Abstract.
We evaluated the therapeutic efficacy of artemether–lumefantrine (AL) fixed-dose combination to treat uncomplicated Plasmodium falciparum malaria in Cruzeiro do Sul, Acre State, in the Amazon region of Brazil. Between December 2015 and May 2016, we enrolled 79 patients, 5–79 years old with fever or history of fever in the previous 48 hours and P. falciparum monoinfection confirmed by microscopy. Attempts were made to provide direct observation or phone reminders for all six doses of AL, and patients were followed-up for 28 days. AL was well tolerated, with no adverse events causing treatment interruption. All but one of the 74 patients who completed the 28-day follow-up had an adequate clinical and parasitologic response = 98.6% (95% CI: 93.2-100%). We could not amplify the one isolate of the case with recurrent infection to differentiate between recrudescence and reinfection. Five (6.3%) patients demonstrated persistent asexual parasitemia on Day 3, but none met definition for early treatment failure. We found no mutations in selected kelch13 gene domains, known to be associated with artemisinin resistance in P. falciparum isolates from Day 0. These results strongly support the continued use of AL as a first-line therapy for uncomplicated P. falciparum malaria in Acre. Routine monitoring of in vivo drug efficacy coupled with molecular surveillance of drug resistance markers remains critical.
BACKGROUND
Although most of the global malaria burden falls on sub-Saharan Africa, malaria remains endemic in the Americas, particularly in the Amazon Basin, with an estimated 800,000 cases reported in 2015.1 Eighteen countries in the region continue to report evidence of active malaria transmission; Venezuela, Brazil, and Peru had most of the malaria cases in 2015.1 According to the Brazil National Malaria Control Program (NMCP), there were a total of 138,127 cases of malaria in Brazil in 2015, 99.9% (137,934) of which occurred in the Amazon region.2 Most of the malaria cases (88%) in Brazil was due to Plasmodium vivax monoinfection (121,330); Plasmodium falciparum monoinfection accounted for 11% (14,761) of cases in 2015. In Acre, Brazil’s westernmost state, a total of 26,632 malaria cases were registered in 2015, 19.3% of the total cases in the country. Cruzeiro do Sul is the second largest city in Acre State with about 11% of the state’s population (82,000 inhabitants); however, over half of the state’s malaria cases (13,989) occurred in this city in 2015. Both P. vivax and P. falciparum are endemic in the region, with approximately 20% of the cases due to P. falciparum.
Since 2006, the Brazilian NMCP has recommended artemisinin-based combination therapy (ACT) as the first-line treatment of uncomplicated P. falciparum malaria in Acre State, initially with artesunate–mefloquine, later replaced by artemether–lumefantrine (AL) in 2014. Although there has not been any evidence of P. falciparum resistance to ACTs in Acre State to date, the NMCP follows the World Health Organization (WHO) guidelines to evaluate antimalarial efficacy routinely every 2 to 3 years as part of the Amazon Network for the Surveillance of Antimalarial Drug Resistance and Amazon Malaria Initiative (Pan-American Health Organization, 2012).3,4 Of concern are reports of suspected artemisinin resistance in neighboring countries: increased incidence of Day 3 parasitemia in Suriname and presence of Kelch propeller (K13) gene mutations associated with artemisinin resistance reported in Guyana.5,6 The regions in Brazil bordering these countries tend to be sparsely populated, thus rendering recruitment and follow-up of patients for a drug efficacy study logistically challenging. As part of the national antimalarial efficacy monitoring, the Brazilian NMCP decided to conduct a 28-day therapeutic efficacy study of AL for the treatment of uncomplicated P. falciparum malaria in Cruzeiro do Sul, given its relatively high disease burden. In addition, resistance marker testing was performed, focusing on mutations in the K13 gene, which have been associated with delayed parasite clearance and artemisinin resistance, and the Pfmdr-1 gene recently implicated in resistance to lumefantrine, the partner drug.7–9
METHODS
Study timing and location.
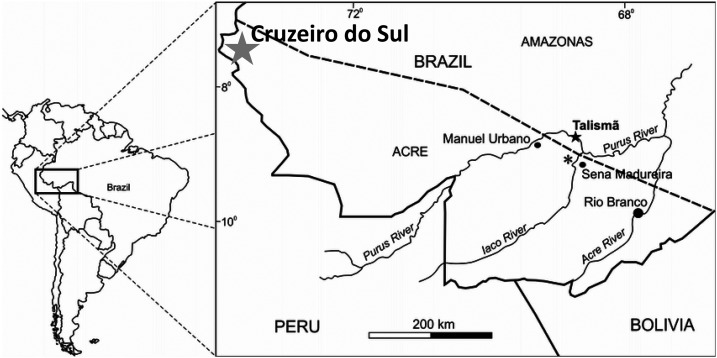
This single-arm prospective study was conducted during the peak malaria transmission season in Cruzeiro do Sul in the western Brazilian Amazon (Figure 1). In Brazil, malaria diagnosis and treatment are offered free of charge at malaria diagnostic outposts in local health centers and hospitals, and it is common practice for patients to seek care for malaria symptoms at these facilities. Participants for this study were recruited at such malaria outposts in Hospital Regional do Juruá (HRJ), the main referral hospital in the area, as well as 11 other local health centers in Cruzeiro do Sul. HRJ has a 24-hour malaria diagnosis laboratory and registers the highest number of malaria diagnoses in the city (19,331 patients in 2014).
