Abstract.
Flubendazole (FLBZ) is a potent and efficacious macrofilaricide after parenteral administration. Studies in animal models and one trial in patients infected with Onchocerca volvulus revealed that FLBZ elicits minimal effects on microfilariae (mf). Severe complications after ivermectin (IVM) treatment of patients with high Loa loa microfilaraemia are of great concern. We examined the potential of FLBZ to rapidly kill L. loa mf, the phenomenon proposed to underlie the complications. Mf of L. loa were exposed to FLBZ, its reduced metabolite, albendazole, or IVM in vitro. Viability of L. loa mf was unaffected by FLBZ (10 μM, 72 hours); similar results were obtained with mf of Brugia malayi. We also measured the effects of FLBZ on transmission of mf. Aedes aegypti were fed FLBZ-exposed B. malayi mf and dissected 24 hours or 14 days postfeeding to count mf that crossed the midgut and developed to infective L3. FLBZ impaired the ability of mf to cross the midgut, regardless of duration of exposure (≥ 2 hours). FLBZ also prevented the development of mf to L3s, irrespective of duration of exposure or concentration. FLBZ is not microfilaricidal under these conditions; however, it blocks transmission. These results support the possibility that FLBZ may be a useful macrofilaricide in loiasis regions and may limit transmission from treated individuals.
BACKGROUND
Infections with filarial nematodes of the family Onchocercidae are among the most debilitating of the neglected tropical diseases and are estimated to affect > 150 million people.1,2 Wuchereria bancrofti and Onchocerca volvulus, the causative agents of lymphatic filariasis (LF) and onchocerciasis, respectively, are of greatest concern because infection can result in significant debilitation and is a major hindrance to socioeconomic development in endemic regions. Loa loa has received less attention. Although generally asymptomatic, loiasis can be rather disconcerting, causing pruritus, Calabar swelling, and subconjunctival migration of adult worms.3 However, a main concern regarding L. loa is the unacceptable incidence of severe adverse events (SAEs) observed after administration of ivermectin (IVM) for the treatment of onchocerciasis in co-endemic regions; high microfilarial loads of L. loa (> 30,000 mf/mL) are associated with cases of IVM-induced SAEs, including encephalopathy and death.4,5 The death of L. loa microfilariae (mf) after IVM treatment is thought to be responsible for SAEs, as IVM treatment is known to rapidly decrease circulating mf.6,7
Because of the limitations imposed by IVM-associated SAEs in L. loa patients, mass drug administration (MDA) programs have been suspended or limited in areas that are highly endemic for L. loa.7–9 This has led the World Health Organization (WHO) to propose alternative strategies that primarily focus on interrupting transmission in these areas.1,2 The recommended strategy involves administration of albendazole twice yearly combined with vector control9–11; however, the length of time necessary for this strategy to be effective has not been defined. Filariasis control programs would be greatly enhanced by a macrofilaricidal drug which elicited no or slow killing of mf, thereby lessening the chances of SAEs. Flubendazole (FLBZ), a benzimidazole anthelmintic approved for the treatment of infections due to gastrointestinal nematodes of livestock11 and humans,12–14 is an appealing candidate to address these concerns.15
As a general feature, FLBZ elicits much greater effects on adults and developmental stages of filariae than mf in animal models after parenteral administration16–18 and in a single O. volvulus human trial.19 Human trials were restricted because of problems related to the route of administration; macrofilaricidal effects are only observed with parenteral administration as available formulations of the drug have very limited oral bioavailability. Injection-based drugs have limited appeal for use in field settings, and patients in the human trial experienced injection site reactions that precluded further development. Fortunately, Johnson & Johnson have undertaken preclinical development of FLBZ20 to repurpose the drug by developing an oral formulation that provides high bioavailability. A highly bioavailable, orally administered macrofilaricide with limited microfilaricidal activity would be an important contribution to campaigns to eliminate filariases as public health concerns. Considering that FLBZ has limited effects on mf in many animal models, it is important to confirm these observations by directly measuring the effects of FLBZ exposure on L. loa mf. This is especially true in light of the fact that orally bioavailable formulations provide much higher systemic exposure than achieved with parenteral administration,21,22 and the acute effects of exposure to high levels of FLBZ on mf have not been reported.
