Abstract.
We present an autochthonous Histoplasma infection in Israel. The patient presented with hoarseness and weight loss. Pathology findings and molecular tests confirmed the diagnosis, and the patient responded to antifungal therapy. Because the patient had an atypical presentation of histoplasmosis, it is likely that other more typical cases have gone unreported. To our knowledge, no other cases have been previously reported from Israel or the Middle East region.
CASE REPORT
A 53-year-old, previously healthy, female presented to her primary care physician with progressive hoarseness, sore throat, cough, purulent sputum, and unexplained weight loss of 5 kg over the previous four months. The patient was a resident of a village in the Galilee region of northern Israel and had never left the country. She did not recall any cave exploration or direct contact with bats. Her neighbors owned parrots, but she had never been exposed to the pets or their cage. The patient did not smoke or drink, nor did she use illicit drugs, and she had no known exposure to patients with tuberculosis.
Her vital signs and physical examination were unremarkable. Blood count revealed normocytic anemia with hemoglobin levels 10 gr/dL consistent with chronic illness, leukopenia of 3,500 cells/µL, and lymphopenia with an absolute lymphocyte count of 350 (normal range 1,500–5,000 cells/µL). Eight years before the diagnosis, the patient had unexplained lymphopenia (1,100 cells/µL). Since then, her lymphocyte count gradually decreased in seven consecutive tests. Levels of thyroid stimulating hormone, ferritin, vitamin B12, and folic acid were normal. Creatinine, liver enzymes, electrolytes, lactate dehydrogenase, and haptoglobin were all normal. C-reactive protein level was 50 (normal range 0–5 mg/L). Serologic tests for infection with human immunodeficiency viruses 1 and 2, and hepatitis B and C viruses were negative. The patient declined further workup; therefore, flow cytometry was not performed.
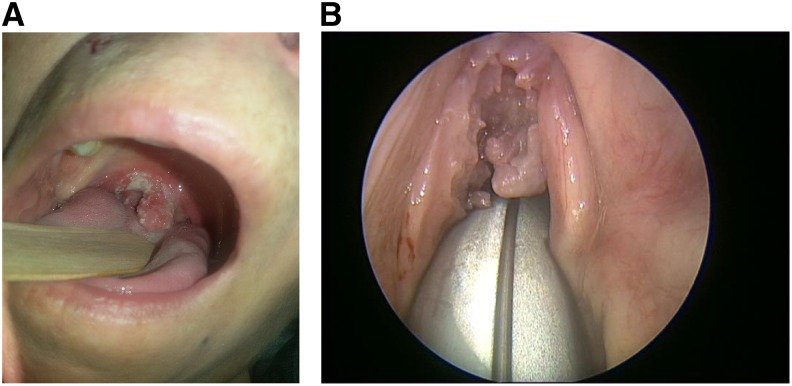
Physical examination revealed a red mucosal lesion with leukoplakia affecting the uvula and extending to the right soft palate and the anterior pillar of the right tonsil (Figure 1A). Laryngeal fiber-optic examination revealed granulation tissue located on the anterior commissure of the glottis and extending down the glottis opening, hindering closure of the glottis (Figure 1B). A primary laryngeal tumor was suspected, and a sample for pathologic examination was taken. Cervical and chest CT scan revealed a 4-mm lesion in the right upper lobe of the lung without any evidence of lymphadenopathy or other masses.
Figure 1.
(A) Mucosal lesion. (B) Granulation lesion of the glottis. This figure appears in color at www.ajtmh.org.
