Abstract.
Sabah is a Malaysian state situated in the northern part of Borneo, and it is endemic for malaria. The incidence of malaria is the lowest (0.05/1,000 population) in Penampang districts of Sabah. In June 26, 2012, two Plasmodium falciparum malaria cases were notified to public health department from a village in Penampang. Immediate investigation was initiated to identify the risk factors and to institute control measures. We performed active case finding by asking household members of all houses in the village regarding malaria symptoms and by examining blood smears. Environmental investigation was performed by collecting samples to detect mosquito breeding sites and to identify malaria transmitting vector mosquitoes. A case–control study with a ratio of 1:4 (11 cases and 44 controls) was conducted using self-administered questionnaire. The microscopic examination of blood smear for malarial parasite and entomology sampling was carried out. The malarial attack rate was 2.3%, 6/11 smears have gametocyte, and the case fatality rate was 9.1%. One case was a migrant rubber tapper from Indonesia which happened to be the first case with gametocyte positive. Overall, the incidence of malaria was higher (6/11) among rubber tappers. The odds of cases for those living nearby stagnant water were 7.3 [95% confidence interval: 1.2–43.5] times higher. In conclusion, an outbreak of P. falciparum malaria was introduced into a malaria-free village by a migrant rubber tapper, by whom the imported parasite was introduced to the community via vector Anopheles balabacensis. Living near stagnant water bodies was the risk factor in this outbreak.
INTRODUCTION
A joint report by the World Health Organization and the United Nations Children’s Fund showed that the number of deaths from malaria has fallen from 839,000 in 2000 to an estimated 438,000 in 2015.1 The number of cases of malaria has also fallen but not by such a large amount, largely because there are more cases than deaths. In 2000, there were 262 million cases of malaria, whereas in 2015 the estimated number was 214 million. Most cases (89%) and deaths (91%) occur in sub-Saharan Africa. Malaysia belongs to the western pacific region where more than 1.2 million cases occur per year. In 2015, in Malaysia, 2,311 confirmed cases of malaria were detected, among which 435 was imported.2
Malaria is a public health challenge in Malaysia, especially in the state of Sabah, Sarawak, and in the interior central regions of Peninsular Malaysia where Perak, Pahang, and Kelantan share their borders and where the population is made up of aborigines. In recent years, however, there are malaria outbreak in states previously known to be free of malaria, such as Penang and Negeri Sembilan in 2008.3 Based on the incidence of malaria cases, Malaysia belongs to the elimination phase (0.25/1,000 population). Sabah contributes half of the total cases reported from Malaysia each year. All areas of Sabah are not equally affected by malaria. The incidence of malaria in Penampang district of Sabah is only 0.05/1,000 of the population and hence was gazetted as medium stratification under the malaria elimination plan.3
No malaria case was notified from Kimoligan (K) village from January 2009 through May 2012. On June 26, 2012, Penampang district health clinic and the Queen Elizabeth Hospital (QEH), Kota Kinabalu, reported the Penampang public health department of two cases of malaria from K village. Malaria is a mandatory notifiable disease in Malaysia and notification should be done within 1 week of confirmed diagnosis. On the same day, a rapid assessment team from Penampang public health department was sent for investigation. The objectives of the study were to confirm the presence of outbreak, to determine the possible risk factors, to implement control measures, and to suggest measures to prevent the future outbreak.
METHODOLOGY
K village is in Penampang district and is situated about 10 km from the center of the district headquarters in Donggongon town. The population of the village was 470 of the indigenous Kadazan ethnicity. The people were mainly involved in agricultural activity. About 70% of the village was surrounded by forests. Two levels of investigation were conducted, that is, case investigation and environmental survey.
On notification, immediately the rapid assessment team headed by the district health officer went to verify the two malaria cases in the clinic and hospital. The details of the case, such as home address and malaria diagnosis, were verified by examining medical records of QEH and health clinic and interviewing the case and acquaintances. We met the head of K village to brief him on the needs of investigation and sought for village demography data. The outbreak operational room was activated, and a rapid response team was formed. District health officer briefed the outbreak and organized 10 teams comprising health inspectors for active case finding in K village. We circulated a malaria outbreak alert to district health clinics and asked them to call immediately to the operational room in case of any suspected malaria case from K village. Operational room officer was ordered to get previous surveillance data on registered malaria cases from K village. We did active case finding by searching door-to-door in all houses. Each team screened all household members by asking them about malaria symptoms and examining blood smears.
