Abstract.
Trichuris trichiura is a soil-transmitted helminth infecting human populations globally. Human cases caused by Trichuris suis and Trichuris vulpis have also been reported. Molecular identifications of Trichuris species infecting human populations in Lao PDR and Myanmar are lacking. Here, we explored molecular data obtained from Trichuris eggs recovered from human fecal samples from these countries and compared these with new and existing data from Thailand. Nuclear ribosomal DNA (18S and ITS2) sequences were amplified from Trichuris eggs and sequenced. Forty-one samples showed 99–100% similarity in their 18S sequences to published sequences of T. trichiura and one sample showed 99% similarity to a sequence of T. suis. Similarly, 41 samples showed 92–100% similarity in their ITS2 sequences to published sequences of T. trichiura and one sample showed 94–97% similarity to sequences of T. suis. This study is the first molecular confirmation of human infection with T. suis in northeast Thailand and the first molecular confirmation of the species of Trichuris infecting humans in Lao PDR and Myanmar.
INTRODUCTION
Human trichuriasis, caused by Trichuris trichiura, is a soil-transmitted helminth infection grouped among important neglected tropical diseases by the World Health Organization.1,2 An estimated 600–800 million people are infected worldwide.3 Various Trichuris species have been found in mammalian hosts, i.e., Trichuris suis (in swine), Trichuris vulpis (in canines), Trichuris ovis (in sheep), Trichuris skrjabini (in goats), and Trichuris muris (in mice).4 Among these, it is thought that only T. vulpis5 and T. suis6,7 can establish persistent active infection in man. Many human cases of trichuriasis present only mild symptoms or are asymptomatic. Persons with heavy infections can experience diffuse colitis, chronic diarrhea, abdominal cramps, rectal tenesmus, and rectal prolapse,3,8 some of which are important health consequences.9 Prevalences of human trichuriasis are high in Central Africa, southern India, and Southeast Asia10 and can be as high as 95% in children,8 particularly school-age children (5–14 years).11 In the lower Greater Mekong Subregion, the following prevalences of trichuriasis have been reported: Lao PDR, 8.5%12; Myanmar, 57%13; Cambodia, 4.1%14; and in pregnant women in southern Thailand, 6.3%.15 Usually, trichuriasis is diagnosed by identification of Trichuris eggs in fecal specimens.16 Eggs of different species of Trichuris are very similar, making it difficult to identify species based on the features of eggs.17
Molecular techniques are increasingly being used as supportive tools for identification at the species level.4,7 Several molecular markers are useful for the identification of Trichuris spp., such as nucleotide sequences of internal transcribed spacers 1 and 2 (ITS1 and ITS2), nuclear small subunit rRNA (18S rRNA), mitochondrial large ribosomal subunit (rrnL), and cytochrome c oxidase subunit 1 (cox1).18–20 However, molecular evidence of the species of Trichuris infecting human populations in Lao PDR and Myanmar have not been reported yet. Here, we aim to explore such molecular evidence from Trichuris eggs recovered from human trichuriasis cases in these countries. We used 18S rRNA sequences to evaluate the phylogenetic relationships of the recovered species from Thailand. The results will provide a better understanding of the molecular epidemiology and a better means for diagnosing human trichuriasis in this region. We placed our data in a global context using sequences of those Trichuris spp. publicly available in the GenBank database. We also sequenced the ITS2 region to confirm identities of the recovered Trichuris species.
MATERIALS AND METHODS
Study area and sample collection.
