult mice were subjected to transient MCA occlusion. Compared with controls, delayed treatment with YC-1 (25 mg/kg) at 3 h, though not at 1, 2 or 4 h, significantly reduced brain infarction at 24 h post-insult (Fig. 3; P<0.05). The infarct volumes are expressed as a percentage of the contralateral hemisphere.
Figure 3.
YC-1 reduced brain infarction in mice subjected to transient middle cerebral artery occlusion. Delayed treatment of YC-1 (25 mg/kg) at 3 h only, significantly reduced brain infarction when compared with controls. The infarct volumes are expressed as a percentage of the contralateral hemisphere (control n=10; YC-1 1 h, n=10; YC-1 2 h, n=7; YC-1 3 h, n=9; YC-1 4 h, n=7). *P<0.05 vs. control (polyethylene glycol 400-treated). YC-1, 3-(5′-Hydroxymethyl-2′-furyl)-1-benzylindazole; n, number of mice.
YC-1 attenuates the glutamate-induced rise in (Ca2+)(i) in cultured neurons
Ratio image detection for (Ca2+)(i) concentrations demonstrated that adding 300 µM glutamate induced an abrupt rise in (Ca2+)(i) levels to ≥1,000 nM in the control group. Compared with controls, treatment with 30 µM YC-1 effectively inhibited this glutamate-induced rise in (Ca2+)(i) (Fig. 4; P<0.05).
Figure 4.
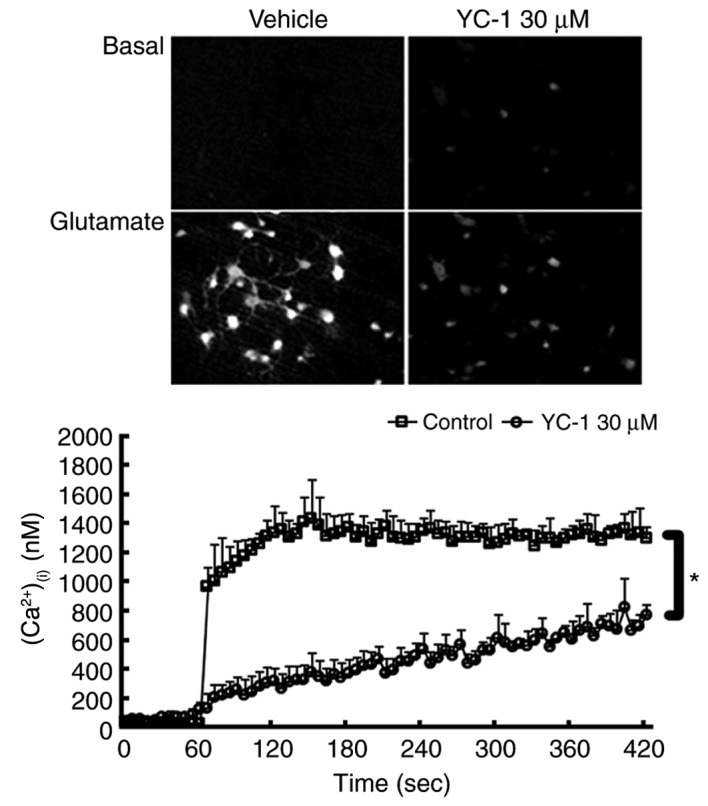
YC-1 attenuated glutamate-induced a rise in (Ca2+)(i) influx in cultured neurons. Ratio image detection for (Ca2+)(i) concentrations demonstrated that treatment with 30 µM YC-1 effectively inhibited the rise in (Ca2+)(i) induced by glutamate exposure. *P<0.05, as indicated (n=4/group; magnification, ×200). YC-1, 3-(5′-Hydroxymethyl-2′-furyl)-1-benzylindazole; (Ca2+)(i), intracellular calcium; n, number of rats.
Immediate treatment with YC-1 following glutamate exposure causes a marked change in (pH)(i)
BCECF loaded single cortical neurons exposed to 300 µM glutamate then immediately treated with YC-1 (30 µM), demonstrated a marked reduction in (pH)(i), when compared with glutamate only, YC-1 only and delayed treatment with YC-1 at 2 and 4 h (Fig. 5; P<0.05).
