Abstract.
Leishmania infantum causes visceral leishmaniasis (VL) in Brazil. We previously observed that VL is more common in males than females living in endemic neighborhoods, despite similar exposure. Using a larger sample, we document that VL is more common in males than females, but only after puberty. BALB/c and C57BL/6 mouse models confirmed that there is a biological basis for male susceptibility to symptomatic VL, showing higher parasite burdens in males than females. Female C57BL/6 mice generated more antigen-induced cytokines associated with curative responses (interferon-γ, interleukin [IL]-1β). Males expressed higher levels of IL-10 and tumor necrosis factor, which are linked to exacerbated disease. Different parasite lines entered or survived at a higher rate in macrophages of male- than female-origin. These results suggest that males are inherently more susceptible to L. infantum than females and that mice are a valid model to study this sex-dependent difference.
Leishmania spp. protozoa cause a spectrum of human diseases, the most severe of which is visceral leishmaniasis (VL), the second most common parasitic cause of death.1,2 Despite its severity, most people infected with the Leishmania spp. causing VL are asymptomatic.1 A better understanding of the host determinants underlying the development of asymptomatic versus symptomatic infection might highlight immune pathways that could become new therapeutic targets.
The importance of sex as a biological variable is being recognized in many inflammatory, infectious, and malignant diseases.3–5 In some cases, this gender bias may be affected by social/behavioral differences in exposure or by healthcare access.3–5 However, in many instances, it is likely that biological differences contribute to the imbalanced disease prevalence.4–7
Epidemiological evidence for sex differences in the prevalence of VL caused by Leishmania donovani or Leishmania infantum vary. Studies of L. donovani infection in Bihar or Kolkata, India, show no sex bias for VL but male predominance of the dermal complication, post kala azar dermal leishmaniasis in Kolkata only.8,9 A meta-analysis of 18 studies of L. infantum infection in South America revealed a significant male bias in the Leishmania skin test (LST), a measure of asymptomatic or cured infection. Similar to other reports, these studies could not discriminate whether the higher incidence of infection in males was due to biological differences or gender-related exposure.10 When documented, a male bias in VL has often been attributed to higher exposure risk among men.11
In the vicinity of Natal, northeast Brazil, L. infantum transmission is peridomestic and vector exposure occurs mostly in the evening when families are in or around the house.12 Analyzing data collected from subjects in neighborhoods with ongoing VL transmission, we previously reported that positive LST rates are equal between males and females, suggesting similar rates of infection. Nonetheless, males were more likely to progress to symptomatic VL.13 Herein, we examined the hypothesis that sex is a biological factor underlying disease susceptibility among individuals infected with L. infantum.
A study of VL patient families and their immediate neighbors was conducted between 1996 and 2004 and first published in 2004.13 Human studies were approved by institutional review boards of the Federal University of Rio Grande do Norte, the University of Iowa, and the National Institutes of Health.13 In the original study, subjects or legal guardians of minors signed an informed consent, and minors aged 12–17 years signed an assent. Individuals were categorized by age, sex, and L. infantum phenotype (VL or LST+). Anonymous data from this study were reanalyzed in the current study and included a larger set of subjects than the original report. There were no subjects encountered for this reanalysis. Human data were analyzed in R Studio using χ2 for age categorical variables and t test for the continuous age variable.
Statistical analysis of the reorganized data (Table 1) showed an age-dependent male predominance in subjects with VL but not other categories. Males older than 10 years, presumptively older than the age of puberty, accounted for significantly more cases of VL than prepubertal children (P = 0.00052). In contrast, there was no significant difference between the male:female ratio in LST+ subjects, in all subjects with known phenotypes, or in the entire dataset (Table 1). The data suggest that despite equal or lower exposure to the parasite, adult males were more likely to develop VL than females. This supports our hypothesis that males have an inherent risk for symptomatic L. infantum infection.
Table 1.
Number (percent) of individuals belonging to different Leishmania infantum infection phenotypes
| LST+ | VL* | Data known | All | |||||
|---|---|---|---|---|---|---|---|---|
| Age group | Under 10 | 10 or older | Under 10 | 10 or older | Under 10 | 10 or older | Under 10 | 10 or older |
| Male | 27 (54) | 164 (47) | 57 (50) | 45 (79) | 84 (51) | 209 (52) | 221 (48) | 414 (43) |
| Female | 23 (46) | 183 (53) | 57 (50) | 12 (21) | 80 (49) | 195 (48) | 244 (52) | 540 (57) |
These values were extracted from data collected as part of a neighborhood study of L. infantum infection in the vicinity of Natal, reported by our group.13 LST+ = positive Leishmania skin test; VL = symptomatic visceral leishmaniasis; Data known: phenotype and gender information was available; All: all subjects in the database, regardless of information available.