Figure 1.
Map of Cruzeiro do Sul, Acre State, Brazil.
Sample size.
The minimum sample size needed to detect a 95% efficacy rate with an absolute precision of 5% on each side of this estimate (90–100%) and a confidence interval (CI) of 95% was 73.10 Estimating a 10% loss to follow-up in a 28-day period, the final sample size was increased to 81 patients.
Study procedures.
Two clinical teams, each comprising a study nurse, a nursing assistant, and a microscopist, were involved in the study. One team was based at the HRJ and the other was a mobile team responsible for enrolling patients from other health centers. The teams were supervised by a study physician based at the HRJ. The protocol for this in vivo study was based on the standard WHO methods for surveillance of antimalarial drug efficacy with modifications for low-transmission settings.3,11 Participants who presented with febrile illness to the aforementioned study sites were approached for recruitment if they had a malaria blood smear positive for P. falciparum monoinfection. Study inclusion criteria were age ≥ 5 years, body weight < 120 kg, hemoglobin level ≥ 8 g/dL, fever (axillary temperature ≥ 37.5°C) or history of fever in the previous 48 hours, parasite density between 250 and 200,000 asexual parasites/μL, and willingness to return to clinic or accept home visits during the 28-day follow-up. Exclusion criteria included presence of malaria danger signs or signs of severe malaria. Pregnant women, those with history of chronic or severe underlying diseases, including malnutrition, as well as those with history of allergy to AL and use of any antimalarial drug in the previous 30 days were also excluded.
After obtaining informed consent, demographic and clinical information was collected using standard clinical report forms. At enrollment, blood was drawn by venous puncture for diagnostic confirmation by microscopy, hemoglobin determination, pregnancy test (for females 10–49 years old), and for preparation of dried blood spots (DBS) on Whatman 903 Protein Saver Cards (Sigma Aldrich, St. Louis, MO).
AL tablets (20 mg artemether/120 mg lumefantrine) used in the study (Lumiter, Macleods Pharmaceuticals, India) were obtained from the Brazilian Ministry of Health stockpiles, which are procured respecting pre-certification processes. Before study initiation, an independent quality assurance evaluation of the AL was ascertained at the Centers for Disease Control and Prevention (CDC) laboratory in Atlanta, U.S., from the lot of drugs used for the study. Three randomly selected tablets from the lot were analyzed for active ingredients by high-performance liquid chromatography (HPLC) as described in the International Pharmacopoeia 4th edition.12 Briefly, pulverized tablet material was weighed, dissolved in acidified methanol, and sonicated for 20 minutes. A filtered portion was injected into an HPLC system and the components separated using a reverse-phase column and a mobile phase of 65% acetronirile and 35% 0.05 M sodium perchlorate buffer. Detection was accomplished using ultraviolet absorbance. Except for one tablet that contained 114% of artemether active pharmaceutical ingredient (API), all tablets were within the acceptable criteria of 90–110% API.
Treatment with AL was administered with water and cookies following manufacturer’s prescribed weight-based twice-daily dosing (5–14 kg: 1 tablet; 15–24 kg: 2 tablets; 25–34 kg: 3 tablets; > 34 kg: 4 tablets) for 3 days. Participants received the first dose of AL under direct observation, and they were observed for 30 minutes after each subsequent dose to monitor for vomiting or other adverse events. Those who vomited a dose were given a second dose and observed for additional 30 minutes. Inability to tolerate the second dose resulted in removal from the study and admission to the hospital for parenteral treatment.
Patients were seen on Day 0 (enrollment), then daily from Days 1 through 3, then on Day 7, and weekly thereafter until Day 28. Patients were also asked to return to clinic if they were ill or had any complaints. During these visits on Days 1 and 2, one of the daily doses of AL was dispensed under direct observation. Effort was made to observe every second daily AL dose as well, but for evening doses occurring after 8 pm, patients were provided the dose to take at home on their own. In such cases, a team member followed up over the phone to remind them before when the dose was due and to remind them to monitor for side effects. The Day 3 visit was conducted as close to 72 hours from the first dose as possible to accurately assess for delayed parasite clearance. At each follow-up visit, the team administered a standard symptom questionnaire and conducted a physical examination, including measurement of axillary temperature and blood was collected by finger prick for thick and thin smears. If patients were found to be parasitemic after Day 3, regardless of symptoms, patients were called back for DBS collection to conduct genotyping. Hemoglobin levels were measured on Days 0, 14, and 28 (Hemocue Inc., Cypress, CA).
