Abstract
In a previous study using a microRNA (miRNA/miR) microarray assay, we demonstrated that miR-133b-5p was upregulated in response to hypoxic preconditioning (HPC). The present study was designed to investigate the role of the miR-133b-5p in HPC-induced cardioprotection and the underlying mechanisms involving caspase-8 and caspase-3 apoptotic signaling. Adult rats were subjected to myocardial ischemia/reperfusion (I/R) injury with or without ischemic preconditioning (IPC), and the level of miR-133b-5p in myocardium was measured. Neonatal rat cardiomyocytes were isolated and subjected to hypoxia/reoxygenation (H/R) injury, with or without HPC. miR-133b-5p antagomir was transfected into the cardiomyocytes to observe whether it could block HPC-induced cardioprotection. Cellular injury was evaluated by detecting cell viability, lactate dehydrogenase (LDH) activity and apoptotic rate. Reverse transcription-quantitative polymerase chain reaction was used to measure the level of miR-133b-5p. The activation of caspase-8 and caspase-3 were measured by western blot analysis to detect the cleaved fragments as well as a colorimetric assay. Following myocardial I/R injury, the expression of miR-133b-5p was decreased in myocardium, while this decrease was restored by IPC. HPC protected neonatal rat cardiomyocytes against H/R injury by increasing cell viability, while reducing LDH release and cell apoptosis. These protective effects were coupled with the upregulation of miR-133b-5p. However, the knockdown of miR-133b-5p in the cardiomyocytes blocked HPC-mediated cardioprotection as reflected by the aggravation of cell injury and apoptosis. HPC upregulated miR-133b-5p level was markedly suppressed by the antagomir. In addition, the cleavage and activities of caspase-8 and caspase-3 were inhibited by HPC while reversed by knockdown of miR-133b-5p. Upregulation of miR-133b-5p contributes to HPC-mediated cardioprotection in cardiomyocytes, and the mechanism may be associated with inhibition of caspase-8 and caspase-3 apoptotic signaling.
Keywords: microRNA, cardiomyocyte, hypoxic preconditioning, apoptosis, caspase
Introduction
Reperfusion after cardiac ischemia increases cell death and infarct size (IS), called myocardial ischemia/reperfusion (I/R) injury, which is the main cause of myocardial injury during the cardiac surgery particularly in coronary artery bypass graft surgery (1,2). Hypoxia/reoxygenation (H/R) as well as hypoxic preconditioning (HPC) is widely used for simulating in vivo myocardial I/R and ischemic preconditioning (IPC) in a cell culture model. In addition, inhaled hypoxic air preconditioning including intermittent hypoxia has been suggested a potential non-pharmacological therapeutic intervention to improve some cardiovascular diseases such as hypertension, myocardial infarction. Such HPC may enhance cellular tolerance to subsequent hypoxia-induced oxidative stress, simulating the protective effects of IPC, taking advantage of convenient and non-invasive intervention (3,4). In recent years, HPC has been extensively studied and established to be cardioprotective against H/R injury, which involves many factors including oxygen transport, energy metabolism, redox balance, stress protein and protein kinase signaling, adenosine release, ATP-sensitive potassium channels, mitochondrial function, control of calcium levels, and nitric oxide production (5–7). However, the detailed mechanisms underlying HPC are not fully understood.
MicroRNAs (miRNAs/miRs) are endogenous, single-stranded, small (approximately 22 nucleotides in length) noncoding RNA molecules that play an important and ubiquitous role in regulating genes expression. MiRNAs typically bind to the 3′ untranslated region (UTR) of their mRNA targets and downregulate gene expression via mRNA degradation or translational inhibition (8–10). So, miRNAs may influence many cellular activities. Indeed, miRNAs have been shown to be actively involved in the coordination of almost all major cellular processes, such as differentiation (11), proliferation (12), metabolism, and apoptosis (13). Also, cumulating evidence suggests critical roles for miRNAs in various types of heart disease, including cardiac hypertrophy (14,15), heart failure (16), and myocardial infarction (17–19).
miR-133 is one of the most studied and best characterized miRNAs, specifically expressed in cardiac and skeletal muscle (20). The miR-133 family contains two members of miR-133a and miR-133b, sharing an almost identical sequence. miR-133a is known as an important anti-apoptotic miRNA in myocardial I/R where it attenuates apoptosis of cardiomyocytes by targeting the apoptosis-related gene caspase-9 (21,22). The mechanism of miR-133b regulating apoptosis in the myocardium, however, has yet to be elucidated. In the present study, we knocked down the expression of miR-133b-5p in cardiomyocytes by using its specific antagomir, in order to examine the role of miR-133b-5p in HPC-mediated cardioprotection and the underlying mechanisms involving caspase apoptotic signaling.
Materials and methods
Animals
All animal experiments were approved by the Institutional Animal Care and Use Committee of Anhui Medical University. Adult Sprague-Dawley (SD) rats (250±30 g) were maintained on a 12 h light/12 h dark cycle at an ambient temperature of 22±2°C, with food and water available ad libitum. All animal experimental procedures were performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH publication no. 85–23, revised 1996).
In vivo I/R injury
Myocardial I/R was performed in vivo by 30 min of ligation of the left anterior descending coronary artery followed by 2 h of reperfusion, as previously described (23). IPC was performed by three cycles of 5 min of ischemia intermitted by 5 min of reperfusion before I/R injury. At the end of reperfusion, heart slices were stained by 1% triphenyl tetrazolium chloride (TTC) for measuring IS. The total volumes of the left and right ventricles (LV+RV), the area at risk (AAR), and IS were measured by a blinded investigator using Image J software (NIH, USA). In other animals, heart tissue samples were taken from left ventricular myocardium for miRNA detection by RT-qPCR.