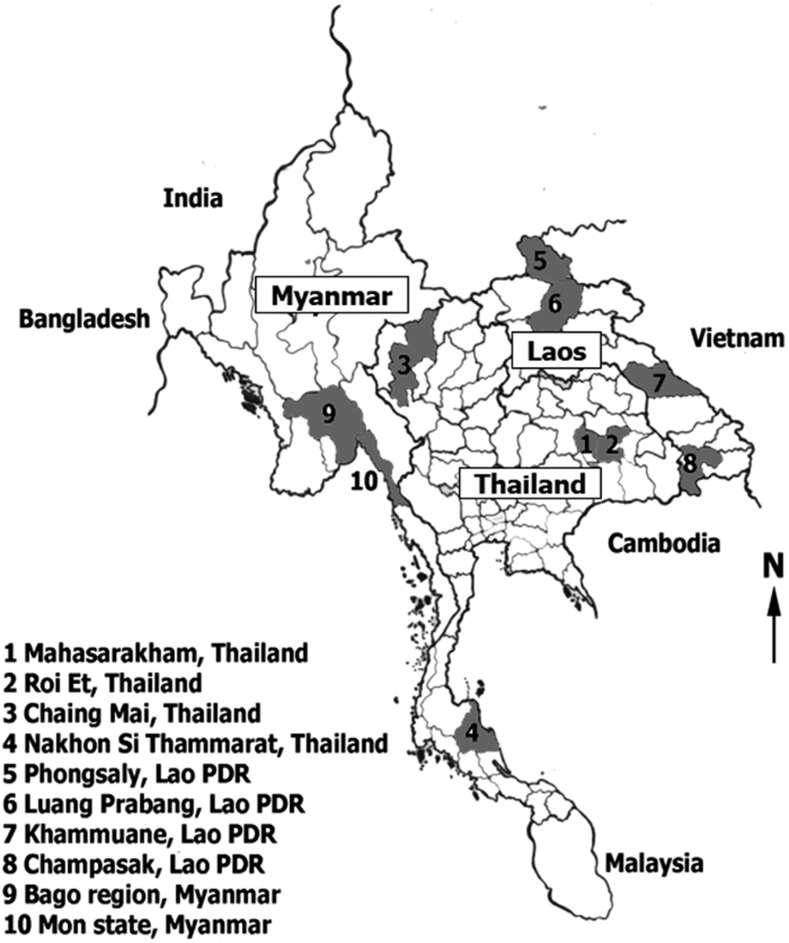
Forty-two fecal samples were collected from different geographical locations in Thailand (northeast [N = 2], north [N = 10], and south [N = 15]); Lao PDR (Luang Prabang [N = 1], Phongsaly [N = 1], Champasak [N = 2], and Khammouane [N = 1]); and Myanmar (Mon State [N = 6] and Bago Region [N = 4]) (Figure 1). The fecal samples containing Trichuris eggs were preserved in 95% alcohol in 15 mL tubes. This study was approved by the Khon Kaen University Ethics Committee for Human Research (HE581292, HE581396, and HE591391). Each participant was informed of the study methods, risks, and benefits of the process. Before enrolment, written consent was obtained from all adult participants and from parents or legal guardians of minors.
Figure 1.
Map of the study areas in Myanmar, Lao PDR, and Thailand. Numbers and shading indicate study provinces in each country.
Preparation of Trichuris species eggs.
Trichuris eggs were collected using the Mini Parasep method (Apacor, Wokingham, UK). Briefly, 2 grams of positive feces were transferred to a Mini Parasep tube and prepared following the manufacturer’s instructions. The Trichuris eggs were collected from the fecal sediment in the Mini Parasep tube under a stereomicroscope. Fifty Trichuris eggs were collected from each sample and transferred into a 1.5-mL microcentrifuge tube. All specimens were stored at −20°C until used for DNA extraction.
DNA extraction, amplification, and sequencing.
Genomic DNA was extracted from Trichuris spp. eggs using the NucleoSpin® tissue kit (Macherey-Nagel GmbH & Co., Duren, Germany), following the manufacturer’s instructions. Each PCR assay was performed in a 25-μL reaction volume consisting of 0.625 U of Taq polymerase (Roche Applied Science, Mannheim, Germany), 2.0 mM of MgCl2, 0.2 mM of dNTP mixture (Vivantis, Selangor Darul Ehsan, Malaysia), 0.2 µM of each primer (Sigma-Aldrich, Singapore), and 5 μL of the DNA sample. The PCR conditions and primers are shown in Table 1. The 18S rRNA and ITS2 gene regions were amplified using the GeneAmp PCR System 9700 (Applied Biosystems, Singapore). PCR products were separated in a 1% agarose gel to verify amplification and measure the product length and quantity. DNA sequencing was performed in both directions, using the PCR primers as sequencing primers, with an Applied Biosystems 3730 + I DNA analyzer and an ABI Big Dye version 3.1 cycle sequencing kit (Foster City, CA). All sequences of Trichuris spp. were confirmed using a nucleotide BLAST search through the National Center for Biotechnology Information (NCBI).