Another important consideration is that it can take weeks to months for FLBZ to produce macrofilaricidal effects.16–19 In the meantime, circulating mf not killed by FLBZ are available to be ingested by the mosquito host, furthering the reinfection cycle. IVM treatment of infected jirds suppresses the development of Brugia malayi and Brugia pahangi mf within a mosquito host that has fed on treated animals,23 suggesting that drugs which are not directly microfilaricidal may have effects on mf that are only detectable in a model that requires development. Therefore, we conducted studies to determine if FLBZ exposure in culture elicited direct microfilaricidal activity and affected the capacity of mf to develop in a mosquito host.
METHODS
Ethics statement.
Loa loa.
Microfilaremic blood was acquired from experimentally infected baboons24 using animal procedures in accordance with animal care and use protocols at the National Institutes of Health (USA). Ethical approval for the use of baboons in this study was obtained from the Ministry of Scientific Research and Innovation of Cameroon. The use of nonhuman primates for research was approved by the Committee on the Ethical Use of Animals in Research within the Research Foundation for Tropical Diseases and Environment (REFOTDE), Cameroon.
Brugia malayi.
Microfilariae and mosquitoes were supplied by the Filariasis Research Reagent Repository Center at the University of Georgia, Athens, GA. All animal protocols were reviewed and approved by the University of Georgia Institutional Animal Care and Use Committee (Approval No. A2013 11-009) and complied with U.S. Department of Agriculture regulations (USDA Assurance No. A3437-01).
Parasites.
Loa loa.
Mf were obtained from experimentally infected baboons (Mandrillus sp.). Blood samples were collected in EDTA-coated tubes and transported to REFOTDE at the University of Buea on ice. Blood constituents were separated on a Percoll gradient (40%, 50%, 65%) centrifuged at 4,000 g × 10 minutes. Mf are found in the top layers containing platelets and lymphocytes. The top layers were removed, passed through a 5-μm syringe filter to remove cells, and placed in a Petri dish filled with Dulbecco’s modified Eagle’s medium (DMEM; Wisent, St-Bruno, QC, Canada) supplemented with penicillin (100 U/mL) and streptomycin (100 μg/mL; Invitrogen) to allow mf to migrate into the media. Mf were then concentrated by centrifuging at 2,000 g × 10 minutes.
Brugia malayi.
Mf were isolated via lavage from the peritoneal cavity of jirds (Meriones unguiculatus) > 120 days postinfection as described.25,26 Mf for infectivity assays were isolated from peritoneal washes via passage through a 5-μm membrane filter and overnight incubation in DMEM medium supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin (Sigma-Aldrich, Oakville, ON, Canada) to allow mf to migrate into the media.
Culture.
Mf were distributed among various treatment groups; FLBZ (10 μM, 1 μM, 100 nM; Epichem Pty Ltd, Bentley, WA, Australia), reduced FLBZ (10 μM, 1 μM, 100 nM; Epichem Pty Ltd), albendazole (10 μM, 1 μM, 100 nM; Sigma-Aldrich), IVM (1 μM; Sigma-Aldrich), or 5% DMSO as a positive control for mf immobilization. All drugs were prepared in 100% DMSO and diluted in media with a final concentration of 0.1% DMSO. Incubations in drug-containing media were conducted for a maximum of 72 hours.
For infectivity assays, mf were exposed to FLBZ (10 μM, 1 μM, 100 nM), FLBZ-R (1 μM) or IVM (1 μM) for 2, 6, or 24 hours before washing in serum-free media.
Motility.
Motility was scored every 24 hours using a 4-point scoring system: 3—highly active, 2—sluggish, 1—twitching of head/tail, and 0—immotile. Percent motility was determined using the following formula:
Mortality was determined as the percent of mf with motility score = 0.
Mosquito feeding.