Isolation of neonatal cardiomyocytes and treatment
Neonatal rat cardiomyocytes were isolated from the hearts of 1–3 days old Sprague-Dawley (SD) rats. The newborn rats were killed by cervical dislocation. The isolated cardiomyocytes were obtained and cultured using the method described previously by Suh et al (24). Briefly, hearts excised from the rats were minced into pieces, and then digested with 0.125% trypsin and 0.1% type II collagenase. The cell suspensions were collected and incubated for 2 h to reduce fibroblast contamination. Then, the non-adherent cardiomyocytes were seeded to dishes and cultured in Dulbecco's modified Eagle's medium/F12 (DMEM/F12) containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin. The medium was supplemented with 100 µmol/l 5-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich, St. Louis, MO, USA) in order to increase the purity of cardiomyocyte.
H/R injury in cardiomyocytes
To induce H/R injury, neonatal rat cardiomyocytes were incubated in glucose-free Tyrode's solution containing (in mmol/l) 139 NaCl, 4.7 KCl, 0.5 MgCl2, 1.0 CaCl2, and 5 HEPES, pH 7.4, and exposed to 95% N2 + 5% CO2 for 4 h using a hypoxia chamber (Stemcell Technologies, Inc., Vancouver, BC, Canada) followed by 2 h reoxygenation under normal culture conditions. The cells cultured under a normoxic atmosphere served as a control. HPC was carried out by 10 min hypoxia and 30 min reoxygenation before the lethal hypoxia.
Transfection of miR-133b-5p antagomir in neonatal cardiomyocytes
Neonatal rat myocardial cells were cultured in DMEM-F12 medium containing 10% FBS, with transfection of miR-133b-5p antagomir or antagomir negative control (NC; Guangzhou RiboBio Co., Ltd., Guangzhou, China) using Lipofectamine RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to procedures the manufacturer recommended (25). After 24 h transfection, the medium was changed and the cells were then subjected to H/R injury with or without HPC.
Cell viability and lactate dehydrogenase (LDH) activity assessment
Neonatal rat cardiomyocytes were plated in the 96-well plates. Cell viability was evaluated by Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan), and the extent of cell injury was determined by detecting the release of LDH in culture medium using a LDH Activity Assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Cell viability and injury were assayed according to the manufacturer's instructions.
Annexin V-fluorescein isothiocyanate (FITC)/ prodium iodide (PI) flow cytometry
Cell apoptosis was detected by Annexin V-FITC/PI staining kit (Biouniquer Technology, Beijing, China) as described before (26). After treatment, the myocytes were collected, washed twice with ice-cold phosphate-buffered solution (PBS). Then, 500 µl of binding buffer was added to resuspend the cells and 5 µl Annexin V-FITC and 5 µl PI were added and mixed. The reaction was conducted in dark at room temperature for 15 min and analyzed with a flow cytometer (Beckman Coulter, Inc., Brea, CA, USA) and FCS Express V3.0 software.
RT-qPCR
The total RNA was extracted from the heart tissues or cardiomyocytes using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) and quantified by spectrophotometry. miR-133b-5p level was measured by All-in-One™ miRNA RT-qPCR Detection kit (GeneCopoeia, Inc., Guangzhou, China) as previously reported (26). The stably expressed U6 was used as the endogenous control. The PCR analysis was performed in a real time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. In each experimental run, double-distilled H2O was served as the NC. Relative quantization was expressed as fold-induction compared with control conditions. Dissociation curves were generated after each run to confirm amplification of specific transcripts. Relative gene expression levels were calculated by the 2−∆∆Cq (27).
Western blot analysis
After treatment, the cells were harvested and the total protein was extracted by standard procedures and then quantified by the bicinchoninic acid (BCA) protein assay (Pierce; Thermo Fisher Scientific, Inc.). A total of 25 µg total protein was subjected to 10% SDS-PAGE gel followed by electrotransfer onto polyvinylidene difluoride (PVDF) membranes (EMD Millipore, Billerica, MA, USA). Nonspecific antibody binding was blocked in Tris-buffered saline solution/0.1 Tween-20 (TBST) containing 5% skimmed milk. Membranes were incubated overnight at 4°C with primary antibodies against β-actin (Abcam, Cambridge, UK), Fas (Abcam), caspase-3 and cleaved caspase-3 (Cell Signaling Technology, Inc., Danvers, MA, USA) or caspase-8 (EMD Millipore), followed by incubation with secondary antibodies for 1 h at room temperature. After washing three times with TBST, the immunoreactive proteins were visualized by ECL (Amersham Pharmacia Biotech, Amersham, UK) and autoradiography. Densitometric analysis was carried out with Image J2× software (NIH, Bethesda, MD, USA).
Caspase-8 and caspase-3 activity assay
The activities of caspase-8 and caspase-3 were determined by caspase-8 and caspase-3 Colorimetric Assay kit (RayBiotech, Norcross, GA, USA) as the manufacturer's instructions. The cells were collected and lysed by cell lysis buffer followed by centrifuging at 10,000 g for 5 min at 4°C. After assaying protein concentration, 100 µg of protein was added in 50 µl cell lysis buffer. Next, the reaction buffer and 5 µl of IETD-ρNA substrate (caspase-8) or DEVD-ρNA substrate (caspase-3) were added to the lysis buffer. The reaction mixtures were incubated at 37°C for 2 h followed by detecting the activities of caspase-8 and caspase-3 at 405 nm in a microtiter plate reader.
Statistical analysis
Data are expressed as mean ± SEM based on at least three independent experiments. Statistical analysis was performed using one-way ANOVA with a Tukey's test by SPSS 10.0 for Windows (SPSS, Inc, Chicago, IL, USA). P<0.05 was considered significant.
