Abstract.
The establishment of a sylvatic reservoir of Zika virus (ZIKV) in the Americas is dependent on the susceptibility of primates of sufficient population density, the duration and magnitude of viremia, and their exposure to the human mosquito-borne transmission cycle. To assess the susceptibility of squirrel (Saimiri sp.) and owl monkeys (Aotus sp.) to infection, we inoculated four animals of each species with ZIKV from the current epidemic. Viremia in the absence of detectible disease was observed in both species and seroconversion occurred by day 28. ZIKV was detected in the spleen of three owl monkeys: one at 7 days postinoculation (dpi) and two at 14 dpi. This study confirms the susceptibility to ZIKV infection of two Neotropical primate species that live in close proximity to humans in South America, suggesting that they could support a widespread sylvatic ZIKV cycle there. Collectively, establishment of a ZIKV sylvatic transmission cycle in South America would imperil eradication efforts and could provide a mechanism for continued exposure of humans to ZIKV infection and disease.
INTRODUCTION
Unlike any other arbovirus, Zika virus (ZIKV) is now known to be the cause of severe neurologic damage in an estimated 1–40% of infants born to mothers with primary infection in pregnancy.1–3 The true magnitude of in utero neurologic damage attributable to ZIKV is not yet known, but the 20-fold increase in microcephaly reported in Brazil during 2015 may significantly underestimate its impact. A critical factor for the development of public health strategies to mitigate the risk of continued human ZIKV disease is whether a sylvatic cycle of ZIKV transmission is likely to become established in South America, as occurred centuries ago for yellow fever virus (YFV) after its importation from Africa.4 Although many factors contribute to the potential for enzootic transmission of ZIKV (e.g., susceptible sylvatic vectors, vector density, proximity to humans with ZIKV infection, and environmental factors), the susceptibility of Neotropical primates to ZIKV infection and the duration of viremia they generate remain unknown and potentially one of the most important factors.5 Given the broad diversity of Neotropical primates in South America, including more than 190 different species and subspecies, the large numbers living in the Amazon Basin as well as tropic forests extending to the north and south, and the proximity of some species (e.g., Saimiri) to human activities, understanding the susceptibility of Neotropical primates to ZIKV infection is critical for predicting future ZIKV circulation.6
Until recently, the data on experimental infections of laboratory animals with ZIKV was limited to a small number of articles that were published in the two decades after its discovery.7 Recent and ongoing studies of ZIKV infection in Old World primates8–11 and rodents12–15 provide potential in vivo models for testing of prevention and treatment strategies, but additional models are necessary to fully characterize pathogenesis in the developing embryo and to broaden our understanding of the potential for establishment of a sylvatic cycle in South America. Neotropical primates have been used as model systems for numerous infectious diseases and are well suited for the study of ZIKV because of the similarity of their placental biology and parturition process with those of humans and other primates. Their gestation periods are 130–152 days, just a few weeks shorter than those of rhesus macaques. Furthermore, if the enhanced susceptibility of Neotropical (Catarrhini) versus Old World primates (Platyrrhini) to YFV and the differential susceptibility to YFV within the various species of Neotropical primates are observed with ZIKV, then future studies can be directed to identify host-specific susceptibility and resistance factors using modern genomic techniques.16,17
We hypothesized that Neotropical primates would support ZIKV replication and develop viremia levels that could support mosquito-borne, sylvatic transmission. In this study, we characterized the dynamics of ZIKV infection in pregnant squirrel monkeys (Saimiri spp.) and nonpregnant owl monkeys (Aotus spp.), Neotropical primates that could be both natural hosts of ZIKV in South America and appealing nonhuman primate models for studies of ZIKV infection. This study provides important new insights into the potential for the environmental maintenance of ZIKV in South America among Neotropical primates.
MATERIALS AND METHODS
Ethics statement.
