Abstract
Patient: Female, 63
Final Diagnosis: Diabetic macular edema
Symptoms: Visual disturbance
Medication: —
Clinical Procedure: Treatment with sodium glucose transporter 2 inhibitor
Specialty: Ophthalmology
Objective:
Unusual or unexpected effect of treatment
Background:
Diabetic macular edema (DME) causes serious visual impairments in diabetic patients. The standard treatments of DME are intra-vitreous injections of corticosteroids or anti-vascular endothelial growth factor antibodies and pan-photocoagulation. These treatments are unsatisfactory in their effects and impose considerable physical and economic burdens on the patients.
Case Report:
A 63-year-old woman was diagnosed as type 2 diabetes with retinopathy 7 years ago. Before the initiation of an SGLT2 inhibitor, the dipeptidyl peptidase-4 inhibitor, sitagliptin (50 mg daily), and metformin (250 mg daily) were used for her glycemic control. The level of her hemoglobin A1c had been controlled around 7%. She began to feel decreased visual acuity and blurred vision of her left eye 8 months before the visit to our clinic. She was diagnosed as DME, which turned out to be corticosteroid-resistant. Her visual acuity further decreased to 20/50. Metformin was changed to ipragliflozin (25mg/day). Her left visual acuity started to improve after 4 weeks of treatment with ipragliflozin and improved to 20/22 after 24 weeks. The macular edema did not change until 12 weeks of the treatment, however, it decreased prominently after 16 weeks.
Conclusions:
In our patient with steroid-resistant DME, her visual symptoms and macular edema recovered after the initiation of an SGLT2 inhibitor. SGLT2 inhibitors might be a potential candidate for the DME treatment.
MeSH Keywords: Diabetes Complications, Diabetic Retinopathy, Sodium-Glucose Transporter 2
Background
Diabetic retinopathy is among the most common causes of visual disabilities including blindness in patients with diabetes mellitus. The diabetic macular edema (DME) is also important pathologic change of diabetic retinopathy [1]. DME attacks diabetic patients’ eye in dependent of the stages of their diabetic retinopathy [2–4]. The mechanisms underlying DME are explained by VEGF-induced inflammations of retina and following leakages of fluid from retinal capillaries. These pathological changes result in retinal edema and, in a clinical point of view, visual disturbances [1–3]. The visual symptoms experienced by the patients with DME are blurred vision, floaters, double vision and gradual loss of visual acuity. The gradual visual deterioration due to DME ends up with blindness and gives heavy physical and mental burden on patients with diabetic mellitus.
Intra-vitreous injections of corticosteroid or antibodies/agents against vascular endothelial growth factor (VEGF) are 2 major therapeutic strategies in the treatment of DME [3,5]. The injection procedures usually need to be repeated every 3 months since the effects of the treatment is transient and limited [5]. Since the cost of these agents are high, the treatment is not only troublesome but also cost-ineffective. Panretinal photo-coagulation with laser beam is another potential strategy for the treatment of DME. However, the concern is that the procedures possibly impair visual acuity in some patients [6].
Sodium glucose transporter 2 (SGLT2) inhibitors have emerged as a standard treatment of type 2 diabetes. Beyond the control of blood glucose levels, additional preferable effects of SGLT2 inhibitors are now accumulating: such as prevention of cardiovascular events and protection against diabetic nephropathy [7–9]. We reported that some of these beneficial effects of SGLT2 could be attributed to the direct action of the agents of SGLT2 on mesangial cells and retinal pericytes [10]. We also reported the presence of SGLT2 in mesangial cells and retinal pericytes [11–15] and suggested SGLT2 in both cells is the main causes of occurrence of diabetic nephropathy and retinopathy [16]. Although it is considerable clinical interest whether SGLT2 inhibitors have any therapeutic effect on diabetic retinopathy including DME, no clinical studies have been reported up to now. We here present a woman with type 2 diabetes who recovered from DME both in clinical and pathological aspects after introduction of an SGLT2 inhibitor for the purpose of glycemic control.
Case Report
A 63-year-old woman was diagnosed as type 2 diabetes with retinopathy 7 years ago. The treatment was started with insulin followed by administrations of the dipeptidyl peptidase-4 (DPP4) inhibitor, sitagliptin, and metformin. The level of her hemoglobin A1c (HbA1c) had been controlled around 7%. She had undergone photocoagulation therapy for the proliferative retinopathy of her right eye. Since then, the visual acuity of her right eye has been maintained 20/20. She began to feel decreased visual acuity and blurred vision of her left eye approximately 8 months before the visit to our clinic. The symptoms were progressive. The ophthalmologist (Dr. T.E.) made diagnosis of DME.
Intra-vitreous injections of corticosteroid injections were performed several times, with no measurable improvement, which lead us to consider that her DME was corticosteroid-resistant. Her visual acuity further decreased to 20/50.
