Abstract
Drug abuse and addiction remain major public health issues, exemplified by the opioid epidemic currently devastating the US. Treatment outcomes across substance use disorders remain unacceptably poor, wherein drug discovery/development for this multifaceted neuropsychiatric disorder focuses on single molecular-level targets. Rather, our opinion is that a systems-level neuroimaging perspective is crucial for identifying novel therapeutic targets, biomarkers to stratify patients, and individualized treatment strategies. Focusing on tobacco use disorder, we advocate a brain systems-level perspective linking two abuse-related facets (i.e., state-like withdrawal and trait-like addiction severity) with specific neurocircuitry (insula- and striatum-centered networks). To the extent that precise neurocircuits mediate distinct facets of abuse, treatment development must adopt not only a systems-level perspective, but also multi-intervention rather than mono-intervention practices.
Keywords: cigarettes, withdrawal, addiction, insula, striatum, anterior cingulate cortex
DRUG ADDICTION TREATMENT OUTCOMES REMAIN POOR
Humans have been using, abusing, and have become addicted to a wide variety of exogenous substances for thousands of years. Opioids, psychostimulants, alcohol, and nicotine remain a common problem across civilizations and epochs in that some individuals who start experimenting with drugs are unable to quit. While these drugs do not share a common pharmacological classification, they do share an abuse liability. Despite enormous advances in understanding the cellular and molecular mechanisms of their action, treatment outcomes remain stubbornly low across all drug classes. The significance of this clinical failure is perhaps no more seriously manifest than the current tragic opioid epidemic in the United States, which leads the world in opioid consumption and opioid-related deaths. While acute overdose is not generally a factor in tobacco use disorder (TUD), long-term cigarette smoking is the largest source of preventable deaths in the country [1].
The current algorithm in addiction medicine (along with supportive family and individual behavioral interventions) is the search for better pharmacotherapies in pursuit of evermore specific and selective receptor-based agents. The overriding hypothesis is that a better ‘silver bullet’ will engage with a/the key receptor-based system to prevent further drug use and thus reversing addiction [2]. However, even following long-term abstinence (e.g., via incarceration), drug addicts recidivate at disturbingly high rates [3, 4]. Unfortunately, this observation highlights the often-mistaken assumption that the absence of the behavior (drug-taking) reflects the absence of the disease (addiction).
In this opinion piece, we offer an alternative hypothesis for why this approach has failed to improve treatment outcomes and posit that the solution may not lie in identifying better molecular-level medicinal chemistry, but rather, in establishing a systems-level neurobiological approach to the disease. We base this view on the well-known complexity of this neuropsychiatric disease that presents with compulsive drug-seeking and –using behaviors, deficits in cognitive processes (e.g., working memory, decision-making, reward processing, inhibitory control), and affective dysregulation. Optimal execution of these mental operations involves multiple complex neurocircuits and brain networks. Taken together as a syndrome, we posit that it seems unlikely that a single molecular target will be able to successfully address all facets of the disease. Focusing on nicotine and TUD, we highlight recent developments that advocate a systems-level perspective for the treatment of substance use disorders (SUDs).
THE NEED FOR BRAIN-BASED BIOMARKERS IN ADDICTION
While the initiation of drug use leads to increases in the local concentration of dopamine within the mesocorticolimbic (MCL) system and reinforces continued drug-seeking and -using, chronic long-term drug intake can induce a state of dependence and/or addiction that no longer resides simply within the MCL circuit [5]. Indeed, most addiction neuroscientists and clinicians agree that SUDs can be characterized by dysregulated cognitive, affective, and reward processing that are reflective of widespread and long-lasting changes in the brain [6].
If the disease is indeed separate from the cause, then attempting to treat the addicted individual by blocking (via receptor antagonists) or directly stimulating the receptors (via agonists) that initially reinforced drug-taking behaviors, may be doomed to failure. Indeed, neither agonist therapy (e.g. nicotine replacement [transdermal patch], opiate replacement [methadone]), nor antagonist agents (e.g. dopamine antagonists) have proven highly efficacious, likely because the disease has ‘moved’ from the initiation source and neurocircuits, to reside in connected but more distributed brain regions and networks [7, 8]. It is our opinion that a complex, multifaceted neuropsychiatric disease such as SUDs, with its high psychiatric comorbidity (e.g. depression, anxiety, psychoses, other drugs) will require a systems-level, brain-based treatment approach along with a quantitative, brain-based biomarker of disease severity. Neither of these currently exist. While many studies have shown behavioral, personality, and even neuroimaging differences between those with SUDs versus healthy individuals, all have failed to serve as useful predictors of treatment success [9]. This has resulted in the inability to tailor treatments most likely to be successful for a given individual and importantly, to follow the progress of treatment (e.g. much like a sphygmomanometer registers blood pressure level changes during treatment for hypertension).
With respect to nicotine and TUD, we highlight recent developments in the field linking two facets of the disorder, namely, state-like withdrawal symptoms and trait-like addiction severity (see Box 1), to distinct neurocircuits centered on the insula and striatum, respectively. We hypothesize that if there are indeed distinct facets of SUDs, they must be mediated via distinct neurobiological systems, and thus, treatment development might be best served by more fully appreciating not only a brain systems-level perspective, but also by a multi-intervention (‘silver buckshot’) as opposed to the more commonly practiced mono-intervention (‘silver bullet’) approach. It is perhaps surprising that such a multi-intervention approach has been resisted in the SUD field despite its common embodiment in oncology and immunology. Based on our position that no single receptor, neurotransmitter system, neurocircuit, cognitive system, or behavior can fully capture the multifaceted nature of the disease, seeking a better mono-therapy would likely, and indeed has repeatedly, failed to treat the disease in its totality, leading to extraordinary recidivism rates.
Box 1. State- versus Trait-related aspects of tobacco use disorder (TUD).
Drug Abuse is the habitual, pathological use of drugs leading to drug dependence and addiction. Herein, we consider TUD to be a multifaceted neuropsychiatric disorder composed of withdrawal-related and addiction-related aspects.
Drug dependence is an adaptive state that develops from repeated drug administration, and which results in withdrawal upon cessation of use. Withdrawal signs can be combinations of physical (e.g., nausea, vomiting, diarrhea induced following opiate abstinence) or psychological symptoms (e.g., anxiety, craving, anhedonia). Dependence is also characterized by tolerance, a need to increase the dose of a drug over time to receive the same effect (or diminished drug effects while maintaining a constant dose over time).
Drug addiction is the chronic relapsing condition involving the compulsive use of drugs and the inability to stop using despite harmful consequences to self, family, or society. It is possible to be drug dependent without being addicted.
After the establishment of drug addiction, the level or severity of the disease remains relatively stable over time and can be considered as a long-term trait of the individual. In contrast, following acute abstinence, individuals often begin to exhibit withdrawal symptoms, which change qualitatively and/or quantitatively as a function of time since last drug administration reflecting the current pharmacologic state of the individual.