This study was carried out in strict accordance with the recommendations described in the Guide for the Care and Use of Laboratory Animals and in accordance with the recommendation and requirement of the Office of Laboratory Animal Welfare and the United States Department of Agriculture. All animal work was approved in advance by the University of Texas MD Anderson Cancer Center’s (MDACC) Institutional Animal Care and Use Committee, in Houston, TX (Protocol #0001528-RN00), and all studies were carried out at the Association for Assessment and Accreditation of Laboratory Animal Care accredited Michale E. Keeling Center for Comparative Medicine and Research in Bastrop, TX (Keeling Center). Fetal ultrasound examinations were performed using manual restraint, and all other procedures were carried out under ketamine anesthesia by trained personnel under the supervision of veterinary staff and every effort was made to ameliorate the welfare and to minimize animal suffering in accordance with the “Weatherall report for the use of nonhuman primates” recommendations. All animals were socially housed in pair groups, under controlled conditions of humidity, temperature, and light (12-hour light/12-hour dark cycles) in an animal biosafety level 2-qualified research room at the Comparative Medicine Research Building. Animals were fed commercial monkey chow twice daily and water was available ad libitum. Animal health and welfare was monitored twice daily, and treats and fruit were provided daily by trained personnel. All nonhuman primate euthanasia procedures were carried out in accordance with the AVMA Guidelines for the Euthanasia of Animals: 2013 Edition.
ZIKV.
ZIKV (Strain Mex_1_44) obtained from the current South/Central American outbreak was provided by the World Reference Center for Emerging Viruses and Arboviruses at the University of Texas Medical Branch.18 The virus was cultured in Vero cells (two passages) and diluted to the working concentration of 10e5 plaque-forming units (PFU)/100 μL in normal saline for subcutaneous inoculation.
Clinical and laboratory evaluations.
Animals were observed and evaluated by study veterinarians daily for the first 2 weeks after inoculation and then weekly until the conclusion of the study. Laboratory assessments (hematology and chemistry profiles) were performed at the Michale E. Keeling Center for Comparative Medicine and Research at MDACC using an Advia 120 Hematology System and an Olympus AU400e Serum Chemistry Analyzer, respectively.
Virologic assessments.
Virologic assessments (blood, urine, and saliva specimens for quantitative polymerase chain reaction [qPCR] detection of ZIKV) were performed using established techniques.19 The volume of blood, urine, and saliva required for each virologic assessment was 200 μL. Blood collections in the protocol were minimized (< 3 mL/week) to avoid iatrogenic anemia. Necropsies were performed at the protocol-specified time points for the owl monkeys and tissue specimens were collected in appropriate media for virus cultivation in vitro, DNA extraction, electron microscopy, and routine histologic studies. Tissue viral loads were determined in fresh-frozen tissues obtained at necropsy using established assays.19 The detection of ZIKV was reported as focus-forming units (FFU) per milliliter for the various body fluids because the standards for reverse transcription (RT)-qPCR were titered in vitro based on the FFU/mL. A “conversion factor” for FFU to genome copies was recently reported as ∼600 vRNA copies/FFU.20
Cytokine assays.
Cytokines were measured in the plasma samples from ethylenediaminetetraacetic acid (EDTA) preserved whole blood using Nonhuman Primate Cytokine kit (PRCCYTOMAG-40K/PCYTMG-40K-PX23) with interferon (IFN)-γ, interleukin (IL)-1b, IL-2, IL-4, IL-6, IL-10, IL-13, and IL-17A from Millipore Corporation (Billerica, MA). Plasma concentrations of cytokines were determined using the cytokine bead array methodology according to the manufacturers’ protocols, as previously described.21,22 Briefly, EDTA-preserved plasma samples were centrifuged (14,000 × g for 5 minutes) and aliquots were frozen at −80°C until used. Before assay, once-thawed plasma samples were precleared by centrifuging at 14,000 × g for 5 minutes. The 96-well filter plate was blocked with assay buffer for 10 minutes at room temperature, washed, and 25 μL of standard or control samples were dispersed in appropriate wells. After adding 25 μL of beads to each well, plate was incubated on a shaker overnight at 4°C. After washing twice, the plate was incubated with detection antibody for 1 hour at room temperature and again incubated with 25 μL of streptavidin–phycoerythin for 30 minutes at room temperature. After washing twice, 150 μL of sheath fluid was added and multianalyte profiling was performed on the Bio-Plex 200 system (Luminex X MAP technology, Austin, TX). Calibration microspheres for classification and reporter readings as well as sheath fluid, assay, and wash buffer were also purchased from Bio-Rad (Hercules, CA). Acquired fluorescence data were analyzed by using the Bio-Plex manager 5.0 (from Bio-Rad). All incubations were performed on a gentle shaker. The minimum detectable concentration was calculated by the Multiplex Analyst immunoassay analysis software from Millipore. The minimum detectable concentrations in picograms per milliliter for the various cytokines are as follows: IFN-γ (2.2), IL-2 (0.7), IL-4 (2.7), IL-6 (0.3), IL-10 (6.2), IL-13 (5.8), and IL-17A (1.3).