On Day 28, all participants received a single low-dose primaquine (15 mg per tablet) for its gametocidal activity against P. falciparum per the following weight-based dosing: 5–14 kg half tablet; 15–24 kg 1 tablet; 25–34 kg, 1 1/2 tablet; ≥ 35 kg, 3 tablets. This deviated from the standard Brazil NMCP treatment guidelines, which recommend administering the primaquine on Day 3. However, to evaluate the antimalarial effect of AL alone for the purposes of this study, the primaquine dose was held until the end of follow-up.
Microscopic blood examination.
Before study initiation, the study microscopists were trained on appropriate staining and microscopy procedures by expert microscopists from Instituto Evandro Chagas, a Brazilian national public health institute. Slides initially obtained by malaria diagnosis outpost staff were used for screening purposes to identify potential participants with P. falciparum monoinfection. During enrollment, a new study slide (thin and thick smears) was made and stained for 40 minutes using diluted Giemsa stain (1:50, vol/vol). Each study slide was read by one study microscopist on the same day to verify the diagnosis and parasite density. A second microscopist, blinded to the first reading, reviewed the slide within 24 hours. A third microscopist read the smear as a tie-breaker if there was discordance in species determination or if asexual parasite density estimates differed by more than 50%.
Parasite density was measured by counting the number of asexual parasites against 200 white blood cells (WBCs) in the thick smear, based on an estimated WBC count of 6,000 WBC/μL, which was found to be appropriate in the Brazilian Amazon malaria epidemiologic context.13 If the parasite count was < 100 parasites per 200 WBCs, then counting continued until 500 WBCs. A total of 1,000 WBCs were counted before a blood smear was considered negative. Asexual parasite density was reported as a geometric mean, whereas gametocytemia was reported as arithmetic mean, both as counts per microliter, of the two concordant results.
Study outcomes.
The WHO definition of therapeutic responses was used to classify patient outcomes.3 Early treatment failure (ETF) was defined as having signs of severe malaria on Days 1–3, parasitemia on Day 2 higher than on Day 0, parasitemia on Day 3 with axillary temperature ≥ 37.5°C, or parasitemia on Day 3 ≥ 25% of Day 0. Late treatment failure was classified as either late clinical failure (LCF) or late parasitological failure (LPF). LCF was defined as parasitemia in the presence of either danger signs/symptoms of severe malaria or axillary temperature ≥ 37.5°C on any day between Day 4 and 28 in patients who did not previously meet criteria for ETF. LPF was defined as presence of parasitemia on any day between Day 7 and 28 in the absence of fever or any criteria for ETF or LCF. Adequate clinical and parasitologic response (ACPR) was defined as absence of parasitemia on Day 28, irrespective of axillary temperature, in patients who did not previously meet any of the criteria for ETF, LCF, or LPF. Participants who met criteria for ETF, LCF, or LPF were removed from further study participation and treated with a rescue regimen (quinine and doxycycline) per the Brazilian malaria treatment guidelines. Loss to follow-up occurred when a participant could not be found despite reasonable efforts to locate him/her at home or work after a missed follow-up visit.
Molecular testing.
Molecular confirmation of P. falciparum infection was done using a multiplex Plasmodium sp./P. falciparum photo-induced electron transfer–polymerase chain reaction (PET-PCR) assays on all Day 0 isolates as previously described.14 Paired DBS samples from Day 0 and day of disease recurrence were used to differentiate between reinfection and recrudescence. Seven neutral microsatellite loci spanning six chromosomes (TA1, chromosome 6; poly α, chromosome 4; PfPK2, chromosome 12; TA109, chromosome 6; 2490, chromosome 10; C2M24, chromosome 2; and C3M69, chromosome 3) were used to assess the relatedness of P. falciparum isolates.15,16 Alleles with a length difference of at least two base pairs were considered different. Paired samples with at least one different microsatellite were considered heterologous haplotypes (i.e., different strains of infection and therefore a reinfection as opposed to a recrudescence).
