Abstract.
Children in the Bolivian Andes are exposed to endemic infections and meager nourishment, and live under poor hygienic conditions. The prevention of children malnutrition is a priority in many countries including Bolivia. In this study, the health status of schoolchildren in Taraco, a Puna district, at 4,000 meters above sea level (masl) and in Caranavi, at 650 masl in the wealthier subtropical valleys, was compared. The weight, height, and hematological and biochemical parameters in blood, parasites in stool, and clinical information in 120 children from rural Taraco and in 96 from semi-urban Caranavi, both predominantly of Aymara ethnicity, were registered. Eleven percent of Taraco children were undernourished compared with 3% in Caranavi. Instead, 41% of the children in Caranavi were obese or overweight, compared with 8% in Taraco. Anemia was found in 74% of the children in Taraco compared with 7% in Caranavi. Albumin levels were normal in all samples, albeit lower in Taraco. Similar and normal serum zinc levels were measured in both groups. Approximately 60% of the children in both locations showed insufficient vitamin D levels, with lower levels in Taraco children. Hymenolepis nana and Entamoeba coli, parasites determinant of poor hygienic conditions, were respectively detected in 78% and 21% of fecal samples from Taraco, and in 29% and 8% of samples from Caranavi. We show increased anemia, nutritional deficiencies, and indications of poor hygienic conditions in highlands compared with lowlands. The prevalence of obesity in the lowlands demands addressing diverse nutritional deficiencies in the regions of Bolivia.
INTRODUCTION
Although geographically dispersed, the global number of children living at high altitude is substantial. Child mortality remains high in many of the high-altitude regions. Across the world, the challenges to health are similar, related to hypoxia, other environmental conditions, and sociocultural vulnerabilities.1 Bolivia is one of the most poorly developed Latin American countries, with the highest prevalence of undernutrition among the Andean countries.2 Data from Peru and Bolivia show that neonatal and infant mortality increase with higher altitude.3 The low-oxygen concentration in high altitude may on its own have a negative influence on children’s growth. However, ethnic groups living in the higher parts of the world have developed defined genetic and physiological changes to high-altitude hypoxia, particularly in the regulatory systems of oxygen respiration and blood circulation.4 Moreover, some of the environmental hardships at high altitude include, but are not limited, to decreased ambient oxygen tension, increased solar radiation, extreme diurnal ranges in temperature, arid climate, and poor soil quality.5 Child growth retardation has also been associated with social and economic status rather than with genetic or physiologic adaptations to hypobaric hypoxia.6
Indigenous groups (Aymaras, Quechuas, and others) still represent most of the people living in Bolivia. This population is overwhelmingly concentrated in high and cold areas with low agricultural productivity. As a consequence, with a relatively narrow range of foods and low nutritional content, children in the highlands may be exposed to food insecurity and nutritional deficiencies.
Here, we measured key determinants of health in school children from Taraco, a rural district typical of the Bolivian highlands (Puna at 4,000 masl) and compared them to determinants from children living in Caranavi, a semi-urban area in the Yungas at lower altitude (600 masl). Differences in these determinants are key in the implementation of health policies to improve life quality in both regions.
MATERIAL AND METHODS
Ethics.
This study was approved by the Ethics Committee of CEI-UMSA (Universidad Mayor de San Andrés) in Bolivia. Individual written informed consents were approved by children’s parents. School teachers and health professionals from the Health Center of each location were also informed and authorized the project.
Study site.
The study was conducted in the Taraco district situated on the shores of Lake Titicaca at an altitude between 3,810 and 4,050 masl, with a permanent cold climate (7.9°C mean yearly temperature) and dry vegetation, characteristics of the Puna ecoregion. The total population is 6,700 habitants of which 99.9% are Aymara. Health and basic service conditions are regular to inappropriate; 55% have piped water supply, barely 30% have a flush toilet or a latrine, and most (99%) of the households lack piped sewers.7 The economy in Puna relies on fishing, cattle raising, and agriculture.
The second study site, Caranavi, is located in the subtropical region of the Yungas, at an altitude of 600–980 masl. The climate is warmer (23°C mean yearly temperature) with tropical vegetation. Caranavi has 51,307 habitants of which 87% are Aymara, 12% are Quechua, and the rest are of mixed origin. Health conditions are better than in Puna, 60% of the population have piped water supply, 60% have flush toilets or latrines, and 33% have sanitary sewers.7 Coffee production, agriculture, and trading constitute the major economic activities.
Participants.
Samples and information from school children aged 6–12 years were collected from October to November 2014 in Taraco, and November 2015 in Caranavi. A total of 120 school children in Taraco were enrolled in the study compared with 96 from Caranavi.