Cultured B. malayi mf were concentrated by centrifugation (2,000 g × 5 minutes) and added to heparinized dog blood (FR3, University of Georgia) at 24–32 mf/μL. Mosquitoes were allowed to feed on infected blood maintained at 37°C using an artificial membrane feeding apparatus for 2 hours. Blood-fed mosquitoes were maintained in the FR3 insectary at the University of Georgia at 27°C and 80% humidity. Mosquitoes were collected from the insectary 24 hours and 14 days after feeding for either immediate dissection or fixation in 90% ethanol for later dissection.
Mosquito dissection.
Freshly collected mosquitoes were kept on ice until dissection. After the removal of the wings and legs, the mosquitoes were placed on a glass slide with a drop of RPMI 1640 (Sigma-Aldrich). Midguts were carefully excised with fine forceps, taking care not to rupture the blood-engorged midgut. The midguts were coverslipped and the number of mf counted under ×400 magnification.
Dissections of ethanol-fixed mosquitoes were conducted following WHO-suggested methods with a few modifications.27 Mosquitoes were washed in descending dilutions of ethanol for 30 minutes (70%, 55%, 25%) followed by a final 30-minute wash in distilled water.
Midguts: The midguts were carefully excised from 24-hour fixed mosquitoes and stained whole in Mayer’s haemalum for 1 hour before washing in distilled water for 15 minutes. Individual midguts were spread on glass slides and left to dry overnight before enumerating mf under ×600 magnification.
L3: Fourteen-day mosquitoes were stained in Mayer’s haemalum at room temperature for 7 days. The mosquitoes were washed for 3 days in distilled water and stored in glycerol until dissection. The head, thorax, and abdomen were separated and placed into individual drops of glycerol. Fine needles were used to tease apart tissues to enumerate L3s under a stereoscopic dissecting microscope at ×8 to ×35 magnification.
Statistical analysis.
Statistical analyses were performed using a two-way analysis of variance in the GraphPad Prism 6 package. All statistical tests were interpreted at the 5% level of significance.
RESULTS AND DISCUSSION
Effects of flubendazole on mf of L. loa.
Limitations of filaricidal drugs used in Africa center on the risk of IVM-induced SAEs in L. loa patients. Chemotherapeutic efforts would be greatly enhanced by a macrofilaricide that elicited little or no damage to mf of L. loa. Because FLBZ is a highly effective macrofilaricide, it is important to understand its effects on mf at concentrations which might be obtained in plasma with an orally bioavailable formulation if it is to be used in the field.
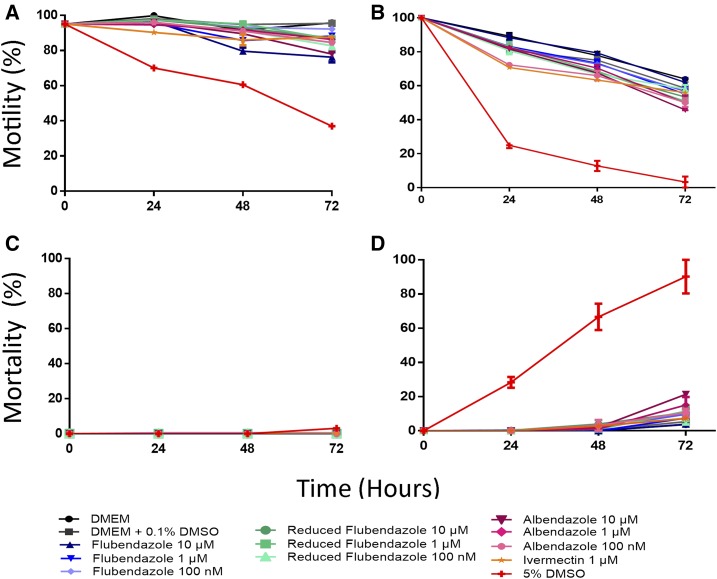
Our results confirm the limited effect of FLBZ on mf reported in previous studies.16–18,28,29 Neither in utero fully stretched mf nor circulating mf exhibited detrimental effects associated with FLBZ treatment. In this study, B. malayi mf obtained from the jird peritoneal cavity were exposed to FLBZ. Lack of motility was considered indicative of mf death. All drug-treated groups had motility similar to that of the control (Figure 1A), with only slight motility impairment. FLBZ had no observable microfilaricidal activity (Figure 1C).