Drug resistance genotyping was attempted on all Day 0 and day of disease recurrence isolates by analyzing single nucleotide polymorphisms in the Kelch 13 propeller domain (K13, associated with resistance to artemisinin), P. falciparum multidrug-resistant protein-1 (Pfmdr-1, associated with resistance to lumefantrine), and P. falciparum chloroquine-resistant (Pfcrt, associated with resistance to chloroquine) genes. The K13 propeller domain was sequenced as recently described.17 The Pfmdr-1 gene was genotyped at codons 86, 184, 1042, and 1246, and the Pfcrt gene was amplified and sequenced for codons 72–76 as previously described.18 Sequencing of the purified PCR products was performed using Big Dye Terminator v3.1 cycle sequencing kit on an iCycler thermal cycler (Bio-Rad, Hercules, CA). The reactions were precipitated in 70% ethanol to clean up dye terminators, rehydrated in 10 μL HiDi formamide, and then sequenced on a 3130xl ABI Genetic Analyzer (Applied Biosystems, Foster City, CA). Sequence analysis was performed using Geneious R7 (Biomatters, Auckland, New Zealand).
Statistical analysis.
Patient data were double entered into an EpiInfo 7.2.0.1 (CDC, Atlanta, GA) database and data cleaning and analysis were conducted using SAS version 9.3 (SAS Institute, Cary, NC). The primary efficacy endpoint was the Day 28 uncorrected and PCR-corrected ACPR for per-protocol analysis. For this, the denominator was limited to patients with ACPR or treatment failure and was exclusive of those with loss to follow-up or protocol violation. We also calculated the cumulative success rate, estimated by Kaplan–Meier analysis, considering all participants with any length of follow-up. Patient information collected until the day of censoring, the last day the patient was observed, was included in the analysis.
Ethical considerations.
Written informed consent was obtained from each participant as well as permission from a legal guardian for participants under age 18. All minors 7–17 years old provided an age-appropriate written assent. The study was reviewed and approved by the institutional review board at the CDC in Atlanta, GA (protocol 2015-204), and the Fundação Hospital Estadual do Acre (protocol 1.286.464). This study was also registered on ClinicalTrials.gov (NCT02600767).
RESULTS
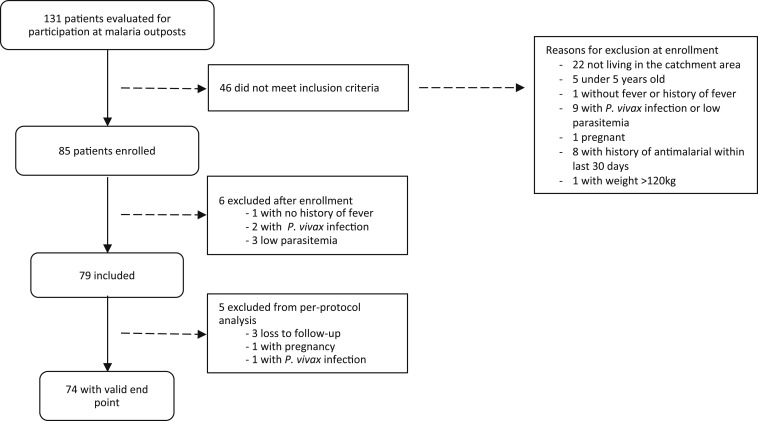
A total of 131 patients diagnosed with P. falciparum infection at the malaria diagnosis outposts were evaluated for participation from December 2015 through May 2016; 46 did not meet the inclusion criteria (Figure 2). A total of 85 patients were conditionally enrolled, 42 from HRJ and 43 from other health centers. However, six of these 85 were later excluded based on lack of fever or history of fever (one patient), parasite density < 250 parasites/μL (three), and P. vivax infection (two)—one by microscopy review and the other by PCR confirmation of species. As a result, 79 patients were included in the Kaplan–Meier analysis for calculation of cumulative success rate. In addition, five additional patients were excluded from per-protocol analysis: three (3.8%) because of loss to follow-up, one (1.3%) presented with P. vivax infection on Day 28, and one (1.3%) with a positive pregnancy test on Day 21. In the end, 74 participants completed Day 28 follow-up.
Figure 2.
Screening, enrollment, and follow-up of study participants, Cruzeiro do Sul, Brazil.
Patient characteristics at enrollment are shown in Table 1. The median age of participants was 30 years (range: 5–79); 42 (53.2%) patients were male. The mean weight of the participants was 62.9 kg (standard deviation 17.9). The geometric mean asexual parasitemia on Day 0 was 3,918 parasites/μL. The arithmetic mean gametocitemia on Day 0 was 95.4 gametoctes/μL, with 19 (24.1%) of the participants having gametocites. On subsequent days, 13 (16.5%), 11 (13.9%), and 7 (8.9%) participants had gametocites on Days 2, 3, and 7, respectively (Table 2). No participant had any gametocites on Days 14 and 21, and two participants (2.5%) had gametocites on Day 28, one with 87.5 gametocites/μL and another with 5.5 gametocites/μL. In 35 separate occasions (6% of all microscopy reads) during the 28-day follow-up of the study, a third microscopy read was needed to serve as a tie-breaker due to discordance in asexual parasite density by over 50%.
