Abstract.
Chikungunya virus is a mosquito-borne alphavirus that causes an acute febrile illness with severe polyarthralgia. The first local transmission of chikungunya virus in the Western Hemisphere was reported in December 2013. In the following year, the virus spread throughout much of the Americas and the number of cases among travelers increased substantially. We reviewed the epidemiology of chikungunya virus disease cases reported among U.S. travelers from 2014 to 2016. A total of 3,941 travel-acquired cases were reported from 49 states and the District of Columbia; 3,616 (92%) reported travel to other countries or territories in the Americas; the remaining 8% reported travel to Asia, Africa, or the Western Pacific. The most commonly reported travel destinations were the Dominican Republic, Puerto Rico, and Haiti. The largest number of cases (N = 2,780, 71%) had illness onset in 2014, followed by 2015 (N = 913, 23%) and 2016 (N = 248, 6%). Cases occurred in every month, but 70% of case-patients had illness onset from April to September, the months when mosquitoes are most likely to be active in the continental United States. Travel-acquired chikungunya cases will likely continue to occur and present a risk of introduction of the virus to locations in the continental United States. Clinicians and public health officials should be educated about the recognition, diagnosis, management, and timely reporting of chikungunya cases.
INTRODUCTION
Chikungunya virus is a mosquito-borne alphavirus that causes an acute febrile illness with polyarthralgia.1 Mortality is rare but the joint pains can be severe and debilitating. Chikungunya virus is transmitted primarily by Aedes aegypti and Ae. albopictus mosquitoes, the same vectors that transmit dengue and Zika viruses.1 These mosquito species are found throughout much of the Americas, including large areas of the United States.2 Humans are the primary amplifying host for chikungunya virus, meaning that they develop high enough levels of viremia to infect mosquitoes that bite them.3–6 Therefore, infected travelers can potentially introduce the virus into nonendemic areas, leading to local transmission and outbreaks of the disease.7
Chikungunya outbreaks have historically occurred in countries in Africa, Asia, Europe, and the Indian and Pacific oceans. In December 2013, the World Health Organization reported the first local transmission of chikungunya virus in the Western Hemisphere, with autochthonous cases identified in Saint Martin.8 By the end of 2016, local transmission had been identified in more than 40 countries or territories throughout the Americas, with more than 2.3 million confirmed and suspected cases reported to the Pan American Health Organization from affected areas.9
Before 2006, chikungunya virus disease was rarely identified in U.S. travelers.10 From 2006 to 2013, studies identified an average of 28 people per year in the United States with positive tests for recent chikungunya virus infection (range 5‒65 per year).10,11 All were travelers visiting or returning to the United States from affected areas in Asia, Africa, or the Indian Ocean. None of these imported cases resulted in local virus transmission. Beginning in 2014, chikungunya virus disease cases were reported among U.S. travelers returning from affected areas in the Americas, and local transmission was identified in Florida, Texas, Puerto Rico, and the U.S. Virgin Islands.12,13 We reviewed the epidemiology of chikungunya virus disease cases reported in U.S. travelers from 2014 through 2016.
METHODS
Case definition.
We defined a case as an individual reported by U.S. state and local health departments to ArboNET with probable or confirmed chikungunya virus disease acquired during travel with illness onset in 2014‒2016. Cases reported by U.S. territories (i.e., Puerto Rico, U.S. Virgin Islands, and American Samoa) with chikungunya virus local transmission were excluded from the analysis. Chikungunya virus disease was not a nationally notifiable disease in 2014, but states could voluntarily report cases to ArboNET, the national surveillance system for arthropod-borne viral diseases. The Centers for Disease Control and Prevention (CDC) encouraged jurisdictions choosing to report cases to ArboNET to apply the 2014 case definition for arboviral diseases.14 In 2015, chikungunya virus disease became a nationally notifiable condition under the same case definition.
Data collection.
Data routinely collected in ArboNET include patient age, sex, county and state of residence, date of illness onset, case status (e.g., confirmed, probable, or suspect), and outcomes (hospitalization and/or fatality). In addition, data on likely location of infection, diagnostic laboratory testing, and clinical symptoms can be reported through ArboNET. In this report, the laboratory evidence of infection is reported using the following hierarchy: reverse transcriptase-polymerase chain reaction (RT-PCR) positive, ≥ 4-fold rise in virus-specific neutralizing antibodies, immunoglobulin (Ig) M antibody positive confirmed using the plaque reduction neutralization test (PRNT), and IgM antibody positive without additional testing. If a case had laboratory evidence of infection using multiple tests, it was only included in the highest category of the hierarchy.