Figure 1.
Effect of flubendazole on the viability of microfilariae of Loa loa (B, D) and Brugia malayi (A, C). Motility (A, B) was scored on a 4-point scale and percent motility calculated using the formula in 5.3.4. Mortality (C, D) was calculated as the percent of microfilariae which were completely immotile and presumed dead. This figure appears in color at www.ajtmh.org.
Loa loa mf were less fit under these culture conditions than B. malayi (Figure 1B), with the motility of mf in all treatments decreasing over time. Some differences in motility were observed between the treated groups and the control, but the greatest difference was observed in the 5% DMSO group, which served as a positive control for motility impairment. Importantly, FLBZ had no microfilaricidal effect on L. loa mf. Increased mortality of drug-treated mf was only observed at the 72-hour timepoint and was < 25%. These results are promising as they suggest that FLBZ is not rapidly lethal to L. loa mf, which may prevent SAEs in patients with high microfilaraemia. In patients, treatment with IVM leads to a sharp decrease in mf to 40% of the original L. loa mf load 24 hours after treatment and to 20% of the original load by 72 hours.30
Slight motility impairment was observed in drug-treated mf compared with the controls. A dramatic loss of motility was not seen, nor was there a striking microfilaricidal effect of the drugs tested (Figure 1B and D), which could have been expected with a potential SAE-inducing drug. However, this assay only measures direct microfilaricidal activity, and it is known that IVM, for instance, is not lethal to mf in culture.31 Instead, IVM-induced microfilaricidal effects require, or are greatly enhanced by, a host immune component.32,33 The limitation of our studies on the effect of FLBZ on L. loa mf in vitro lies in the absence of host components in the system; we have not demonstrated that exposure of mf in vivo to FLBZ at high concentrations may lead to effects similar to those observed with IVM or diethylcarbamazine. However, in human onchocerciasis and animal models for LF, FLBZ elicits macrofilaricidal effects over long durations.16,19 In addition, O. volvulus mf counts in skin did not diminish in human onchocerciasis patients given FLBZ parenterally until several months posttreatment. It still must be proven that an oral formulation would not provide sufficiently high exposure to produce a drastically different response on mf in vivo.
Effects of flubendazole on infectivity of mf to mosquitoes.
FLBZ requires weeks to months to produce macrofilaricidal effects.16–19 In the meantime, circulating mf are available to be ingested by the mosquito host, potentially furthering the infection cycle. MDA programs would benefit greatly by having a drug which is not directly microfilaricidal, and therefore potentially safe to use in L. loa co-endemic areas. We therefore examined the transmission-limiting ability of FLBZ by determining the infectivity of treated mf for the mosquito host, including development to the infective L3 stage.
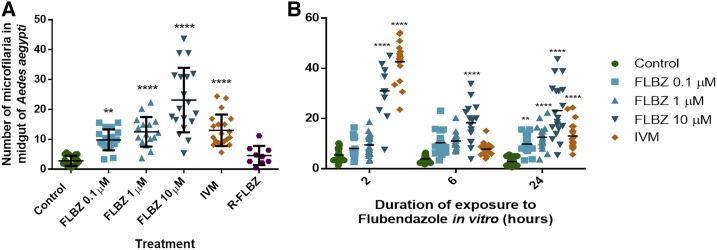
FLBZ exposure (24 hours) led to significantly fewer mf crossing the mosquito midgut in a concentration-dependent manner (Figure 2A). Significantly more FLBZ-exposed mf were retained in the mosquito midgut compared with the control. The 10-μM FLBZ group had the greatest impairment at all timepoints of exposure (2, 6, and 24 hours; Figure 2B). The number of mf retained in the midgut after 2- and 6-hour exposure to lower concentrations (100 nM, 1 μM) was not different from the control (Figure 2B). Interestingly, FLBZ-R (1 μM) had no effect on midgut penetration. It is important to note that this metabolite produces histological damage to adult B. malayi28 and is known to be bioactive.34
Figure 2.