Before introduction of an SGLT2 inhibitor, her prescription regimen for diabetes was sitagliptin (50mg daily) and metformin (250 mg daily). Metformin was changed to ipragliflozin (25 mg/day) for the better glycemic control.
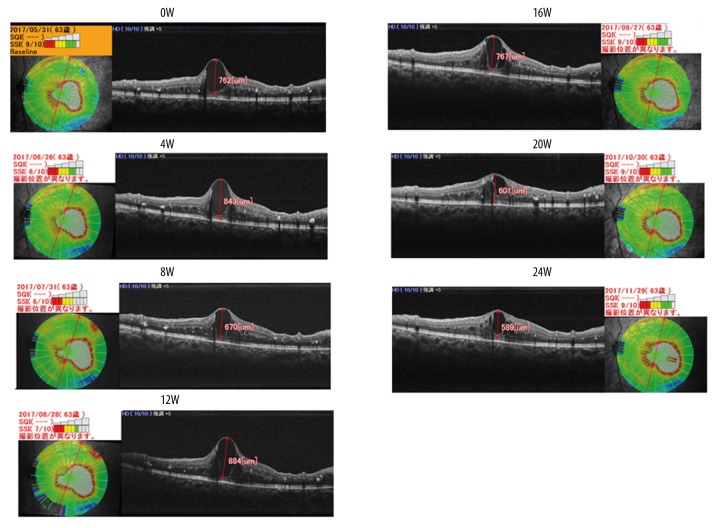
Her left visual acuity started to improve after 4 weeks of treatment with ipragliflozin and improved to 20/22 after 24 weeks (Table 1) with subjective amelioration of blurred vision disappeared. The treatment had no influence on her right visual acuity. Although the macular edema measured with optical coherence tomography images (OCT) using an RS-3000 (Nidek Co., LTD, Aichi, Japan) did not change until 12 weeks of the treatment, the size of the cyst decreased prominently after 16 weeks (Figure 1.).
Table 1.
Time course of clinical parameters of the patient.
| 0W | 4W | 8W | 12W | 16W | 20W | 24W | |
|---|---|---|---|---|---|---|---|
| Body weight | 47.9 | 47.2 | 47.1 | 46.2 | 45.2 | 46.2 | 46.3 |
| BMI | 25.4 | 24.1 | 24 | 23.6 | 23.1 | 23.6 | 23.6 |
| Blood pressure (mmHg) | 132/60 | 124/60 | 132/74 | 124/60 | 126/64 | 124/60 | 126/60 |
| Diabetic nephropathy | Stage 1 | Stage 1 | Stage 1 | Stage 1 | Stage 1 | Stage 1 | Stage 1 |
| Diabetic neuropathy | None | None | None | None | None | None | None |
| Visual acuity | 0.4 (20/50) | 0.8 (20/25) | 0.8 (20/25) | 0.7 (20/28) | 0.7 (20/28) | 0.9 (20/22) | 0.9 (20/22) |
| Chemistry | |||||||
| Cr (mg/dl) | 0.58 | 0.57 | 0.6 | 0.59 | 0.62 | 0.61 | 0.65 |
| eGFR (ml/m/1.73 m2) | 80 | 81 | 76 | 80 | 77 | 75 | 70 |
| ACR (mg/gcr) | 7.5 | 18.8 | |||||
| Glucose (mg/dl) | 128 | 105 | 124 | 100 | 127 | 106 | 103 |
| HbA1c (%) | 7 | 6.7 | 6.6 | 6.5 | 6.5 | 6.5 | 6.7 |
| Urinalysis | |||||||
| Protein | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| Sugar | (−) | (3+) | (4+) | (4+) | (4+) | (4+) | (4+) |
| Ketone body | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
Clinical course of body weight (BW), body mass index (BMI), visual acuity, creatinine (Cr), estimated glomerular filtration rate (eGFR), urine albumin creatinine ratio (ACR; urine albumin/urine g creatinine), blood sugar level, and hemoglobin A1c (HbA1c; NGSP) in our patient.
Visual acuity is expressed using the Landolt ring method, which is generally used in Japan, and using the American method. Blood sugar was measured using a glucose oxidase method, and HbA1c was measured using HPLC method.
Figure 1.
Clinical course of macular edema in our patient before and after the administration of the SGLT2 inhibitor until 24 weeks. Cyst heights were 762 μm (0W), 843 μm (4W), 670 μm (8W), 884 μm (12W), 767 μm (16W), 601 μm (20W), and 589 μm (24W).