Results
IPC upregulates the expressional level of miR-133b-5p in rat myocardium after I/R injury
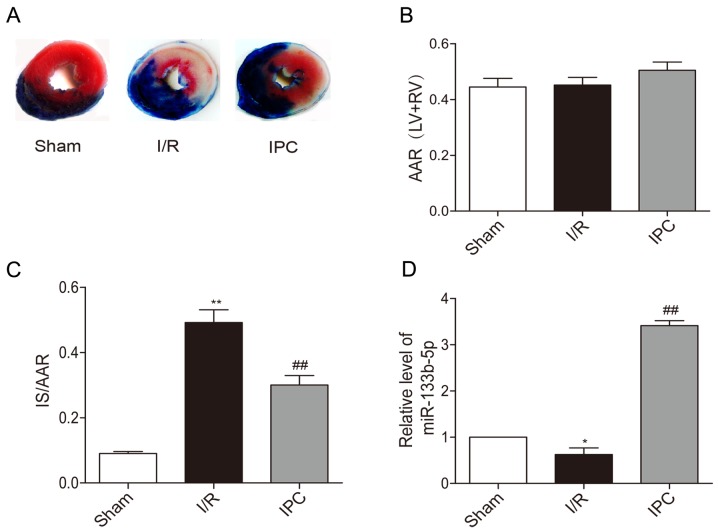
Representative photographs of heart sections stained by TTC in each group are presented. The non-ischemic area stained blue and AAR was brick red while IS was white (Fig. 1A). There were no significant difference in AAR/(LV+RV) between each group in rats (Fig. 1B). IPC significantly reduced IS/AAR caused by I/R injury (Fig. 1C). Upon the exposure of rat hearts to I/R, the expression of miR-133b-5p was decreased in myocardium, while IPC markedly upregulated the level of miR-133b-5p (Fig. 1D).
Figure 1.
Effects of IPC on the infarct size and expressions of miR-133b-5p of in vivo rat hearts. (A) Heart sections were stained by TTC. (B) AAR/ (LV+RV) and (C) IS/ARR were calculated by Image J software (n=6). (D) The levels of miR-133b-5p was detected by quantitative RT-PCR (n=3). Data are presented as mean ± SEM, *P<0.05, **P<0.01 vs. Sham; ##P<0.01 vs. I/R. IPC, ischemia preconditioning; TTC, triphenyltetrazolium chloride; AAR, area at risk; LV, left ventricular; RV, right ventricular; IS, infarct size; I/R, ischemia/reperfusion; miR, microRNA.
HPC-mediated cardioprotection against H/R injury is accompanied with the upregulation of miR-133b-5p
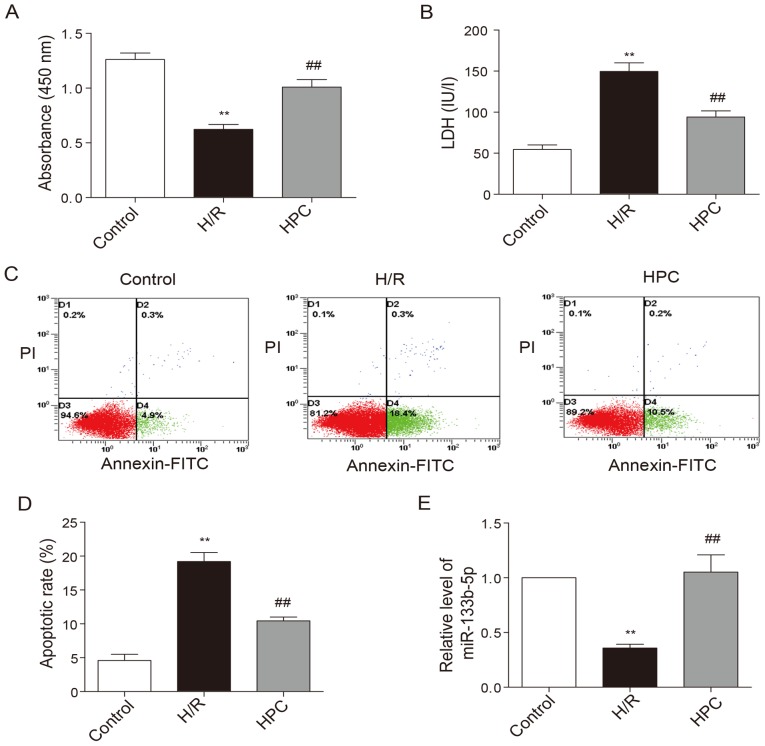
Upon the exposure of neonatal cardiomyocytes to H/R injury, the viability of cardiomyocytes was noticeably decreased (Fig. 2A) while the LDH level elevated (Fig. 2B). In addition, the percentage of cells at early apoptotic stage as shown in the lower right quadrant was increased following H/R injury (Fig. 2C and D). HPC significantly increased cell viability, reduced LDH levels as well as early apoptotic rate. The level of miR-133b-5p was markedly reduced due to H/R injury, but restored by HPC (Fig. 2E).
Figure 2.
Effects of HPC on H/R injury and the level of miR-133b-5p. (A) The viability of cardiomyocytes was measured by Cell Counting Kit-8 method. (B) Cell injury was determined by lactate dehydrogenase release in the culture medium. (C) Cell apoptosis was measured by Annexin V-FITC/PI flow cytometry and (D) the apoptotic rate is expressed as the percentage of cells at early apoptotic stage (the lower right quadrant). (E) The level of miR-133b-5p was detected by quantitative RT-PCR. Data are presented as mean ± SEM (n=3), **P<0.01 vs. control; ##P<0.01 vs. H/R. HPC, hypoxic preconditioning; H/R, hypoxia/reoxygenation; miR, microRNA; FITC, fluorescein isothiocyanate; PI, prodium iodide.