Venous blood samples were taken in the morning (between 8:00 and 12:00 am), all children were fasting until blood sample was drawn and plasma and blood cells were separated. Samples were immediately analyzed for hematological parameters and placed at −20°C until other tests were performed. Fecal samples were collected for copro-parasitological analysis. Children that had not abstained from eating were excluded.
Anthropometric determinations.
Height was measured with mounted mobile stadiometer (Seca. Germany). Weight was measured using a portable mechanical scale (Seca). All measurements were performed and instruments were calibrated according to the Centers for Disease Control and Prevention (CDC) Anthropometry Procedures Manual of the National Health and Nutrition Examination Program by trained study staff. Body mass index (BMI) (kg/m2) was calculated as weight divided by the square of length. Age- and sex-specific percentiles and the corresponding z-scores were determined by using the CDC and World Health Organization (WHO) Child Growth Standards for both height-for-age and BMI-for-age.8
These reference growth charts were used to estimate the prevalence of underweight, overweight, and obesity. A BMI-for-age ≤ 5th percentile classified children as underweight, from 85th to 95th represented overweight, and children with values ≥ 95th were considered obese. Height-for-age (H/A) < −2 z score represented stunting, and a z score < −3 represented severely stunting, according to WHO and CDC. Children anthropometric parameters were calculated using WHO Anthroplus 1.0.4 (WHO Anthroplus software).
Nutritional questionnaire.
A nutritional questionnaire was conducted, using a modified version of the Mediterranean diet quality index for children and adolescents (KIDMED) evaluation test.9 Modifications to the KIDMED done considering the availability of local sources of nutrients, were minor (Table 1). The index ranges from 0 to 7 and is based on an 11-questionnaire test. A score-based classification of the diet was done as following: ≤ 3 low-quality diet (LQD), 4–6 needs to improve diet (ID), and 7 optimal diet (OD).
Table 1.
Modified KIDMED test to assess diet quality in Bolivian populations
| Scoring | |
|---|---|
| +1 | Eats a fruit or drinks fruit juice every day |
| +1 | Consumes fresh or cooked vegetables regularly once a day |
| +1 | Consumes fish regularly (at least one to two times per week) |
| −1 | Consumes more than once a week any fast food (hamburger, fried chicken) |
| +1 | Consumes pasta or rice almost every day (four or more times per day) |
| +1 | Consumes cereals or bread for breakfast |
| −1 | Skips breakfast |
| +1 | Consumes a dairy product for breakfast (yoghurt, milk, etc.) |
| −1 | Consumes biscuits or pastries for breakfast |
| +1 | Consumes cheese at least three times a week |
| −1 | Consumes candies several times per day |
Score: 7 optimal diet, 3–6 needs improvement, < 3 low-quality diet.
Hematological and biochemical parameters.
Blood groups.
ABO and Rhesus (Rh) blood groups were determined by a hemagglutination technique using specific antibodies.
Hemoglobin.
Hemoglobin (Hb) was measured by a HemoCue analyzer (HemoCueHb 201, Sweden). WHO references with different ranges related to the altitude were used. In Puna, anemia was considered mild at 14.5–14.9 g/dL; moderate 11.5–14.4 g/dL, or severe < 11.5 g/dL according to published levels for healthy residents in altitude. In the Yungas, mild anemia was defined as 11–11.4 g/dL, moderate 8–10.9 g/dL, and severe < 8 g/dL.
Hematocrit.
After mixing heparinized blood samples, capillary tubes were centrifuged for 5 minutes at 2,500 g. Microhaematocrit tubes and a reader (Hawksley & Sons Ltd., Sussex, United Kingdom) were used to calculate the final percentage.
Vitamin D, albumin, and zinc.
Levels of 25(OH)-vitamin D (vitD) in serum were measured using a commercial enzyme-linked immunosorbent assay kit (Immundiagnostik AG, Bensheim, Germany). Subjects were classified as vitD sufficient if > 75 nmol/L; insufficient 30–75 nmol/L; and deficient < 30 nM.
Albumin and zinc were measured in samples using a Cobas 8000 modular analyser (Roche). Tested normal range used for albumin was 3.6–4.8 g/dL and for zinc 11–18 μM.10
Copro-parasitological analysis.
Parasitological analysis to determine helminths and protozoa in fecal samples in children from both locations was made using the Ritchie and Lumbreras tests.11 The presence of the helminth Hymenolepis nana and the protozoa Entamoeba coli, considered as indicators of oral–fecal contamination, were recorded.
Statistics.
Descriptive statistics, including frequency distributions for categorical variables (e.g., age, sex) and mean (±SD) and median for continuous variables are presented. A χ2 test was used to compare frequencies between different categories (e.g., severe or moderate anemia) or to compare frequencies of events in the case of noncontinuous variables.
For comparisons of different parameters stratified by age or gender between the locations a two-way analysis of variance (ANOVA) was used. The Spearman rank correlation test was used to test the correlation between parameters. All tests were performed using GraphPad Prism 6.0 (GraphPad Software Inc.). The mean ± SD of normally distributed parameters was calculated and depicted.