Discussion
In the presented case, the visual acuity and subjective symptoms started to improve as early as 4 weeks after the initiation of the SGLT2 inhibitor, ipragliflozin, followed by morphological amelioration of the macula. The effect of the SGLT2 inhibitor is not likely to be mediated by glycemic control due to the following reasons; First, the amplitude of reduction of HbA1c level was rather small (0.3%). Second, the time course of the visual improvement was too rapid. Third, it has not been reported that DME can be improved by proper glycemic control attained by other measures in a short time course. Delayed response to intravitreous steroid injection and spontaneous remission of DME are not common. These observations led us to consider that the improvement of DME appeared to be mediated by SGLT2 through its specific pharmacological action.
Recently, reports of the beneficial effects of SGLT2 on cardiovascular events and diabetic nephropathy are accumulating [7–9]. The inhibitory effect of SGLT2 on albuminuria was independent of glycemic control [17], which also suggests SGLT2 inhibitors have a direct effect on diabetes complications [10].
The clinical doses of ipragliflozin are 25 mg to 100 mg as determined by the Japan Ministry of Health, Labor and Welfare. We prescribed 25 mg of ipragliflozin to the patients in order to minimize its adverse effects such as dehydration, polyuria, and genital infections, considering her age and body weight. Indeed, this small amount of ipragliflozin resulted in glycosuria and reduction of hemoglobin A1c levels, indicating that the concentration of the SGLT2 inhibitor in renal proximal tubules was sufficient in this case. Moreover, the concentration of drug in the blood circulation of the patient seems to be high enough to inhibit SGLT2 in the body other than in the renal proximal tubules.
At present, intravitreous injections of corticosteroids, anti-VEGF antibodies, or agents or photocoagulation are regard as gold standard therapies for the treatment of DME [3–5]. VEGF is reported to be one of the endogenous substances responsible for the development of DME [1,5]. Among these treatments, anti-VEGF antibody therapy is proved to be superior to the photocoagulation therapy. However, anti-VEGF antibodies have been reported to have serious adverse effects such as nonfatal cerebrovascular accidents and myocardial infarction [5]. In addition, anti-VEGF antibodies and agents are expensive and need frequent (several times per year) and long-term administration. Together with their ineffectiveness in some patients, these results show that novel therapeutic measures are urgently needed. The present case suggests that SGLT2 inhibitors are candidate drugs for this purpose.
Capillaries consist of pericytes and endothelial cells. Pericytes play important roles such as structural maintenance of the capillary and regulation of blood flow in capillary nets [18]. DME is manifested as focal or diffuse leakage from retinal dilated capillaries and micro-aneurysms, probably caused by retinal barrier dysfunction [2,3,6]. It is likely that damage to retinal pericytes leads to the retinal barrier dysfunction. In diabetic retinopathy, retinal pericyte swellings and loss are key pathological changes, followed by retinal micro-aneurysms, bleeding, and proliferative changes [19]. Likewise, high-glucose-induced dysfunction of retinal pericytes may be involved in the development of DME, since retinal pericytes are reported to produce VEGF in diabetic retinopathy [20].
We reported the presence of SGLT2 in mesangial cells and retinal pericytes [11,12,15]. Long-term high-glucose conditions impair contractile responses of mesangial cells and lead to the loss of retinal pericytes. These are considered as major changes observed in diabetic nephropathy and retinopathy [19]. The non-selective SGLT inhibitor, phlorizin, prevented the impaired contractility of retinal pericytes and mesangial cells under high-glucose conditions [14,15]. Thus, it is plausible to consider that SGLT2 inhibitors protect pericytes against high-glucose-induced damages. Although speculative, similar mechanisms may be working in the recovery of DME treated with an SGLT2 inhibitor. Another possible mechanism to be considered is that ipragliflozin reversed DME through the inhibition of VEGF production. Since retinal pericytes are reported to produce VEGF in diabetic retinopathy [20], an SGLT2 inhibitor may attenuate DME directly through the inhibition of VEGE production.
The prices of SGLT2 inhibitors differ among countries, ranging from $1.8 to $14.0 a day and $657 to $5110 per year. Prices of anti-VEGF antibodies or agents differ from $1800 to $2000 per year. The mean injection times were reported to be 9–12 times per year in the VISTA and VIVID studies and 7–8 times per year for ranibizumab [21]. On the other hand, the mean intravitreous injections are reported to be about 3.1 times per year (range, 1–17) in 2-year follow-up [22]. From these data, the cost of only drugs for the treatment of DME is in the range of $5580–$21 600 per year. The incidence of DME is 3.8% to 6.81% in diabetic patients [23]. It is estimated there are about 746 000 patients suffering from DME in the US population aged 40 years or older in 2010 [23]. Considering the large number of patients with DME worldwide, SGLT2 inhibitors may have a crucial effect on the economic and physical burdens on patients and society. In addition, SGLT2 inhibitors have other beneficial effects, such as glycemic control and protection of vital organs like the heart and kidneys, which makes their influence on personal and public health even greater. One of the adverse effects of SGLT2 inhibitors is volume depletion, which is more prominent in the elderly (≥75 years old) with higher doses of INVOKANA© (from the Brief Summary of Prescribing Information). If a smaller dose of SGLT2 inhibitor is effective on DME, as observed in our case, the medical cost would further be reduced, especially in the elderly.