The microscopic examination of blood smears was read within 24 hours after taking blood sample. The staining techniques and blood film examination for malaria parasite detection were conducted in accordance with a standard operating procedure set by the Public Health Laboratory. Briefly, peripheral blood was collected by using a finger prick using disposable blood lancet, and then thick and thin films were made. After being air-dried in horizontal position, the thin blood films were fixed in methanol for 10–15 seconds. Then, smears were stained with 3% Giemsa solution for 45–60 minutes. Each slide was examined microscopically under oil immersion lens by experienced technicians who were trained on malaria diagnosis and species identification. The examination used qualitative method. The thin smear was used to identify the Plasmodium species. If no Plasmodium was found after observing hundred fields it was considered as negative. For quality control, each slide was examined separately by technicians from two laboratories without knowing each other’s result.
Risk factors were determined by odds ratio in a case–control study with a ratio of 1:4 (11 cases and 44 controls). The number of controls was determined by using a power of 80% with a significance level of < 0.05. All the cases and controls were interviewed using a self-administered questionnaire. A case was defined as any individual with or without fever, chills, and headache and was previously well, working or residing in K village, and was tested positive for malarial parasites on blood smear examination. Control was defined as any healthy individual working or residing in K village and tested negative for malarial parasites on blood smear examination. The control samples were randomly sampled among 79 households with exclusion criteria for those who arrived in the village within less than a week before. The list of households was collected from the village leaders, and it was used as a sampling frame. A simple random sampling method was used to select household registry using a table of random numbers, and when the selected household was inconvenient, the households before or after that were sampled. All individuals who were members of randomly selected households and those who slept there on the previous night of the survey were included in the study. Relatives who were present in those households during the study and individuals who were not willing to take part in the study were excluded from the study. Collected data were checked, any incomplete or misfiled questionnaire was sent back to the respective data collector for correction. The laboratory investigation result for each participant was attached with the respective questionnaire.
Data were analyzed by using statistical analysis software package Statistical Package for the Social Sciences version 16.0 (IBM Corporation). Statistical analysis was conducted using descriptive methods and bivariable analysis for individual risk estimation. To estimate the risk factor while controlling other factors, a multiple logistic regression was conducted. We used the odds ratios with 95% confidence interval (CI) as the effect measurement. The relative contribution of each selected variables to the outcome of interest was determined using multivariate logistic regression. The significant level was considered at P < 0.05.
Environmental investigation was performed by visiting sites and collecting samples to detect mosquito breeding sites and by identifying malaria transmitting vectors. Adult mosquitoes were collected by direct catches of mosquitoes from baits. Written informed consent was taken from the volunteers, and malaria prophylaxis was given to the volunteers before collecting mosquitoes. Larvae were collected using the dipping method.4 The location of the study subject’s house and potential breeding sites was tagged with a GPS device for analysis. The map data were obtained from Google and Digital Globe.
RESULTS
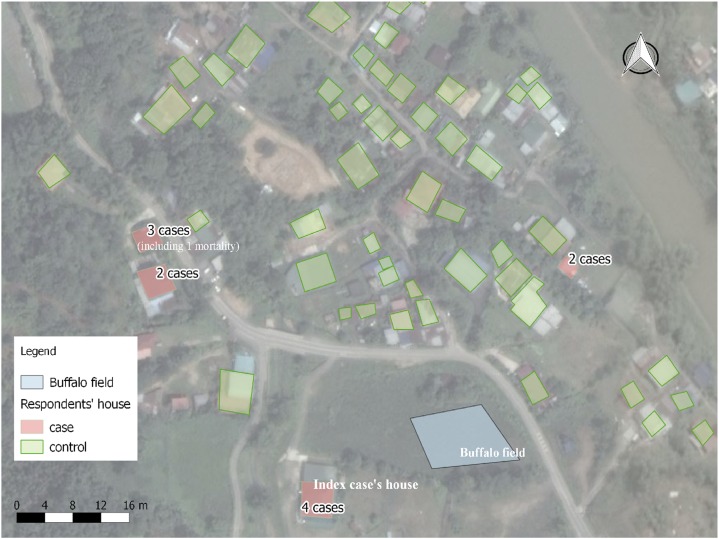
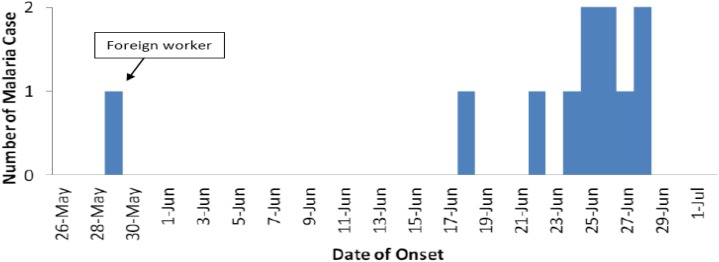