Data analysis.
Categorical variables were described as proportions, and continuous variables were described using median and interquartile range (IQR). Data were analyzed using SAS statistical software, version 9.3 (SAS Institute, Cary, NC). The map was generated using ArcGIS version 10.3 (ESRI, Redlands, CA).
RESULTS
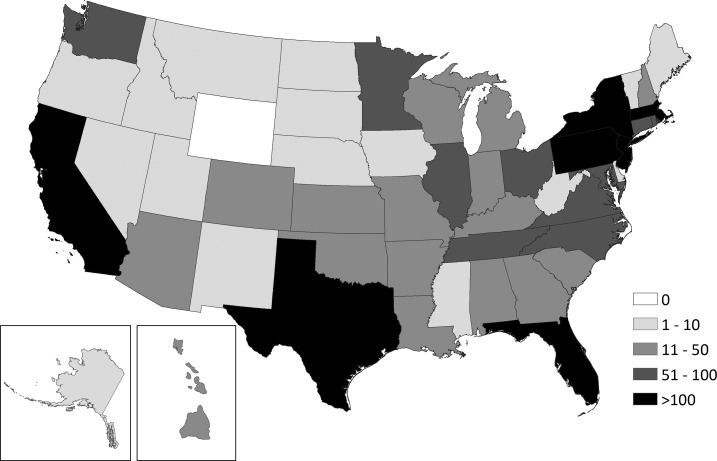
A total of 3,941 travel-acquired chikungunya virus disease cases were reported to ArboNET with illness onset from 2014 through 2016. Case-patients were reported from 49 states and the District of Columbia; seven states (California, Florida, Massachusetts, New Jersey, New York, Pennsylvania, and Texas) reported more than 100 cases (Figure 1). The largest number of cases (N = 2,780, 71%) had illness onset in 2014, followed by 2015 (N = 913, 23%) and 2016 (N = 248, 6%). Cases occurred in every month, but 2,769 (70%) case-patients had illness onset from April to September, the months when Ae. albopictus and Ae. aegypti are most likely to be active in the continental United States (Table 1).
Figure 1.
Travel-acquired chikungunya virus disease reported to ArboNET by state—United States, 2014–2016.
Table 1.
Characteristics of travel-acquired chikungunya virus disease cases—United States, 2014–2016
| Cases (N = 3,941) | ||
|---|---|---|
| No. | (%) | |
| Gender | ||
| Female | 2,602 | (66) |
| Male | 1,339 | (34) |
| Age group (years) | ||
| < 10 | 87 | (2) |
| 10–19 | 293 | (7) |
| 20–29 | 392 | (10) |
| 30–39 | 565 | (14) |
| 40–49 | 817 | (21) |
| 50–59 | 847 | (21) |
| 60–69 | 589 | (15) |
| 70+ | 349 | (9) |
| Unknown | 2 | (< 1) |
| Month of illness onset | ||
| January–March | 284 | (7) |
| April–June | 864 | (22) |
| July–September | 1,905 | (48) |
| October–December | 888 | (23) |
| Hospitalized | ||
| Yes | 714 | (18) |
| No | 3,053 | (77) |
| Unknown | 174 | (4) |
| Fatality | ||
| Yes | 4 | (< 1) |
| No | 3,761 | (95) |
| Unknown | 176 | (5) |
| Region where infection was acquired | ||
| Caribbean | 2,458 | (62) |
| Central America | 645 | (16) |
| North America | 259 | (7) |
| South America | 254 | (6) |
| Asia | 196 | (5) |
| Oceania/Pacific Islands | 52 | (1) |
| Africa | 17 | (< 1) |
| Unknown | 60 | (1) |
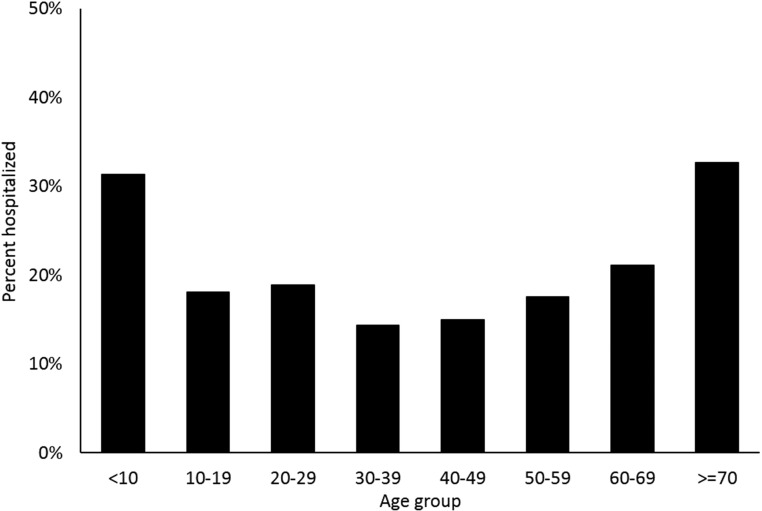