Knockdown of miR-133b-5p blocks the protective effects of HPC
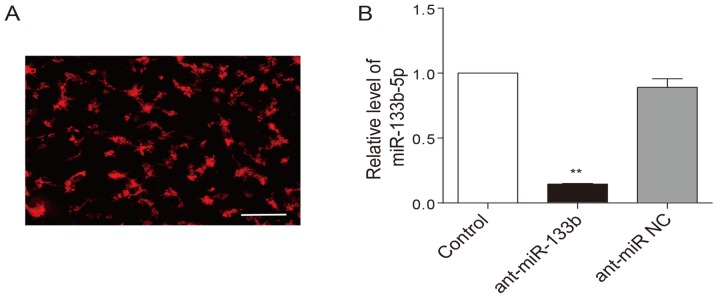
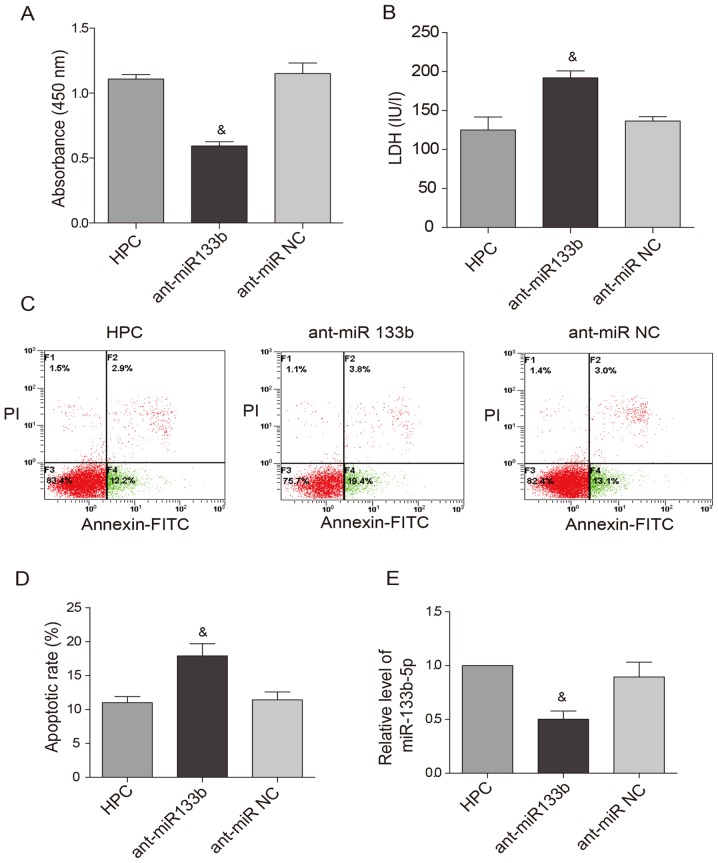
To examine whether HPC-induced cardioprotection is dependent on miR-133b-5p, the antagomir that mediates specific silencing of endogenous miR-133b-5p were transfected into neonatal cardiomyocytes. The transfection efficiency of the antagomir was above 80%, which was monitored with a Cy3-labelled (red) control (Fig. 3A). As expected, the miR-133b-5p antagomir largely decreased the level of miR-133b-5p in the cardiomyocytes (Fig. 3B). When the expression of miR-133b-5p was knocked down by the antagomir, the cardioprotective effects of HPC were obviously attenuated. Cell viability was reduced (Fig. 4A) while LDH level (Fig. 4B) and apoptotic rate (Fig. 4C and D) were raised. Meanwhile, the upregulation of miR-133b-5p by HPC was markedly suppressed by the miR-133b-5p antagomir (Fig. 4E).
Figure 3.
Verification of miR-133b-5p antagomir transfection in cariomyocyets. (A) The transfection efficiency was monitored with a Cy3-labelled negative control. Bar, 100 µm. (B) The level of miR-133b-5p was detected by quantitative RT-PCR and normalized to U6 RNA. Transfection of the miR-133b-5p antagomir successfully knocked down the expression of miR-133b-5p in neonatal rat cardiomyocytes. Data are presented as mean ± SEM (n=3), **P<0.01 vs. control. miR, microRNA; ant-miR-133b, miR-133b-5p antagomir; ant-miR NC, antagomir negative control.
Figure 4.
Knockdown of miR-133b-5p blocked HPC-induced cardioprotection. (A) The viability of cardiomyocytes was measured by Cell Counting Kit-8 method. (B) Cell injury was determined by lactate dehydrogenase release in the culture medium. (C) Cell apoptosis was measured by Annexin V-FITC/PI flow cytometry and (D) the apoptotic rate is expressed as the percentage of cells at early apoptotic stage (the lower right quadrant). (E) The level of miR-133b-5p was detected by quantitative RT-PCR. Data are presented as mean ± SEM (n=3). &P<0.05 vs. HPC. HPC, hypoxic preconditioning; miR, microRNA; ant-miR-133b, miR-133b-5p antagomir; ant-miR NC, antagomir negative control; FITC, fluorescein isothiocyanate; PI, prodium iodide.