RESULTS
Anthropometric measurements.
The BMI-for-age mean presented in z-scores in children from Taraco was −0.24 considerably lower than in Caranavi (0.74) (Figure 1A).
Figure 1.
Body mass index (BMI)-for-age and height-for-age in school children from Taraco and Caranavi. The individual and mean BMI-for-age of school children in Taraco and Caranavi are depicted (A). Differences between groups are significant at *P < 0.05 (unpaired Student’s t test). Using Centers for Disease Control and Prevention age and gender BMI-for-age charts, children were classified as undernourished (UN); overweight (OW); and obese (OB). The frequency of UN, OW, and OB children is depicted (B). Children classified as normal are not shown. Differences between both populations are significant at *P < 0.05 and ****P < 0.0001; χ2 test. BMI-for-age was stratified by gender (C) and age (D), differences are significant ****P < 0.0001; Student t test. Height-for-age z-scores (H/A) are represented as a distribution curve from each population (nonlinear fit, goodness of fit 0.97 and 0.96 in Taraco and Caranavi). Differences between the frequencies of H/A in both population are significant (P < 0,0001 unpaired Student’s t test) (E). The difference in the frequencies of stunted children in both localities was not statistically significant (P < 0.08 χ2 test) (F).
According to the CDC growth chart, 11% of children in Taraco were undernourished compared with 3% in Caranavi. Seven percent of children in Taraco were overweight and 1% was classified as obese. This can be compared with 18% children in Caranavi that were overweight and an alarming 23% classified as obese (Figure 1B).
A lower BMI-for-age in Taraco compared with Caranavi was seen in all age groups (6–7, 8–10, and 11–12 years old) and in both genders (Figure 1C, D).
Height-for-age z-scores in children from both populations were calculated to define stunting. The height-for-age in children from Taraco was lower than those from Caranavi (Figure 1E). In Taraco, 5.0% of children were stunted and 1.7% severely stunted, whereas 1.1% of children were stunted and no severe cases were found in Caranavi (Figure 1F).
Blood parameters.
The information from a population census indicates that children from both sites are likely to share a common Aymaran ancestry. In support of this information we found that 97% and 94% of children in Taraco and Caranavi, respectively, were blood group O. Blood group A was found in 2% of the children in Taraco and in 5% of those in Caranavi. Blood group B was only detected 1% of the children at both sites. The Rh factor was expressed by all samples from Taraco and in 99% from Caranavi. Blood group O Rh+ is expressed in most of the American indigenous populations.
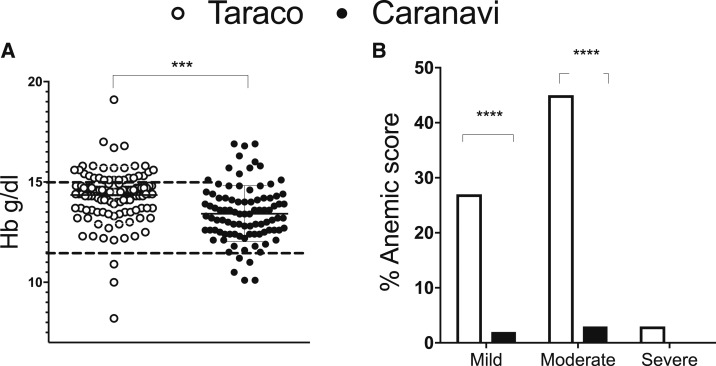
The Hb levels were, as expected by the elevation, higher in Taraco (Figure 2A). However, after correction because of altitude 26% of the children in Taraco were classified with mild; 45% moderate, and 3% with severe anemia (Figure 2B). The frequency of children with anemia was higher in all age groups and in both genders in Taraco compared with those in Caranavi (Figure 2B, Table 2). The hematocrit (Ht) levels positively correlated (r = 0.60 P < 0.0001) with Hb values (data not shown).
Figure 2.
Frequency of anemia in school children from Taraco and Caranavi. The hemoglobin levels in individual blood samples the mean per group are depicted (A). The different cutoff for normal levels in both populations is indicated. Differences between groups are significant at ***P < 0.001; Student’s t test. The frequencies of mild, moderate, and severe anemia are depicted (B). Differences are significant at P < 0.0001; χ2 test.
Table 2.