Noninvasive human neuroimaging (see Box 2) has the ability to identify differences in brain structure and function between groups of individuals with SUDs and healthy controls, as well as monitor individuals along the course of treatment to identify brain regions/networks that change with treatment-induced prolonged abstinence [10]. Specifically, imaging biomarkers have the potential to determine whether structural and/or functional brain differences among individuals with SUDs remit to a (presumed) pre-addicted state or whether alternative brain regions/networks compensate for those dysregulated in addiction. For example, specific cortico-striatal circuits are altered among individuals with cocaine use disorder (vs. controls), and these differences also correlate with compulsive behaviors [11]. How such dysregulated circuits change (or are compensated for by other circuits) during treatment is currently unknown. Furthermore, one can make post hoc predictions of treatment outcome by using neuroimaging data and post-treatment outcomes [9, 12], thereby providing insight into brain mechanisms important for recovery. Candidate imaging markers of treatment outcomes could also be tested in predictive clinical trials. Lastly, neuroimaging may facilitate identification of circuit/network-based intermediate phenotypes (endophenotypes) that might be used to stratify individuals and potentially identify personalized treatments with higher probabilities of outcome success [e.g., 13]. Indeed, the ultimate goal of this strategy includes developing a system to individualize predictions of health outcomes based on models developed from group studies [9].
Box 2. Structural and functional Magnetic Resonance Imaging (MRI).
Brain structure (e.g. gray matter density, cortical thickness, and white matter tract microstructure and integrity), biochemical constituents (using MR spectroscopy [MRS]), and function (functional MRI [fMRI]) can be measured using MRI techniques. Changes in brain function are inferred from changes in blood flow, blood volume, and oxygenation, the so-called blood-oxygenation level-dependent (BOLD) signal. Brain activity can be assessed while individuals perform specific cognitive and emotional-laden tasks, thus linking behavioral performance of addiction-related processes (e.g. working memory, attention, and inhibitory control) with the localization and magnitude of brain activity [e.g., 73].
fMRI data can also be acquired in the absence of a directed task, that is at ‘rest’ [74]. Application of resting-state functional connectivity (rsFC) assessments of so-called resting-state fMRI (rs-fMRI) data has demonstrated specific brain connections (i.e. neurocircuits and networks) exist in the absence of a directed task, with the ‘strength’ of such connections at ‘rest’ able to predict the magnitude of subsequent task activation and behavioral performance success [75, 76]. Differences in resting brain circuits may reflect neuropsychiatric disease, including TUD [32, 46]. One commonly employed rsFC analysis strategy, “seed-based” correlation analysis, is a hypothesis-driven approach involving the selection of one or more regions of interest as “seeds”, followed by the computation of correlations between the rs-fMRI time series of the seed and all other brain voxels.
Most neuroimaging studies are inherently correlative, precluding causative processes to be identified. Nevertheless, designs that incorporate a parametric manipulation of a task or a pharmacological intervention (i.e. dose response), allows for more precise interpretations. Noninvasive brain stimulation (e.g., transcranial magnetic stimulation [TMS] and transcranial direct current stimulation [tDCS]) may allow more direct probes of putative neurocircuit plasticity. TMS is an emerging FDA-approved treatment for refractory depression and several small, and in some cases, open label studies suggest that TMS may be efficacious for SUDs [77–79] including smoking [80–82]. Many of the brain regions and circuits discussed herein are connected to and modulated by TMS delivered over the dorsolateral PFC [83]. However, very few TMS studies have also considered fMRI signals before and after SUD treatment [84]. As such, an important future research direction will be to examine how brain networks like those described herein are modulated over the course of TMS SUD treatment.
TOWARD BRAIN NETWORK-LEVEL BIOMAKERS OF NICOTINE WITHDRAWAL
Anxiety, irritability, tobacco craving, and difficulty concentrating are nicotine withdrawal symptoms that make short-term cessation difficult for most smokers [14]. Nicotine administration ameliorates abstinence-induced emotional [15] and cognitive dysfunction [16] indicating that early relapse occurs, in part, to relieve such symptoms, thereby maintaining smoking by negative reinforcement [17]. Available smoking cessation aids (bupropion, nicotine replacement, varenicline), although efficacious for only some smokers, to some degree reduce such aversive symptoms. For example, varenicline, acting at nicotinic acetylcholine receptors (nAChRs) aids cessation by ameliorating abstinence-induced withdrawal while also attenuating nicotine-induced effects following re-exposure [18]. In nicotine’s absence, varenicline acts as a partial agonist at α4β2 nAChRs, producing ~50–60% the action of nicotine as assessed by cellular patch clamp techniques, as well as in vitro and in vivo assessment of dopamine release in rat nucleus accumbens [18]. Of note, in the nucleus accumbens, dopamine release is a common molecular substrate of all addictive drugs [18b]. In the presence of nicotine, varenicline also acts as an antagonist binding with higher affinity than nicotine, thus preventing full nicotine-induced receptor activation, as assessed in rodent models [18, 19]. Accordingly, varenicline has been reported to reduce withdrawal-induced affective and cognitive disturbances in human smokers as well as the subjective rewarding aspects of cigarette smoking [20]. As this ‘dual action’ (i.e., partial agonist/antagonist) profile may explain varenicline’s improved efficacy over other cessation aids [21], we leveraged these neuropharmacologic properties to delineate brain regions and neurocircuits associated with withdrawal by utilizing varenicline and nicotine as pharmacologic probes in a neuroimaging research program [22–27].
Specifically, we administered varenicline and nicotine, both alone and in combination, to overnight-abstinent smokers and nonsmokers in a two-drug, double-blind, placebo-controlled design [22–27]. In a counter-balanced order, participants underwent a regimen of varenicline and placebo pill administration (17-days each; pill factor) and completed fMRI scans near the end of each period, wearing on different days, a transdermal nicotine or placebo patch (patch factor) [22]. Indicative of brain regions and neurocircuits linked with nicotine withdrawal, we anticipated: (i) a specific varenicline (pill) and nicotine (patch) interaction effect across dependent variables; these would be derived from preclinical data regarding varenicline’s neuropharmacological properties (Fig.1A), and (ii) that such drug effects would not be observed among nonsmokers who, by design, were not in a state of nicotine withdrawal (i.e., a negative control group). By assessing heart rate before and after patch application, we first confirmed that the drug manipulations produced an observable physiological response in line with varenicline’s dual action profile [22]. That is, nicotine, fully, and varenicline alone and partially, increased heart rate, while varenicline in combination with nicotine partially antagonized nicotine’s effect (Fig.1B) [22].
Figure 1. Neurocircuits linked to withdrawal identified using pharmacologic probes.