Consequence of flubendazole (FLBZ) exposure on midgut penetration of Brugia malayi microfilariae to Aedes aegypti mosquitoes. (A) Number of microfilariae retained in the mosquito midgut 24 hours after feeding on microfilariae which were exposed to FLBZ, reduced FLBZ or ivermectin (IVM) for 24 hours in vitro. (B) Number of microfilariae retained in the mosquito midgut 24 hours after feeding on microfilariae which were exposed to FLBZ or IVM for 2, 6, or 24 hours in vitro. **P value ≤ 0.01 ****P value ≤ 0.0001. This figure appears in color at www.ajtmh.org.
Although FLBZ exposure inhibited the ability of mf to cross the midgut, a proportion of these mf crossed into the abdominal cavity. The fate of these mf was assessed by determining if mf that had penetrated the midgut developed to the infective L3 stage.
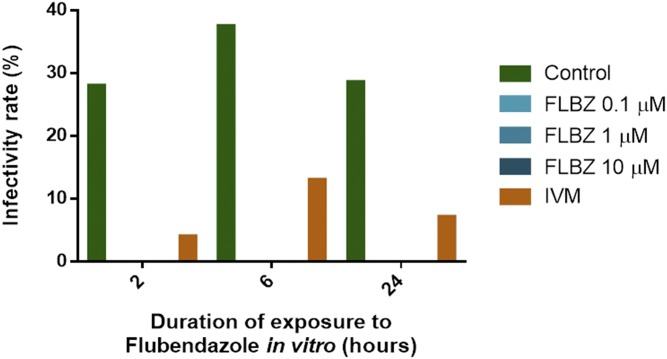
Infectivity in the controls was < 37.5% (Figure 3). Strikingly, exposure to FLBZ at all concentrations and for all durations completely abolished the ability of mf to develop to infective L3s (Figure 3).
Figure 3.
Infectivity rate of Aedes aegypti fed on flubendazole-treated Brugia malayi microfilariae. Infectivity rate was calculated as the percent of mosquitoes which harbored infective L3 larvae 14 days after feeding. This figure appears in color at www.ajtmh.org.
The midgut wall of the mosquito host acts as a physical barrier to invasion by mf. Invasion into the mosquito hemocoel was once thought to require the mf to first lose their sheath before rupturing the membranes of the midgut wall using a cephalic hook.35 Subsequent studies reported that mf lost their sheath as they migrate through the midgut.36,37
Whether the loss of the sheath occurs before or during midgut migration, this step is an obvious requirement for development to later larval stages.
Although the factors leading to exsheathment are unknown, the process is thought to be developmentally regulated38,39 and can be stimulated by calcium-dependent proteases as well as other proteases or endopeptidases.23,40,41 Given that these factors need to be secreted from the worm to break down the sheath, it is possible that FLBZ blocks the secretion of such factors. BZs42,43 including FLBZ,42 inhibit protein secretion from gastrointestinal nematodes and B malayi mf. It would not be surprising if a similar phenomenon occurs in other filarial larval stages. We did not investigate the presence or absence of sheaths on mf that penetrated the midgut. If, at lower FLBZ concentrations, mf penetrated the midgut without losing their sheath, this could account for their inability to migrate and develop to the infective stage.
CONCLUSIONS
FLBZ did not show rapid killing of L. loa mf in vitro. If rapid death of L. loa mf is responsible for the severe complications observed in IVM-treated patients, it is tempting to speculate on the safety of FLBZ in loiasis areas as it does not lead to rapid mf death. Further experiments are needed to explore the consequence of FLBZ exposure in vivo. Although not directly microfilaricidal, FLBZ completely abolished the development of Brugia sp. mf to infective L3s in Aedes aegypti. Consequently, FLBZ treatment could block the transmission of filariae to another host. However, we do not know if mf can recover from FLBZ exposure, as is the case with adult B. malayi.44
These results suggest that FLBZ could be a safe macrofilaricide to use in regions co-endemic for O. volvulus and L. loa owing to an absence of rapid microfilaricidal effects, although having the added benefit of blocking further transmission.