Two-thirds of case-patients were female (Table 1). The median age of case-patients was 47 years (IQR 33–59 years); 10% were aged < 20 years. Overall, 714 (18%) case-patients were hospitalized and four (< 1%) died. The hospitalization rate for males (23%) was higher than that for females (17%) (P < 0.01). Hospitalization rates were highest among the youngest (< 10 years; 26/83, 31%) and oldest (≥ 70 years; 112/343, 33%) case-patients (Figure 2). Six (55%) of the 11 children ≤ 1 year of age were hospitalized. All four fatal cases were aged ≥ 63 years; two were female and two were male. The median time from illness onset to death was 57 days (range 36–61 days).
Figure 2.
Hospitalization rates by age group among travel-acquired chikungunya virus disease cases—United States, 2014–2016.
Data on clinical symptoms were reported for 3,146 (80%) case-patients (Table 2). The most commonly reported clinical symptoms included fever or chills (94%) and arthralgia (84%). Eighty one percent of case-patients had both fever and arthralgia. Myalgia, rash, and headache were reported in about half of the case-patients. Gastrointestinal symptoms, including nausea, vomiting, or diarrhea, occurred in 721 (23%) case-patients with reported symptoms. Arthritis was reported in 309 (10%) case-patients.
Table 2.
Common clinical symptoms among travel-acquired chikungunya virus disease cases*—United States, 2014–2016
| Cases (N = 3,146) | ||
|---|---|---|
| No. | (%) | |
| Fever or chills | 2,961 | (94) |
| Arthralgia | 2,642 | (84) |
| Myalgia | 1,741 | (55) |
| Rash | 1,711 | (54) |
| Headache | 1,434 | (46) |
| Nausea or vomiting | 589 | (19) |
| Arthritis | 309 | (10) |
| Diarrhea | 253 | (8) |
Data on clinical symptoms reported for 3,146/3,941 (80%) reported cases.
The type of laboratory (i.e., commercial or public health) providing evidence of infection was reported for 2,921 (74%) cases. Of those, 2,308 (79%) were tested at a commercial laboratory, 475 (16%) at a public health laboratory, and 138 (5%) had testing at both commercial and public health laboratories. Data on the laboratory evidence of chikungunya virus infection were reported for 2,440 (62%) cases. Chikungunya viral RNA was detected using RT-PCR in 642 (26%) of those cases; RT-PCR–positive specimens were collected a median of 2 days after illness onset (IQR 1–4 days). Twenty-one (1%) cases had a ≥ 4-fold rise in anti-chikungunya virus neutralizing antibodies between acute- and convalescent-phase specimens. One hundred thirty-four (5%) cases were serum IgM positive and confirmed using PRNT. For 1,643 (67%) cases, the diagnosis was determined on the basis of the detection of anti-chikungunya virus IgM antibodies in a single serum specimen without neutralizing antibody testing performed.