Table 1.
Participant characteristics at study enrollment (Day 0), Cruzeiro do Sul (N = 79)
| Characteristic | Value |
|---|---|
| Age, years (median, range) | 30 (5–79) |
| Male, n (%) | 42 (53.2%) |
| Weight (kg) (mean, SD) | 62.9 (17.9) |
| Asexual parasite density (parasites/μL) (geometric mean, range, geometric SD) | 3,918.4 (272–14,068, 398.6) |
| Percentage of patients with gametocites | 24.1% |
| Gametocitemia (gametocites/μL) (arithmetic mean, range, SD) | 95.4 (11.5–608, 31.9) |
Table 2.
Gametocitemia by day of follow-up, Cruzeiro do Sul (N = 79)
| Treatment day | Number of patients with gametocites (%) | Mean arithmetic gametocitemia (gametocites/μL) (SD) |
|---|---|---|
| Day 2 | 13 (16.5) | 34.5 (74.6) |
| Day 3 | 11 (13.9) | 30.3 (36.1) |
| Day 7 | 7 (8.9) | 22.9 (19.9) |
| Day 14 | 0 | n/a |
| Day 21 | 0 | n/a |
| Day 28 | 2 (2.5) | 46.5 (58.0) |
n/a = not applicable.
Clinical presentation.
The median duration of fever at time of enrollment was 3 days (range: 1–15 days) (Table 3). Seventeen (21.5%) patients had a documented temperature ≥ 37.5°C on the Day 0 visit, but only two (2.5%) still had documented fever at the Day 1 follow-up visit. The most common clinical complaint on Day 0 was headache with 71 (89.9%) reporting this symptom, and 16 reported it on Day 7. The second and third most common symptoms on Day 0 were body aches (41.8%) and stomachache (6.3%). The mean hemoglobin levels were 13.6, 12.8, and 13.3 g/dL on Days 0, 14, and 28, respectively.
Table 3.
Patient clinical characteristics, Cruzeiro do Sul (N = 79)
| Clinical or laboratory characteristic | Value |
|---|---|
| Mean duration of fever at time of enrollment (range) | 3 days (1–15 days) |
| Common clinical complaints (%) | |
| Day 0 (N = 79) | Headache 71 (89.9), body aches 33 (41.8), stomach ache 5 (6.3) |
| Day 7 (N = 78) | Headache 16 (20.5), body aches 0, stomach ache 3 (3.8) |
| Mean hemoglobin (g/dL) (SD) | |
| Day 0 | 13.6 (1.9) |
| Day 14 | 12.8 (1.4) |
| Day 28 | 13.3 (1.4) |
| Number of patients with anemia (Hb ≤ 11.0 g/dL) (%) | |
| Day 0 | 7 (8.9) |
| Day 14 | 11 (13.9) |
| Day 28 | 8 (10.1) |
Safety and tolerability.
Overall, both AL and primaquine were well tolerated by the participants. There were no serious adverse events reported with either medication. No patient required re-dosing because of vomiting. Ten patients (12.7%) reported rash or itchiness, but these episodes were self-limited and clinically assessed to be unrelated to AL by the study physician. No patient required discontinuation of medication because of side effects.
Treatment efficacy.
On Day 3, five participants (6%, 95% CI: 1.9–13.2%) had persistent parasitemia with geometric mean asexual parasite density of 16.8 parasites/μL (range: 4–28.8 parasites/μL). These patients did not have any notable clinical symptoms and did not meet criteria for ETF because they were not febrile and did not have parasitemia ≥ 25% of Day 0. Of note, the median total dose among those with delayed clearance on Day 3 was 8.4 mg/kg for the artemether component and 50.4 mg/kg for the lumefantrine component, which was comparable with the median total AL dose among all patients, which was 8.0 mg/kg for artemether (range: 4.7–15 mg/kg) and 48.2 mg/kg for lumefantrine (range: 28.2–90 mg/kg).
There was one patient who presented with new-onset fever on Day 28 who had LCF with low P. falciparum asexual parasitemia (mean: 11 parasites/μL) confirmed independently by three microscopists. However, repeated attempts at PCR amplification for P. falciparum were negative, so microsatellite genotyping could not be performed to differentiate between recrudescence and reinfection. As such, this patient was considered a case of LCF for uncorrected analysis, but was excluded from PCR-corrected analysis per WHO guidelines.3 Thus, the ACPR was 98.6% (95% CI: 93.2–100%) in uncorrected analysis and 100% (95% CI: 95.4–100%) in PCR-corrected analysis for both per-protocol and Kaplan–Meier analyses.