Levels of Hemoglobin and hematocrit (Ht) in blood and the frequency of anemic school children in Taraco and Caranavi
| Taraco (Puna) | Caranavi (Yungas) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | |||||||
| Stratification | N° | Hemoglobin (g/dL) * | Ht (% volume) | Anemia N° (%) | N° | Hemoglobin (g/dL)* | Ht (% volume) | Anemia N° (%) |
| Gender | ||||||||
| Male | 59 | 14.34 ± 0.14*** | 44.21 ± 0.65**** | 46 (78) # | 42 | 13.67 ± 0.23 | 39.52 ± 0.40 | 3 (7) |
| Female | 60 | 14.37 ± 0.22 **** | 43.91 ± 0.63**** | 42 (70)# | 51 | 13.23 ± 0.17 | 38.85 ± 0.33 | 4 (8) |
| Age group | ||||||||
| 6–7 | 25 | 13.9 ± 0.18 ** | 42.68 ± 0.95**** | 21 (84)# | 28 | 13 ± 0.25 | 38.07 ± 0.57 | 3 (11) |
| 8–10 | 59 | 14.6 ± 0.18**** | 44.32 ± 0.66**** | 41 (69)# | 53 | 13.4 ± 0.18 | 39.22 ± 0.28 | 4 (8) |
| 11–12 | 35 | 14.2 ± 0.25 | 44.31 ± 0.87*** | 26 (74)# | 12 | 14.3 ± 0.48 | 41.17 ± 0.50 | 0 (0) |
| Total | 119 | 14.35 ± 0.13 | 44.06 ± 0.45**** | 88 (74)# | 93 | 13.43 ± 0.14 | 39.16 ± 0.26 | 7 (8) |
Differences between schoolchildren in Taraco and Caranavi are significant at **P < 0.01; ***P < 0.001 and ****P < 0.0001 student t test and analysis of variance analysis. Differences in frequencies of anemic children between Taraco and Caranavi are significant at #P < 0.0001 Fisher’s exact test.
Albumin, zinc and vitamin D.
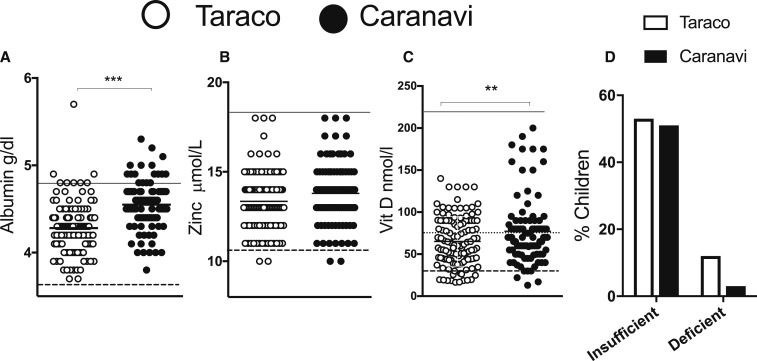
Albumin and zinc levels were within normal ranges in both samples (Figure 3A, B and Table 3). However, the albumin levels were lower in children from Taraco than in those from Caranavi in all age groups tested, and for both genders (Table 3).
Figure 3.
Albumin, zinc and vitamin D (vitD) levels in plasma from children in Taraco and Caranavi. The mean ± SEM in each population is depicted. The normal ranges in plasma for albumin (A), the cut off for Zn (B) and the levels in which vitD (C) is considered normal, deficient or insufficient are shown. Differences between the albumin and vitD concentration in both populations are significant at P < 0.001; Student’s t test. The frequency of children with insufficient and deficient vitD levels in Tarcao and Caranavi are reported (D).
Table 3.
Levels of albumin, zinc and vitamin D (vitD) in plasma samples from schoolchildren from the Taraco and Caranavi stratified by age and gender
| Taraco (Puna) | Caranavi (Yungas) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | |||||||
| Stratification | N° | Albumin (g/dL) | Zinc (μmol/L) | VitD (nmol/L) | N° | Albumin (g/dL) | Zinc (μmol/L) | Vit D (nmol/L) |
| Gender | ||||||||
| Male | 55 | 4.28 ± 0.05**** | 13.44 ± 0.23 | 64.5 ± 3.54 | 41 | 4.56 ± 0.04 | 13.88 ± 0.26 | 72.76 ± 5.88 |
| Female | 57 | 4.26 ± 0.03**** | 13.16 ± 0.22 | 65.82 ± 4.15* | 50 | 4.52 ± 0.04 | 13.72 ± 0.26 | 84.96 ± 7.10 |
| Age group (y) | ||||||||
| 6–7 | 22 | 4.16 ± 0.04**** | 13.2 ± 0.25 | 71.82 ± 6.09 | 26 | 4.47 ± 0.06 | 13.6 ± 0.27 | 84.92 ± 6.35 |
| 8–10 | 58 | 4.31 ± 0.04**** | 13.2 ± 0.28 | 64.76 ± 4.20 | 53 | 4.5 ± 0.03 | 14.03 ± 0.30 | 79.27 ± 7.00 |
| 11–12 | 32 | 4.30 ± 0.05**** | 13.4 ± 0.31 | 61.26 ± 4.05 | 12 | 4.6 ± 0.07 | 13.7 ± 0.41 | 69.42 ± 12.41 |
| Total | 112 | 4.28 ± 0.03**** | 13.62 ± 0.26 | 65.98 ± 2.67** | 91 | 4.55 ± 0.03 | 13.79 ± 0.18 | 79.43 ± 4.76 |
Differences between samples in Taraco and Caranavi are significant at **P < 0.01; ****P < 0.0001 student’s t test and analysis of variance.