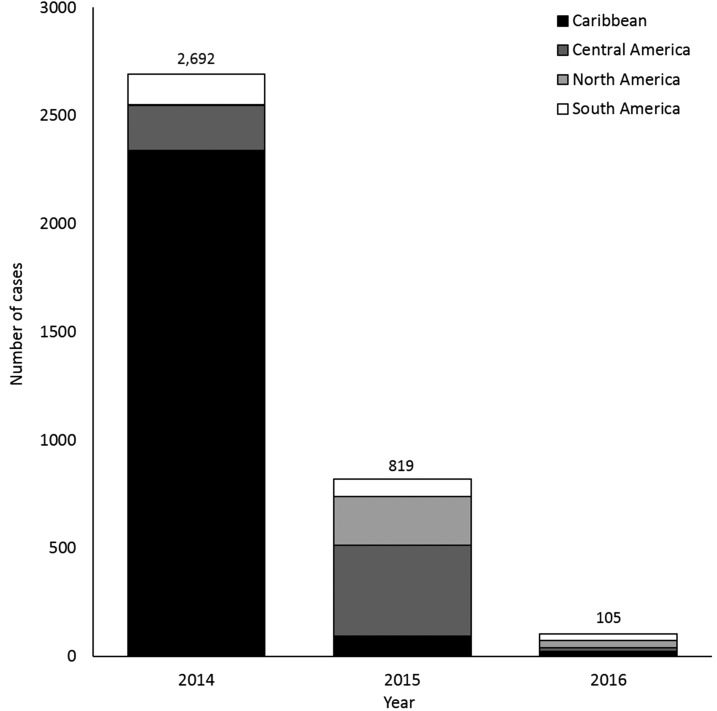
Of the 3,881 case-patients with known travel history, 3,616 (93%) reported travel to destinations in the Western Hemisphere, including the Caribbean (N = 2,458, 63%), Central America (N = 645, 17%), North America (N = 259, 7%), and South America (N = 254, 7%). The most commonly reported travel destinations were the Dominican Republic (N = 975, 25%), Puerto Rico (N = 489, 13%), and Haiti (N = 454, 12%). The predominant region of travel for cases from the Americas shifted over time. In 2014, 2,339 (87%) of the 2,692 cases were travelers to the Caribbean whereas in 2015, 643 (79%) of the 819 cases were travelers to Central America and Mexico (Figure 3). Relatively few cases reported travel to destinations outside of the Americas. One hundred ninety-six (5%) case-patients reported travel to Asia, 52 (1%) to Oceania or the Pacific Islands, and 17 (< 1%) to Africa.
Figure 3.
Region of the Americas where reported travel-associated chikungunya virus disease cases were acquired, by year—United States, 2014–2016.
DISCUSSION
Before 2014, the diagnosis of chikungunya virus infection in a U.S. traveler was relatively rare.10,11 Coincident with large outbreaks in the Caribbean followed by other regions in the Americas, reported cases increased sharply in 2014. These imported cases present a potential risk for the introduction and initiation of local transmission of chikungunya virus in the continental United States. This risk was demonstrated by the first autochthonous transmission cases in the continental United States in Florida and Texas.13,15 However, despite large numbers of imported cases and a substantial proportion with laboratory evidence suggestive of viremia, local transmission was limited (i.e., 12 reported cases in Florida in 2014 and one case in Texas in 2015). The future of chikungunya virus in the United States, particularly the risk of local transmission in U.S. states, is uncertain, but similar patterns to that seen for dengue virus might be expected, given the similar ecology and vectors. From 2010 to 2014, an average of 1.5 million cases of dengue were reported annually in the Americas.16 In the contiguous United States, an average of 24 locally transmitted dengue cases (range: 2–58 cases) were reported per year over the same time.17,18
The observed patterns in seasonality and countries of origin probably reflect both travel patterns and the progression of the outbreak in the Americas where most infections were acquired. Most of the case-patients were aged 40–69 years, likely mirroring the traveler population to affected regions. There was a higher predominance of females in this group of cases than previously reported,10,11,19 but this difference is not likely due to differences in infection rates or traveler population demographics. This difference could be due to women having more severe or persistent symptoms; findings of previous studies that have evaluated gender differences in disease severity and persistence have been inconsistent.20–28 The higher hospitalization rate among females reported here does not support increased severity among women but could be consistent with a higher proportion of women presenting to health care. It is also possible that this difference is due to some other unknown factor.
Reported symptoms among these travel-associated cases were similar to those reported in previous outbreaks, with most of the patients reporting fever and arthralgia.4,20,29–31 Hospitalization rates were high, but this likely is an artifact of passive surveillance. Case-patients with more severe disease manifestations more often present to health care, undergo diagnostic testing, and are reported to public health authorities. Fatalities were rare; given the limited clinical information available in ArboNET, it was not possible to assess the causal relationship between chikungunya virus infection and death in those circumstances, but all occurred > 1 month after illness onset.