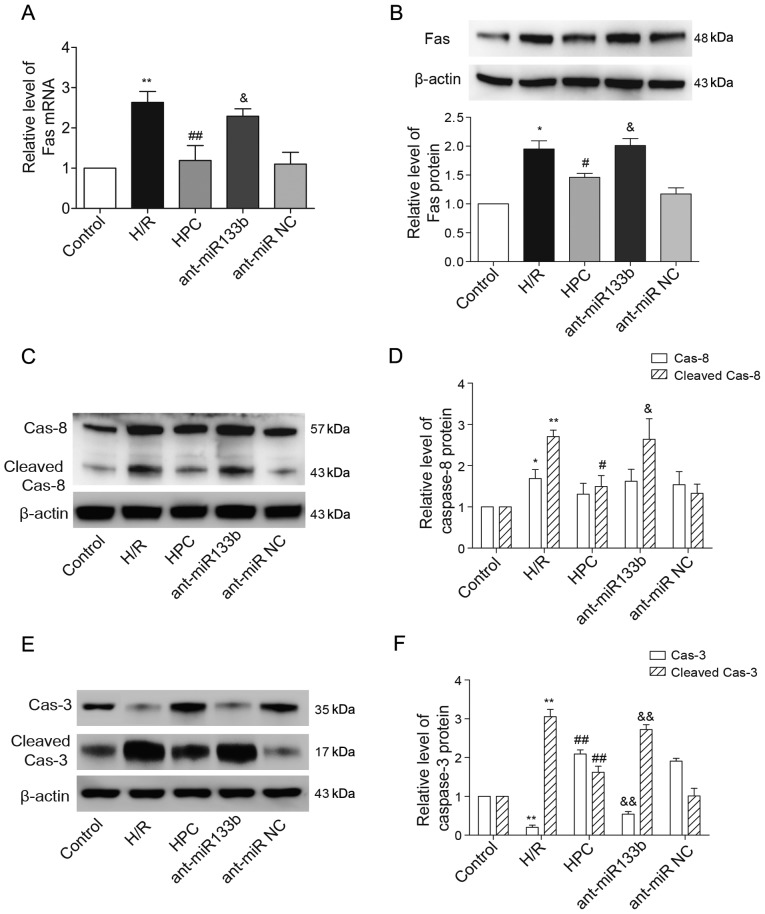
Effects of miR-133b-5p antagomir on the levels of Fas mRNA/protein and the cleavages of caspase-8 and caspase-3
As one of the target genes of miR-133b-5p, the expressional levels of Fas mRNA and protein that decreased by HPC were both reversely raised due to the knockdown of miR-133b-5p (Fig. 5A and B). We next examined whether the apoptosis regulators of caspase-8 and caspase-3, downstream of Fas death receptor, were regulated by miR-133b-5p. Using western blot method, we detected the cleaved fragments of caspase-8 and caspase-3, which represents their activation. As shown in Fig. 5C and D, the full length caspase-8 (57 kDa) was strongly upregulated upon H/R injury, while its level was not affected by HPC or miR-133b-5p antagomir. The enhanced cleavage of caspase-8 (43 kDa) was suppressed by HPC but reversed by the miR-133b-5p antagomir. Interestingly, the level of full length caspase-3 (35 kDa) was reduced but the cleaved caspase-3 (17 kDa) was markedly strengthened in response to H/R injury. However, the pro-caspase-3 (full length) level was restored while the activated caspase-3 (cleaved) was weakened by HPC, and these effects were abolished by knockdown of miR-133b-5p (Fig. 5E and F).
Figure 5.
Effects of miR-133b-5p antagomir on Fas mRNA/protein expression and the cleavage of caspase-8 and caspase-3. (A) Fas mRNA detected by quantitative RT-PCR. (B) The level of Fas protein expression was examined by western blot and the intensities of Fas protein bands were normalized to β-actin. (C) The full-length (57 kDa) and cleaved caspase-8 (43 kDa) were detected by western blot and (D) the intensities of full-length and cleaved caspase-8 were normalized to β-actin, respectively. (E) The full-length (35 kDa) and cleaved caspase-3 (17 kDa) were detected by western blot and (F) the intensities of full-length and cleaved caspase-3 were normalized to β-actin, respectively. Data are presented as mean ± SEM (n=3). *P<0.05, **P<0.01 vs. Control; #P<0.05, ##P<0.01 vs. H/R; &P<0.05, &&P<0.01 vs. HPC. miR, microRNA; H/R, hypoxia/reoxygenation; HPC, hypoxic preconditioning; ant-miR-133b, miR-133b-5p antagomir; ant-miR NC, antagomir negative control; Cas-8, caspase-8; Cas-3, caspase-3.
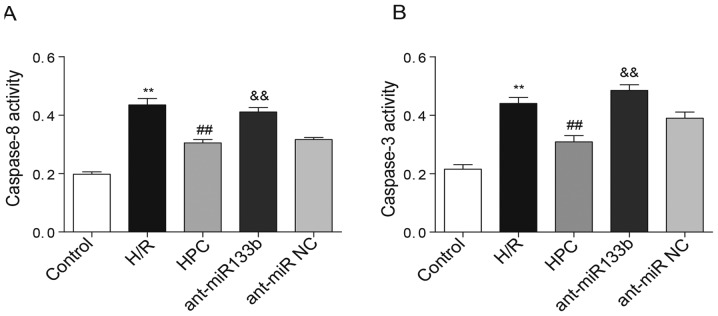
Effects of miR-133b-5p antagomir on the activities of caspase-8 and caspase-3
The activation of caspase-8 and caspase-3 was further detected by colorimetric assay in which the activities of caspase-8 and caspase-3 were determined by their cleavage capability of the labeled substrates. According to Fig. 6A and B, we found that both caspase-8 and caspase-3 were activated following H/R injury, whereas their activities were significantly inhibited by HPC. However, knockdown of miR-133b-5p recovered the activities of both caspase-8 and caspase-3 in cardiomyocytes.
Figure 6.