Resistance markers.
Our findings showed no mutations in the K13 gene in the 72 isolates from Day 0 that were successfully sequenced for this gene. All of the Day 0 isolates sequenced for the Pfcrt gene associated with chloroquine resistance harbored the mutant SVMNT haplotype (77 out of 77 typed isolates), which has been previously reported in 100% of isolates from Brazil.19 For the pfmdr1-gene, isolates that were successfully sequenced harbored the Y184F (68/68), S1034C (71/71), N1042D (71/71), and D1246Y (71/71) mutations.
DISCUSSION
AL remains highly efficacious for the treatment of uncomplicated P. falciparum malaria in Cruzeiro do Sul, with Day 28 uncorrected APCR of 98.7% and 100% after PCR correction. This finding is consistent with previous treatment efficacy studies in the Brazilian Amazon and surrounding countries, which showed continued effectiveness of ACTs.20–22 Of concern, however, is the higher-than-expected prevalence of Day 3 parasitemia in five (6.3%) participants, although no K13 mutation was detected in our population.
The significance of Day 3 parasitemia prevalence in detecting parasite resistance to artemisinin depends on factors such as level of initial parasitemia, timing of sampling, sample size, and the efficacy of the partner drug.23 To ensure precise timing of Day 3 clearance, our study team conducted the Day 3 follow-up exactly 72–74 hours after the first dose of AL. Although the clinical teams attempted to provide directly observed therapy of all AL doses, they were unable to observe evening doses that occurred after 8 pm as home visits were logistically challenging at night. In these cases, phone calls served in lieu of direct observation, so adherence could not be assured for these doses, which may have resulted in suboptimal drug intake and delayed parasite clearance. Of the five patients with Day 3 parasitemia, two had direct observation of the evening doses and three had phone reminders. It should also be noted that the presence of Day 3 parasitemia had no correlation with lower dose per weight. Other factors could have affected drug levels, such as decreased drug absorption. We did not measure plasma drug or metabolite levels; thus, we do not have pharmacodynamic data to correlate with parasite clearance phenotype. Last, we also did not measure the parasite clearance time, which is the gold standard for measuring efficacy of the artemisinin component. As such, although we observed higher-than-expected prevalence of Day 3 parasitemia, we cannot definitively conclude there was reduced efficacy.
Experience from the Greater Mekong Subregion of Southeast Asia, where artemisinin resistance and K13 mutations are widespread, showed that although Day 3 parasitemia > 3% is worrisome, it does not rule in presence of resistance.23 Rather, it is an indication that additional studies are needed to further understand and characterize artemisinin resistance, such as focused artemisinin monotherapy trials complemented by genetic testing for K13 mutations, as recommended by the Technical Expert Group on Drug Efficacy and Response.24 Currently, the WHO recommendation is to use a threshold of ≥ 10% Day 3 parasitemia before artemisinin resistance is suspected.25 However, although this 10% threshold may be useful in Southeast Asia,26 a lower threshold of 5% has been suggested to be more sensitive in a higher-transmission area such as sub-Saharan Africa to detect delayed parasite clearance and artemisinin resistance at an early stage.27
Despite the prevalence of delayed parasite clearance on Day 3 observed in our study, no drug policy changes are warranted in Cruzeiro do Sul at this time per the current WHO recommendations. Delayed parasite clearance has been shown to be significantly associated with K13 mutation, although this relationship is not always well-defined.23,28 Although K13 has been a reliable marker for artemisinin resistance in Southeast Asia, it is unclear if there are other genes associated with resistance in other regions. Thus, continued monitoring of in vivo AL efficacy to assess the prevalence of Day 3 positivity over time, coupled with molecular surveillance of mutations in the K13 gene and other relevant genes associated with resistance to the partner drug, are critical in guiding antimalarial drug policy in Brazil and beyond. Our study showed the K13 gene from Day 0 samples were all wild type, which is reassuring considering the prevalence of Day 3 positivity. Mutations in the pfcrt and pfmdr1-genes were fixed as previously demonstrated in isolates from this region.29 The N86F184D1246 haplotype was recently implicated as a marker of lumefantrine failure,30 but we did not observe this haplotype in our study samples that mostly harbored the mutant 1246Y. Continued monitoring of molecular markers of resistance, together with in vivo efficacy studies in this region, is necessary.
Our study results were limited by the inability to classify the one case of LCF as either recrudescence or reinfection by microsatellite genotyping. It is unclear if this case of LCF truly represented treatment failure because repeated attempts to amplify the isolate were unsuccessful. This situation could have been explained by a false-positive microscopy reading, although our study microscopists were highly experienced and three independent microscopists confirmed the presence of low-level P. falciparum parasitemia (mean asexual parasite density of 11 parasites/μL, which is equivalent to approximately one parasite per 500 leukocytes) in a patient with recurrence of fever at the Day 28 visit. The negative PCR result may also reflect limitations in molecular testing, such as problems with DNA extraction from filter paper or the PCR amplification.