Figure 5.
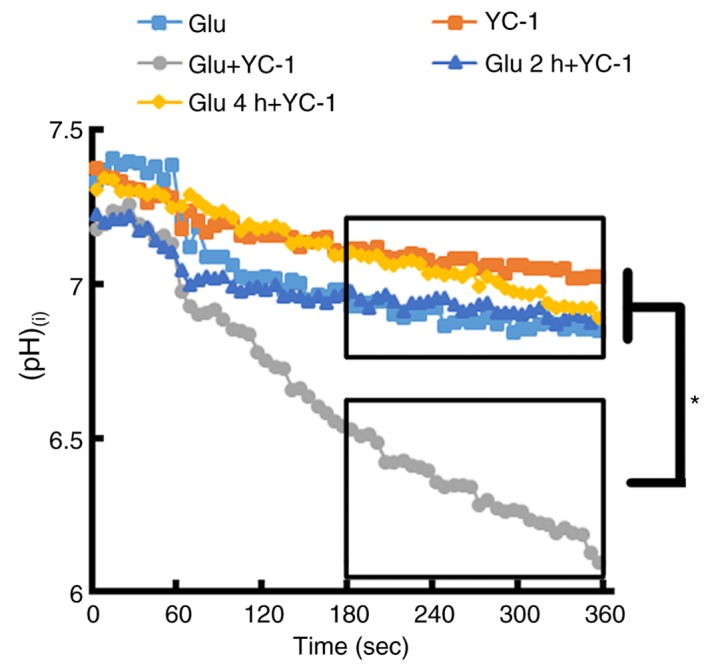
BCECF-loaded single cortical neurons were exposed to 300 µM Glu. Following the exposure of cortical neurons to 300 µM Glu, immediate treatment with YC-1 (30 µM) demonstrated a marked reduction in (pH)(i) when compared with Glu only, YC-1 only, and delayed treatment with YC-1 at 2 and 4 h. (Glu n=11; YC-1 n=10; Glu+YC-1 n=9; Glu 2 h+YC-1 n=9; Glu 4 h+YC-1 n=10). *P<0.05, as indicated. BCECF, 2′,7′-Bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein; YC-1, 3-(5′-Hydroxymethyl-2′-furyl)-1-benzylindazole; Glu, glutamate; (pH)(i), intracellular pH; n, number of rats.
Discussion
The results of the present study indicated that YC-1 demonstrated neurotoxicity at a concentration >100, and 10–30 µM YC-1 achieved potent cytoprotection against glutamate-induced neuronal damage. Additionally, 30 µM YC-1 effectively attenuated the rise in (Ca2+)(i) levels in cultured neurons exposed to glutamate. However, only the cultured neurons administered YC-1 (30 µM) at 4 h following exposure to glutamate demonstrated protection against glutamate-induced neuronal damage and cell swelling. Immediate treatment of YC-1 (30 µM) following the exposure of cortical neurons to glutamate induced a marked reduction in the (pH)(i). In this animal stroke model, only delayed treatment of YC-1 at 3 h post-insult had neuroprotective effects in mice subjected to transient focal cerebral ischemia.
Hypoxia-ischemia increases extracellular glutamate resulting in the cytotoxicity of neurons. Excessive glutamate will stimulate NMDARs and increase the influx of (Ca2+)(i) (11,19). Excessive Ca2+ influx overwhelms the compensatory mechanisms of neurons, causing osmotic volume expansion (swelling). If the impact is limited and small, it may be reversible if appropriate therapeutic actions are taken (20). A previous study demonstrated that pretreatment with 3–30 µM YC-1 could decrease the damaging effect of glutamate on PC12 cells, a cell line derived from a rat with adrenal medulla pheochromocytoma (21). In addition, treatment with 10–50 µM YC-1 to neutrophils stimulated with cyclopiazonic acid produced a concentration-dependent reduction of (Ca2+)(i) (21). In agreement with this previous report (21), the present study demonstrated that YC-1 (10–30 µM) also effectively reduced glutamate-induced neuronal damage in primary cortical neurons. YC-1 (30 µM) also effectively attenuated the rise in (Ca2+)(i) levels in cultured neurons exposed to glutamate. These results indicated that the neuroprotection produced by YC-1 may be mediated via its ability to limit glutamate-induced excitotoxicity.