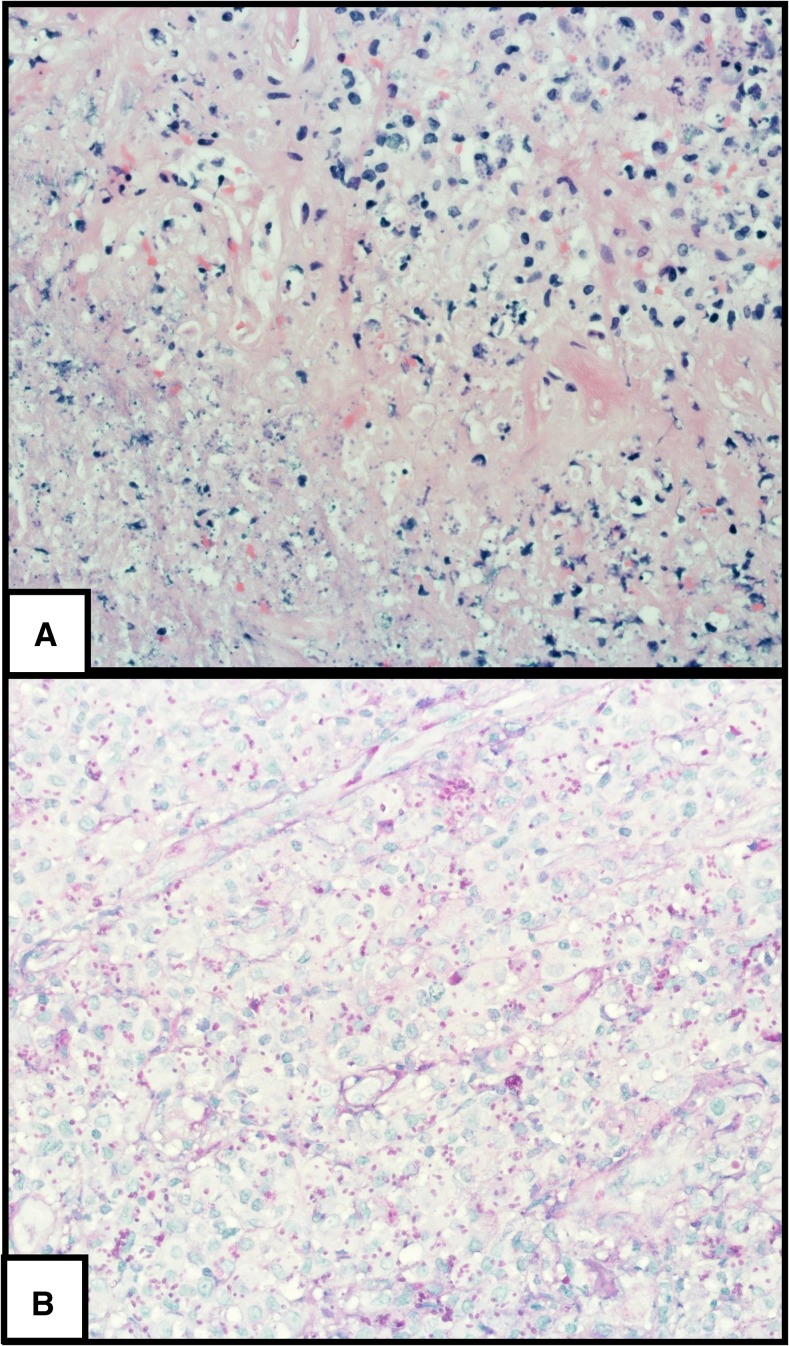
Biopsy from the lesion (Figure 2) revealed necrotic tissue with chronic inflammation containing numerous teardrop-shaped buds (Figure 2A), positive for periodic acid-Schiff and silver stains, and suggestive of yeasts (Figure 2B).
Figure 2.
(A) (×400) Hematoxylin and eosin stain shows necrosis (left bottom) and chronic inflammation. Many teardrop-shaped yeasts are seen in histiocytes and in the necrotic area. (B) (×400) Periodic acid-Schiff stain enhances the yeasts. This figure appears in color at www.ajtmh.org.
As culturing formalin-fixed paraffin-embedded tissue is unreliable, we performed a polymerase chain reaction assay, which confirmed a diagnosis of histoplasmosis. An assay designed to detect fungal DNA was used, followed by direct sequencing and matching of the resulting sequence to public databases. DNA was extracted from tissue using the Qiamp DNA mini kit (Qiagen, Hilden, Germany) and was amplified by using two different primer sets specific for the large subunit fungal ribosomal RNA gene and internal transcribed spacer region 1 (ITS1). Each assay included negative and positive controls.
ITS1 and ITS2 region DNA were simultaneously amplified as a single product by using primers ITS1 (5′TCCGTAGGTGAAC CTGCGG) and ITS4 (5′TCCTCCGCTTAT TGATATGC).2 The divergent domain at the 5′ end of the large subunit rDNA gene (6) was symmetrically amplified with primers NL-1 (5′GCATATCAATAAGCGGAGGAAAAG) and NL-4 (5′GGTCCGTGTTTCAAGACGG).1 The resulting amplicons were directly sequenced and comparative analysis of the nucleotide sequence was performed using the BLAST algorithm.1,2
Four months after the onset of symptoms, a serum serologic test (both complement fixation and immunodiffusion) for histoplasmosis was submitted to the Centers for Disease Control and Prevention (CDC) and was negative.