We observed that 10% of patients remained anemic on Day 28 despite a complete cure from malaria. Although the pathophysiology of anemia with P. falciparum infection is complex and multifactorial, it is possible that some patients had other underlying reasons for anemia such as from poor nutrition or iron deficiency. Without knowing the baseline prevalence of anemia in this population, it is difficult to comment on the impact of P. falciparum infection on the hemoglobin levels.
One of the challenges of conducting an in vivo efficacy study in the Americas is the low endemicity of malaria in the region, which makes patient enrollment difficult, especially for P. falciparum, which account for less than 20% of all malaria cases. To meet the desired sample size for our study within the peak transmission season, we increased the number of health facilities in the study, which made follow-up visits more logistically challenging, but nonetheless possible. The study staff received intensive training prior to study initiation to standardize practices and patient follow-up. To decrease noncompliance, follow-up visits were scheduled such that the nurses and nurse assistants could provide directly observed therapy or phone follow-up. The challenges associated with low endemicity often pose barriers for successful in vivo studies, but our study demonstrated their feasibility.
The continued efficacy of AL in Cruzeiro do Sul warrants its use as first-line therapy for uncomplicated P. falciparum malaria. However, close in vivo monitoring and molecular surveillance of mutations in genes associated with artemisinin (K13) and partners drug resistance in the region are imperative given the higher-than-expected prevalence of delayed parasite clearance seen in our study, raising concern for potential artemisinin resistance.
Acknowledgments:
We would like to thank the Ministry of Health of Brazil and the Acre State Health Secretariat for their support for this evaluation, and all the patients and their caregivers who participated. We are indebted to the study team, especially to Simone Daniel, José Maria de Souza do Nascimento, José Mário Veloso Peres, Luciana Silva-Flannery, Francisca Ingrid de Souza Conceição, Michael Green, and Venkatachalam Udhayakumar for their assistance with implementation and sample processing.
REFERENCES
- 1.World Health Organization , 2008. World Malaria Report. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2.Casos de Malária por mês e espécie parasitária - Brasil 2015–2016: Brazil National Malaria Control Program. 2016.
- 3.Fernandes RC, Medina-Acosta E, 2009. Disseminated BCG disease and the full contraindication to BCG vaccination for children exposed to and/or infected by HIV. Int J Tuberc Lung Dis 13: 1188–1189, author reply 1189–1190. [PubMed] [Google Scholar]
- 4.PAHO , 2011. Strategic Orientation Document on Monitoring the Efficacy and Resistance to Antimalarials in the Current Epidemiological Context Available at: http://www.paho.org/hq/index.php?option=com_topics&view=readall&cid=5525&Itemid=40757&lang=en. Accessed October 20, 2017.
- 5.Vreden SG, Jitan JK, Bansie RD, Adhin MR, 2013. Evidence of an increased incidence of day 3 parasitaemia in Suriname: an indicator of the emerging resistance of Plasmodium falciparum to artemether. Mem Inst Oswaldo Cruz 108: 968–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chenet SM, et al. 2016. Independent emergence of the Plasmodium falciparum kelch propeller domain mutant allele C580Y in Guyana. J Infect Dis 213: 1472–1475. [DOI] [PubMed] [Google Scholar]
- 7.Ariey F, et al. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashley EA, et al. Tracking Resistance to Artemisinin Collaboration , 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371: 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sidhu AB, Uhlemann AC, Valderramos SG, Valderramos JC, Krishna S, Fidock DA, 2006. Decreasing pfmdr1 copy number in Plasmodium falciparum malaria heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine, and artemisinin. J Infect Dis 194: 528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lwanga SK, Lemeshow S, 1991. Sample Size Determination in Health Studies: A Practical Manual Available at: http://apps.who.int/iris/bitstream/10665/40062/1/9241544058_%28p1-p22%29.pdf.
- 11.PAHO , 2003. Resistance to Antimalarials Available at: http://www.paho.org/hq/index.php?option=com_content&view=article&id=2405%3Aresistance-antimalarials&catid=1233%3Amalaria-program&Itemid=1912&lang=en. Accessed October 20, 2017.