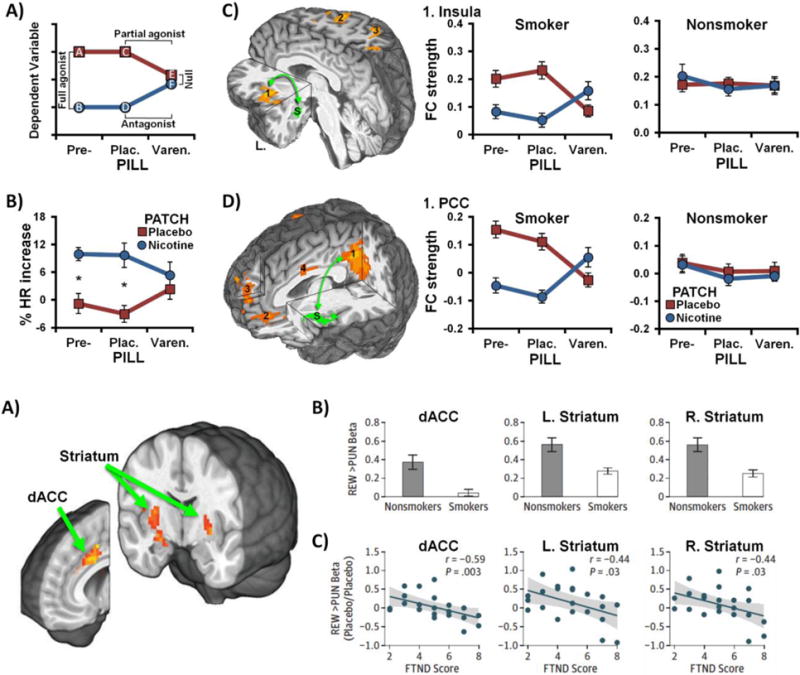
(A) Schematic of the hypothesized varenicline (pill) and nicotine (patch) pharmacologic interaction (adapted from: [22]). Withdrawal-related effects on the dependent variable were anticipated to be greatest in the absence of drug administration (placebo conditions, data points A & C). Nicotine was expected to reduce withdrawal-related elevations (B & D), consistent with a full agonist effect (A vs. B, C vs. D). Varenicline was also expected to reduce the dependent variable (E), consistent with a partial agonist effect (C vs. E). Combined varenicline and nicotine administration (F) was anticipated to produce an attenuated nicotine-induced response, consistent with an antagonist effect (D vs. F). These partial agonist and antagonist effects then yield a null effect of nicotine versus placebo patch (E vs. F) in the presence of varenicline. (B) Impact of drugs on heart rate (HR) among abstinent cigarette smokers (* p < 0.05, adapted from: [22]). (C) Amygdala-insula resting-state functional connectivity (rsFC) (left) was reduced by varenicline and nicotine in a manner consistent with the hypothesized pharmacologic interaction among abstinent smokers (middle), but not nonsmokers (right) (adapted from: [24]). (D) Insula’s rsFC with the posterior cingulate cortex (1, left) and medial prefrontal cortex (2 & 3) was similarly reduced by varenicline and nicotine among smokers (middle), but not nonsmokers (right) [24]. S: seed region (green), Pre-: before beginning study pill administration; Plac.: under placebo pills; Varen.: under active varenicline pills.
Operating under the premise that the amygdala and its interconnected neurocircuitry underlie negative affective states accompanying drug withdrawal [6, 28], we delineated brain regions whose resting-state functional connectivity (rsFC) strengths with an amygdala ‘seed region’ (see Box 2) were altered by varenicline and nicotine [24]. Utilizing an ‘agnostic’ whole-brain search strategy, rsFC in an amygdala-insula neurocircuit was shown to be decreased among abstinent smokers by nicotine and varenicline (vs. placebo) in a manner consistent with varenicline’s partial agonist/antagonist profile (Fig.1C) [24]. Indicative of this neurocircuit’s critical association with the withdrawal state, such drug-induced rsFC modulations were not observed among nonsmokers [24]. To more fully contextualize the insula’s role, another rsFC assessment was performed using this identified insula region as a new seed; both varenicline and nicotine (vs. placebo) again decreased smoker’s rsFC between the insula and –among other regions– components of the so-called default-mode network (DMN), including the parahippocampus/amygdala, posterior cingulate cortex (PCC), ventromedial prefrontal cortex (vmPFC) and dorsomedial PFC (dmPFC) (Fig.1D) [24]. Additional exploratory analyses further indicated that elevated rsFC between the insula and the PCC of smokers – among other regions– was both reduced by varenicline and nicotine and associated with greater subjective measures (self-reported symptoms) and objective measures of withdrawal severity (slower task reaction times) [24]. Regarding implications, these outcomes provide a systems-level perspective centered on the insula regarding the neural underpinnings of nicotine withdrawal symptoms, suggesting one putative neuroimaging biomarker of TUD.
While the above studies represent an exemplar of using pharmacologic probes combined with fMRI to gain insight into the neural mechanisms of a multifaceted neuropsychiatric condition, it is worth placing these ‘withdrawal-related neurocircuitry’ observations in the larger context of TUD. First, the insula is critically involved in maintaining cigarette smoking such that patients with stroke damage impacting this region (vs. patients with brain damage elsewhere), quit smoking more readily [29] and experience fewer and less severe withdrawal symptoms [30]. Accordingly, based on human neuroimaging evidence, the insula, serving an interoceptive monitoring role, is thought to track withdrawal-induced physiologic sensations and, in turn, modify affective, motivational, and cognitive processes via alterations in the functional interactions with other brain regions (e.g., amygdala, vmPFC, dmPFC, PCC, ACC, striatum) [31, 32]. For example, regarding affective processes, elevated insula-amygdala rsFC has been linked to increased self-reported anxiety [33] as well as irritability [31]. In addition, elevated insula-amygdala rsFC has been observed in adolescents and adults with anxiety disorders (vs. health controls) [34, 35] and elevated insula-amygdala rsFC has been shown to positively correlate with greater anxiety severity among both anxiety patients [36] and healthy controls [33].
Regarding motivational processes, whereas the insula monitors interoceptive signals associated with a homeostatic disequilibrium [37], we suggest that insula-DMN interactions are involved in preparing the organism to respond to and alleviate such states. For example, many of the regions whose rsFC with the insula that were modulated by varenicline and nicotine as described above (e.g., PCC, parahippocampal gyrus, vmPFC, dmPFC) have also consistently shown increased activity across human neuroimaging studies of drug cue-reactivity/craving (i.e., increased brain activity following presentation of drug-related [e.g., cocaine, alcohol, smoking] vs. neutral visual stimuli) [38, 39]. Conversely, these same regions consistently exhibit decreased activity following nAChR agonist administration (vs. placebo) as indicated by a recent meta-analytic assessment of pharmacologic fMRI studies [40]. Noteworthy, this same meta-analysis also observed convergent nAChR agonist-induced activity increases in lateral and medial prefrontal and parietal regions (collectively referred to as the executive control network [ECN; 41]). These ECN regions support externally-focused attention and thus, increased activity may account for nicotine’s well-characterized ability to augment cognitive operations (e.g. attention, working memory) particularly among abstinent smokers [16]. In summary, while regions overlapping the DMN tend to show increased activity during drug cue-reactivity/craving [38, 39], these same regions appear to also show decreased activity [40] and reduced rsFC with the insula following nAChR agonist administration [24]. Collectively, these observations provide evidence that functional interactions between the insula and regions of the DMN and/or altered activity within these regions may contribute to aspects of craving and cognitive impairments often associated with withdrawal.