The patient was diagnosed with laryngeal histoplasmosis and treated with oral itraconazole, a loading dose of 200 mg orally three times a day for the first 3 days of therapy and then maintenance therapy with 200 mg orally once a day for 6 months. Within 3 weeks, she felt well, hoarseness and chronic cough resolved, and follow-up laryngoscopy demonstrated complete resolution of the lesions.
DISCUSSION
Histoplasma capsulatum is a dimorphic fungus with a wide geographic distribution. It is most prevalent in the Mississippi and Ohio river valleys in the USA and in Central and South America.3,4 Histoplasmosis also occurs, although less commonly, in Africa, the Indian subcontinent, Southeast Asia, China, and Australia.5–8 In Africa, H. capsulatum var. duboisii coexists with H. capsulatum var. capsulatum.4 Histoplasmosis is acquired through inhalation of the fungus, usually from contaminated soil. The presence of H. capsulatum in the soil is strongly linked to the presence of bird and bat guano.3
Even though the organism was isolated from a soil sample in a cave in northern Israel in 1977, and subsequently from a dead bat from the same cave, it is not considered endemic to the eastern Mediterranean region or the Middle East. The organism recovered was confirmed to be H. capsulatum by the CDC.9
All histoplasmosis infections previously diagnosed in Israel had been contracted in Central or South America, with the exception of a single case acquired in the USA.10
We hypothesize that the patient was infected through contact with bat droppings near her village of residence. She does not recall an obvious source of exposure and has never traveled out of Israel, but she resides near the cave of Yodfat in the Galilee region where the organism was isolated back in 1977.9 It is also possible that the patient inhaled the fungus several months earlier when construction workers were building a new neighborhood near her place of residence. During these construction works, several caves were excavated by the Israel Antiquities Authority and then blown up. The village residents recalled a cloud of dust that resulted from these events. We searched the cave of Yodfat, which is now visited frequently by tourists, and found no bats. Environmental samples from this cave did not contain the fungus. The lack of a clear event of exposure is not surprising, as in some cohorts of histoplasmosis cases, only around 20% of patients recall exposure to caves, bats, or birds.11,12
No cases of autochthonous H. capsulatum infection have been previously reported in Israel, or in the Middle East, although it is likely that cases occurred and went undiagnosed. One case was previously reported from Turkey.12 Laryngeal histoplasmosis is an uncommon presentation of Histoplasma infection, so that it is likely that other cases with pulmonary manifestations went undiagnosed because of lack of awareness. Only serologic studies in residents of the Galilee area would provide an estimate of how common histoplasmosis actually is.
The patient had laryngeal involvement of Histoplasma infection, along with symptoms suggestive of a disseminated disease. Less than 100 cases of laryngeal histoplasmosis have been reported. It is commonly assumed that laryngeal infection is secondary to hematogenous spread to the larynx, although direct infection through inhalation, or secondary infection through respiratory secretions, is also possible. Common presenting symptoms are hoarseness, dysphagia, and cough, as well as systemic symptoms such as weight loss, low-grade fever, and fatigue.13–16 Laryngeal endoscopic examination usually reveals a nodule or an ulcerated mass in the supraglottic area, mimicking laryngeal cancer or tuberculosis.13,15,16
The most prominent risk factor for H. capsulatum infection is immunosuppression, secondary to acquired immune deficiency syndrome, organ transplantation, corticosteroids, or anti-tumor necrosis factor immunomodulators.17–19 Our patient did not have any known immune suppression, but did have an unexplained lymphopenia, which had been present for at least eight years before the current infection. The patient declined further workup for an immune deficiency, but still has leukopenia and lymphopenia after completion of antifungal therapy. We suspect that congenital or acquired lymphocytopenia resulted in an immune deficiency that led to the infection being systemic and atypical.20 Serologic tests for histoplasmosis were inconclusive, which is not an uncommon finding in other cohorts and may result from an inadequate antibody production in a patient with severe lymphocytopenia.10
In conclusion, we report an autochthonous case of histoplasmosis in Israel. The patient had an uncommon clinical presentation of the infection, leading us to hypothesize that other more typical cases may have gone undiagnosed.
Acknowledgments:
We would like to acknowledge the directors of Rambam Healthcare Campus and French Hospital Nazareth for allowing us to report this case.