Pretreatment with YC-1 at 10–30 µM could protect neurons against glutamate-induced neurotoxicity. The results of the present study identified that pretreatment with YC-1 at 30 µM attenuated the glutamate-induced rise in (Ca2+)(i). Pretreatment with YC-1 produced a neuroprotective effect, potentially due to the neurons remaining intact at the time of treatment, and mediated the decrease in (Ca2+)(i) escalation following ischemia. However, post-treatment with YC-1 at 30 µM was protective only when added to the medium at 4 h, though not at 2 h. Similar results were observed in the cell swelling experiment. Post-treatment with YC-1 at 30 µM reduced glutamate-induced cell swelling when added to the medium at 4 h, though not at 2 h. Thus, these data indicated that YC-1 was neurotoxic, potentially due to its effect on aggravating post-ischemic cell swelling and pH within 4 h of ischemia. Conversely, these data clearly indicated that this mechanistic effect diminished following 4 h of ischemia. Therefore, YC-1 regained its neuroprotective effects following 4 h of ischemia.
Immediate treatment with YC-1 at 30 µM demonstrated a marked reduction of (pH)(i) following stroke. The (pH)(i) data from the present study also indicated that YC-1 was neurotoxic to intraischemic cells immediately post-insult, this may be due to its effect in further decreasing (pH)(i). In addition, ischemia induced cellular damage with decreased (pH)(i) up to 4 h. Therefore, post-treatment with YC-1 may aggravate neuronal damage within 4 h of ischemia. Thus, acidosis may serve a critical role in the pathogenesis of brain ischemia. This may also explain how post-treatment with YC-1 at 30 µM protected neurons against glutamate-induced neurotoxicity and cellular swelling when added to the medium at 4 h, though not at 2 h. Although the optimal time point was slightly different between the in vitro (4 h) and the in vivo (3 h) data, the possibility that the in vitro data may not be fully translated to in vivo data cannot be excluded.
Ischemia increased extracellular glutamate resulting in excitotoxic damage and an increased influx of (Ca2+)(i). Although YC-1 could reduce glutamate-induced neuronal damage, cell swelling and increased influx of (Ca2+)(i), the protective effects were not observed in delayed YC-1 treatment at 1–3 h in vitro and 1–2 h in vivo following stroke induction. This also indicated that other factors may interfere with or counteract the therapeutic effect of YC-1. A significant change in (pH)(i) during the early phase of stroke was identified, which may account for the phenomena observed above.
The mechanism of ischemic stroke is quite complex. Following ischemia, oxygen depletion forces the brain to switch to anaerobic glycolysis. The accumulation of lactic acid as a byproduct of glycolysis and protons produced by ATP hydrolysis cause the pH levels to fall in the ischemic brain. During severe ischemia, pH can fall to <6.0 (4). Even small changes in (pH)(i) can affect a considerable number of important mechanisms and induce cell death following stroke. The results of the present study demonstrated that neurons exposed to glutamate or delayed treatment with YC-1 at 2 h then glutamate exposure produced similar changes in (pH)(i) (a reduction of ~0.4 in pH); however, immediate treatment with YC-1 following the exposure of cortical neurons to glutamate resulted in a marked reduction in (pH)(i) (a reduction of ~1.0 in pH). In addition, only neurons treated with YC-1 or delayed treatment with YC-1 at 4 h following glutamate exposure produced minor changes in (pH)(i) (reduction of ~0.2 in pH). These changes in (pH)(i) may be the cause of the protective effect of YC-1 at 4 h in vitro and 3 h in vivo following brain injury.