P < 0.001, Pearson’s χ2 test comparing VL distributions; not significant comparing distributions of LST or overall.
To address the consistency of sex bias over the years, we expanded our study to include all cases of VL reported in the state of Rio Grande do Norte between 1995 and 2010. Age and gender information was available for 1967 individuals. Ages ranged from 17 days to 87 years, with a mean (median) of 15.67 (7.0) years. Figure 1 illustrates the sex bias at different ages. The incidence of VL showed the most equal or the most skewed sex distributions at ages 2–5 or 21–40, respectively. The observation that males had a slightly increased prevalence in children below 1 year might relate to the phenomenon described as “mini-puberty” observed in several infectious diseases, attributed to increased levels of sex steroids.14 Differences in VL were determined to be statistically significant at ages above 10 (P < 2.3 × 10−16, χ2).
Figure 1.
Sex ratios in visceral leishmaniasis (VL) cases reported to the state of Rio Grande do Norte, Brazil. Reported cases of VL to the Ministry of Health of the state of Rio Grande do Norte, an endemic region of Leishmania infantum infection. (A) Percentage of male and female patients. (B) Distribution of patients by age and sex. N = 1,967. For statistical comparisons, all other age groups were compared with the 2–5 years old category. The overall significance level for these data is P < 0.0001, using a χ2 test of contingency for the overall table and χ2 test for each age group vs. the overall gender distribution.
Because of the higher prevalence of VL in males above the age of puberty (Table 1, Figure 1), we examined a possible biological basis for male susceptibility in two murine models of L. infantum infection (BALB/c and C57BL/6 mice, age 4–6 weeks). Protocols for animal work were in accordance with National Institutes of Health guidelines and approved by The University of Iowa and the Iowa City VA Medical Center ACUCs. Statistical analyses were done with GraphPad Prism 7.02.
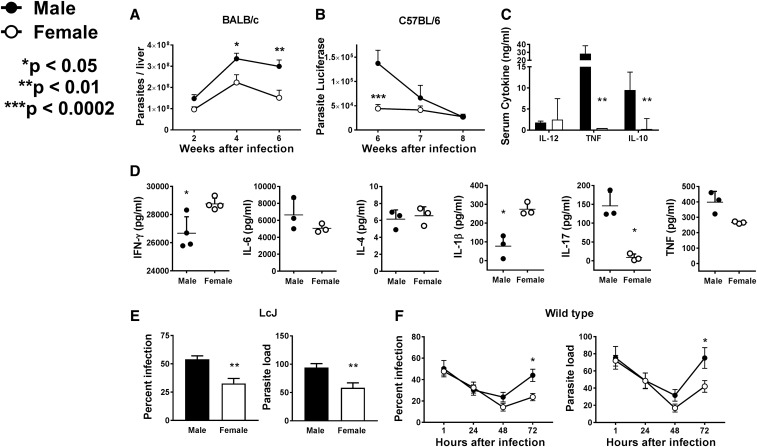
Male and female mice were inoculated intravenously with 1 × 107 stationary phase wild-type (WT) L. infantum promastigotes (Brazilian strain MHOM/BR/00/1669) or promastigotes expressing luciferase. Wright Giemsa–stained touch preparations of livers were used to determine parasite loads. Infections with luciferase-expressing parasites were quantified by in vivo imaging after inoculating luciferin as described.15 Parasite loads were also quantified in total organ DNA by quantitative polymerase chain reaction (qPCR) (kDNA5 marker).16 Parasite loads in males were higher than in female BALB/c mice (Figure 2A). Similarly, liver parasite loads were significantly higher in male versus female C57BL/6 mice when measured by in vivo imaging (Figure 2B). These differences were validated by qPCR in two experiments with 10 mice/group (8.66 ± 3.20 × 107 versus 1.59 ± 0.484 × 107 in males versus females, respectively; P = 0.0420). These results suggest that across different genetic backgrounds, males are more susceptible to L. infantum infection.
Figure 2.