Plaque (focus) neutralization assay.
Sera were assayed to determine the specific neutralizing antibody titers using the plaque reduction neutralization test (PRNT) as previously described.23 Briefly, the PRNT was performed in 24-well plates with subconfluent Vero cell cultures, using a fixed virus inoculum (∼1,500 FFU) against varying serum dilutions (1:20–1:1,280). The PRNT titers were scored as reciprocal of the highest dilution of serum that inhibited 80% of foci (PRNT80). Samples scored with PRNT80 < 20 were considered negative.
RESULTS
Infection of squirrel monkeys.
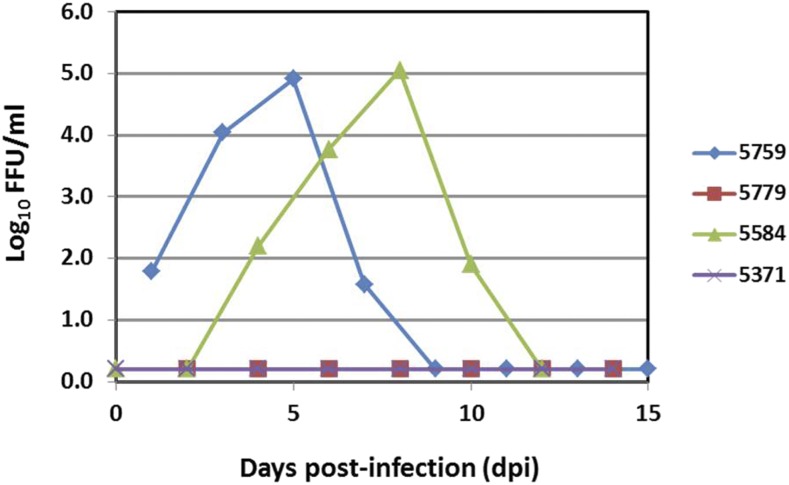
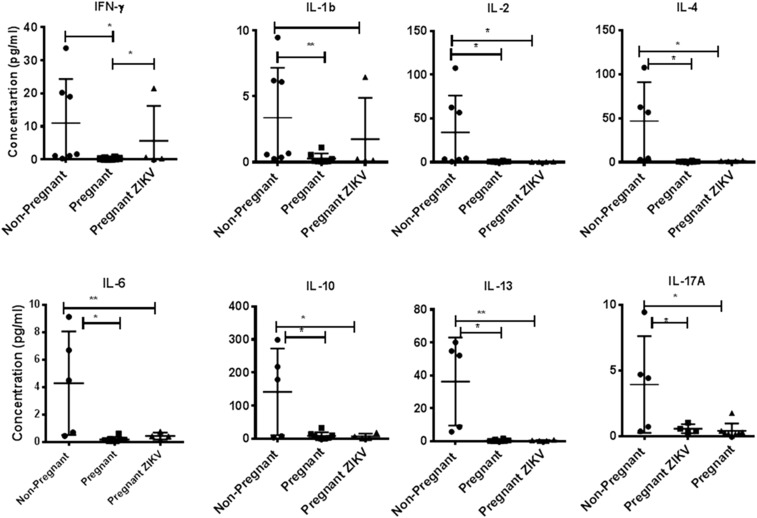
Two Bolivian (Saimiri boliviensis boliviensis) and two Guyanese (Saimiri sciureus sciureus) squirrel monkeys were inoculated subcutaneously between the scapulae in early pregnancy (24–34 days postconception, equivalent to 7–8 weeks human gestation) with 10e5 FFU of ZIKV strain MEX_1_44.18 None of the four animals exhibited signs of clinical disease after ZIKV inoculation and there were no abnormalities in their hematology or chemistry profiles at 5 days postinoculation (dpi) (data not shown). Viremia and viruria were detected in two (one Bolivian squirrel monkey and one Guyanese squirrel monkey) of the four total animals by RT-qPCR. Peak plasma viremia of 4.91 log10 FFU/mL occurred at 5 dpi for NHP#5759 and peak viremia of 5.05 log10 FFU/mL occurred at 10 dpi NHP#5584 as extrapolated from qPCR (Figure 1). Viruria was detected at 9 and 14 dpi for NHP#5759 (1.22 log10 FFU/mL) and NHP#5584 (0.82 log10 FFU/mL), respectively; salivary ZIKV secretion was detected as late as 14 dpi, with peak values of 2.62, 2.48, and 0.11 log10 FFU/mL for NHP#5759, NHP#5584, and NHP#5371, respectively. The limit of detection for this assay was 1.0 FFU/mL. Comparison of plasma cytokines from nonpregnant, pregnant, and ZIKV-exposed pregnant squirrel monkeys during the acute phase of infection (5 