Table 1.
The specific primers used in the present study
| Gene regions | Primers/approximate amplicon size | PCR conditions |
|---|---|---|
| 18S rRNA | For Trichuridae nematodes | 1. Pre-incubation 94°C 5 min |
| 18S965F: 5′-GGCGATCAGATACCGCCCTAGTT-3′ | 2. Amplification for 35 cycles | |
| 18S1573R: 5′-TACAAAGGGCAGGGACGTAGT-3′ (Guardone et al., 2013) | Denaturation 95°C 30 sec | |
| PCR product size was 727 bp | Annealing 59°C 30 sec | |
| Extension 72°C 30 sec | ||
| 3. Final extension 72°C 10 min | ||
| ITS2 | For T. trichiura | 1. Pre-incubation 94°C 5 min |
| ITS2_tt_F2: 5′-GCTCGTAGGTCGTTGAAG-3′ | 2. Amplification for 35 cycles | |
| ITS2_tt_R2: 5′-TAGCCAAGTCGGGTAGT-3′ | Denaturation 95°C 30 sec | |
| For T. suis | Annealing 50°C/54°C* 30 sec | |
| ITS2_tt_F2: 5′-GCTCGTAGGTCGTTGAAG-3′ | Extension 72°C 30 sec | |
| ITS2_tt_R2_new1: 5′-GGGCAGCTTCCGTACT-3′ (newly designed primers) | 3. Final extension 72°C 10 min | |
| PCR product size of T. trichiura was 325 bp and T. suis was 355 bp |
ITS2 = internal transcribed spacers 2; PCR = polymerase chain reaction.
Annealing temperature used was 50°C for T. trichiura and 54°C for T. suis.
Phylogenetic tree analysis.
The 18S rRNA sequences of Trichuris spp. were aligned with reference sequences from the NCBI database using BioEdit software.21 The maximum likelihood (ML) method of tree construction implemented in MEGA version 6.0 was used.22 The Tamura 3-parameter (T92 + G) model was found to be the best-fit substitution model. This model was determined using MEGA version 6.0. All sequences were deposited in the GenBank database (GenBank Accession nos. MF288617–MF288648) and are presented in Table 2.
Table 2.
Trichuris species sequences deposited in the GenBank database
| Countries/number of sample | 18S rRNA | ITS2 | ||
|---|---|---|---|---|
| Thailand (N = 27) | Sequence IDs | Accession numbers | Sequence IDs | Accession numbers |
| Northeastern (N = 2) | NE1 THA* | MF288628* | NE1 THA* | MF288646* |
| NE3 THA | MF288629 | NE3 THA | MF288647 | |
| Southern (N = 15) | 3S THA, 4S THA, 6S THA, 8S THA, 9S THA, 15S THA, 16S THA, 17S THA, 19S THA, 21S THA, 22S THA, 23S THA, 24S THA, 25S THA, and 27S THA | MF288617 | 4S THA, 6S THA, 8S THA, 9S THA, 15S THA, 16S THA, 17S THA, 19S THA, 21S THA, 22S THA, 23S THA, 24S THA, 25S THA, and 27S THA | MF288633 |
| 3S THA | MF288638 | |||
| Northern (N = 10) | N34 THA, N44 THA, N59 THA, N63 THA, N112 THA, N117 THA, N118 THA, N119 THA and N121 THA | MF288619 | N118 THA, N34 THA, N59 THA, N63 THA, N112 THA, N119 THA, N121 THA, and N123 THA | MF288635 |
| N123 THA | MF288627 | N44 THA | MF288644 | |
| N117 THA | MF288645 | |||
| Lao PDR (N = 5) | 9PSL LAO | MF288621 | 9PSL LAO | MF288639 |
| 73CPS LAO | MF288622 | 73CPS LAO | MF288640 | |
| 3LPB LAO | MF288626 | 3LPB LAO | MF288637 | |
| 74KM LAO | MF288623 | 74KM LAO | MF288641 | |
| 81CPS LAO | MF288624 | 81CPS LAO | MF288642 | |
| Myanmar (N = 10) | A049 MMR, A037 MMR, A128 MMR, and A149 MMR | MF288618 | TS35 MMR, TS45 MMR, TS03 MMR, and TS05 MMR | MF288636 |
| TS03 MMR and TS35 MMR | MF288620 | A037 MMR, A049 MMR, A128 MMR, and A149 MMR | MF288634 | |
| KH086 MMR | MF288625 | KH086 MMR | MF288643 | |
| TZY007MMR | MF288632 | TZY007MMR | MF288648 | |
| TS45 MMR | MF288631 | |||
| TS05 MMR | MF288630 | |||
ITS2 = internal transcribed spacers 2.