In Taraco, 11% of children showed vitD deficiency and 52% had insufficient vitD levels, whereas in Caranavi vitD levels were considered deficient in 3% and insufficient in 55% of the children (Figure 3C, D). There was no difference in vitD levels between age groups. Females from Taraco showed lower vitD levels than those from Caranavi, whereas the concentrations of vitD in males in both populations were similar (Table 3). There was no correlation between vitD and the other parameters assessed (not shown).
Fecal contamination, stool consistency, and quality of diet.
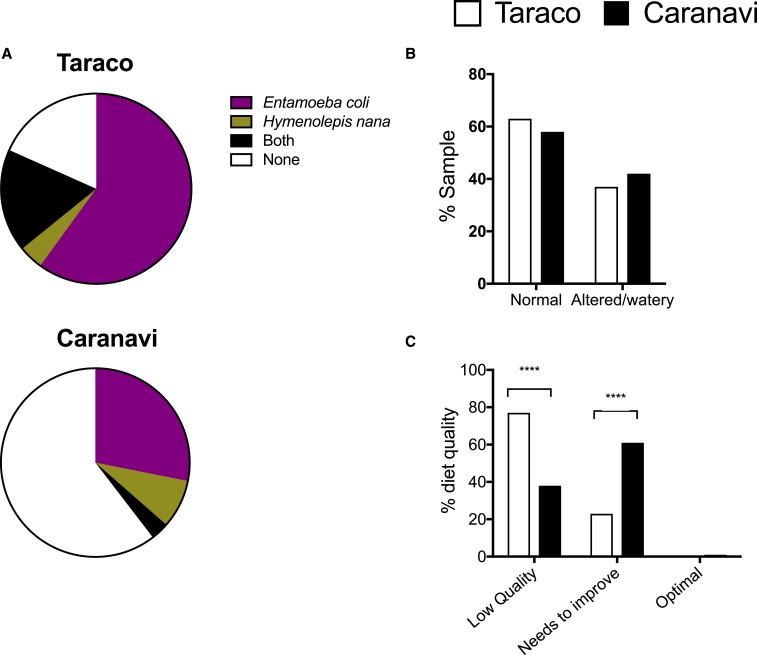
The prevalence of the helminth H. nana and the protozoa E. coli in stool samples were used as markers of ingestion of contaminated water or food. Entamoeba coli and H. nana were found in 82% of the samples in Taraco versus 39% in Caranavi (Figure 4A). Higher frequencies of these parasites were found also in both genders in Taraco and in the different age groups in Taraco compared with Caranavi (Table 4). The frequency of children harbouring both parasites was also higher in Taraco than Caranavi (Table 4). However, the frequency of children with abnormal or watery stool consistence in both localities was similar and high (Figure 4B).
Figure 4.
Faecal contamination, stool consistency and quality of diet in school children from Taraco and Caranavi. Entamoeba coli and Hymenolepis nana prevalence in Puna and Yungas study sites. The proportion of samples containing either E. coli, H. nana, both parasites or none is illustrated in pie charts (A). Differences in the frequency of children with parasites between both locations are significant at P < 0.0001; χ2 test. The percentage of children with watery/altered vs. normal stool consistency in both locations is depicted (B). The frequency of children in both locations with low-quality diet, diet with needs of improvement or optimal diet is depicted (C). Differences between the quality of diets in both locations are significant at P < 0.0001; χ2 test. This figure appears in color at www.ajtmh.org.
Table 4.
Frequencies of parasites that indicate contamination in stool samples from school children from the Bolivian Highlands and Tropical Valleys stratified by age and gender
| Location | N° | No (%) | ||||
|---|---|---|---|---|---|---|
| Entamoeba coli | Hymenolepis nana | E. coli + H. nana | Total positive | |||
| Taraco (Puna) | Gender | |||||
| Male | 59 | 36 (61)*** | 4 (7) | 8 (13) | 48 (81)**** | |
| Female | 61 | 36 (59)** | 1 (2) | 13 (22)** | 50 (82)**** | |
| Age group | ||||||
| 6–7 | 25 | 16 (64)* | 3 (12) | 4 (16)* | 23 (92)**** | |
| 8–10 | 60 | 35 (58)* | 2 (4) | 11 (18)* | 48 (80)**** | |
| 11–12 | 35 | 21 (60)* | 0 (0) | 6 (17) | 27 (77)** | |
| Total | 120 | 72 (60)**** | 5 (4) | 21 (18)*** | 98 (82)**** | |
| Caranavi (Yungas) | Gender | |||||
| Male | 43 | 10 (23) | 6 (14) | 2 (5) | 18 (42) | |
| Female | 53 | 17 (32) | 2 (4) | 1 (2) | 20 (38) | |
| Age group | ||||||
| 6–7 | 28 | 9 (32) | 2 (7) | 0 (0) | 11 (39) | |
| 8–10 | 55 | 15 (27) | 6 (10) | 3 (5) | 24 (44) | |
| 11–12 | 13 | 3 (23) | 0 (0) | 0 (0) | 3 (23) | |
| Total | 96 | 27 (28) | 8 (8) | 3 (3) | 38 (39) | |
Differences in frequencies of parasite containing stools between stratified groups in different localities at *P < 0.05; **P < 0.01; ***P < 0.001 and p < 0.0001 Fisher’s exact test.