dpi) was performed to identify profiles associated with acute ZIKV infection (Figure 2). Although several statistically significant differences were observed between pregnant and nonpregnant animals, only IFN-γ concentrations were significantly different in the cytokine profile of ZIKV-exposed and ZIKV-unexposed pregnant animals. This result was driven by the high concentration of IFN-γ in the plasma of animal #5371, the significance of which is unclear. Three of the four ZIKV-inoculated squirrel monkeys had detectable neutralizing antibody titers of ≥ 1:80 at 28 dpi, as measured by PRNT80. Baseline titers were < 1:20 for all animals. Demographic, clinical, and virologic data from ZIKV-exposed squirrel monkeys and controls are summarized in Table 1. Pregnancy-related data and fetal outcomes will be published in a subsequent article.
Figure 1.
Zika virus viral load (log10 FFU/mL) data from squirrel monkey plasma, as determined by RT-PCR. This figure appears in color at www.ajtmh.org.
Figure 2.
Cytokine bead array analyses of plasma samples. In duplicate wells of the 96-well filter plate, 25 μL of plasma from nonpregnant, pregnant, and Zika virus (ZIKV)-exposed pregnant squirrel monkeys were incubated with 25 μL of cytokine-coupled beads overnight at 4°C followed by washing and staining with biotinylated detection antibody. The plates were read on Bio-Rad 200 using Luminex technology, and the results are expressed as picograms per milliliter concentration. The minimum detectable concentrations in picograms per milliliter for interferon (IFN)-γ (2.2), interleukin (IL)-2 (0.7), IL-4 (2.7), IL-6 (0.3), IL-10 (6.2), IL-13 (5.8), and IL-17A (1.3) are used for considering positive responses. See Methods section for experimental details. The results shown are the average of two separate experiments, and the standard deviation values did not exceed 15% of the mean value. P values were considered significant at P < 0.05.
Table 1.