Effects of miR-133b-5p antagomir on the activities of caspase-8 and caspase-3. (A) The activity of caspase-8 was measured using caspase-8 Colorimetric Assay kits. (B) The activity of caspase-3 was measured using caspase-3 Colorimetric Assay kits. Data are presented as mean ± SEM (n=3). **P<0.01 vs. control; ##P<0.01 vs. H/R; &&P<0.01 vs. HPC. H/R, hypoxia/reoxygenation; HPC, hypoxic preconditioning; ant-miR-133b, miR-133b-5p antagomir; ant-miR NC, antagomir negative control.
Discussion
Cardiomyocyte apoptosis represents a critical event during myocardial ischemia and reperfusion. Growing evidences show that a number of miRNAs can promote or inhibit cardiomyocyte apoptosis via regulating the downstream target genes and signaling pathways (28). In the present study, by using miR-133b-5p antagomir, we demonstrated that miR-133b-5p contributed to HPC-induced cardioprotection against H/R injury in cardiomyocytes by inhibiting the activation of capase-8 and caspase-3 apoptotic signaling.
We first found that the expressional level of miR-133b-5p was reduced following in vivo myocardial I/R injury, while this reduction was restored by IPC treatment, which suggests a potential role of miR-133b-5p in cardioprotection. In neonatal rat cardiomyocytes, HPC conferred cardioprotection against H/R injury by improving cell viability while decreasing LDH release and cell apoptosis. This protective effect was coupled with the upregulation of miR-133b-5p, indicating that HPC may exert cardioprotection, in partly, by upregulating miR-133b-5p. Previous study reported that the plasma levels of miR-133a and miR-133b were upregulated following acute myocardial infarction, and this upregulation was consistent with the increase of cardiac troponin I (cTnI), representing a novel marker for cardiac damage (29,30). In addition, the levels of miR-133a and miR-133b in myocardium have been found changed during cardiac development, myocardial infarction and heart failure (31–33). These make both miR-133a and miR-133b truly valuable therapeutic genes for cardiac injury.
In a previous work, we showed that overexpression of miR-133b-5p in H9c2 cardiomyoblasts protected the cells against H/R injury (26). In the present study, we applied primary neonatal rat cardiomyocytes, which are more suitable for both simulating in vivo condition and transfecting the antagomir effectively. The most important observation of this study is that the cardioprotective effect of HPC against H/R injury in cardiomyocyte was significantly attenuated by transfection of the miR-133b-5p antagomir. Thus, it is reasonable to speculate that HPC induced cardioprotection was mediated by the upregulation of miR-133b-5p. The finding is in agreement with the previous observation on a cardiac protective role of miRNAs (34). Yin et al (35) reported that an ischemic stimulus induced the expression of miR-1, miR-21 and miR-24, and injection of these IPC-derived miRNAs directly into the left ventricle reduced myocardial IS, indicating that preconditioning induced miRNAs produced a protective phenotype following ischemic injury. Inconsistent with our results, however, miR-133b has been reported to promote apoptosis in several carcinoma cells including lung cancer, renal carcinoma, and osteosarcoma (36–38). Moreover, Xia et al (39) recently reported that miR-133b-5p accelerates neuron apoptosis via targeting heat shock protein 70. These discrepancies may due to disease-specific circumstances as well as various cell types applied in different studies.
The activation of caspase-8 and caspapse-3 is reported directly or indirectly regulated by several miRNAs including miR-133 in the process of cardiomyocyte apoptosis (40). Our recent study using dual luciferase reporter gene technique showed that miR-133b-5p directly targeted Fas gene by interacting with the 3′ UTR (26). Thus, there is no wonder that HPC-reduced expression of Fas mRNA/protein was blocked by the miR-133b-5p antagomir. Fas death pathway has been considered as a critical mediator for cardiomyocyte death and myocardial infarction during myocardial I/R (41,42). It is well known that the activation of Fas by extracellular Fas ligand (FasL) in turn activates the pro-apoptotic members of caspase-8 and the downstream apoptosis executors of caspase-3 (43). The cleavage and activities of caspase-8 and caspase-3 were inhibited by HPC but reversed by the miR-133b-5p antagomir. These results suggest that miR-133b-5p antagomir may indirectly influence the activation of caspase-8 and caspase-3 through regulating Fas gene expression. Otherwise, miR-133b-5p may directly target the post-transcription of caspase-8 and caspase-3, although the interaction mechanisms need to be further explored.
The cleavage and activation of caspase-8 and caspase-3 have been confirmed as critical steps in apoptosis signaling pathway, resulting in DNA degradation and cell apoptosis. In this study, the exposure of cardiomyocytes to H/R led to the cleavage and activation of both caspase-8 and caspase-3, which may in turn result in cardiomyocytes apoptosis. Interestingly, the expression of pro-caspase-3 showed a reduction corresponding to its increased cleavage, while the pro-caspase-8 level was not obviously changed following cleavage. Caspase-8 is the most upstream caspase that is well recognized as the initiator caspase, which directly activates the downstream caspase-3. The effect of caspase-8 on caspase-3 can also be amplified by the mitochondrial signaling when initial caspase-8 activation is low (44). This may be the reason why the cleavage of pro-caspase-8 is markedly less than those of pro-caspase-3, and thus no apparent change of pro-caspase-8 level can be observed.
It should be noted that we conducted the experiments in neonatal rat cardiomyocytes, and the significance and implications of the results remain need to be verified in an animal model in vivo. Although we found that the expression of miR-133b-5p was also changed in an in vivo rat model of myocardial I/R injury, its role in IPC-mediated cardioprotection still need to be confirmed in future. In addition, the caspase-8 and caspase-3 apoptotic signaling may be controlled by other miRNAs during cardiomyocyte apoptosis, while we could only focused on the miR-133b-5p in the present study.