This sample was identified as Trichuris suis, whereas other samples were identified as Trichuris trichiura.
RESULTS
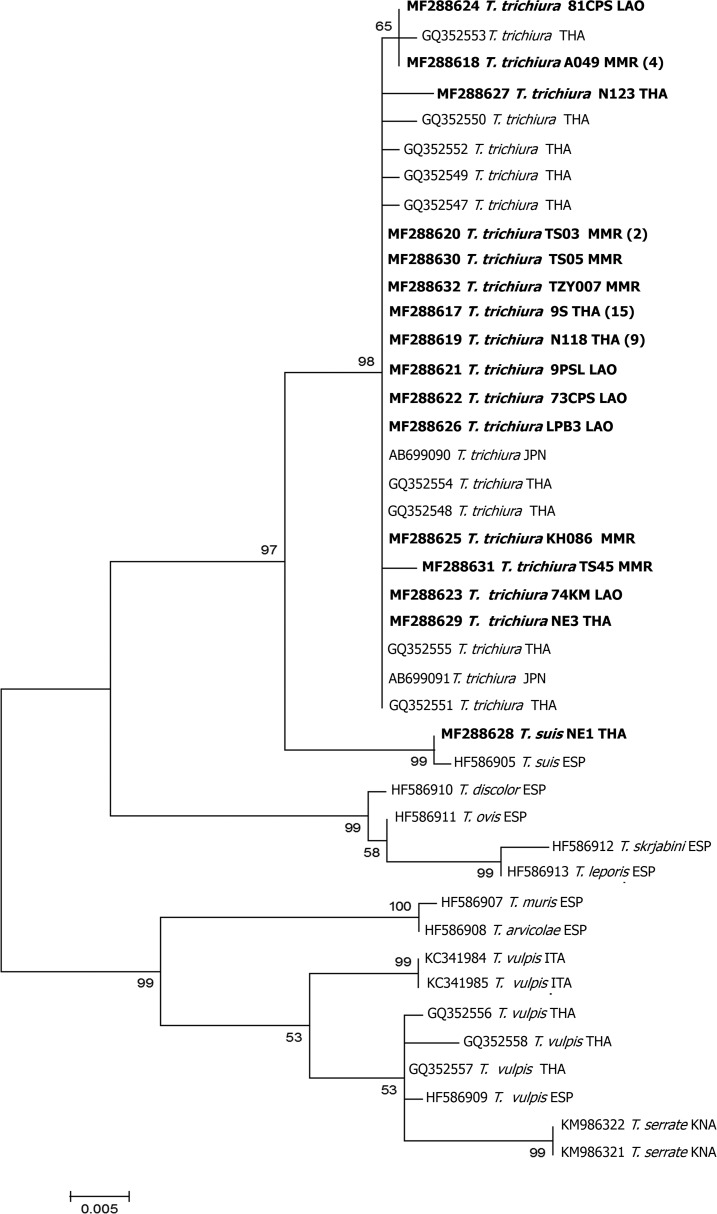
Forty-one of the 42 18S rRNA sequences were very similar (99–100% similarity and 100% query coverage) to various GenBank accessions of T. trichiura from humans; AB699090 from T. trichiura adult worms from a human in Japan and GQ352547–GQ352555 from eggs in feces of trichuriasis patients in Thailand. The remaining sequence (GenBank Accession no. MF288628), derived from a sample from northeast Thailand, exhibited 99% similarity (100% coverage) to the 18S sequence from a T. suis adult from Sus scrofa domestica in Spain (GenBank Accession no. HF586905). This sequence showed only 97–98% similarity to T. trichiura (coverage 100%; GenBank Accession nos. GQ352547–GQ352555). In the phylogenetic tree (Figure 2), 41 samples were grouped in the clade of T. trichiura sequences reported from humans in Japan and Thailand with bootstrap support of 98%. One sample (NE1 THA) (GenBank Accession no. MF288628) was grouped in the clade with T. suis from pig in Spain with bootstrap support of 99%. Trichuris trichiura and T. suis appear as monophyletic sister taxa in the tree (Figure 2).