Based on our modified KIDMED questionnaire we found that the quality of diet in Puna was lower than the one in the Yungas: 77% of children in Taraco had a LQD and the remaining had a regular diet with need for improvement (NI). Instead, in Caranavi, 38% of children reported a LQD, 61% a diet that NI, and 1% reported an optimal diet (Figure 4C). No associations between the presence of E. coli and/or H. nana, stool consistency, and nutritional information were found (data not shown).
DISCUSSION
We compared the health status of Bolivian school children from Puna and the Yungas, by means of anthropometry and different clinical and nutritional parameters. Based on the national census from 2012,7 supported by the blood group antigen typing,12,13 children from both locations likely belong to the Aymara ethnical group. Major differences between the sites include those associated with the altitude as well as a rural environment in Puna compared with a warmer climate and a more urban community in the Yungas.
A major finding in this cross-sectional study is that 11% of children in the highlands showed signs of undernutrition compared with 3% in the lowlands, using CDC growth charts to normalize the BMI determinations for age and gender. Instead, 18% of the children in the Yungas were overweight and 23% obese (compared with 7% and 1%, respectively, in the Puna). Whereas 6.7% of children in Taraco showed stunted growth compared with 1.1% in Caranavi.
The health of children living at high altitude is shaped not only by the low-oxygen environment, but also by population ancestry and sociocultural determinants. Children health can vary from one population group to another because of both genetic adaptations as well as factors such as nutrition, chronic infections, exposure to pollutants and toxins, socioeconomic status, and access to medical care. Although growth restriction is often reported among high-altitude populations, recent studies suggest that slower linear growth relates to socioeconomic status and the availability of adequate nutrition than to hypobaric hypoxia.5,14 A positive trend in height, with improved living conditions in high altitude, supports this notion.6 In our study, children in Taraco highlands had higher prevalence of fecal–oral contamination and a poorer diet than those living in Caranavi. These findings suggest that children in Puna are at higher risk of being infected with intestinal pathogens, related to the poor sanitary conditions, including contaminated water resources and latrine uses.
Urban children are taller and heavier than their rural counterparts in almost all low-income and middle-income countries. These urban–rural differences are largest in Central Andean Latin America.
Up to 20 years back, research on malnutrition in developing countries focused almost exclusively on undernutrition. However, about 7% of the world population is now considered to be obese.15 In agreement with our data, overweight and obesity were mainly found in urban areas,16 and an inverse association between altitude and obesity has been reported in Peru.17 Our comparison highlights the importance of including prevention of weight gain among the public health nutrition policies in the region. Obesity in childhood predicts adverse health outcomes in adulthood, including a higher risk for cardiovascular disease, type II diabetes, the metabolic syndrome, and different types of cancer among other health problems.18 In coincidence with our observation, cross-sectional and secular studies show that the prevalence rates of overweight and obesity have risen alarmingly in many developing countries.19 Whereas hygienic and nutritional conditions are better in the Yungas, the increased caloric uptake in connection with a relatively higher socioeconomic status are very likely determinants of the increased child overweight/obesity frequency observed.
Anemia is widely prevalent among young children in low- and middle-income countries, affecting 43% of school children worldwide.20 A high percentage of children in Taraco were considered to have moderate or mild anemia. Similar results were obtained when evaluating the Ht levels. In concurrence with our results, children at high altitudes showed significantly lower oxygen saturation, more cyanosis, lower systolic blood pressure, and higher Hb levels.21 Anemia can be relatively well tolerated in healthy children, but may constitute a risk factor for pneumonia.22 In the highlands, the availability of iron-rich food is scarce. The diet for children typically consists of carbohydrates with few vegetables; meats only being served occasionally. A systematic review revealed a low average intake of iron and other micronutrients in Andean highlands.23 Although not analyzed in this study, low iron levels may be a major reason for anemia observed in these children. Iron supplementation has shown to revert high levels of anemia in the region.24 Given the high prevalence of anemia found in Puna, prevention and treatment should be a priority for school children in this region.