Regarding cognitive processes, neuroimaging studies have converged on the view that efficient goal-directed cognition is facilitated by activation of some brain areas (e.g., ECN regions) and yet deactivation of others (e.g., DMN regions) [42–44]. Such views regarding large-scale brain network dynamics provide useful heuristic frameworks to appreciate not only optimal cognitive performance [42], but also cognitive disturbances in neuropsychiatric conditions [44]. Inspired by these systems-level perspectives, we previously synthesized a framework on the consequences of nicotine withdrawal revolving around 3 commonly observed brain networks, the DMN, ECN, and the salience network (SN; a network thought to facilitate the switching of attention between external versus internal stimuli, see Box 3) [32, 45–47]. We suggest that in the nicotine-deprived state, the insula (a core SN node) might track withdrawal-induced physiologic sensations and, in turn, might direct attentional resources toward this internal state via increased interactions with the DMN (SN-DMN), thereby biasing activity toward the DMN and away from the ECN. The consequences of such a shift in large-scale brain networks dynamics might result in be increased cravings for and ruminations about drugs (DMN) while concurrently reducing executive functions and top-down cognitive control (ECN). Conversely, acute nicotine administration, might bias processing resources away from the DMN and toward the ECN through increased SN-ECN interactions, thereby focusing attention toward external stimuli. Indeed, a recent study specifically tested this triple network interaction (SN-DMN, SN-ECN) hypothesis in the context of nicotine withdrawal (vs. smoking satiety) and characterized the relationship between abstinence-induced changes in the interactions between these networks and subjective (craving), objective (working memory behavioral performance), and neural functioning measures (working memory brain activity) [48]. In that study, not only was nicotine withdrawal associated with increased SN-DMN and decreased SN-ECN interactions, these network dynamic alterations were also related to clinically-relevant measures, including greater craving, suboptimal behavioral performance, and reduced DMN deactivation during working memory task performance [48].
Box 3. Triple brain network model of nicotine withdrawal and administration.
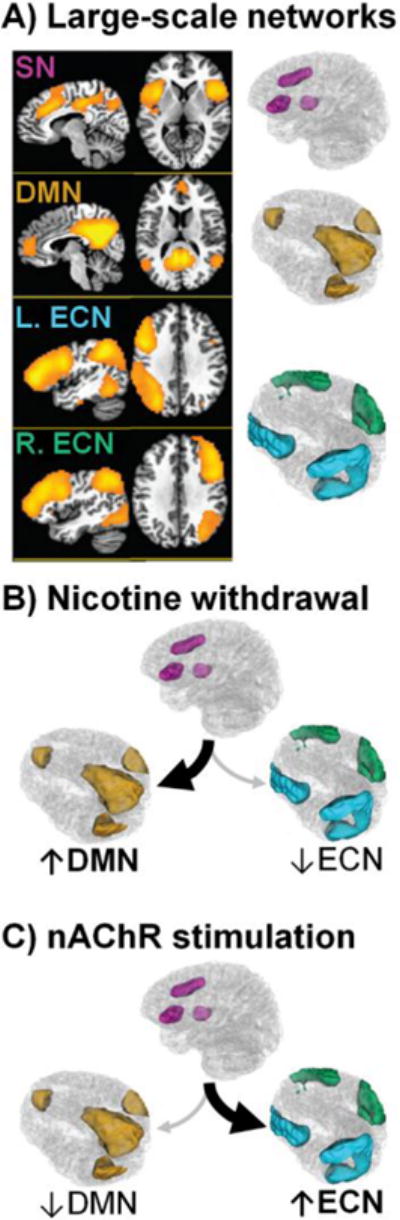
Inspired by brain systems-level perspectives regarding optimal cognition [42] and cognitive disturbances in neuropsychiatric conditions [44], we have advocated a network-level heuristic framework regarding the consequences of nicotine withdrawal and administration revolving around three brain networks, the salience network (SN), default-mode network (DMN), and executive control network (ECN) [32, 45–47]. This framework is centered on the interoceptive role of the insula [37], which along with the anterior cingulate cortex, constitutes core nodes of the SN [41] (Figure 1, Panel A, pink, adapted from: [48]).
The SN is hypothesized to facilitate processing of the currently most homeostatically relevant stimuli arising from internal or external sources by toggling the relative activity between the competitively interacting (i.e., anticorrelated) DMN and ECN [41, 85, 86]. Whereas the DMN (Figure 1, Panel A, gold), anchored by the posterior cingulate cortex, medial PFC, and parahippocampus is generally associated with internally oriented cognitive operations, the ECN, composed of lateral prefrontal and parietal regions (Figure 1 Panel A, blue & green), is generally associated with externally oriented attention and executive processes. Failures to adequately suppress DMN regions [87] or to activate ECN regions [88] as well as maladaptive interactions between nodes of these networks [89] represent systems-level contributors to suboptimal task performance.
Accordingly, during nicotine withdrawal, we suggest that the insula (SN) tracks withdrawal-induced physiological sensations and, in turn, biases processing resources toward this homeostatically relevant state via increased interactions with the DMN at the expense of decreased externally oriented attention mediated by the ECN (Figure 1 Panel B). Conversely, acute nAChR stimulation may bias processing resources away from the DMN and toward the ECN, thereby enhancing executive function and focusing attention toward external stimuli and goal-directed behaviors (Figure 1 Panel C).
Taken together, we propose that insula-centric network dynamics might represent a state-like neuroimaging biomarker of acute nicotine withdrawal. This position is supported by the observations that such network dynamics are: (i) modulated by two pharmacologic smoking cessation aids (varenicline and nicotine) in abstinent smokers [24], (ii) unaltered by the same drugs in nonsmokers [24], (iii) linked with self-reported withdrawal symptoms and tobacco craving [24, 48], and (iv) correlate with abstinence-induced disruption of behavioral performance and task-related brain activation [48]. While requiring further characterization, we speculate that the insula’s interactions with specific brain regions may contribute to distinct withdrawal symptoms, including anxiety and irritability (insula-amygdala) as well as tobacco craving and difficulty concentrating (insula-DMN and/or insula-ECN). As such, a desirable characteristic of efficacious cessation interventions may be the normalization of activity between and/or within these brain networks and thereby the stabilization of affective (e.g., anxiety, irritability), motivational (e.g., craving, cue-reactivity), and cognitive processes (e.g., difficult concentrating, task performance) particularly among those smokers experiencing more pronounced symptoms during the initial stages of quitting.