Figure 2.
Maximum-likelihood reconstruction of phylogeny based on nucleotides (after trimming the primer sequences and reducing the alignment to the length of the shortest published sequence retained in the analysis.) in the 18S rRNA gene of Trichuris suis and T. trichiura. Bootstrap scores (percentages of 1,000 replications) are presented for each node. The sequences of Trichuris species obtained from the GenBank database are indicated with their accession numbers, genus and species, and country codes. Trichuris sequences of this study are presented in bold. The tree is midpoint-rooted. CHN = China; ITA = Italy; JPN = Japan; KNA = Saint Kitts and Nevis; LAO = Lao PDR; MMR = Myanmar; THA = Thailand.
Similarly, 41 of the 42 ITS2 sequences showed 92–100% identity with ITS2 sequences of T. trichiura adults from humans in Uganda (GenBank Accession no. KJ588134, 61–70% query coverage) and China (AM992981 and AM992998, 100% coverage). Sample NE1 THA from northeast Thailand—the sample which had an 18S sequence similar to that of T. suis—showed 92% similarity to T. suis ITS2 sequences from Uganda (GenBank Accession no. JN181780, 62% query coverage) and 94–97% similarity to sequences of this species from China (AM993004, AM993005, AM993011, AM993012, and AM993016, 100% coverage). The same sequence showed at most 85% similarity to T. trichiura (GQ301555, 100% query coverage), most of the similarities lying within the 5.8S gene region. The ITS2 regions downstream of the 5.8S gene differed so much between species that alignment was impossible. However, alignment of sequences from individuals within a species was straightforward.
DISCUSSION
Adults of T. trichiura and T. suis can be discriminated by morphological parameters and biometrical determinations.23 However, differentiation of the species by egg morphology is difficult. Hence, the development of molecular techniques for species identification and evaluation of diversity is of great use.19 For example, in 2013, Guardone and coworkers24 used 18S rRNA and mitochondrial cox1 gene sequences to discriminate several species of Trichuridae infecting dogs, cats, and wild mammals. The ITS1 and ITS2 and mitochondrial DNA have often been useful for discriminating closely related Trichuris species.25 Here, molecular identification to species of Trichuris eggs collected from human fecal samples from Lao PDR, Myanmar, and Thailand has been presented. ITS2 sequences of many Trichuris species differ too much for alignment to be possible. For this reason, we did not attempt to construct a phylogenetic tree from these. However, our sequences closely matched those of T. trichiura and T. suis published previously. Phylogenetic trees of 18S rRNA sequences revealed that 41 and one Trichuris samples were closely related to T. trichiura and T. suis, respectively (Figure 2). Among the species for which sequences are available, these two appeared to be sisters. This result is supported by previous reports from Spain and China that these species are closely related.7,23 It is, therefore, curious that their ITS2 sequences are so different.
Recently, PCR-restriction fragment length polymorphisms have been used as a tool for the detection of soil-transmitted helminthes including T. trichiura in Vietnam, a country located in the lower Greater Mekong Subregion.26 Adult T. trichiura from human and T. suis from swine in China were identified by their ITS1 and ITS2 sequences.7 In Thailand, Areekul and coworkers27 reported molecular evidence of T. trichiura and T. vulpis eggs in human fecal samples using PCR. Here, we have reported the first molecular identification of T. trichiura in humans in Lao PDR and Myanmar and of T. suis from a human in Thailand. A possible human infection with T. suis has been reported from the Massachusetts General Hospital, USA: identification of the worm was by morphological features.6 Successful experimental cross infections of humans with T. suis eggs and of pigs with T. trichiura eggs have been reported.28
Although T. vulpis or T. suis can cause an uncommon zoonosis and are rarely found in human populations,5–7 in this study, human species T. trichiura and T. suis infections were confirmed in humans by molecular identification, but no T. vulpis infection was detected despite when considering living environments of human populations in the study areas and sanitary condition, domestic dogs and pigs are probably kept and local people seem to be frequently infected with T. vulpis than T. suis infection. This reason cannot be accurately explained. This is possibly because of the limited number (N = 42) of samples examined in this study. If high numbers of Trichuris egg samples are examined, confirmation of T. vulpis infection in humans in these areas can possibly be achieved and further observations explored.