A total of 470 persons from 79 households were subjected to blood smear microscopic examination; only 11 (2.3%) cases of malaria were detected. All of them were Plasmodium falciparum. The age distribution of the malaria cases was in the following order: one case in 0–10 years, two in 11–20 years, three each in 31–40 and 41–50 years, and one each in 51–60 and 71–80 years age group. Males (30.4/1,000) had a higher incidence than females (16.7/1,000). The main occupations of the case patients were rubber tapping 6/11 (55.5%) and rearing buffalo 4/11 (36.4%). Of 11 cases, two patients were detected by passive surveillance. One case was a migrant worker from Indonesia whose occupation was rubber tapping. Otherwise there was no significant travel history. An 80-year-old patient died due to cerebral malaria that made the case fatality rate of 9.1%. She was a housewife and as a part of her physical activity, she was doing evening walks for the past 1 month. The gametocyte rate was 55% (6/11), among these, five patients were from house number 8 which is near the buffalo field, and one is the infected migrant worker from Indonesia who worked at this house. The rate means the percentage of blood smear positives that had gametocytes on the smear. All cases had fever; other symptoms present were headache in seven (64%), vomiting in six (55%), and nausea in six (55%) cases. Spot mapping was performed to show the location and surroundings of the affected houses (Figure 1). House number 8 was most affected with four cases, which was next to the buffalo field. However, the fatal case was in house number 4 which was far from buffalo fields. On June 26, 2012, Penampang district health clinic reported the Penampang public health department of a case of malaria from K village. On the same day, QEH also reported a death due to P. falciparum, and the patient was from the same locality. The rapid assessment team from the Penampang public health department plotted the epidemic curve based on when the first symptoms were detected, determined that the first case-patient developed symptoms on May 28, 2012, and noted that the case number slowly increased and peaked on June 25, 2012 (Figure 2). The time of the first symptoms in the deceased case was obtained from hospital records.
Figure 1.
The location of the respondents’ houses during the case–control study and the buffalo field which is one of the breeding sites for vector mosquitoes. This figure appears in color at www.ajtmh.org.
Figure 2.
Epidemic curve of malaria cases in K village based on when the first symptoms were detected. The x axis indicates the number of cases, and the y axis indicates the day when the first symptom was detected. This figure appears in color at www.ajtmh.org.
There were no significant sociodemographic differences between the cases and controls except the number of family size in which 82% of cases have more than five family members compared with only 48% of controls (Table 1). Independently, the most significant factor for malaria cases was living near stagnant water with crude odds ratio (OR) = 6.5 [95% CI: 1.2; 33.7] followed by not using insecticide treated bed net with crude OR = 5.2 [95% CI: 1.2; 22.3] and employment as rubber tappers with crude OR = 4.7 [95% CI: 1.2; 18.8]. However, after adjusting with other variables, only one factor was significant, that is, living nearby stagnant water with adjusted OR = 7.3 [95% CI: 1.2; 43.5] (Table 2).
Table 1.
The demographic characteristics of the respondents of the case–control study
| Factors | Case (N = 11) | Control (N = 44) | |
|---|---|---|---|
| Age | |||
| 0–30 | 3 (27.3%) | 14 (31.8%) | Fisher exact |
| 31–60 | 7 (63.6%) | 22 (50.0%) | P = 0.733 |
| 61–80 | 1 (9.1%) | 8 (18.2%) | – |
| Gender: | |||
| Male | 7 (63.6%) | 20 (45.5%) | χ2 = 1.164 |
| Female | 4 (36.4%) | 24 (54.5%) | P = 0.1410 |
| Education level: | |||
| Not educated | 3 (27.3%) | 10 (22.7%) | Fisher exact |
| Educated | 8 (72.7%) | 34 (77.3%) | P = 0.5137 |
| Family size | |||
| 1–5 | 2 (18.2%) | 23 (52.3%) | χ2 = 4.125 |
| > 5 | 9 (81.8%) | 21 (47.7%) | P = 0.0423 |
| Monthly income | |||
| < RM 500 | 9 (81.8%) | 35 (79.5%) | χ2 = 0.02841 |
| > RM500 | 2 (18.2%) | 9 (20.5%) | P = 0.8662 |
Table 2.