In past years, national reporting of identified chikungunya infections was relatively poor.10,11 This likely was related to the fact that chikungunya was not a nationally notifiable disease condition in the United States until 2015. Recent efforts to improve the completeness and timeliness of reporting for all identified chikungunya virus disease cases might have increased reporting of identified cases; these efforts included improved communications between the commercial laboratory and state and local health departments and outreach to health-care providers regarding recognition, diagnosis, and reporting of cases.
The findings in this report are subject to several limitations. ArboNET is a passive surveillance system that is dependent on clinicians considering the diagnosis of chikungunya, obtaining the appropriate diagnostic test, and having positive results reported to public health authorities. Patients with chikungunya virus infection may not seek medical attention, particularly those patients with mild symptoms. Health-care providers may not recognize the clinical features of chikungunya virus disease or submit specimens for appropriate testing. Diagnostic testing might have been negative but performed outside the ideal timing period (e.g., specimen collected too early in the course of the illness before the development of detectable IgM antibodies or too late to detect using PCR). In addition, the sensitivity of chikungunya diagnostic tests is not 100%. Therefore, diagnosis and reporting of chikungunya virus infections are incomplete. In addition, many of the reported travel-associated cases of chikungunya were diagnosed using a single IgM test. Studies have shown that anti-chikungunya virus IgM antibodies can persist for more than a year21,32; therefore, some positive results may reflect past rather than recent infections. There is also the potential for false-positive IgM results for chikungunya due to cross-reactivity to other alphaviruses, such as Mayaro virus, which can be found in some regions with chikungunya virus activity. In such situations, the true etiologic agent cannot be determined without further confirmatory testing using PRNT. There is no cross-reactivity between chikungunya virus and flaviviruses, such as Zika and dengue viruses. Finally, ArboNET does not collect information about coinfections. It is possible that some of the patients described here may have had coinfections, but coinfections have been found to be rare even in areas with documented cocirculation of two viruses.33–35
Chikungunya virus transmission has continued to occur in the Western Hemisphere in 2017 but at lower levels than was first seen after the introduction of the virus into the region.36 This is likely due to the exposure and development of protective antibodies in susceptible populations. It is expected that chikungunya cases among travelers visiting or returning to the United States from affected areas will continue to be identified. These imported cases could result in additional local spread of the virus in the continental United States. Clinicians and public health officials should continue to be educated about the recognition, diagnosis, management, and timely reporting of chikungunya cases. Chikungunya virus infection should be considered in patients with acute onset of fever and polyarthralgia, especially those who have recently traveled to destinations with ongoing outbreaks or where chikungunya is considered to be endemic. Because chikungunya, dengue, and Zika viruses can circulate in the same area and cause similar clinical illnesses, these patients also should be evaluated for these and other etiologies based on travel itinerary, symptoms, timing of illness onset in relation to travel, and potential exposures.37 Until dengue can be ruled out, aspirin and other nonsteroidal anti-inflammatory drugs should be avoided to reduce the risk of hemorrhage, and patients should be managed for possible dengue virus infection as proper clinical management can improve outcomes.38 Health-care providers are encouraged to report suspected chikungunya infections to their state or local health department to facilitate diagnosis and to mitigate the risk of local transmission. Public health officials should perform surveillance for chikungunya cases in returning travelers and be aware of the risk of possible local transmission in areas where Aedes species mosquitoes are currently present. Further research is needed to better quantify the clinical, economic, and public health impact of travel-associated cases of chikungunya virus disease.
REFERENCES
- 1.Staples J, Breiman R, Powers A, 2009. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin Infect Dis 49: 942–948. [DOI] [PubMed] [Google Scholar]
- 2.Darsie R, Ward R, 2005. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. Gainesville, FL: University Press of Florida. [Google Scholar]
- 3.Panning M, Grywna K, van Esbroeck M, Emmerich P, Drosten C, 2008. Chikungunya fever in travelers returning to Europe from the Indian Ocean region, 2006. Emerg Infect Dis 14: 416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rezza G, et al. 2007. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet 370: 1840–1846. [DOI] [PubMed] [Google Scholar]
- 5.Lanciotti RS, Kosoy OL, Laven JJ, Panella AJ, Velez JO, Lambert AJ, Campbell GL, 2007. Chikungunya virus in US travelers returning from India, 2006. Emerg Infect Dis 13: 764–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laurent P, Le Roux K, Grivard P, Bertil G, Naze F, Picard M, Staikowsky F, Barau G, Schuffenecker I, Michault A, 2007. Development of a sensitive real-time reverse transcriptase PCR assay with an internal control to detect and quantify chikungunya virus. Clin Chem 53: 1408–1414. [DOI] [PubMed] [Google Scholar]
- 7.Jupp PG, McIntosh BM, 1988. Chikungunya virus disease. Monath TP, ed. The Arboviruses: Epidemiology and Ecology, Vol. 2. Boca Raton, FL: CRC Press, 137–157. [Google Scholar]
- 8.World Health Organization , 2013. Chikungunya in the French Part of the Caribbean Isle of Saint Martin. Disease Outbreak News Available at: http://www.who.int/csr/don/2013_12_10a/en/. Accessed September 1, 2015.