TOWARD BRAIN NETWORK-LEVEL BIOMAKERS OF ADDICTION SEVERITY
While modulating withdrawal-related neurocircuits may be necessary for promoting short-term abstinence, it may not be sufficient for long-term cessation as other neurocircuits and their addiction-related plasticity likely contribute to different facets of SUDs (and vice versa). Accordingly, poor smoking cessation outcomes are perhaps related to a singular focus on the results (i.e., withdrawal symptoms) rather than the cause of the problem (e.g., addiction neuroplasticity). In contrast to the state-like withdrawal-related neurocircuitry outlined above, trait-like addiction-related neurocircuits are anticipated to not be modulated by acute nAChR agonist administration (e.g., many smokers continue to smoke while wearing ‘the patch’), but rather, to correlate with measures of addiction severity [49]. Regarding smoking, addiction severity is typically quantified via the Fagerström Test for Nicotine Dependence (FTND). The FTND is a validated clinical measure that is highly heritable [50] and routinely utilized as a primary phenotype in studies associating smoking behaviors with nAChR and other genetic variants [51]. As such, delineating relations between precise neurocircuits and FTND scores may yield endophenotypic markers of addiction severity that could be leveraged to track intervention outcomes. Below we consider evidence supporting our hypothesis that the trait of nicotine addiction severity may be linked with aberrant activity within, and interactions between, the anterior cingulate cortex (ACC) and the striatum.
The role of the striatum (and more generally, the MCL dopaminergic circuit) in processing reinforcing stimuli, including those associated with drugs of abuse, is well established [7, 52, 53]. Accordingly, the striatum and its interconnected neurocircuitry play a prominent role in SUD-related processes, with ventral areas being linked to reward learning, and dorsal areas, to compulsive drug-seeking behavior [6]. Neuroimaging evidence suggests that dysregulated reward processing associated with an extended smoking history, is mediated by neuroadaptations (and/or preexisting vulnerabilities) in the striatum, and manifest as both hypersensitivity to drug-related reward (e.g., drug cues) [39] and hyposensitivity to nondrug-related reward (e.g., money) [54–56]. As such, the impact of varenicline and nicotine on the neural correlates of nondrug-related reward sensitivity (i.e., brain activity following receipt of rewarding vs. punishing outcomes) were recently probed among abstinent smokers and nonsmokers [27]. Utilizing a probabilistic reversal learning task and the same participants from the withdrawal-related neurocircuitry study described above [24], hypo-activation within the bilateral dorsal striatum and dorsal ACC (dACC; another SN node) was observed following the receipt of rewards (Fig.2A) among abstinent smokers versus nonsmokers (Fig.2B) [27]. In stark contrast to the drug-induced modulations of withdrawal-related neurocircuitry (Fig.1), these blunted reward-receipt responses were not modulated by varenicline or nicotine, but rather, were negatively correlated with addiction severity (FTND) scores (Fig.2C) [27]. Juxtaposing these null drug effects when considering blunted reward-receipt brain responses (Fig.2) with the drug-modulated insula-centric rsFC positive results above (Fig.1) provides compelling evidence that some facets of TUD are not targeted by nAChR agents, which might partly account for their inability to help the vast majority of smokers quit.
Figure 2. Brain regions linked to addiction severity identified via a reward processing task.
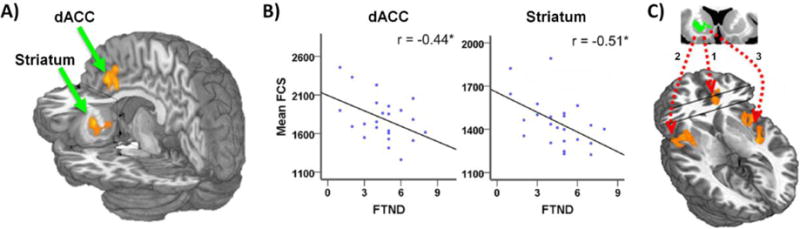
(A) Smoker versus nonsmoker group differences following reward receipt were observed in the dorsal anterior cingulate cortex (dACC) and bilateral striatum. (B) Abstinent smokers (white) relative to nonsmokers (grey) showed reduced brain activity following receipt of rewarding outcomes (win a dollar) versus punishing outcomes (lose a dollar) in the dACC, left striatum, and right striatum. In stark contrast to the outcomes in Fig. 1, these reward-sensitivity hypo-activations among smokers were not modulated by varenicline or nicotine. (C) Rather, more severe reductions in reward sensitivity negatively correlated with greater levels of addiction severity (higher FTND scores) among abstinent smokers. REW: reward (win a dollar); PUN: punishment (lose a dollar); FTND: Fagerström Test of Nicotine Dependence. Figure adapted from: [27].
Placing these observations in the larger context of TUD, accumulating evidence links an extended smoking history with striatal hypoactivity during monetary-reward processing tasks [25, 55, 57–61], albeit, not consistently [62, 63]. More severe striatal hypoactivity to aspects of monetary reward has been associated with poorer outcomes among smokers when considering the number of cigarettes smoked per day [64], craving severity [56], their ability to briefly refrain from smoking [59], and, ultimately, relapse rates [58]. Noteworthy, the aforementioned studies have often focused on reward anticipation as opposed to reward receipt (as in Fig.2). Specifically, when considering reward anticipation in a monetary incentive delay task, striatal and dACC hypoactivation among abstinent smokers following monetary reward-predicting cues can be modulated by acute nicotine administration, in effect, ‘normalizing’ such activity to levels observed among nonsmokers [25]. In contrast, other studies have documented reduced striatal responses following nondrug-reward receipt among smokers versus nonsmokers, which was not ameliorated by nicotine administration, but rather, negatively correlated with years of daily smoking [57]. Collectively, although further studies are warranted, these emerging results suggest a potential distinction for striatal and dACC responses during reward anticipation versus reward receipt. Specifically, reduced activity during reward anticipation may be linked with the pharmacological state of withdrawal and be potentially ameliorated with acute nicotine intake, while activity during reward receipt may be linked to an addiction trait (FTND scores), not normalized by acute nicotine administration.
Recent evidence similarly connects rsFC between the striatum and dACC with FTND scores. For example, one study reported individual differences in the rsFC strength of multiple cingulate subregions as a function of addiction severity and acute nicotine administration among smokers [49]. This study described an ‘addiction-related circuit’ involving the dACC and striatum such that weaker rsFC between these regions negatively correlated with FTND scores [49]. Critically, this dACC-striatum circuit was not impacted by acute nicotine administration, supporting our hypothesis that such rsFC may reflect an addiction trait as opposed to a pharmacological state. Although not identical, a similar circuit was subsequently attributed to a variation in an α5 nAChR subunit gene [65]. Specifically, the α5 nAChR smoking-related ‘risk allele’ was associated with reduced rsFC between the dACC and striatum (extending into adjacent limbic areas), where again, a weaker circuit predicted greater FTND scores [65], thus suggesting a potential brain circuit linkage to this known genetic smoking risk (i.e., an endophenotype) [66]. Extending these observations, another study assessed the functional coupling of the dACC and bilateral insula among both non-psychiatrically ill smokers and smokers diagnosed with schizophrenia [67]. Operating under the premise that alterations in dACC- and insula-centered neurocircuitry might contribute to elevated rates of smoking in schizophrenia, rsFC differences were characterized as a function of addiction severity, nicotine administration, and diagnosis. Across all subjects, dACC-striatum and insula-striatum rsFC negatively correlated with addiction severity accounting for half of the variance in FTND scores [67]. Furthermore, such rsFC was again unaltered by nicotine administration. In a separate study, dACC-striatum rsFC was similarly linked with addiction severity and schizophrenia diagnosis [68]. Collectively, these studies implicate dACC-striatum coupling not only as a potential endophenotypic marker of addiction severity among healthy smokers, but also connect such altered neurocircuitry with high smoking rates among schizophrenic patients.