Risk factors for malaria cases, and the cases are compared with the controls
| Case (N = 11) | Control (N = 44) | Unadjusted OR | Adjusted OR | |
|---|---|---|---|---|
| Living near stagnant water | ||||
| No | 2 (18.2%) | 26 (59.1%) | 1 | 1 |
| Yes | 9 (81.8%) | 18 (40.9%) | 6.5 (1.2–33.7) | 7.3 (1.2–43.5) |
| Use of ITN | ||||
| Yes | 3 (27.3%) | 29 (65.9%) | 1 | 1 |
| No | 8 (72.7%) | 15 (34.1%) | 5.2 (1.2–22.3) | 4.1 (0.8–21.0) |
| Rubber tapper | ||||
| No | 5 (45.5%) | 35 (79.5%) | 1 | 1 |
| Yes | 6 (54.5%) | 9 (20.5%) | 4.7 (1.2–18.8) | 3.5 (0.7–17.7) |
| House type* | ||||
| Permanent | 10 (90.9%) | 41 (93.2%) | 1 | – |
| Temporary | 1 (9.1%) | 3 (6.8%) | 1.3 (0.1–14.5) | – |
OR = odds ratio; ITN = insecticide treated net.
Permanent indicates houses constructed by brick, concrete, and other durable materials, houses constructed by other than these materials are designated as temporary.
Our entomology investigation showed that Anopheles balabacensis, Culex sp., Mansonia sp., and Armigeres sp. were circulating in the area. The A. balabacensis biting peak between 8 and 9 pm, both indoor and outdoor types were detected. Larvae survey in stagnant water near the buffalo fields found larvae of An. balabacensis. These larvae were also found in limited number of mud footprint of buffalo (Figure 3).
Figure 3.
Clockwise: The road toward the buffalo field. Water pools in the buffalo field. A small pond situated in the buffalo field. The buffalos are grazing in the buffalo field. This figure appears in color at www.ajtmh.org.
DISCUSSION
No malaria case was reported from K Village from 2009 through May 2012. Then, there was an imported malaria outbreak into the area by a migrant worker which was similar as in developed countries.5 Rapid response was able to halt the outbreak through active case detection and vector control intervention. The initiatives taken to contain an outbreak were mass blood smear screening of everyone in K village, hospitalization of malaria cases for treatment until their blood film for malarial parasite becomes negative with a follow-up for 6 months. Analysis of the epidemic curve suggests that the outbreak was due to importation of P. falciparum by a migrant rubber tapper. Risk factors were noted for being near to a breeding site and being male. A total of 235 insecticide-treated mosquito nets were supplied to all houses in K village, and the nets were treated again in June and December 2012. We also conducted indoor residual spraying in all houses using commercially available deltamethrine and repeated the spraying 6 months later. At the buffalo fields, we did chemical larvacidal treatment with commercially available temephose to stagnant waters. Malaria volunteers were appointed, and they were actively doing malaria screening in their own villages. They also organized malaria awareness campaign to their villagers and were supported by local political leaders. Until now no malaria case has been reported from these villages. Our findings highlighted that migrant laborers may have reintroduced malaria in areas where competent vectors exist. Malaria was possibly endemic in communities of migrant rubber tappers, who are often mobile and pose a challenge for controlling malaria in the region. We recommended the Sabah Industrial Rubber Board and landowners to inform the health authority of new migrant workers within 2 weeks of arrival for free malaria screening. It has been shown that rather than forest-dependent spreading, malaria is mainly affected by population movement either for economic or security reasons.6 This mode of transmission could seriously threaten the success of malaria elimination programs in Malaysia. Further studies are needed to evaluate malaria epidemiology in migrant populations to determine how best to adopt strategies to control malaria transmission.