In summary, our results revealed that HPC induced cardioprotection in cardiomyocyte was, at least partly, dependent on the upregulation of miR-133b-5p, and the mechanisms may be associated with inhibition of caspase-8 and caspase-3 apoptotic signaling. The finding may provide new insight into potential therapeutic application for ischemic cardiac injury.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81471145), and the Natural Science Foundation of Higher Education Institutions of Anhui Province (KJ2014ZD16 and KJ2017A172). The authors thank Professor Tak-Min Wong (University of Hong Kong, Hong Kong, China) for the assistance in editing and revising the manuscript.
References
- 1.Arslan F, Smeets MB, O'Neill LA, Keogh B, McGuirk P, Timmers L, Tersteeg C, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 2010;121:80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- 2.Gong D, Zhang Y, Zhang H, Gu H, Jiang Q, Hu S. Aldehyde dehydrogenase-2 activation during cardioplegic arrest enhances the cardioprotection against myocardial ischemia-reperfusion injury. Cardiovasc Toxicol. 2012;12:350–358. doi: 10.1007/s12012-012-9179-6. [DOI] [PubMed] [Google Scholar]
- 3.Verges S, Chacaroun S, Godin-Ribuot D, Baillieul S. Hypoxic conditioning as a new therapeutic modality. Front Pediatr. 2015;3:58. doi: 10.3389/fped.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrington JH, Chrismas BCR, Gibson OR, Tuttle J, Pegrum J, Govilkar S, Kabir C, Giannakakis N, Rayan F, Okasheh Z, et al. Hypoxic air inhalation and ischemia interventions both elicit preconditioning which attenuate subsequent cellular stress In vivo following blood flow occlusion and reperfusion. Front Physiol. 2017;8:560. doi: 10.3389/fphys.2017.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das DK, Maulik N. Cardiac genomic response following preconditioning stimulus. Cardiovasc Res. 2006;70:254–263. doi: 10.1016/j.cardiores.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106:360–368. doi: 10.1016/j.amjcard.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, Clarke SC, Shapiro LM, Schofield PM, O'Sullivan M, Dutka DP. Cardiac remote ischemic preconditioning in coronary stenting (CRISP stent) study: A prospective, randomized control trial. Circulation. 2009;119:820–827. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 10.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 11.Gagan J, Dey BK, Layer R, Yan Z, Dutta A. MicroRNA-378 targets the myogenic repressor MyoR during myoblast differentiation. J Biol Chem. 2011;286:19431–19438. doi: 10.1074/jbc.M111.219006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng S, Cong S, Zhang X, Bao X, Wang W, Li H, Wang Z, Wang G, Xu J, Du B, et al. MicroRNA-192 targeting retinoblastoma 1 inhibits cell proliferation and induces cell apoptosis in lung cancer cells. Nucleic Acids Res. 2011;39:6669–6678. doi: 10.1093/nar/gkr232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 14.Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 15.Song XW, Li Q, Lin L, Wang XC, Li DF, Wang GK, Ren AJ, Wang YR, Qin YW, Yuan WJ, Jing Q. MicroRNAs are dynamically regulated in hypertrophic hearts, and miR-199a is essential for the maintenance of cell size in cardiomyocytes. J Cell Physiol. 2010;225:437–443. doi: 10.1002/jcp.22217. [DOI] [PubMed] [Google Scholar]
- 16.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, et al. MicroRNAs in the human heart: A clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 17.Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284:29514–29525. doi: 10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian L, Van Laake LW, Huang Y, Liu S, Wendland MF, Srivastava D. miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J Exp Med. 2011;208:549–560. doi: 10.1084/jem.20101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Zhang X, Ren XP, Chen J, Liu H, Yang J, Medvedovic M, Hu Z, Fan GC. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation. 2010;122:1308–1318. doi: 10.1161/CIRCULATIONAHA.110.964684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wystub K, Besser J, Bachmann A, Boettger T, Braun T. miR-1/133a clusters cooperatively specify the cardiomyogenic lineage by adjustment of myocardin levels during embryonic heart development. PLoS Genet. 2013;9:e1003793. doi: 10.1371/journal.pgen.1003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He B, Xiao J, Ren AJ, Zhang YF, Zhang H, Chen M, Xie B, Gao XG, Wang YW. Role of miR-1 and miR-133a in myocardial ischemic postconditioning. J Biomed Sci. 2011;18:22. doi: 10.1186/1423-0127-18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li AY, Yang Q, Yang K. miR-133a mediates the hypoxia-induced apoptosis by inhibiting TAGLN2 expression in cardiac myocytes. Mol Cell Biochem. 2015;400:173–181. doi: 10.1007/s11010-014-2273-2. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Irwin MG, Wong TM. Remifentanil preconditioning protects against ischemic injury in the intact rat heart. Anesthesiology. 2004;101:918–923. doi: 10.1097/00000542-200410000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Suh JH, Choi E, Cha MJ, Song BW, Ham O, Lee SY, Yoon C, Lee CY, Park JH, Lee SH, Hwang KC. Up-regulation of miR-26a promotes apoptosis of hypoxic rat neonatal cardiomyocytes by repressing GSK-3β protein expression. Biochem Biophys Res Commun. 