Further evidence highlighting the relevance of dACC-striatum coupling for smoking behaviors in general, and addiction severity in particular, comes from a study examining variation in the hepatic enzyme cytochrome P450 (CYP2A6) gene which regulates nicotine metabolism rates [69]. Smokers with the reduced CYP2A6 genotype (i.e., slow and intermediate metabolizers), exhibit higher levels and/or longer durations of nicotine in plasma and presumably brain; they start smoking earlier in life, become addicted more quickly, but smoke fewer cigarettes relative to those with the normal genotype [70–72]. Given these dissociable smoking behaviors, a data-driven rsFC graph theory metric (functional connectivity strength [FCS]) derived from smokers and nonsmokers was utilized to identify brain regions and neurocircuits underlying these different smoker phenotypes [69]. Employing an ‘agnostic’ whole-brain search, a two-way ANOVA with factors for genotype (slow vs. intermediate vs. normal) and smoking (smoker vs. nonsmoker) identified a significant interaction effect only in the dACC and striatum (Fig.3A), such that slow metabolizers showed reduced FCS in both regions when considering smokers, but critically, not in nonsmokers [69]. In the slow metabolizing smoker subgroup, these FCS values were negatively correlated with FTND scores (Fig.3B) [69]. To delineate the precise neurocircuits contributing to this FCS genotype (CYP2A6) by environment (smoking) interaction, a standard rsFC assessment was performed using the identified dACC and striatum regions as seeds. Both seeds identified the bilateral insula as driving the striatum and dACC FCS interaction (Fig.3C) [69]. These outcomes are noteworthy in that they: (i) demonstrate that nicotine metabolism and, in turn, the concentration of nicotine delivered to the brain can sculpt neurocircuits, and (ii) are remarkably coincident with the addiction-related neurocircuitry studies described above. The fact that this collection of studies [27, 49, 65, 67–69] converged on similar brain regions while employing independent participant samples and data sets, different experimental designs, and unique analytic strategies increases confidence in the critical role of the dACC and striatum (and their functional connections with each other and the insula) regarding addiction severity.
Figure 3. Neurocircuits linked to addiction severity identified via nicotine metabolism genetics.
(A) Human whole-brain analysis identified the dorsal anterior cingulate cortex (dACC) and striatum as the only regions displaying a genotype (slow vs. intermediate vs. normal metabolizer) by smoking (smoker vs. nonsmoker) interaction when considering functional connectivity strength (FCS). Unlike nonsmokers, smokers with the slow metabolizing CYP2A6 genotype demonstrated reduced FCS in these brain regions. (B) Among the slow metabolizing smoker subgroup, FCS values in both the dACC (left) and the left striatum were negatively correlated with addiction severity (FTND) scores. (C) A standard rsFC assessment employing the striatal region as a seed identified specific neurocircuits driving the FCS genotype by environment (smoking) interaction in (A), including striatum-dACC (1) and striatum-insula (2 & 3) neurocircuits. Figure adapted from [69].
Taken together, we posit that ACC-striatum network dynamics represent a trait-like biomarker of nicotine addiction severity. Emerging results suggest that altered activity within and/or rsFC between these regions are linked to addiction severity as such brain measures are: (i) inversely correlated with FTND scores in smokers [27, 49, 65, 67, 69], (ii) not impacted by acute nAChR agonist administration [27, 49, 67], (iii) are modulated by nAChR [65] and nicotine metabolism genetics [69], and (iv) are related to a neuropsychiatric condition where smoking rates are disproportionately high [68]. Given that this addiction-related neurocircuitry does not appear to be modulated by nAChR agonists, other classes of pharmacologic agents may be needed to target this facet of TUD (Box 4). In summary, we surmise that a viable intervention target necessitating further investigation may be the enhancement of activity within and/or between the dACC and striatum particularly among those smokers who are more severely addicted.
Box 4. Glutamatergic drugs and smoking cessation.
Moving beyond nAChR agonists, the impact of N-Acetylcysteine (NAC; a glutamatergic agent that reduces abuse-related dysfunction of the glial glutamate transporter [GLT-1]), on striatal rsFC has been evaluated among abstinent smokers [90]. This study observed that NAC administration (vs. placebo) decreased smoking behaviors and increased rsFC between the striatum and medial PFC regions [90]. By restoring GLT-1 functioning in the striatum and decreasing synaptic glutamate levels, NAC is thought to reduce intrusive thoughts, in this instance, presumably related to cigarettes [91]. However, mixed findings have been reported regarding the impact of NAC on smoking behaviors [92–94]. While evidence suggests that NAC can reduce the number of cigarettes smoked per day, this drug does not appear to alleviate withdrawal symptoms [92, 93] like other ‘frontline’ treatments do (i.e., varenicline and nicotine). According to our hypothesis, NAC may target a different facet of nicotine abuse (e.g., linked to the striatum) and is perhaps best viewed as a ‘second-line’ or combination intervention providing greatest benefit only after withdrawal symptoms (e.g., linked to the insula) have been managed.
CONCLUDING REMARKS
The studies above highlight the utility of considering a brain systems-level perspective to provide enhanced insight into the neurobiology of a multifaceted psychiatric disorder. With addiction treatment success rates barely improving and the percent of the US population that continue to smoke, it is a public health imperative to develop more efficacious smoking cessation interventions (Box 5). The addiction field in general and smoking in particular, has lagged behind peripheral organ disease medicine in the development of biologically based disease markers, which has greatly advanced therapeutic development and subsequent treatment success in those organ system pathologies. Greater appreciation of a brain systems-level perspective via neuroimaging, coupled with ‘big data’ computational advances such as machine learning, are expected to ‘bend the curve’ in treatment outcomes. It is intriguing to speculate that should brain based biomarkers be developed that can quantify aspects of TUD and predict treatment outcome, it may also be possible to develop peripheral biomarkers (e.g., stress hormone or cytokine levels relating to insula function) that could be more easily measured in a physician’s office. Such a measure would greatly aid ‘point of care’ assessment and potentially identify those who require additional intervention prior to a lapse or relapse event. One important caveat to this prediction and a major limitation to virtually all studies summarized, is that of ecological validity and subsequent predictability based on the extant literature. Smoking (and other SUDs) does not exist as an independent disease entity, but is most often found comorbid with other neuropsychiatric diseases. Moreover, individuals with TUD also use and/or abuse other drugs (see also: Outstanding Questions). Yet, most research studies employ cohorts based on relatively unique disease presentation (e.g. ‘pure’ smokers). Whether these data will generalize to the general smoker population or represent aspects of brain function common across SUDs are important and yet open questions for future research.