Differential parasite load and cytokine response between males and females. Age-matched male and female BALB/c or C57BL/6 mice were inoculated intravenously with 1 × 107 Leishmania infantum promastigotes and used for experiments A–D. (A) At the indicated time points, BALB/c mice were euthanized and their livers used to determine parasite load by microscopic examination. Shown are the means ± SE of five mice per group of three repeat experiments. *P < 0.05, **P < 0.01, two-way analysis of variance (ANOVA) with Sidak’s multiple comparison test. (B) Parasite loads were measured in C57BL/6 mice by in vivo imaging of mice infected with luciferase-expressing L. infantum and quantified in photons/second. Data represent the mean ± SE of two experiments with five mice per group. ***P < 0.0002, two-way ANOVA with Sidak’s multiple comparison test. (C) After 8 weeks of infection, blood samples were obtained from C57BL/6 mice and serum concentrations of interleukin (IL)-12, IL-10, and tumor necrosis factor (TNF) (ng/mL) measured using Bioplex. Data represent the mean ± SD of five mice per group. **P < 0.01, unpaired, two-tailed t test with Welch’s correction. (D) C57BL/6 mice were euthanized and their splenocytes stimulated for 48 hours with soluble L. infantum antigen (5 µg/mL). Supernatants were collected and the concentrations of interferon-γ, IL-6, IL-4, IL-1β, IL-17, and TNF measured using Bioplex. Data represent the mean ± SD of four mice per group (*P < 0.05 paired t test). (E) Bone marrow macrophages from either male or female C57BL/6 mice were infected at a 1:10 ratio with LcJ promastigotes, a partly attenuated clonal line of L. infantum. One hour after infection, the samples were fixed, stained with Wright Giemsa, and examined by light microscopy. (F) Bone marrow macrophages derived from either male or female C57BL/6 mice were infected at a 1:10 ratio with wild type L. infantum promastigotes. At the indicated times, samples were fixed, stained with Wright Giemsa, and examined by light microscopy. For both panels (E) and (F), percent of infected macrophages and parasite load are shown in left and right panels, respectively. Data represent the mean ± SE of three (E) or two (F) experiments, each with triplicate conditions. For (E), **P < 0.01, two-tailed unpaired t test with Welch’s correction. For (F), *P < 0.05, two-way ANOVA with Sidak’s multiple comparison test.
Studies of serum cytokine concentrations in infected mice showed higher levels of interleukin (IL)-12 in females, whereas males had significantly higher levels of tumor necrosis factor (TNF) and IL-10 (Figure 2C). In addition, female C57BL/6 splenocytes produced significantly higher levels of antigen-induced IL-1β and interferon-γ, which are associated with type 1 protective responses, than male splenocytes.1 In contrast, male splenocytes produced higher amounts of TNF and IL-17, which are associated with inflammation or suppression of cure1 (Figure 2D).
Among the earliest interactions between Leishmania spp. and a mammalian host is recognition and uptake of the parasite via macrophage receptors. We previously showed that there are differences between the mechanisms of macrophage entry of attenuated versus virulent promastigotes.17 Consistently, Figure 2E shows that whereas macrophage uptake of the attenuated parasite line LcJ differs between male and female macrophages, this difference is not evident using virulent parasites (Figure 2F, 1 hour). Nonetheless, male macrophages supported higher survival and replication of WT L. infantum than female macrophages (Figure 2F). Published reports document differential expression of many transcripts including surface receptors and signaling proteins by macrophages of male- versus female-origin.3,6,7 Thus, it is reasonable to hypothesize that the previously mentioned results could be explained by distinct surface interactions affecting the route of Leishmania entry and different intracellular killing mechanisms between the sexes.17 Furthermore, differences have been found between innate, humoral, and cell-mediated immune responses of males versus females, which might underlie sex differences in disease susceptibility.3–5
Data in this report underscore the fact that in addition to other risk factors, there is a sex-related risk for progression to symptomatic VL that may be attributed to biological differences. Sex biases have already been reported in experimental models of cutaneous leishmaniasis. Male hamsters are more susceptible than females to infection with Leishmania panamensis or Leishmania guyanensis.18 Male DBA/2 mice are more susceptible to Leishmania mexicana but females are more susceptible to Leishmania major.19 Hence, studies in cutaneous leishmaniasis have shown that the particular combination of parasite and host determine whether there is a sex bias and which sex has higher morbidity.
Our data document differences in prevalence of VL in male versus female Brazilians infected with L. infantum. Parallel differences have not been reported in India, where most VL is caused by L. donovani. Different observations between the countries could be attributed to differences between parasite biological characteristics, human genetics, and/or behavioral or cultural factors.20
The previously mentioned results extend the sex bias observed in the epidemiological data from Brazil to show an increased risk of progression to symptomatic infection in males infected with L. infantum. Mouse models showed that compared with their female counterparts, males carry a higher burden of L. infantum parasites and fail to develop a protective T cell response. Although these are not equivalent steps in disease pathogenesis, it is reasonable to use mouse models to dissect immune pathways underlying the sex bias. Pathways that differentially affect immunity in males and females may aid in the design of new therapeutic targets in the host immune response to better control this infection.
Acknowledgments:
We are grateful to the Ministry of Health of the state of Rio Grande do Norte for making available the data for this study. We also thank Yani Chen, Bayan Zhanbolat, and Melissa Kurtz for their help with animal procedures and parasite cultures.