In conclusion, molecular evidence of T. trichiura and T. suis in humans in Lao PDR, Myanmar, and Thailand have been presented. Clearly, awareness needs to be raised of the zoonotic potential of T. suis in Thailand. Molecular data, for systematic, taxonomic, and diagnostic studies in human populations associated with T. trichiura and T. suis, are important for continuing epidemiological investigations, population genetics of T. trichiura and T. suis, and prevention and control programs to reduce animal-to-human transmission in this region. A monitoring scheme should be established, taking into account the role of reservoir hosts (i.e., pig) in the natural background of human trichuriais caused by T. suis.28
Acknowledgments:
We would like to thank David Blair for his valuable suggestions and assistance with the presentation of this article through the Khon Kaen University Publication Clinic.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript or in the submission of the paper for publication.
REFERENCES
- 1.Hotez PJ, Fenwick A, Savioli L, Molyneux DH, 2009. Rescuing the bottom billion through control of neglected tropical diseases. Lancet 373: 1570–1575. [DOI] [PubMed] [Google Scholar]
- 2.Dunn JC, Turner HC, Tun A, Anderson RM, 2016. Epidemiological surveys of, and research on, soil-transmitted helminths in southeast Asia: a systematic review. Parasit Vectors 27: 9–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ, 2006. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367: 1521–1532. [DOI] [PubMed] [Google Scholar]
- 4.Liu GH, Gasser RB, Nejsum P, Wang Y, Chen Q, Song HQ, Zhu XQ, 2013. Mitochondrial and nuclear ribosomal DNA evidence supports the existence of a new Trichuris species in the endangered françois’ leaf-monkey. PLoS One 8: e66249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Márquez-Navarro A, García-Bracamontes G, Alvarez-Fernández BE, Ávila-Caballero LP, Santos-Aranda I, Díaz-Chiguer DL, Sánchez-Manzano RM, Rodríguez-Bataz E, Nogueda-Torres B, 2012. Trichuris vulpis (Froelich, 1789) infection in a child: a case report. Korean J Parasitol 50: 69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kradin RL, Badizadegan K, Auluck P, Korzenik J, Lauwers GY, 2006. Iatrogenic Trichuris suis in a patient with Crohn disease. Arch Pathol Lab Med 130: 718–720. [DOI] [PubMed] [Google Scholar]
- 7.Liu GH, Zhou W, Nisbet AJ, Xu MJ, Zhou DH, Zhao GH, Wang SK, Song HQ, Lin RQ, Zhu XQ, 2014. Characterization of Trichuris trichiura from humans and T. suis from pigs in China using internal transcribed spacers of nuclear ribosomal DNA. J Helminthol 88: 64–68. [DOI] [PubMed] [Google Scholar]
- 8.Stephenson LS, Holland CV, Cooper ES, 2000. The public health significance of Trichuris trichiura. Parasitology 121: S73–S95. [DOI] [PubMed] [Google Scholar]
- 9.Lenk EJ, Redekop WK, Luyendijk M, Rijnsburger AJ, Severens JL, 2016. Productivity loss related to neglected tropical diseases eligible for preventive chemotherapy: a systematic literature review. PLoS Negl Trop Dis 10: e0004397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L, 2003. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol 19: 547–551. [DOI] [PubMed] [Google Scholar]
- 11.Azira NMS, Zeehaida M, 2012. Severe chronic iron deficiency anaemia secondary to Trichuris dysentery syndrome - a case report. Trop Biomed 29: 626–631. [PubMed] [Google Scholar]