Box 5. CLINICIAN’S CORNER.
Whereas 68% of smokers want to quit and 55% try to do so within a given year, only ~7% achieve sustained cessation [95]. This disparity between the desire to stop smoking and actual quit rates highlights a need for novel approaches to expedite the evolution of cessation interventions.
More severe withdrawal symptoms and higher levels of addiction represent major barriers to quitting. Recent human brain imaging research has provided enhanced insight into precise brain circuits that may contribute to such withdrawal- and addiction-related barriers to cessation.
Brain circuits centered primarily on the insula, a brain region that if damaged leads to greater rates of smoking cessation, appear to be critically involved with nicotine withdrawal-related processes perpetuating smoking.
Simultaneously, brain circuits centered primarily on the striatum, a brain region contributing to the processing of reinforcing stimuli including those associated with drugs of abuse, appear to be critically involved with addiction-related processes.
These dissociable brain circuitries linked with distinct barriers to quitting and likely impacted by distinct pharmacotherapies warrants consideration of multi-therapy as opposed to mono-therapy approaches in practice to improve cessation rates.
Highlights.
One reason for low smoking quit rates is a still limited appreciation of the neurocircuitry underlying distinct aspects of tobacco use disorder (TUD). Neuroimaging is providing enhanced insight into the multifaceted nature of TUD by considering activity within and interactions between brain regions.
We highlight emerging distinctions between neurocircuitry underlying 2 facets of the TUD, namely those linked to state-like pharmacological factors (withdrawal-related) and to trait-like addiction severity factors (addiction-related).
An emerging concept is that insula-centric neurocircuit dynamics may represent a state-like biomarker of acute withdrawal and that dynamics between the anterior cingulate cortex and striatum represent a trait-like biomarker of chronic addiction severity.
Pharmacologically targeting both neurocircuits simultaneously may improve outcomes.
Acknowledgments
MTS is supported by grants from the National Institutes of Health (NIDA: K01DA037819, R01DA041353 and NIMHD: U54MD012393, sub-project 5378). EAS is supported by the NIDA Intramural Research Program of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
GLOSSARY
- Anterior Cingulate Cortex (ACC)
mammalian brain region located in the middle frontal lobe surrounding the corpus callosum; associated with several mental operations often implicated in SUDs including executive functions, emotion regulation, reward processing, error detection, and decision-making
- Default Mode Network (DMN)
large-scale brain network that is most active when an individual is not focused on any external task, but rather, during internally focused thought (e.g., day dreaming and mind wandering) (Box 3)
- Endophenotype
(or intermediate phenotype); quantifiable biological marker present most often among individuals with a disease than in healthy individuals. Identification of endophenotypes represents one strategy to link genes to behavior and involves characterizing brain systems impacted by risk gene variants and, in turn, can infer how brain function relates to complex aspects of behaviors implicated in psychiatric disease
- Executive Control Network (ECN)
large-scale brain network most active during externally oriented, attentionally demanding cognitive tasks (e.g., working memory); generally anti-correlated with regions of the DMN (Box 3).
- Fagerström Test of Nicotine Dependence (FTND)
6 question self-report instrument used to quantify the level of nicotine addiction. Scores from 0-4 suggest very low to low levels of addiction severity, and scores from 6-10 indicate high to very high addiction severity
- Functional Connectivity Strength (FCS)
data driven, graph theory metric applied to resting state fMRI data. It is calculated as the correlation coefficient of every voxel against all other brain voxels and is thought to index the intrinsic functional organization of the brain
- Insula
mammalian brain region located within the lateral sulcus, the fissure dividing the temporal, parietal, and frontal lobes. The insula is associated with several mental operations often implicated in SUDs, including emotional processing, self-awareness, error detection, drug craving, and homeostatic regulation
- Interoceptive signals
information from the peripheral nervous system regarding the internal physiological state of the body
- Homeostatic disequilibrium
physiological body state that motivates an organism to execute behaviors alleviating such a state and facilitating a return to a homeostatic set point. Examples may include the subjective experience of thirst, hunger, and drug use urges
- Large-Scale Brain Network
group of spatially distinct, interacting brain regions that display highly correlated temporal signal fluctuations. They are generally identified using resting state (task independent) fMRI data and an analytical approach such as group Independent Component Analysis (gICA) or seed-based rsFC analyses
- Monetary Incentive Delay (MID) task
neuroimaging paradigm to assess the neurobiological mechanisms of reward anticipation and/or reward receipt using a series of symbols presented serially indicating the probability of winning or losing (valence) various amounts (magnitude) of money if a button is pressed within a given time window
- Neurocircuit
two or more brain regions that appear functionally related as identified through an analysis strategy
- Probabilistic Reversal Learning (PRL) task
a neuroimaging paradigm to assess the neurobiological mechanisms of cognitive flexibility and behavioral inhibition wherein subjects must learn an association rule between two, generally abstract stimuli. The rule contingencies change in a probabilistic (rather than deterministic) fashion during the task unbeknownst to the subject
- Resting-state functional connectivity (rsFC)
evaluates the interaction between two or more brain regions. It is generally assessed during the ‘resting’ (i.e. non-task) condition and measured as the correlation coefficient of the low frequency (<0.1 Hz) spontaneous fluctuations in the fMRI (blood-oxygenation level dependent [BOLD]) signal. The term functional coupling is used herein as a synonym
- Salience Network (SN)
large-scale brain network thought to dynamically regulate or switch processing between the DMN and ECN following detection of a physiologically relevant stimuli or conditions to best process externally vs. internally oriented cognition (Box 3)
- Striatum
group of mammalian subcortical brain structures including the caudate, putamen, and nucleus accumbens. It is associated with several mental operations often implicated in SUDs including motor and action planning, motivation, reinforcement, reward processing, and decision-making
- Systems-level neurobiological approach
understanding the relationship between the activity within and between large-scale brain networks that is quantifiable by non-invasive neuroimaging and mental operations such brain activity may support (e.g., working memory, reward processing, and inhibitory control)
- Tobacco use disorder (TUD)
a Diagnostic and Statistical Manual of Mental Disorders (DSM-5) diagnosis assigned to individuals who are addicted to the drug nicotine due to use of tobacco products. TUD is the most common substance use disorder in the US
- Varenicline
the currently most efficacious pharmacologic smoking cessation aid. A partial agonist at the α4β2 nicotinic receptor subtype that acts as a ‘weak’ agonist in nicotine’s absence and an antagonist in its presence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.U.S. Department of Health and HumanServices. The health consequences of smoking - 50 years of progress: A reports of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Revention and Health Promotion, office on smoking and Health; 2014. [Google Scholar]