REFERENCES
- 1.Wilson ME, Jeronimo SM, Pearson RD, 2005. Immunopathogenesis of infection with the visceralizing Leishmania species. Microb Pathog 38: 147–160. [DOI] [PubMed] [Google Scholar]
- 2.de Freitas EO, Leoratti FM, Freire-de-Lima CG, Morrot A, Feijo DF, 2016. The contribution of immune evasive mechanisms to parasite persistence in visceral leishmaniasis. Front Immunol 7: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein SL, Flanagan KL, 2016. Sex differences in immune responses. Nat Rev Immunol 16: 626–638. [DOI] [PubMed] [Google Scholar]
- 4.Markle JG, Fish EN, 2014. SeXX matters in immunity. Trends Immunol 35: 97–104. [DOI] [PubMed] [Google Scholar]
- 5.vom Steeg LG, Klein SL, 2016. SeXX matters in infectious disease pathogenesis. PLoS Pathog 12: e1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansen R, et al. 2014. Sex differences in the human peripheral blood transcriptome. BMC Genomics 15: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayne BT, Bianco-Miotto T, Buckberry S, Breen J, Clifton V, Shoubridge C, Roberts CT, 2016. Large scale gene expression meta-analysis reveals tissue-specific, sex-biased gene expression in humans. Front Genet 7: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das VN, Pandey RN, Siddiqui NA, Chapman LA, Kumar V, Pandey K, Matlashewski G, Das P, 2016. Longitudinal study of transmission in households with visceral leishmaniasis, asymptomatic infections and PKDL in highly endemic villages in Bihar, India. PLoS Negl Trop Dis 10: e0005196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukhopadhyay D, Mukherjee S, Ghosh S, Roy S, Saha B, Das NK, Chatterjee M, 2016. A male preponderance in patients with Indian post kala-azar dermal leishmaniasis is associated with increased circulating levels of testosterone. Int J Dermatol 55: e250–e255. [DOI] [PubMed] [Google Scholar]
- 10.Belo VS, Werneck GL, Barbosa DS, Simoes TC, Nascimento BW, da Silva ES, Struchiner CJ, 2013. Factors associated with visceral leishmaniasis in the Americas: a systematic review and meta-analysis. PLoS Negl Trop Dis 7: e2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das A, Karthick M, Dwivedi S, Banerjee I, Mahapatra T, Srikantiah S, Chaudhuri I, 2016. Epidemiologic correlates of mortality among symptomatic visceral leishmaniasis cases: findings from situation assessment in high endemic foci in India. PLoS Negl Trop Dis 10: e0005150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lima ID, et al. 2012. Leishmania infantum chagasi in northeastern Brazil: asymptomatic infection at the urban perimeter. Am J Trop Med Hyg 86: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeronimo SM, et al. 2004. An emerging peri-urban pattern of infection with Leishmania chagasi, the protozoan causing visceral leishmaniasis in northeast Brazil. Scand J Infect Dis 36: 443–449. [DOI] [PubMed] [Google Scholar]
- 14.Guerra-Silveira F, Abad-Franch F, 2013. Sex bias in infectious disease epidemiology: patterns and processes. PLoS One 8: e62390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thalhofer CJ, Chen Y, Sudan B, Love-Homan L, Wilson ME, 2011. Leukocytes infiltrate the skin and draining lymph nodes in response to the protozoan Leishmania infantum chagasi. Infect Immun 79: 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weirather JL, et al. 2011. Serial quantitative PCR assay for detection, species discrimination, and quantification of Leishmania spp. in human samples. J Clin Microbiol 49: 3892–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez NE, Gaur Dixit U, Allen LA, Wilson ME, 2011. Stage-specific pathways of Leishmania infantum chagasi entry and phagosome maturation in macrophages. PLoS One 6: e19000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Travi BL, Osorio Y, Melby PC, Chandrasekar B, Arteaga L, Saravia NG, 2002. Gender is a major determinant of the clinical evolution and immune response in hamsters infected with Leishmania spp. Infect Immun 70: 2288–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snider H, Lezama-Davila C, Alexander J, Satoskar AR, 2009. Sex hormones and modulation of immunity against leishmaniasis. Neuroimmunomodulation 16: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weirather JL, Duggal P, Nascimento EL, Monteiro GR, Martins DR, Lacerda HG, Fakiola M, Blackwell JM, Jeronimo SM, Wilson ME, 2017. Comprehensive candidate gene analysis for symptomatic or asymptomatic outcomes of Leishmania infantum infection in Brazil. Ann Hum Genet 81: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]