More than 80% of the vitD requirement comes from sunlight exposure.25 However, vitD deficiency is prevalent worldwide, including in some countries with sunny climates.26 Where harsh climate commands, and infants and young children are kept indoors or completely covered when outdoors, rickets may become prevalent. For example, in a community of Tibetan children, 66% had clinical signs of rickets, and vitD levels were deficient in a subsample.14 Low vitD intake and high rates of poverty have been associated with hypovitaminosis D.25,27 Although the perceived importance of vitD was initially limited to bone health and calcium homeostasis, over the past decades its function in other tissues has been appreciated. VitD may be involved in the etiology of type 2 diabetes28 and may play a role in the homeostasis of the immune system and the susceptibility to infections.29
In few studies, vitD insufficiency was shown to be highly prevalent in South American children, suggesting that vitD intake is of concern in these communities. Studies from the Argentinean highlands, which included children, found lower vitD levels in the highland population compared with those from the lowlands.30 In our study, vitD levels were reduced in the highlands, where vitD deficiency was more prevalent as compared with the lowlands. The diet in the Bolivian highlands relies heavily on potato and other tubers and grains. The extremely low dietary fat intakes (approximately 3–9% of dietary energy from fat) may prevent adequate absorption of fat-soluble vitamins as well as lead to deficiencies of essential fatty acids.
Zinc deficiency is still a common problem in poor populations and impairs the overall immune function and resistance to infections. In particular, zinc intake in children and adults in the Andean region of Bolivia has been reported to be low.23 However, normal zinc levels in serum were found in children from both sites. Although it may be argued that serum zinc is not a reliable marker of transient zinc status, serum zinc levels indicate that no long term or severe depletion was evident in these children.
The parasites E. coli and H. nana were used as markers of low sanitary conditions. These non-pathogenic parasites that are transmitted via the oral–fecal route are frequent commensal parasites in the human digestive tract. These were frequently observed in stool samples from Taraco, demonstrating the poor sanitary conditions in this region. The presence of E. coli or H. nana in stools was not associated with a watery consistence.
Several limitations are important to consider for proper interpretation of our data. Future studies comparing rural and urban locations in lowlands and highlands will contribute to the understanding of the relative importance of altitude versus socioeconomic and cultural conditions in the differences observed. Better defined individual sociodemographic information would have improved the study. It was not possible to collect the samples at the same time in both locations; despite this, samples were collected at the same season in consecutive years. The nutritional questionnaire used, although adapted to local conditions, has not been validated, although we believe is appropriate for the study.
Altogether, we showed that children in the highlands have severe nutritional concerns including undernourishment, anemia and a moderate to severe hypovitaminosis D. The lack of proper hygienic conditions, probably amplify the deprived condition. However, many of the school children in Yungas were considered overweight or obese, probably because of increased consumption of high levels of carbohydrates, sugar, and saturated fat contained in fast food, with low levels of vitamins, fibers, or proteins. Our data highlight the different challenges to improve the nutritional and hygienic conditions in children living in the highlands and lowlands of the region.
Acknowledgments:
We would like to thank Reynaldo Jimenez and the Health Center of Taraco staff for their help in coordinating activities in the primary schools.
REFERENCES
- 1.Niermeyer S, Andrade Mollinedo P, Huicho L, 2009. Child health and living at high altitude. Arch Dis Child 94: 806–811. [DOI] [PubMed] [Google Scholar]
- 2.Larrea C, Freire W, 2002. Social inequality and child malnutrition in four Andean countries. Rev Panam Salud Publica 11: 356–364. [DOI] [PubMed] [Google Scholar]
- 3.Edmonston B, Andes N, 1983. Community variations in infant and child mortality in Peru. J Epidemiol Community Health 37: 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beall CM, et al. 1998. Hemoglobin concentration of high-altitude Tibetans and Bolivian Aymara. Am J Phys Anthropol 106: 385–400. [DOI] [PubMed] [Google Scholar]
- 5.Leonard WR, 1989. Nutritional determinants of high-altitude growth in Nunoa, Peru. Am J Phys Anthropol 80: 341–352. [DOI] [PubMed] [Google Scholar]
- 6.Pawson IG, Huicho L, Muro M, Pacheco A, 2001. Growth of children in two economically diverse Peruvian high-altitude communities. Am J Hum Biol 13: 323–340. [DOI] [PubMed] [Google Scholar]
- 7.Instituto Nacional de Estaditica , 2012. Censo Nacional de Poblacion y Vivienda Available at: http://www.censosbolivia.bo/. Accessed February 7, 2017.
- 8.National Center for Health Statistics , 2009. Clinical Growth Charts. Available at: https://www.cdc.gov/growthcharts/clinical_charts.htm. Accessed February 7, 2017.