- 12.Laymanivong S, Hangvanthong B, Keokhamphavanh B, Phommasansak M, Phinmaland B, Sanpool O, Maleewong W, Intapan PM, 2014. Current status of human hookworm infections, ascariasis, trichuriasis, schistosomiasis mekongi and other trematodiases in Lao People’s Democratic Republic. Am J Trop Med Hyg 90: 667–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montresor A, Zin TT, Padmasiri E, Allen H, Savioli L, 2004. Soil-transmitted helminthiasis in Myanmar and approximate costs for countrywide control. Trop Med Int Health 9: 1012–1015. [DOI] [PubMed] [Google Scholar]
- 14.Yong TS, et al. 2014. Prevalence of intestinal helminths among inhabitants of Cambodia (2006–2011). Korean J Parasitol 52: 661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liabsuetrakul T, et al. 2009. Epidemiology and the effect of treatment of soil-transmitted helminthiasis in pregnant women in southern Thailand. Southeast Asian J Trop Med Public Health 40: 211–222. [PubMed] [Google Scholar]
- 16.Ok KS, Kim YS, Song JH, Lee JH, Ryu SH, Lee JH, Moon JS, Whang DH, Lee HK, 2009. Trichuris trichiura infection diagnosed by colonoscopy: case reports and review of literature. Korean J Parasitol 47: 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghai RR, Simons ND, Chapman CA, Omeja PA, Davies TJ, Ting N, Goldberg TL, 2014. Hidden population structure and cross-species transmission of whipworms (Trichuris sp.) in humans and non-human primates in Uganda. PLoS Negl Trop Dis 8: e3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutillas C, de Rojas M, Ariza C, Ubeda JM, Guevara D, 2007. Molecular identification of Trichuris vulpis and Trichuris suis isolated from different hosts. Parasitol Res 100: 383–389. [DOI] [PubMed] [Google Scholar]
- 19.Dolezalova J, et al. 2015. How many species of whipworms do we share? Whipworms from man and other primates form two phylogenetic lineages. Folia Parasitol (Praha) 3: 62. [DOI] [PubMed] [Google Scholar]
- 20.Meekums H, Hawash MB, Sparks AM, Oviedo Y, Sandoval C, Chico ME, Stothard JR, Cooper PJ, Nejsum P, Betson M, 2015. A genetic analysis of Trichuris trichiura and Trichuris suis from Ecuador. Parasit Vectors 19: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall TA, 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41: 95–98. [Google Scholar]
- 22.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S, 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cutillas C, Callejón R, de Rojas M, Tewes B, Ubeda JM, Ariza C, Guevara DC, 2009. Trichuris suis and Trichuris trichiura are different nematode species. Acta Trop 111: 299–307. [DOI] [PubMed] [Google Scholar]
- 24.Guardone L, Deplazes P, Macchioni F, Magi M, Mathis A, 2013. Ribosomal and mitochondrial DNA analysis of Trichuridae nematodes of carnivores and small mammals. Vet Parasitol 197: 364–369. [DOI] [PubMed] [Google Scholar]
- 25.Callejón R, Nadler S, De Rojas M, Zurita A, Petrášová J, Cutillas C, 2013. Molecular characterization and phylogeny of whipworm nematodes inferred from DNA sequences of cox1 mtDNA and 18S rDNA. Parasitol Res 112: 3933–3949. [DOI] [PubMed] [Google Scholar]
- 26.George S, et al. 2016. The molecular speciation of soil-transmitted helminth eggs collected from school children across six endemic countries. Trans R Soc Trop Med Hyg 110: 657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Areekul P, Putaporntip C, Pattanawong U, Sitthicharoenchai P, Jongwutiwes S, 2010. Trichuris vulpis and T. trichiura infections among schoolchildren of a rural community in northwestern Thailand: the possible role of dogs in disease transmission. Asian Biomed 4: 49–60. [Google Scholar]
- 28.Beer RJ, 1976. The relationship between Trichuris trichiura (Linnaeus 1758) of man and Trichuris suis (Schrank 1788) of the pig. Res Vet Sci 20: 47–54. [PubMed] [Google Scholar]