Summary of demographic, clinical, and virologic data on ZIKV-exposed and control animals
| Animal | NHP# | Species | Age (years), sex | Viremia | Peak viremia (log10 FFU/mL) | Viruria | Salivary secretion | ZIKV PRNT80 |
|---|---|---|---|---|---|---|---|---|
| 1 | 5759 | Saimiri boliviensis | 3, F | + | 4.91 | + | + | 1:80 at 28 dpi |
| 2 | 5779 | S. boliviensis | 3, F | − | − | − | +* | 1:80 at 28 dpi |
| 3 | 5371 | Saimiri sciureus | 5, F | − | − | − | − | < 1:20 at 28 dpi |
| 4 | 5584 | S. sciureus | 4, F | + | 5.05 | + | + | 1:160 at 28 dpi |
| 5—control | 5834 | S. sciureus | 3, F | − | − | − | − | < 1:20 at 28 dpi |
| 6—control | 5817 | S. sciureus | 3, F | − | − | − | − | < 1:20 at 28 dpi |
| 7 | 85864 | Aotus nancymaae | 6, F | − | − | − | − | < 1:40 at 7 dpi |
| 8 | 86480 | A. nancymaae | 16, M | − | − | − | − | < 1:40 at 7 dpi |
| 9 | 85939 | A. nancymaae | 14, M | + | 2.55 | − | − | 1:320 at 14 dpi |
| 10 | 86101 | A. nancymaae | 11, F | + | 0.94 | − | − | 1:160 at 14 dpi |
dpi = days postinoculation; FFU = focus-forming units; PRNT = plaque reduction neutralization test; ZIKV = Zika virus.
Salivary secretion of 1.92 log10 FFU/mL at 22 dpi.
Infection of owl monkeys.
Two male and two nonpregnant female owl monkeys (Aotus nancymaae) were inoculated with 2 × 10e5 FFU of ZIKV strain MEX_1_44, as described previously. Blood, urine, and saliva specimens were collected on alternating days from pairs of male and female owl monkeys so that iatrogenic blood collections did not exceed weekly limits. All four animals remained clinically well after inoculation and routine hematology and serum chemistry analyses were normal at 4 dpi, with the exception of a modest increase in Alanine aminotransferase over baseline for animal #86480 (data not shown). Two of the four owl monkeys had ZIKV viremia: animal #86101 had viremia at 2 and 4 dpi by PCR and focus assay, and animal #85939 had viremia at 4 dpi by PCR and focus assay. Peak viral loads in the ZIKV-infected owl monkeys were 2.55 log10 FFU/mL at 4 dpi for animal #85939 and 0.94 log10 FFU/mL at 2 dpi for animal #86101. ZIKV was detected in the plasma of animals #85939 and #86101 by inoculation on Vero cells, with peak virus titers of 200 and 600 FFU/mL, respectively, at 4 dpi. Both animals that were euthanized at 14 dpi seroconverted based on PRNT80. ZIKV was not detected in urine, salivary secretions, or excretions from owl monkeys by culture or qPCR assays. At necropsy, there were no gross anatomic abnormalities and histologic evaluation revealed lymphoid hyperplasia in spleen of the two animals euthanized at 7 dpi. No other histologic abnormalities were found (data not shown). ZIKV was detected at low levels by RT-qPCR in the spleen of three owl monkeys: #86480, #85939, and #86101, with estimated viral loads of 4.25, 0.18, and 0.17 FFU/μg of RNA, respectively. The absolute limit of detection in this assay was < 0.1 FFU per well. All other tissues, including multiple areas of brain, were negative for ZIKV by RT-qPCR.
DISCUSSION
This study provides evidence of the susceptibility of squirrel and owl monkeys to infection with Asian-lineage ZIKV, supporting the hypothesis that Neotropical primates could serve as reservoir and amplification hosts should a sylvatic cycle of ZIKV be established in South America. ZIKV has been detected at low levels by RT-PCR in wild-caught and pet capuchin monkeys and marmosets in northeastern Brazil,24 but whether the virus titers in these species are adequate to support zoonotic transmission and/or sylvatic cycles of ZIKV is unknown.