Acknowledgments:
We are thankful to the Fonds Quebecois de la Recherche sur la Nature et les Technologies (FQRNT) for support of the Centre for Host-Parasite Interactions and to the Canada Research Chairs.
REFERENCES
- 1.Zoure H, Wanji S, Noma M, Amazigo UV, Diggle PJ, Tekle AH, Remme JH, 2011. The geographic distribution of Loa loa in Africa: results of large scale implementation of the Rapid Assessment Procedure for Loiasis (RAPLOA). PLoS Negl Trop Dis 5: e1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization , 2015. Investing to overcome the global impact of neglected tropical diseases. Third WHO Report on Neglected Tropical Diseases. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 3.Metzger WG, Mordmüller B, 2014. Loa loa—does it deserve to be neglected? Lancet Infect Dis 14: 353–357. [DOI] [PubMed] [Google Scholar]
- 4.Gardon J, Kamgno J, Folefack G, Gordon-Wendel N, Bouchite B, Boussinesq M, 1997. Marked decreaes in the Loa loa microfilaraemia six and 12 months after a single dose of ivermectin. Trans R Soc Trop Med Hyg 91: 593–594. [DOI] [PubMed] [Google Scholar]
- 5.Boussinesq M, Gardon J, Gardon-Wendel N, Chippaux J, 2003. Clinical picture, epidemiology and outcome of Loa-associated serious adverse events related to mass ivermectin treatment of onchocerciasis. Filaria J 2: S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardon J, Gardon-Wendel N, Demanga N, Kamgno J, Chippaux J-P, Boussinesq M, 1997. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet 350: 18–22. [DOI] [PubMed] [Google Scholar]
- 7.Boussinesq M, Gardon J, Kamgno J, Pion S, Gardon-Wendel N, Chippaux J, 2001. Relationships between the prevalence and intensity of Loa loa infection in the central province of Cameroon. Ann Trop Med Parasitol 95: 495–507. [DOI] [PubMed] [Google Scholar]
- 8.Boussinesq M, Gardon J, Gardon-Wendel N, Kamgno JPN, Chippaux J, 1998. Three probable cases of Loa loa encephalopathy following ivermectin treatment for onchocerciasis. Am J Trop Med Hyg 58: 461–469. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization , 2012. Provisional strategy for interrupting lymphatic filariasis transmission in loiasis-endemic countries. Report of the Meeting on Lymphatic Filariasis, Malaria and Integrated Vector Management. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 10.World Health Organization, 2014. Report of the Seventh Meeting of the Strategic and Technical Advisory Group for Geglected Tropical Diseases. Available at: http://www.who.int/neglected_diseases/NTD_STAG_report_2014.pdf.