The shape of the epidemic curve suggested a common source outbreak. The first case–patient developed the symptoms on May 28, 2012, the outbreak started after the onset of the second case 20 days later, and the spreading peaked within a week, and the cases slowly peaked by June 25, 2012. The index case was possibly a migrant rubber tapper. The presence of P. falciparum gametocyte in his blood film for malaria parasite slide, the presence of the same parasite in all the cases, and the incubation period of 9–14 days of this Plasmodium along with his arrival 1 month back to this locality, all suggested introduction of the P. falciparum to the locality. In the interiors of Penampang, during the past 3 years, rubber cultivation had increased due to escalating rubber price and government initiatives. The impact was the influx of migrant workers, mostly undocumented immigrants from Indonesia. A large reservoir of infections would make the gametocytes available to the malaria vectors leading to stable and continuous transmission. In this outbreak, the high gametocytes prevalence rates indicated that the reservoir of malaria infections in the locality was higher. Under permissive climatic conditions, the infectious vector population could increase, leading to higher rates of malaria prevalence.7
This study also highlighted the importance of the environmental condition that was conducive for mosquitoe breeding (stagnant water) remains to be an important factor for malaria outbreak even in a low endemicity area as shown in previous studies.8,9 The vector An. balabacensis females mainly fed outdoors, with peak activity between 2200 and 0200 hours, but will also feed indoors and rest outdoors afterward, although females have also been observed feeding outdoors throughout the night with a peak activity between 1900 and 2000 hours, whereas indoor feeding peaked between 2200 and 2300 hours.10,11 The larvae of An. balabacensis was found in the stagnant water near the buffalo fields and in water containing the footprints of the buffaloes. Most likely the villagers were infected while passing by the stagnant water (the footprint of the buffaloes) or stayed in houses near the buffaloes fields. Although the vector was different, a study in Gambia showed that malaria vector density was affected by the distance of a settlement from the water source.12
The factors affecting the transmission of malaria include human behavior, endurance of malaria parasites, conditions of social and health facilities, knowledge, and attitude of the community toward malaria agents, transmission, treatment-seeking behavior, and presence of mosquitoes control activities.13 Despite living near stagnant water increases the risk of getting malaria infection, the human behavior also plays an important role in preventing malaria. Okech et al.14 have shown that using mosquito reduction measures, including environmental management, the usage of mosquito repellent and smoke, insecticide canister sprays, window and door screens, and the use of an insecticide-treated bed nets resulted in 30% reduction of malaria cases in 4 years. Similarly, our findings have shown that a person that did not use the insecticide-treated bed nets was 4.1 times more likely to get malaria infection. The impact of malaria reduction using insecticide-treated bed nets was significant and more effective especially if combined with other control methods, such as larval habitat source reduction and residual spray.15,16
The outbreak of malaria exists in this locality with the overall attack rate of 23.4 per 1,000 of the population. However, in this outbreak, an outdoor activity, such as rubber tapping and buffalo rearing increased the risk of malarial infection. Therefore, most of the cases were adults, and the male gender was predominant. The case fatality rate for this outbreak was 9.1% which was comparable with reports elsewhere at 7.1%.17 The locations of affected houses were near to the buffaloes fields. Even though the fatality case house was far from the buffalo fields, she owned a few buffaloes at the fields and was always visiting them in the early evenings. All rubber tappers have to pass by the buffalo fields to go to the rubber plantations.
In elimination programs, potential transmission foci are identified from data reported by passive case detection.18 Therefore, we think passive case detection should be continued in Malaysia. However, more awareness is needed among the health care personnel, so that early detection can be done to avoid morbidity and mortality. Many reports suggest that very low parasitemia and asymptomatic carriage that can still cause on going transmission.19 In this study, we found that all cases had symptoms, this was consistent with higher parasitemias having fever and being detected by microscopy. The fact that there have been no further reports of transmission in the village suggest that asymptomatic infections were not important in the transmission cycle in this setting, even if they were present and missed by our microscopy.
The limitation of this study was that we could not use genotyping for malaria which may have helped with incrimination of the source of the outbreak. The strength of the study was that we have detected that immigrant laborers are one of the challenges in the elimination stage of malaria control in Malaysia. We also managed to find out the risk factors and vectors contributing to malaria transmission in Sabah.
In conclusion, an outbreak of P. falciparum was detected in K village which was possibly transmitted from a migrant worker and spread by the vector An. balabacensis. Risk factors were rubber tapping activity and passing by stagnant water bodies. Integrated vector management, malaria screening, social mobilization, and campaign were instituted. Cooperation from the Rubber Board and landowners is required to prevent future outbreak.