A recent modeling analysis concluded that establishment of a sylvatic ZIKV transmission cycle is dependent on a range of biologically plausible parameters, such as host and vector population sizes, host birthrates, and ZIKV force of infection.5 The proximity of Neotropical primates to humans in many areas of South America where ZIKV is circulating in the urban cycle suggests that zoonotic transmission could occur frequently, potentially initiating epidemic cycles of ZIKV disease in humans for decades. Whether sylvatic ZIKV might impact the biodiversity of Neotropical primates in South America remains to be determined. Based on the subclinical nature of the infections observed in this study, the impact on biodiversity of Neotropical primate species would likely depend on the long-term consequences of congenital infections on survival of infected infant monkeys as they grow and mature.
In addition to confirming the susceptibility of Neotropical primates to ZIKV infection, this study defined the approximate 50% infectious dose for ZIKV MEX1_44 in these species at a dose of ∼10e5 PFU, based on seroconversion of five of six animals at 14–28 dpi and the detection of viremia in four of eight animals. This dose is similar to that used in several studies of Thai and Puerto Rican ZIKV in rhesus (Macaca mulatta) and cynomolgus macaques (Macaca fascicularis),8,10 but much less than the virus titer used in a recent report of severe neurologic damage in an infant pig-tailed macaque (Macaca nemestrina) infected with a Cambodian ZIKV strain later in pregnancy.9 It is also slightly higher than estimated titers of ZIKV found in the saliva of infected mosquitoes.25 The variability in the incidence of viremia, viruria, salivary excretion, and seroconversion observed among the animals in this study is not unexpected as similar variability has been seen with experimental infection of dengue virus and ZIKV in rhesus macaques.26,27 Consistent with the data from observational studies in humans, the discordant outcomes from infection with different ZIKV strains in different species of nonhuman primates (NHP) suggest that both host and virologic factors likely influence the outcome of ZIKV infection in NHPs. As such, variability in the infectivity and productivity of ZIKV in different NHPs may influence the magnitude of the contribution of a particular species or subspecies in supporting a sylvatic cycle of ZIKV. In addition, the dynamics of ZIKV infection may differ in pregnant and nonpregnant squirrel monkeys, a hypothesis that will be addressed in future studies. As published studies of ZIKV in Old World primates, we did not observe significant cytokine derangements that would offer clues to the innate or adaptive signaling required to control ZIKV infection.26 As such, additional studies of NHPs that include the study of fetal tissues in the weeks and months after maternal inoculation with ZIKV will be necessary to fully understand the pathogenesis of congenital ZIKV syndrome and to define the utility of various NHP models of ZIKV infection and disease.
In summary, this study confirms the susceptibility of two Neotropical primate species to ZIKV infection and viremia levels potentially sufficient to support mosquito-borne enzootic transmission. These results offer the opportunity to advance the understanding of ZIKV neuropathogenesis and its neurodevelopmental consequences in well-studied Neotropical primates that could be used for preclinical studies of candidate vaccines and antiviral agents to prevent and/or treat ZIKV infection. Additional studies of ZIKV in Neotropical primates with varying doses and timing of infection during pregnancy, as well as neonatal ZIKV infection, will be necessary to fully understand the pathogenesis of ZIKV infection in humans and nonhuman primates. Moreover, several arboreal New World mosquitoes involved in the enzootic transmission of YFV (reviewed in Hanley and others27) could serve as enzootic ZIKV vectors and should be evaluated experimentally. Importantly, establishment of a ZIKV sylvatic transmission cycle in the Americas, which could potentially be widespread throughout the neotropics such as YFV, would render future eradication efforts practically impossible, and also might inhibit our ability to control the ongoing outbreak of congenital Zika syndrome.
Acknowledgments:
We acknowledge the technical and support staff of the Keeling Center for their assistance with this project.