REFERENCES
- 1.Kurtzman CP, Robnett CJ, 1997. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol 35: 1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen YC, Eisner JD, Kattar MM, Rassoulian-Barrett SL, Lafe K, Bui U, Limaye AP, Cookson BT, 2001. Polymorphic internal transcribed spacer region 1 DNA sequences identify medically important yeasts. J Clin Microbiol 39: 4042–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cano MV, Hajjeh RA, 2001. The epidemiology of histoplasmosis: a review. Semin Respir Infect 16: 109–118. [DOI] [PubMed] [Google Scholar]
- 4.Baddley JW, et al. 2011. Geographic distribution of endemic fungal infections among older persons, United States. Emerg Infect Dis 17: 1664–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gugnani HC, Muotoe-Okafor F, 1997. African histoplasmosis: a review. Rev Iberoam Micol 14: 155–159. [PubMed] [Google Scholar]
- 6.Gopalakrishnan R, Nambi PS, Ramasubramanian V, Abdul Ghafur K, Parameswaran A, 2012. Histoplasmosis in India: truly uncommon or uncommonly recognised? J Assoc Physicians India 60: 25–28. [PubMed] [Google Scholar]
- 7.McLeod DS, Mortimer RH, Perry-Keene DA, Allworth A, Woods ML, Perry-Keene J, McBride WJ, Coulter C, Robson JM, 2011. Histoplasmosis in Australia: report of 16 cases and literature review. Medicine (Baltimore) 90: 61–68. [DOI] [PubMed] [Google Scholar]
- 8.Pan B, Chen M, Pan W, Liao W, 2013. Histoplasmosis: a new endemic fungal infection in China? Review and analysis of cases. Mycoses 56: 212–221. [DOI] [PubMed] [Google Scholar]
- 9.Ajello L, Kuttin ES, Beemer AM, Kaplan W, Padhye A, 1977. Occurrence of Histoplasma capsulatum Darling, 1906 in Israel, with a review of the current status of histoplasmosis in the Middle East. Am J Trop Med Hyg 26: 140–147. [DOI] [PubMed] [Google Scholar]
- 10.Segel MJ, Rozenman J, Lindsley MD, Lachish T, Berkman N, Neuberger A, Schwartz E, 2015. Histoplasmosis in Israeli travelers. Am J Trop Med Hyg 92: 1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheat LJ, 2006. Histoplasmosis: a review for clinicians from nonendemic areas. Mycoses 49: 274–282. [DOI] [PubMed] [Google Scholar]
- 12.Ashbee HR, Tintelnot K, 2008. Histoplasmosis in Europe: report on an epidemiological survey from the European Confederation of Medical Mycology Working Group. Med Mycol 46: 57–65. [DOI] [PubMed] [Google Scholar]
- 13.Durand ML, Lin DT, Juliano AF, Sadow PM, 2014. Case 32-2014. A 78-year-old woman with chronic sore throat and a tonsillar mass. N Engl J Med 371: 1535–1543. [DOI] [PubMed] [Google Scholar]
- 14.Antonello VS, Zaltron VF, Vial M, Oliveira FM, Severo LC, 2011. Oropharyngeal histoplasmosis: report of eleven cases and review of the literature. Rev Soc Bras Med Trop 44: 26–29. [DOI] [PubMed] [Google Scholar]
- 15.O’Hara CD, Allegretto MW, Taylor GD, Isotalo PA, 2004. Epiglottic histoplasmosis presenting in a nonendemic region: a clinical mimic of laryngeal carcinoma. Arch Pathol Lab Med 128: 574–577. [DOI] [PubMed] [Google Scholar]
- 16.Ramadan O, 2016. Laryngeal histoplamosis overview. Otolaryngol Open J. 2: 141–149. [Google Scholar]
- 17.McKinsey DS, McKinsey JP, 2011. Pulmonary histoplasmosis. Semin Respir Crit Care Med 32: 735–744. [DOI] [PubMed] [Google Scholar]
- 18.Cuellar-Rodriguez J, Avery RK, Lard M, Budev M, Gordon SM, Shrestha NK, van Duin D, Oethinger M, Mawhorter SD, 2009. Histoplasmosis in solid organ transplant recipients: 10 years of experience at a large transplant center in an endemic area. Clin Infect Dis 49: 710–716. [DOI] [PubMed] [Google Scholar]
- 19.Smith JA, Kauffman CA, 2009. Endemic fungal infections in patients receiving tumour necrosis factor-alpha inhibitor therapy. Drugs 69: 1403–1415. [DOI] [PubMed] [Google Scholar]
- 20.Kortsik C, Elmer A, Tamm I, 2003. Pleural effusion due to Histoplasma capsulatum and idiopathic CD4 lymphocytopenia. Respiration. 70: 118–122. [DOI] [PubMed] [Google Scholar]