- 9.Pan American Health Organization Chikungunya: PAHO/WHO Data, Maps and Statistics Available at: http://www.paho.org/hq/index.php?option=com_topics&view=rdmore&cid=6917&Itemid=40931&lang=en. Accessed May 22, 2017.
- 10.Gibney KB, Fischer M, Prince HE, Kramer LD, St George K, Kosoy OL, Laven JJ, Staples JE, 2011. Chikungunya fever in the United States: a fifteen year review of cases. Clin Infect Dis 52: e121–e126. [DOI] [PubMed] [Google Scholar]
- 11.Lindsey NP, Prince HE, Kosoy O, Laven J, Messenger S, Staples JE, Fischer M, 2015. Chikungunya virus infections among travelers – United States, 2010–2013. Am J Trop Med Hyg 92: 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention , 2014. Notes from the field: chikungunya virus spreads in the Americas—Caribbean and South America, 2013–2014. MMWR Morb Mortal Wkly Rep 63: 500–501. [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Chikungunya Virus in the United States Available at: http://www.cdc.gov/chikungunya/geo/united-states.html. Accessed November 9, 2015.
- 14.Centers for Disease Control and Prevention Arboviral Diseases, Neuroinvasive and Non-neuroinvasive: 2014 Case Definition Available at: http://wwwn.cdc.gov/nndss/conditions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive/case-definition/2014/. Accessed November 9, 2015.
- 15.Kendrick K, Stanek D, Blackmore C, 2014. Notes from the field: transmission of chikungunya virus in the continental United States–Florida, 2014. MMWR Morb Mortal Wkly Rep 63: 1137. [PMC free article] [PubMed] [Google Scholar]
- 16.Pan American Health Organization Dengue: PAHO/WHO Data, Maps and Statistics: Annual Cases Reported of Dengue Available at: http://www.paho.org/hq/index.php?option=com_topics&view=rdmore&cid=6290&Itemid=40734&lang=en. Accessed January 5, 2016.
- 17.U.S. Geologic Survey Arboviral Disease Maps (Legacy Site) Available at: http://diseasemaps.usgs.gov/2013/index.html. Accessed January 6, 2016.
- 18.U.S. Geologic Survey Arboviral Disease Maps Available at: http://diseasemaps.usgs.gov/mapviewer/. Accessed January 6, 2016.
- 19.Sharp TM, et al. 2014. Chikungunya cases identified through passive surveillance and household investigations–Puerto Rico, May 5–August 12, 2014. MMWR Morb Mortal Wkly Rep 63: 112–118. [PMC free article] [PubMed] [Google Scholar]
- 20.Staikowsky F, Talarmin F, Grivard P, Souab A, Schuffenecker I, Le Roux K, Lecuit M, Michault A, 2009. Prospective study of chikungunya virus acute infection in the Island of La Réunion during the 2005–2006 outbreak. PLoS One 4: e7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borgherini G, Poubeau P, Jossaume A, Gouix A, Cotte L, Michault A, Arvin-Berod C, Paganin F, 2008. Persistent arthralgia associated with chikungunya virus: a study of 88 adult patients on Reunion Island. Clin Infect Dis 47: 469–475. [DOI] [PubMed] [Google Scholar]
- 22.Couturier E, Guillemin F, Mura M, Léon L, Virion JM, Letort MJ, De Valk H, Simon F, Vaillant V, 2012. Impaired quality of life after chikungunya virus infection: a 2-year follow-up study. Rheumatol 51: 1315–1322. [DOI] [PubMed] [Google Scholar]
- 23.Gérardin P, Fianu A, Malvy D, Mussard C, Boussaïd K, Rollot O, Michault A, Gaüzere BA, Bréart G, Favier F, 2011. Perceived morbidity and community burden after a chikungunya outbreak: the TELECHIK survey, a population-based cohort study. BMC Med 9: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kularatne SA, Weerasinghe SC, Gihan C, Wickramasinghe S, Dharmarathne S, Abeyrathna A, Jayalath T, 2012. Epidemiology, clinical manifestations, and long-term outcomes of a major outbreak of chikungunya in a hamlet in Sri Lanka, in 2007: a longitudinal cohort study. J Trop Med 2012: 639178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohd Zim MA, Sam IC, Omar SF, Chan YF, AbuBakar S, Kamarulzaman A, 2013. Chikungunya infection in Malaysia: comparison with dengue infection in adults and predictors of persistent arthralgia. J Clin Virol 56: 141–145. [DOI] [PubMed] [Google Scholar]