- 9.Sahingoz SA, Sanlier N, 2011. Compliance with mediterranean diet quality index (KIDMED) and nutrition knowledge levels in adolescents. A case study from Turkey. Appetite 57: 272–277. [DOI] [PubMed] [Google Scholar]
- 10.Labmedicin Skåne , 2017. Analysportalen: Zink. Available at: http://www.analysportalen-labmedicin.skane.se/viewAnalys.asp?Nr=2462. Accessed February 7, 2017.
- 11.Centers for Disease Control and Prevention , 2005. Stool Specimens. Diagnostic Procedures. Laboratory Identification of Parasitic Diseases of Public Health Concern. Available at: http://www.cdc.gov/dpdx/diagnosticProcedures/stool/specimencoll.html. Accessed February 7, 2017.
- 12.Best WR, Layrisse M, Bermejo R, 1962. Blood group antigens in Aymara and Quechua speaking tribes from near Puno, Peru. Am J Phys Anthropol 20: 321–329. [DOI] [PubMed] [Google Scholar]
- 13.Matson GA, Swanson J, Robinson A, 1966. Distribution of hereditary blood groups among Indians in South America. 3. In Bolivia. Am J Phys Anthropol 25: 13–33. [DOI] [PubMed] [Google Scholar]
- 14.Harris NS, Crawford PB, Yangzom Y, Pinzo L, Gyaltsen P, Hudes M, 2001. Nutritional and health status of Tibetan children living at high altitudes. N Engl J Med 344: 341–347. [DOI] [PubMed] [Google Scholar]
- 15.Smith KB, Smith MS, 2016. Obesity statistics. Prim Care 43: 121–135. [DOI] [PubMed] [Google Scholar]
- 16.Morales R, Aguilar AM, Calzadilla A, 2004. Geography and culture matter for malnutrition in Bolivia. Econ Hum Biol 2: 373–389. [DOI] [PubMed] [Google Scholar]
- 17.Woolcott OO, Gutierrez C, Castillo OA, Elashoff RM, Stefanovski D, Bergman RN, 2016. Inverse association between altitude and obesity: a prevalence study among andean and low-altitude adult individuals of Peru. Obesity (Silver Spring) 24: 929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raman RP, 2002. Obesity and health risks. J Am Coll Nutr 21: 134S–139S. [DOI] [PubMed] [Google Scholar]
- 19.Bhurosy T, Jeewon R, 2014. Overweight and obesity epidemic in developing countries: a problem with diet, physical activity, or socioeconomic status? Sci World J 2014: 964236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, Pena-Rosas JP, Bhutta ZA, Ezzati M; Nutrition Impact Model Study Group , 2013. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health 1: e16–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moschovis PP, et al. 2013. Childhood anemia at high altitude: risk factors for poor outcomes in severe pneumonia. Pediatrics 132: e1156–e1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lozano JM, 2001. Epidemiology of hypoxaemia in children with acute lower respiratory infection. Int J Tuberc Lung Dis 5: 496–504. [PubMed] [Google Scholar]
- 23.Berti PR, Fallu C, Cruz Agudo Y, 2014. A systematic review of the nutritional adequacy of the diet in the central Andes. Rev Panam Salud Publica 36: 314–323. [PubMed] [Google Scholar]
- 24.Berger J, Aguayo VM, Tellez W, Lujan C, Traissac P, San Miguel JL, 1997. Weekly iron supplementation is as effective as 5 day per week iron supplementation in Bolivian school children living at high altitude. Eur J Clin Nutr 51: 381–386. [DOI] [PubMed] [Google Scholar]
- 25.Holick MF, 2007. Vitamin D deficiency. N Engl J Med 357: 266–281. [DOI] [PubMed] [Google Scholar]
- 26.Peters BS, dos Santos LC, Fisberg M, Wood RJ, Martini LA, 2009. Prevalence of vitamin D insufficiency in Brazilian adolescents. Ann Nutr Metab 54: 15–21. [DOI] [PubMed] [Google Scholar]
- 27.Moore CE, Murphy MM, Holick MF, 2005. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr 135: 2478–2485. [DOI] [PubMed] [Google Scholar]
- 28.Liu E, Meigs JB, Pittas AG, McKeown NM, Economos CD, Booth SL, Jacques PF, 2009. Plasma 25-hydroxyvitamin d is associated with markers of the insulin resistant phenotype in nondiabetic adults. J Nutr 139: 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwalfenberg GK, 2011. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res 55: 96–108. [DOI] [PubMed] [Google Scholar]
- 30.Hirschler V, Maccallini G, Molinari C, Aranda C; San Antonio de los Cobres Study Group , 2013. Low vitamin D concentrations among indigenous Argentinean children living at high altitudes. Pediatr Diabetes 14: 203–210. [DOI] [PubMed] [Google Scholar]