A previous study indicated that a change in (pH)(i) affects the acid-induced increase of (Ca2+)(i), membrane depolarization and acidosis-mediated neuronal injury (22). Acidosis serves a critical role in brain ischemia. Acid-sensing ion channels (ASICs) are a key mediator of acidosis-induced neuronal injury (23). A previous study indicated that ASIC1a is involved in synaptic plasticity, learning/memory and fear conditioning (4). In addition, a previous study indicated that the function of ASICs is modulated by extracellular pH and also (pH)(i) (5). In the results from the present study, treatment with YC-1 immediately following neuronal exposure to glutamate resulted in a marked reduction in (pH)(i). However, whether such a change is associated with ASICs remains unclear. Thus, further studies on whether YC-1 affects ASICs in early stroke are required.
In conclusion, YC-1 offers neuroprotection against glutamate-induced neuronal damage in mice subjected to transient focal cerebral ischemia. Although YC-1 treatment appears to be a potential strategy for clinical therapy, the timing of treatment was crucial in the present study and so further studies are required to elucidate its potential.
References
- 1.O'Bryant Z, Vann KT, Xiong ZG. Translational strategies for neuroprotection in ischemic stroke-focusing on acid-sensing ion channel 1a. Transl Stroke Res. 2014;5:59–68. doi: 10.1007/s12975-013-0319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee EJ, Chen HY, Hung YC, Chen TY, Lee MY, Yu SC, Chen YH, Chuang IC, Wu TS. Therapeutic window for cinnamophilin following oxygen-glucose deprivation and transient focal cerebral ischemia. Exp Neurol. 2009;217:74–83. doi: 10.1016/j.expneurol.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 3.Demaerschalk BM, Yip TR. Economic benefit of increasing utilization of intravenous tissue plasminogen activator for acute ischemic stroke in the United States. Stroke. 2005;36:2500–2503. doi: 10.1161/01.STR.0000185699.37843.14. [DOI] [PubMed] [Google Scholar]
- 4.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: Blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Li MH, Leng TD, Feng XC, Yang T, Simon RP, Xiong ZG. Modulation of acid-sensing ion channel 1a by intracellular pH and its role in ischemic stroke. J Biol Chem. 2016;291:18370–18383. doi: 10.1074/jbc.M115.713636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mutch WA, Hansen AJ. Extracellular pH changes during spreading depression and cerebral ischemia: Mechanisms of brain pH regulation. J Cereb Blood Flow Metab. 1984;4:17–27. doi: 10.1038/jcbfm.1984.3. [DOI] [PubMed] [Google Scholar]
- 7.von Hanwehr R, Smith ML, Siesjö BK. Extra- and intracellular pH during near-complete forebrain ischemia in the rat. J Neurochem. 1986;46:331–339. doi: 10.1111/j.1471-4159.1986.tb12973.x. [DOI] [PubMed] [Google Scholar]
- 8.Mari Y, Katnik C, Cuevas J. ASIC1a channels are activated by endogenous protons during ischemia and contribute to synergistic potentiation of intracellular Ca(2+) overload during ischemia and acidosis. Cell Calcium. 2010;48:70–82. doi: 10.1016/j.ceca.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Szatkowski M, Attwell D. Triggering and execution of neuronal death in brain ischaemia: Two phases of glutamate release by different mechanisms. Trends Neurosci. 1994;17:359–365. doi: 10.1016/0166-2236(94)90040-X. [DOI] [PubMed] [Google Scholar]