Conclusions
In our patient with steroid-resistant DME, the visual symptoms improved soon after the initiation of an SGLT2 inhibitor. SGLT2 inhibitors might be a potential treatment for DME.
Footnotes
Conflicts of interest
None.
References:
- 1.Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350(1):48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 2.The DCCT/EDIC Research Group: Frequency of evidence-based screening for retinopathy in type 1 diabetes. N Engl J Med. 2017;376(16):1507–16. doi: 10.1056/NEJMoa1612836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simó R, Hernández C. Advances in the medical treatment of diabetic retinopathy. Diabetes Care. 2009;32(8):1556–62. doi: 10.2337/dc09-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serban R, Cioboata M, Chiotan C, et al. Visual acuity outcome in patients with diabetic maculopathy. J Med Life. 2014;7(Spec No.2):71–75. [PMC free article] [PubMed] [Google Scholar]
- 5.The Diabetic Retinopathy Clinical Research Network: Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193–203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: A systematic review. JAMA. 2007;298(8):902–16. doi: 10.1001/jama.298.8.902. [DOI] [PubMed] [Google Scholar]
- 7.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in Type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 8.Wanner C, Inzucchi SE, Lachin JM, et al. EMPA-REG OUTCOME investigators, empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–34. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 9.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 10.Wakisaka M. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(18):1799–800. doi: 10.1056/NEJMc1611290. [DOI] [PubMed] [Google Scholar]
- 11.Wakisaka M, He Q, Spiro MJ, et al. Glucose entry into rat mesangial cells is mediated by both Na (+)-coupled and facilitative transporters. Diabetologia. 1995;38(3):291–97. doi: 10.1007/BF00400633. [DOI] [PubMed] [Google Scholar]
- 12.Wakisaka M, Yoshinari M, Yamamoto M, et al. Na+-dependent glucose uptake and collagen synthesis by cultured bovine retinal pericytes. Biochim Biophys Acta. 1997;1362(1):87–96. doi: 10.1016/s0925-4439(97)00071-9. [DOI] [PubMed] [Google Scholar]
- 13.Wakisaka M, Yoshinari M, Asano T, et al. Normalization of glucose entry under the high glucose condition by phlorizin attenuates the high glucose-induced morphological and functional changes of cultured bovine retinal pericytes. Biochim Biophys Acta. 1999;1453(1):83–91. doi: 10.1016/s0925-4439(98)00087-8. [DOI] [PubMed] [Google Scholar]
- 14.Wakisaka M, Kitazono T, Kato M, et al. Sodium-coupled glucose transporter as a functional glucose sensor of retinal microvascular circulation. Circ Res. 2001;88(11):1183–88. doi: 10.1161/hh1101.091265. [DOI] [PubMed] [Google Scholar]
- 15.Wakisaka M, Nagao T, Yoshinari M. Sodium glucose cotransporter 2 (SGLT2) plays as physiological glucose sensor and regulates cellular contractility in rat mesangial cells. PLoS One. 2016;11:e0151585. doi: 10.1371/journal.pone.0151585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakisaka M, Nagao T. Sodium glucose cotransporter 2 in mesangial cells and retinal pericytes and its implications for diabetic nephropathy and retinopathy. Glycobiology. 2017;27(8):691–95. doi: 10.1093/glycob/cwx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherney D, Lund SS, Perkins BA, et al. The effect of sodium glucose co-transporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia. 2016;59(9):1860–70. doi: 10.1007/s00125-016-4008-2. [DOI] [PubMed] [Google Scholar]
- 18.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7(4):452–64. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kador PF, Akagi Y, Terubayashi H, et al. Prevention of pericyte ghost formation in retinal capillaries of galactose-fed dogs by aldose reductase inhibitors. Arch Ophthalmol. 1988;106(8):1099–102. doi: 10.1001/archopht.1988.01060140255036. [DOI] [PubMed] [Google Scholar]
- 20.Amano S, Yamagishi Si, Kato N, et al. Sorbitol dehydrogenase overexpression potentiates glucose toxicity to cultured retinal pericytes. Biochem Biophys Res Commun. 2002;299(2):183–88. doi: 10.1016/s0006-291x(02)02584-6. [DOI] [PubMed] [Google Scholar]
- 21.Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122(10):2044–52. doi: 10.1016/j.ophtha.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Fong DS, Luong TQ, Contreras R, et al. Treatment an analysis from large U.S. integrated health care system. Retina. :2017. doi: 10.1097/IAE.0000000000001790. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132(11):1334–40. doi: 10.1001/jamaophthalmol.2014.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]