REFERENCES
- 1.Unicef , World Health Organization , 2015. Achieving the Malaria MDG Target: Reversing the Incidence of Malaria 2000–2015 Available at: www.who.int/malaria/publications/atoz/9789241509442/en. Accessed September 2015.
- 2.World Health Organization , 2016. World Malaria Report. License: CC BY-NC-SA 3.0 IGO. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 3.Ministry of Health Malaysia , 2011. National Strategic Plan for Elimination of Malaria, 2011–2020. Putrajaya. [Google Scholar]
- 4.World Health Organization , 2002. Social Mobilization and Training Team: Malaria Entomology and Vector Control. WHO/CDS/CPE/SMT/2002.18 Rev.1 2v. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 5.White NJ, Breman JG, Osler W, 2012. Chapter 210. Malaria. Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds Harrison’s Principles of Internal Medicine, 18th edition. New York, NY: McGraw-Hill. [Google Scholar]
- 6.Nigoon Jitthai, 2013. Chapter 4 Migration and malaria, in Mekong malaria III monograph towards malaria elimination in the greater Mekong subregion. The Southeast Asian Journal of Tropical Medicine and Public Health 44 (Suppl 1): SEAMEO TropMed, Bangkok, Thailand, 166–200; discussion 306–167.24159832 [Google Scholar]
- 7.Wanjala CL, Waitumbi J, Zhou G, Githeko AK, 2011. Identification of malaria transmission and epidemic hotspots in the western Kenya highlands: its application to malaria epidemic prediction. Parasit Vectors 4: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giha HA, Rosthoj S, Dodoo D, Hviid L, Satti GM, Scheike T, Arnot DE, 2003. The epidemiology of febrile malaria episodes in an area of unstable and seasonal transmission. Trans R Soc Trop Med Hyg 94: 645–651. [DOI] [PubMed] [Google Scholar]
- 9.Alemu A, Tsegaye W, Golassa L, Abebe G, 2011. Urban malaria and associated risk factors in Jimma town, south-west Ethiopia. Malar J 10: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohani A, Lokman Hakim S, Hassan AR, Chan ST, Ong YF, Abdullah AG, Lee HL, 1999. Bionomics of Anopheles balabacensis baisas, the principal malaria vector in Ranau, Sabah. Trop Biomed 2: 31–38. [Google Scholar]
- 11.Sinka ME, et al. 2011. The dominant anopheles vector of human malaria in the Asia Pacific region: occurrence data, distribution maps and bionomic precis. Parasite Vectors 4: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindsay SW, Alonso PL, Armstrong Schellenberg JR, Hemingway J, Adiamah JH, Shenton FC, Jawara M, Greenwood BM, 1993. A malaria control trial using insecticide-treated bed nets and targeted chemoprophylaxis in a rural area of the Gambia, west Africa. 7. Impact of permethrin-impregnated bed nets on malaria vectors. Trans R Soc Trop Med Hyg 87 (Suppl 2): 45–51. [DOI] [PubMed] [Google Scholar]
- 13.Tilaye T, Deressa W, 2007. Prevalence of urban malaria and associated factors in Gondar town, northwest Ethiopia. Ethiop Med J 45: 151–158. [PubMed] [Google Scholar]
- 14.Okech BA, Mwobobia IK, Kamau A, Muiruri S, Mutiso N, Nyambura J, Mwatele C, Amano T, Mwandawiro CS, 2009. Use of integrated malaria management reduces malaria in Kenya. PLoS One 3: e4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yakob L, Yan G, 2009. Modeling the effects of integrating larval habitat source reduction and insecticide treated nets for malaria control. PLoS One 4: e6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Protopopoff N, Wright A, West PA, Tigererwa R, Mosha FW, Kisinza W, Kleinschmidt I, Rowland M, 2015. Combination of insecticide treated nets and indoor residual spraying in northern Tanzania provides additional reduction in vector population density and malaria transmission rates compared to insecticide treated nets alone: a randomised control trial. PLoS One 10: e0142671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reyburn H, et al. 2005. Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA 293: 1461–1470. [DOI] [PubMed] [Google Scholar]
- 18.Rundi C, 2011. Malaria Elimination in Malaysia Third annual APMEN technical and business meeting, May 9–12, 2011; Kota Kinabalu, Malaysia. Available at: http://apmen.org/apmen-iii-meeting-proceedings/. Accessed May 3, 2012.
- 19.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L, 2013. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther 11: 623–639. [DOI] [PubMed] [Google Scholar]