- 10.Dubinsky JM. Intracellular calcium levels during the period of delayed excitotoxicity. J Neurosci. 1993;13:623–631. doi: 10.1523/JNEUROSCI.13-02-00623.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WT, Lin MH, Lee EJ, Hung YC, Tai SH, Chen HY, Chen TY, Wu TS. Magnolol reduces glutamate-induced neuronal excitotoxicity and protects against permanent focal cerebral ischemia up to 4 hours. PLoS One. 2012;7:e39952. doi: 10.1371/journal.pone.0039952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang TL, Hung HW, Kao SH, Teng CM, Wu CC, Cheng SJ. Soluble guanylyl cyclase activator YC-1 inhibits human neutrophil functions through a cGMP-independent but cAMP-dependent pathway. Mol Pharmacol. 2003;64:1419–1427. doi: 10.1124/mol.64.6.1419. [DOI] [PubMed] [Google Scholar]
- 13.DeNiro M, Al-Halafi A, Al-Mohanna FH, Alsmadi O, Al-Mohanna FA. Pleiotropic effects of YC-1 selectively inhibit pathological retinal neovascularization and promote physiological revascularization in a mouse model of oxygen-induced retinopathy. Mol Pharmacol. 2010;77:348–367. doi: 10.1124/mol.109.061366. [DOI] [PubMed] [Google Scholar]
- 14.Yan J, Zhou B, Taheri S, Shi H. Differential effects of HIF-1 inhibition by YC-1 on the overall outcome and blood-brain barrier damage in a rat model of ischemic stroke. PLoS One. 2011;6:e27798. doi: 10.1371/journal.pone.0027798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tai SH, Hung YC, Lee EJ, Lee AC, Chen TY, Shen CC, Chen HY, Lee MY, Huang SY, Wu TS. Melatonin protects against transient focal cerebral ischemia in both reproductively active and estrogen-deficient female rats: The impact of circulating estrogen on its hormetic dose-response. J Pineal Res. 2011;50:292–303. doi: 10.1111/j.1600-079X.2010.00839.x. [DOI] [PubMed] [Google Scholar]
- 16.DeLorenzo RJ, Sun DA, Blair RE, Sombati S. An in vitro model of stroke-induced epilepsy: Elucidation of the roles of glutamate and calcium in the induction and maintenance of stroke-induced epileptogenesis. Int Rev Neurobiol. 2007;81:59–84. doi: 10.1016/S0074-7742(06)81005-6. [DOI] [PubMed] [Google Scholar]
- 17.Trenholm S, Baldridge WH. The effect of aminosulfonate buffers on the light responses and intracellular pH of goldfish retinal horizontal cells. J Neurochem. 2010;115:102–111. doi: 10.1111/j.1471-4159.2010.06906.x. [DOI] [PubMed] [Google Scholar]
- 18.Tai SH, Chen HY, Lee EJ, Chen TY, Lin HW, Hung YC, Huang SY, Chen YH, Lee WT, Wu TS. Melatonin inhibits postischemic matrix metalloproteinase-9 (MMP-9) activation via dual modulation of plasminogen/plasmin system and endogenous MMP inhibitor in mice subjected to transient focal cerebral ischemia. J Pineal Res. 2010;49:332–341. doi: 10.1111/j.1600-079X.2010.00797.x. [DOI] [PubMed] [Google Scholar]
- 19.Papadia S, Soriano FX, Léveillé F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alzheimer C. Na+ channels and Ca2+ channels of the cell membrane as targets of neuroprotective substances. Adv Exp Med Biol. 2002;513:161–181. doi: 10.1007/978-1-4615-0123-7_5. [DOI] [PubMed] [Google Scholar]
- 21.Yang X, Wang Y, Luo J, Liu S, Yang Z. Protective effects of YC-1 against glutamate induced PC12 cell apoptosis. Cell Mol Neurobiol. 2011;31:303–311. doi: 10.1007/s10571-010-9622-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang WZ, Chu XP, Li MH, Seeds J, Simon RP, Xiong ZG. Modulation of acid-sensing ion channel currents, acid-induced increase of intracellular Ca2+, and acidosis-mediated neuronal injury by intracellular pH. J Biol Chem. 2006;281:29369–29378. doi: 10.1074/jbc.M605122200. [DOI] [PubMed] [Google Scholar]
- 23.Jiang N, Wu J, Leng T, Yang T, Zhou Y, Jiang Q, Wang B, Hu Y, Ji YH, Simon RP, et al. Region specific contribution of ASIC2 to acidosis-and ischemia-induced neuronal injury. J Cereb Blood Flow Metab. 2017;37:528–540. doi: 10.1177/0271678X16630558. [DOI] [PMC free article] [PubMed] [Google Scholar]