REFERENCES
- 1.Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL, 2016. Zika and the risk of microcephaly. N Engl J Med 375: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellington SR, et al. 2016. Estimating the number of pregnant women infected with Zika virus and expected infants with microcephaly following the Zika virus outbreak in Puerto Rico, 2016. JAMA Pediatr 170: 940–945. [DOI] [PubMed] [Google Scholar]
- 3.Brasil P, et al. 2016. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med 375: 2321–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryant JE, Holmes EC, Barrett AD, 2007. Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathog 3: e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Althouse BM, Vasilakis N, Sall AA, Diallo M, Weaver SC, Hanley KA, 2016. Potential for Zika virus to establish a sylvatic transmission cycle in the Americas. PLoS Negl Trop Dis 10: e0005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rylands AB, Mittermeier RA, 2009. The diversity of the New World primates (Platyrrhini): an annotated taxonomy. Garber PA, Estrada A, Bicca-Marques JC, Heymann EW, Strier KB, eds. South American Primates: Comparative Perspectives in the Study of Behavior, Ecology, and Conservation New York, NY: Springer, 23–54. [Google Scholar]
- 7.Dick GW, Kitchen SF, Haddow AJ, 1952. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 46: 509–520. [DOI] [PubMed] [Google Scholar]
- 8.Osuna CE, et al. 2016. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med 22: 1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams Waldorf KM, et al. 2016. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med 22: 1256–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley DM, et al. 2016. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun 7: 12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aliota MT, et al. 2016. Heterologous protection against Asian Zika virus challenge in rhesus macaques. PLoS Negl Trop Dis 10: e0005168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sapparapu G, et al. 2016. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature 540: 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Govero J, et al. 2016. Zika virus infection damages the testes in mice. Nature 540: 438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miner JJ, et al. 2016. Zika virus infection in mice causes panuveitis with shedding of virus in tears. Cell Rep 16: 3208–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, Langsjoen RM, Paessler S, Vasilakis N, Weaver SC, 2016. Characterization of a novel murine model to study Zika virus. Am J Trop Med Hyg 94: 1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ministério da Saúde B , 2005. Manual de Vigilância de Epizootias em Primatas Não-Humanos. Brasilia: Brasilia Ministério da Saúde.
- 17.Vasconcelos PF, 2003. Yellow fever. Rev Soc Bras Med Trop 36: 275–293. [DOI] [PubMed] [Google Scholar]
- 18.Guerbois M, et al. 2016. Outbreak of Zika virus infection, Chiapas State, Mexico, 2015, and first confirmed transmission by Aedes aegypti osquitoes in the Americas. J Infect Dis 214: 1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR, 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14: 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll T, et al. 2017. Zika virus preferentially replicates in the female reproductive tract after vaginal inoculation of rhesus macaques. PLoS Pathog 13: e1006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nehete PN, Hanley PW, Nehete BP, Yang G, Ruiz JC, Williams L, Abee CR, Sastry KJ, 2013. Phenotypic and functional characterization of lymphocytes from different age groups of Bolivian squirrel monkeys (Saimiri boliviensis boliviensis). PLoS One 8: e79836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nehete PN, Nehete BP, Chitta S, Williams LE, Abee CR, 2017. Phenotypic and functional characterization of peripheral blood lymphocytes from various age- and sex-specific groups of owl monkeys (Aotus nancymaae). Comp Med 67: 67–78. [PMC free article] [PubMed] [Google Scholar]
- 23.Vasilakis N, Durbin AP, da Rosa AP, Munoz-Jordan JL, Tesh RB, Weaver SC, 2008. Antigenic relationships between sylvatic and endemic dengue viruses. Am J Trop Med Hyg 79: 128–132. [PubMed] [Google Scholar]
- 24.Favoretto S, Araujo D, Oliveira D, Duarte N, Mesquita F, Zanotto P, Durigon E, 2016. First detection of Zika virus in Neotropical primates in Brazil: a possible new reservoir. bioRxiv, 10.1101/049395. [Google Scholar]
- 25.Roundy CM, et al. 2017. Variation in Aedes aegypti mosquito competence for Zika virus transmission. Emerg Infect Dis 23: 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch AJ, et al. 2017. Zika virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog 13: e1006219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanley KA, Monath TP, Weaver SC, Rossi SL, Richman RL, Vasilakis N, 2013. Fever versus fever: the role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect Genet Evol 19: 292–311. [DOI] [PMC free article] [PubMed] [Google Scholar]