2012;423:404–410. doi: 10.1016/j.bbrc.2012.05.138. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura T, Kuroi M, Fujiwara Y, Warashina S, Sato Y, Harashima H. Small-sized, stable lipid nanoparticle for the efficient delivery of siRNA to human immune cell lines. Sci Rep. 2016;6:37849. doi: 10.1038/srep37849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He SF, Zhu HJ, Han ZY, Wu H, Jin SY, Irwin MG, Zhang Y. MicroRNA-133b-5p is involved in cardioprotection of morphine preconditioning in rat cardiomyocytes by targeting fas. Can J Cardiol. 2016;32:996–1007. doi: 10.1016/j.cjca.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Louch WE, Sheehan KA, Wolska BM. Methods in cardiomyocyte isolation, culture, and gene transfer. J Mol Cell Cardiol. 2011;51:288–298. doi: 10.1016/j.yjmcc.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss JB, Eisenhardt SU, Stark GB, Bode C, Moser M, Grundmann S. MicroRNAs in ischemia-reperfusion injury. Am J Cardiovasc Dis. 2012;2:237–247. [PMC free article] [PubMed] [Google Scholar]
- 29.D'Alessandra Y, Devanna P, Limana F, Straino S, Di Carlo A, Brambilla PG, Rubino M, Carena MC, Spazzafumo L, De Simone M, et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J. 2010;31:2765–2773. doi: 10.1093/eurheartj/ehq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cortez-Dias N, Costa MC, Carrilho-Ferreira P, Silva D, Jorge C, Calisto C, Pessoa T, Robalo Martins S, de Sousa JC, da Silva PC, et al. Circulating miR-122-5p/miR-133b ratio is a specific early prognostic biomarker in acute myocardial infarction. Circ J. 2016;80:2183–2191. doi: 10.1253/circj.CJ-16-0568. [DOI] [PubMed] [Google Scholar]
- 31.Boštjančič E, Jerše M, Glavač D, Zidar N. miR-1, miR-133a/b, and miR-208a in human fetal hearts correlate to the apoptotic and proliferation markers. Exp Biol Med (Maywood) 2015;240:211–219. doi: 10.1177/1535370214546268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bostjancic E, Zidar N, Stajer D, Glavac D. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology. 2010;115:163–169. doi: 10.1159/000268088. [DOI] [PubMed] [Google Scholar]
- 33.Sucharov C, Bristow MR, Port JD. miRNA expression in the failing human heart: Functional correlates. J Mol Cell Cardiol. 2008;45:185–192. doi: 10.1016/j.yjmcc.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu HJ, Han ZY, He SF, Jin SY, Xu SJ, Fang XD, Zhang Y. Specific MicroRNAs comparisons in hypoxia and morphine preconditioning against hypoxia-reoxgenation injury with and without heart failure. Life Sci. 2017;170:82–92. doi: 10.1016/j.lfs.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 35.Yin C, Salloum FN, Kukreja RC. A novel role of microRNA in late preconditioning: Upregulation of endothelial nitric oxide synthase and heat shock protein 70. Circ Res. 2009;104:572–575. doi: 10.1161/CIRCRESAHA.108.193250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou W, Bi X, Gao G, Sun L. miRNA-133b and miRNA-135a induce apoptosis via the JAK2/STAT3 signaling pathway in human renal carcinoma cells. Biomed Pharmacother. 2016;84:722–729. doi: 10.1016/j.biopha.2016.09.074. [DOI] [PubMed] [Google Scholar]
- 37.Pan JY, Sun CC, Bi ZY, Chen ZL, Li SJ, Li QQ, Wang YX, Bi YY, Li DJ. miR-206/133b Cluster: A weapon against lung cancer? Mol Ther Nucleic Acids. 2017;8:442–449. doi: 10.1016/j.omtn.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ying B, Huang H, Li H, Song M, Wu S, Ying H. Procaine inhibits proliferation and migration and promotes cell apoptosis in osteosarcoma cells by upregulation of microRNA-133b. Oncol Res. 2017;25:1463–1470. doi: 10.3727/096504017X14878518291077. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Xia C, Cai Y, Lin Y, Guan R, Xiao G, Yang J. MiR-133b-5p regulates the expression of the heat shock protein 70 during rat neuronal cell apoptosis induced by the gp120 V3 loop peptide. J Med Virol. 2016;88:437–447. doi: 10.1002/jmv.24355. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Li X, Zhou Y, Shi H, Xu C, He H, Wang S, Xiong X, Zhang Y, Du Z, et al. Downregulation of miR-133 via MAPK/ERK signaling pathway involved in nicotine-induced cardiomyocyte apoptosis. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:197–206. doi: 10.1007/s00210-013-0929-1. [DOI] [PubMed] [Google Scholar]
- 41.Jeremias I, Kupatt C, Martin-Villalba A, Habazettl H, Schenkel J, Boekstegers P, Debatin KM. Involvement of CD95/Apo1/Fas in cell death after myocardial ischemia. Circulation. 2000;102:915–920. doi: 10.1161/01.CIR.102.8.915. [DOI] [PubMed] [Google Scholar]
- 42.Lee P, Sata M, Lefer DJ, Factor SM, Walsh K, Kitsis RN. Fas pathway is a critical mediator of cardiac myocyte death and MI during ischemia-reperfusion in vivo. Am J Physiol Heart Circ Physiol. 2003;284:H456–H463. doi: 10.1152/ajpheart.00777.2002. [DOI] [PubMed] [Google Scholar]
- 43.Kantari C, Walczak H. Caspase-8 and bid: Caught in the act between death receptors and mitochondria. Biochim Biophys Acta. 2011;1813:558–563. doi: 10.1016/j.bbamcr.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 44.Engels IH, Stepczynska A, Stroh C, Lauber K, Berg C, Schwenzer R, Wajant H, Jänicke RU, Porter AG, Belka C, et al. Caspase-8/FLICE functions as an executioner caspase in anticancer drug-induced apoptosis. Oncogene. 2000;19:4563–4573. doi: 10.1038/sj.onc.1203824. [DOI] [PubMed] [Google Scholar]