- 12.World Health Organization , 2006. The International Pharmacopoeia = Pharmacopoea Internationalis. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 13.Alves-Junior ER, Gomes LT, Assis-Oliveira FB, Silverio-Silva LR, Nery AF, Fontes CJ, 2014. Quantification of parasite density in 200 microscopic fields underestimates the parasitemia level in malaria patients. Trop Biomed 31: 387–391. [PubMed] [Google Scholar]
- 14.Lucchi NW, et al. 2014. PET-PCR method for the molecular detection of malaria parasites in a national malaria surveillance study in Haiti, 2011. Malar J 13: 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson TJ, Su XZ, Bockarie M, Lagog M, Day KP, 1999. Twelve microsatellite markers for characterization of Plasmodium falciparum from finger-prick blood samples. Parasitology 119: 113–125. [DOI] [PubMed] [Google Scholar]
- 16.McCollum AM, Mueller K, Villegas L, Udhayakumar V, Escalante AA, 2007. Common origin and fixation of Plasmodium falciparum dhfr and dhps mutations associated with sulfadoxine-pyrimethamine resistance in a low-transmission area in South America. Antimicrob Agents Chemother 51: 2085–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talundzic E, et al. 2015. Selection and spread of artemisinin-resistant alleles in Thailand prior to the global artemisinin resistance containment campaign. PLoS Pathog 11: e1004789. Erratum in: PLoS Pathog 2015;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talundzic E, Chenet SM, Goldman IF, Patel DS, Nelson JA, Plucinski MM, Barnwell JW, Udhayakumar V, 2015. Genetic analysis and species specific amplification of the artemisinin resistance-associated kelch propeller domain in P. falciparum and P. vivax. PLoS One 10: e0136099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gbotosho GO, Folarin OA, Bustamante C, da Silva LH, Mesquita E, Sowunmi A, Zalis MG, Oduola AM, Happi CT, 2012. Different patterns of pfcrt and pfmdr1 polymorphisms in P. falciparum isolates from Nigeria and Brazil: the potential role of antimalarial drug selection pressure. Am J Trop Med Hyg 86: 211–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladeia-Andrade S, de Melo GN, de Souza-Lima Rde C, Salla LC, Bastos MS, Rodrigues PT, Luz F, Ferreira MU, 2016. No clinical or molecular evidence of Plasmodium falciparum resistance to artesunate-mefloquine in northwestern Brazil. Am J Trop Med Hyg 95: 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alecrim MG, Lacerda MV, Mourao MP, Alecrim WD, Padilha A, Cardoso BS, Boulos M, 2006. Successful treatment of Plasmodium falciparum malaria with a six-dose regimen of artemether-lumefantrine versus quinine-doxycycline in the western Amazon region of Brazil. Am J Trop Med Hyg 74: 20–25. [PubMed] [Google Scholar]
- 22.Rahman R, Martin MJ, Persaud S, Ceron N, Kellman D, Musset L, Carter KH, Ringwald P, 2016. Continued sensitivity of Plasmodium falciparum to artemisinin in Guyana, with absence of kelch propeller domain mutant alleles. Open Forum Infect Dis 3: ofw185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodrow CJ, White NJ, 2017. The clinical impact of artemisinin resistance in Southeast Asia and the potential for future spread. FEMS Microbiol Rev 41: 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO , 2016. Minutes of the Technical Expert Group (TEG) on Drug Efficacy and Response 10–11 December 2015 Available at: http://www.who.int/malaria/mpac/mar2016/en/. Accessed February 2, 2017.
- 25.WHO , 2015. Status Report on Artemisinin Resistance, 2015 Available at: http://whatnot/malaria/publications/atoz/update-artemisinin-resistance-sep2015/en/. Accessed October 20, 2016.
- 26.White LJ, et al. 2015. Defining the in vivo phenotype of artemisinin-resistant falciparum malaria: a modelling approach. PLoS Med 12: e1001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dahal P, d’Alessandro U, Dorsey G, Guerin PJ, Nsanzabana C, Price RN, Sibley CH, Stepniewska K, Talisuna AO; WWARN Artemisinin Based Combination Therapy (ACT) Africa Baseline Study Group , 2015. Clinical determinants of early parasitological response to ACTs in African patients with uncomplicated falciparum malaria: a literature review and meta-analysis of individual patient data. BMC Med 13: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tun KM, et al. 2016. Parasite clearance rates in Upper Myanmar indicate a distinctive artemisinin resistance phenotype: a therapeutic efficacy study. Malar J 15: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Legrand E, et al. 2012. Discordant temporal evolution of Pfcrt and Pfmdr1 genotypes and Plasmodium falciparum in vitro drug susceptibility to 4-aminoquinolines after drug policy change in French Guiana. Antimicrob Agents Chemother 56: 1382–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mbaye A, et al. 2016. Selection of N86F184D1246 haplotype of Pfmrd1 gene by artemether-lumefantrine drug pressure on Plasmodium falciparum populations in Senegal. Malar J 15: 433. [DOI] [PMC free article] [PubMed] [Google Scholar]