- 26.Moro ML, et al. 2012. Long-term chikungunya infection clinical manifestations after an outbreak in Italy: a prognostic cohort study. J Infect 65: 165–172. [DOI] [PubMed] [Google Scholar]
- 27.Thiberville SD, Boisson V, Gaudart J, Simon F, Flahault A, de Lamballerie X, 2013. Chikungunya fever: a clinical and virological investigation of outpatients on Reunion Island, Southwest Indian Ocean. PLoS NTD 7: e2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Win MK, Chow A, Dimatatac F, Go CJ, Leo YS, 2010. Chikungunya fever in Singapore: acute clinical and laboratory features, and factors associated with persistent arthralgia. J Clin Virol 49: 111–114. [DOI] [PubMed] [Google Scholar]
- 29.Borgherini G, Poubeau P, Staikowsky F, Lory M, Le Moullec N, Becquart JP, Wengling C, Michault A, Paganin F, 2007. Outbreak of chikungunya on Reunion Island: early clinical and laboratory features in 157 adult patients. Clin Infect Dis 44: 1401–1407. [DOI] [PubMed] [Google Scholar]
- 30.Staikowsky F, Le Roux K, Schuffenecker I, Laurent P, Grivard P, Develay A, Michault A, 2008. Retrospective survey of chikungunya disease in Réunion Island hospital staff. Epidemiol Infect 136: 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakshmi V, Neeraja M, Subbalaxmi MV, Parida MM, Dash PK, Santhosh SR, Rao PV, 2008. Clinical features and molecular diagnosis of chikungunya fever from south India. Clin Infect Dis 46: 1436–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grivard P, et al. 2007. Molecular and serologic diagnosis of chikungunya virus infection. Pathol Biol (Paris) 55: 490–494. [DOI] [PubMed] [Google Scholar]
- 33.Caron M, Paupy C, Grard G, Becquart P, Mombo I, Nso BB, Kassa Kassa F, Nkoghe D, Leroy EM, 2012. Recent introduction and rapid dissemination of chikungunya virus and dengue virus serotype 2 associated with human and mosquito co-infections in Gabon, Central Africa. Clin Infect Dis 55: e45–e53. [DOI] [PubMed] [Google Scholar]
- 34.Nkoghe D, Kassa RF, Bisvigou U, Caron M, Grard G, Leroy EM, 2012. No clinical or biological difference between chikungunya and dengue fever during 2010 Gabonese outbreak. Infect Dis Rep 4: e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Omarjee R, Prat C, Flusin O, Boucau S, Tenebray B, Merle O, Huc-Anais P, Cassadou S, Leparc-Goffart I, 2012. Importance of case definition to monitor ongoing outbreak of chikunugnya virus on a background of actively circulating dengue virus, St. Martin, December 2013 to 2014. Euro Surveill 19: 20753. [DOI] [PubMed] [Google Scholar]
- 36.Pan American Health Organization , 2017. Chikungunya: PAHO/WHO Data, Maps and Statistics Available at: http://www.paho.org/hq/index.php?option=com_topics&view=rdmore&cid=8975&Itemid=40931&lang=en. Accessed May 22, 2017.
- 37.Centers for Disease Control and Prevention , 2017. Chapter 5: Post-travel evaluation CDC Yellow Book 2018: Health Information for International Travel. New York, NY: Oxford University Press. [Google Scholar]
- 38.Centers for Disease Control and Prevention , 2015. Dengue clinical and laboratory guidance. Available at: http://www.cdc.gov/dengue/clinicalLab/. Accessed November 9, 2015.