- 11.Bradley RE, Guerrero J, Becker HN, Michael BF, Newcomb K, 1983. Flubendazole: dose range and efficacy studies against common internal parasites of swine. Am J Vet Res 44: 1329–1333. [PubMed] [Google Scholar]
- 12.Horton RJ, 1990. Benzimidazoles in a wormy world. Parasitol Today 6: 106. [DOI] [PubMed] [Google Scholar]
- 13.Yangco BG, Klein TW, Deresinski SC, Vickery AC, Craig CP, 1981. Flubendazole and mebendazole in the treatment of trichuriasis and other helminthiases. Clin Ther 4: 285–290. [PubMed] [Google Scholar]
- 14.Kan SP, 1983. The anthelmintic effects of flubendazole on Trichuris trichiura and Ascaris lumbricoides. Trans R Soc Trop Med Hyg 77: 668–670. [DOI] [PubMed] [Google Scholar]
- 15.Mackenzie CD, Geary TG, 2011. Flubendazole: a candidate macrofilaricide for lymphatic filariasis and onchocerciasis field programs. Expert Rev Anti Infect Ther 9: 497–501. [DOI] [PubMed] [Google Scholar]
- 16.Zahner H, Schares G, 1993. Experimental chemotherapy of filaiasis: comparative evaluation of the efficacy of filaricidal compounds in Mastomys coucha infected with Litomosoides carinii, Acanthocheilonema viteae, Brugia malayi and B. pahangi. Acta Trop 52: 221–266. [DOI] [PubMed] [Google Scholar]
- 17.Denham DA, Samad R, Cho SY, Suswillo RR, Skippins SC, 1979. The anthelmintic effects of flubendazole on Brugia pahangi. Trans R Soc Trop Med Hyg 73: 673–676. [DOI] [PubMed] [Google Scholar]
- 18.Mak JW, 1981. Antifilarial activity of mebendazole and flubendazole on Breinlia booliati. Trans R Soc Trop Med Hyg 75: 306–307. [DOI] [PubMed] [Google Scholar]
- 19.Dominguez-Vazquez A, Taylor HR, Greene BM, Ruvalcaba-Macias AM, Rivas-Alcala AR, Murphy RP, Beltran-Hernandez F, 1983. Comparison of flubendazole and diethylcarbamazine in treatment of onchocerciasis. Lancet 1: 139–143. [DOI] [PubMed] [Google Scholar]
- 20.Anonymous , 2014. Johnson & Johnson Joins Public and Private Partners in the Largest Coordinated Action to Date to Eliminate or Control Neglected Tropical Diseases. Available at: http://www.jnj.com/news/all/johnson-and-johnson-joins-public-and-private-partners-in-the-largest-coordinated-action-to-date-to-eliminate-or-control-neglected-tropical-diseases.
- 21.Ceballos L, Mackenzie CD, Geary TG, Alvarez L, Lanusse C, 2014. Exploring the potential of flubendazole in filariasis control: evaluation of the systemic exposure for different pharmaceutical preparations. PLoS Negl Trop Dis 8: e2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longo M, Zanoncelli S, Colombo PA, Harhay MO, Scandale I, Mackenzie C, Geary T, Madrill N, Mazué G, 2013. Effects of the benzimidazole anthelmintic drug flubendazole on rat embryos in vitro. Reprod Toxicol 36: 78–87. [DOI] [PubMed] [Google Scholar]
- 23.Rao UR, Kwa BH, Nayar JK, Vickery AC, 1990. Brugia malayi and Brugia pahangi: transmission blocking activity of ivermectin and brugian filarial infections in Aedes aegypti. Exp Parasitol 71: 259–266. [DOI] [PubMed] [Google Scholar]
- 24.Wanji S, Eyong EE, Tendongfor N, Ngwa C, Esuka E, Kengne-Ouafo A, Datchoua-Poutcheu F, Enyong P, Hopkins A, Mackenzie CD, 2015. Parasitological, hematological and biochemical characteristics of a model of hyper-microfilariaemic Loiasis (Loa loa) in the baboon (Papio anubis). PLoS Negl Trop Dis 9: e0004202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno Y, Geary TG, 2008. Stage- and gender-specific proteomic analysis of Brugia malayi excretory-secretory products. PLoS Negl Trop Dis 2: e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennuru S, Semnani R, Meng Z, Ribeiro JM, Veenstra TD, Nutman TB, 2009. Brugia malayi excreted/secreted proteins at the host/parasite interface: stage- and gender-specific proteomic profiling. PLoS Negl Trop Dis 3: e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization , 2013. Lymphatic Filariasis: Practical Entomology. A Handbook for National Elimination Programmes. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 28.O’Neill M, Geary JF, Agnew DW, Mackenzie CD, Geary TG, 2015. In vitro flubendazole-induced damage to vital tissues in adult females of the filarial nematode Brugia malayi. Int J Parasitol Drugs Drug Resist 5: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franz M, Zahner H, Benten P, 1990. Fine-structure alterations in female Brugia malayi and Litomosoides carinii after in vivo treatment with flubendazole. Parasitol Res 76: 401–405. [DOI] [PubMed] [Google Scholar]
- 30.Ducorps M, Gardon-Wendel N, Ranque S, Ndong W, Boussinesq M, Gardon J, Schneider D, Chippaux JP, 1995. Secondary effects of the treatment of hypermicrofilaremic loiasis using ivermectin. Bull Soc Pathol Exot 88: 105–112. [PubMed] [Google Scholar]
- 31.Tompkins J, Stitt L, Ardelli B, 2010. Brugia malayi: in vitro effects of ivermectin and moxidectin on adults and microfilariae. Exp Parasitol 124: 394–402. [DOI] [PubMed] [Google Scholar]
- 32.Moreno Y, Nabhan JF, Solomon J, Mackenzie CD, Geary TG, 2010. Ivermectin disrupts the function of the excretory-secretory apparatus in microfilariae of Brugia malayi. Proc Natl Acad Sci USA 107: 20120–20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGarry H, Plant L, Taylor M, 2005. Diethylcarbamazine activity against Brugia malayi microfilariae is dependent on inducible nitric-oxide synthase and the cyclooxygenase pathway. Filaria J 4: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urbizu L, Confalonieri A, Sánchez Bruni S, Lanusse C, Ignacio Alvarez L, 2012. Nematodicidal activity of flubendazole and its reduced metabolite on a murine model of Trichinella spiralis infection. Chemotherapy 58: 295–298. [DOI] [PubMed] [Google Scholar]
- 35.Esslinger JH, 1962. Behavior of microfilariae of Brugia pahangi in Anopheles quadrimaculatus. Am J Trop Med Hyg 11: 749–758. [Google Scholar]
- 36.Christensen BM, Sutherland DR, 1984. Brugia pahangi: exsheathment and midgut penetration in Aedes aegypti. Trans Am Microsc Soc 103: 423–433. [Google Scholar]
- 37.Agudelo-Silva F, Spielman A, 1985. Penetration of mosquito midgut wall by sheathed microfilariae. J Invertebr Pathol 45: 117–119. [DOI] [PubMed] [Google Scholar]
- 38.Hollanda JC, Denham DA, Suswillo RR, 1982. The infectivity of microfilariae of Brugia pahangi of different ages to Aedes aegypti. J Helminthol 56: 155–157. [DOI] [PubMed] [Google Scholar]
- 39.Griffiths K, Mayhew GF, Zink RL, Erickson SM, Fuchs JF, McDermott CM, Christensen BM, Michalski ML, 2009. Use of microarray hybridization to identify Brugia genes involved in mosquito infectivity. Parasitol Res 106: 227–235. [DOI] [PubMed] [Google Scholar]
- 40.Devaney E, Howells RE, 1979. The exsheathment of Brugia pahangi microfilariae under controlled conditions in vitro. Ann Trop Med Parasitol 73: 227–233. [DOI] [PubMed] [Google Scholar]
- 41.Fuhrman JA, Urioste SS, Hamill B, Spielman A, Piessens WF, 1987. Functional and antigenic maturation of Brugia malayi microfilariae. Am J Trop Med Hyg 36: 70–74. [DOI] [PubMed] [Google Scholar]
- 42.Watts SDM, Rapson EB, Atkins AM, Lee DL, 1982. Inhibition of acetylcholinesterase secretion from Nippostrongylus brasiliensis by benzimidazole anthelmintics. Biochem Pharmacol 31: 3035–3040. [DOI] [PubMed] [Google Scholar]
- 43.Tekwani BL, 1992. Secretory cholinesterase of Ancylostoma ceylanicum: effect of tubulin binding agents and benzimidazole anthelmintics. Life Sci 50: 747–752. [DOI] [PubMed] [Google Scholar]
- 44.O’Neill M, Mansour A, DiCosty U, Geary J, Dzimianski M, McCall SD, McCall JW, Mackenzie CD, Geary TG, 2016. An in vitro/in vivo model to analyze the effects of flubendazole exposure on adult female Brugia malayi. PLoS Negl Trop Dis 10: e4698. [DOI] [PMC free article] [PubMed] [Google Scholar]