- 2.Chiamulera C, et al. Drug discovery for the treatment of substance use disorders: novel targets, repurposing, and the need for new paradigms. Current Opinion in Pharmacology. 2017 doi: 10.1016/j.coph.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 3.McLellan A, et al. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 4.Chandler RK, et al. Treating Drug Abuse and Addiction in the Criminal Justice System: Improving Public Health and Safety. JAMA: the journal of the American Medical Association. 2009;301(2):183–190. doi: 10.1001/jama.2008.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry. 2016;3(8):760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharm. 2010;35(4):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balleine BW, O’Doherty JP. Human and Rodent Homologies in Action Control: Corticostriatal Determinants of Goal-Directed and Habitual Action. Neuropsychopharmacology. 2009;35:48. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabrieli JDE, et al. Prediction as a Humanitarian and Pragmatic Contribution from Human Cognitive Neuroscience. Neuron. 2015;85(1):11–26. doi: 10.1016/j.neuron.2014.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konova AB, et al. Common and distinct neural targets of treatment: changing brain function in substance addiction. Neurosci Biobehav Rev. 2013;37(10 Pt 2):2806–17. doi: 10.1016/j.neubiorev.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Y, et al. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry. 2015;72(6):584–592. doi: 10.1001/jamapsychiatry.2015.1. [DOI] [PubMed] [Google Scholar]
- 12.Adinoff B, et al. Basal Hippocampal Activity and Its Functional Connectivity Predicts Cocaine Relapse. Biological Psychiatry. 2015;78(7):496–504. doi: 10.1016/j.biopsych.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drysdale AT, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nic Tob Res. 2007;9(3):315–27. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- 15.Kassel JD, et al. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- 16.Heishman SJ, et al. Nicotine and smoking: A review of effects on human performance. Exp Clin Psychopharm. 1994;2:345–395. [Google Scholar]
- 17.Baker TB, et al. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Rev. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- 18.Rollema H, et al. Pharmacological profile of the alpha 4 beta 2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 18 b.Pontieri FE, et al. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- 19.Rollema H, et al. Preclinical pharmacology of the alpha 4 beta 2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol. 2009;78(7):813–824. doi: 10.1016/j.bcp.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 20.Patterson F, et al. Varenicline improves mood and cognition during smoking abstinence. Biol Psych. 2009;65(2):144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzales D, et al. Varenicline, an alpha 4 beta 2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation - A randomized controlled trial. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 22.Sutherland MT, et al. Individual differences in amygdala reactivity following nicotinic receptor stimulation in abstinent smokers. Neuroimage. 2013;66:585–593. doi: 10.1016/j.neuroimage.2012.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutherland MT, et al. Insula’s functional connectivity with ventromedial prefrontal cortex mediates the impact of trait alexithymia on state tobacco craving. Psychopharmacol. 2013;228(1):143–55. doi: 10.1007/s00213-013-3018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutherland MT, et al. Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiatry. 2013;74:538–546. doi: 10.1016/j.biopsych.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fedota JR, et al. Reward Anticipation Is Differentially Modulated by Varenicline and Nicotine in Smokers. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carroll AJ, et al. Greater externalizing personality traits predict less error-related insula and anterior cingulate cortex activity in acutely abstinent cigarette smokers. Addict Biol. 2015;20(2):377–89. doi: 10.1111/adb.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lesage E, et al. Neural Signatures of Cognitive Flexibility and Reward Sensitivity Following Nicotinic Receptor Stimulation in Dependent Smokers: A Randomized Trial. JAMA Psychiatry. 2017;74(6):632–640. doi: 10.1001/jamapsychiatry.2017.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nature Neuroscience. 2005;8(11):1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- 29.Naqvi NH, et al. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdolahi A, et al. Damage to the insula leads to decreased nicotine withdrawal during abstinence. Addiction. 2015;110(12):1994–2003. doi: 10.1111/add.13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naqvi NH, Bechara A. The insula and drug addiction: An interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214(5–6):435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutherland MT, et al. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012;62(4):2281–95. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baur V, et al. Resting-state functional and structural connectivity within an insula-amygdala route specifically index state and trait anxiety. Biol Psychiatry. 2013;73(1):85–92. doi: 10.1016/j.biopsych.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Hamm LL, et al. Aberrant amygdala functional connectivity at rest in pediatric anxiety disorders. Biol Mood Anxiety Disord. 2014;4(1):15. doi: 10.1186/s13587-014-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sripada RK, et al. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012;37(4):241–9. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy AK, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2013;52(3):290–299 e2. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Craig AD. How do you feel - now? The anterior insula and human awareness. Nature Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 38.Chase HW, et al. The Neural Basis of Drug Stimulus Processing and Craving: An Activation Likelihood Estimation Meta-Analysis. Biological Psychiatry. 2011;70(8):785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engelmann JM, et al. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60(1):252–62. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutherland MT, et al. Neurobiological impact of nicotinic acetylcholine receptor agonists: an activation likelihood estimation meta-analysis of pharmacologic neuroimaging studies. Biol Psychiatry. 2015;78(10):711–20. doi: 10.1016/j.biopsych.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anticevic A, et al. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16(12):584–92. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu Y, et al. Resting-state glutamate and GABA concentrations predict task-induced deactivation in the default mode network. J Neurosci. 2013;33(47):18566–73. doi: 10.1523/JNEUROSCI.1973-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cog Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Sutherland MT, et al. Beyond functional localization: Advancing the understanding of addiction-related processes by examining brain connectivity. In: Wilson SJ, editor. The Wiley-Blackwell Handbook on the Neuroscience of Addiction. Wiley-Blackwell; 2015. [Google Scholar]
- 46.Fedota JR, Stein EA. Resting-state functional connectivity and nicotine addiction: prospects for biomarker development. Ann N Y Acad Sci. 2015;1349:64–82. doi: 10.1111/nyas.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pariyadath V, et al. Resting state functional connectivity analysis for addiction medicine: From individual loci to complex networks. Prog Brain Res. 2016;224:155–73. doi: 10.1016/bs.pbr.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 48.Lerman C, et al. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. 2014;71(5):523–30. doi: 10.1001/jamapsychiatry.2013.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hong LE, et al. Association of Nicotine Addiction and Nicotine’s Actions With Separate Cingulate Cortex Functional Circuits. Arch Gen Psychiat. 2009;66(4):431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vink JM, et al. Heritability of smoking initiation and nicotine dependence. Behav Genet. 2005;35(4):397–406. doi: 10.1007/s10519-004-1327-8. [DOI] [PubMed] [Google Scholar]
- 51.Bidwell LC, et al. Genome-wide single nucleotide polymorphism heritability of nicotine dependence as a multidimensional phenotype. Psychol Med. 2016;46(10):2059–69. doi: 10.1017/S0033291716000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delgado MR. Reward-related responses in the human striatum. Ann N Y Acad Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- 53.Wise RA. Roles for nigrostriatal–not just mesocorticolimbic–dopamine in reward and addiction. Trends Neurosci. 2009;32(10):517–24. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balodis IM, Potenza MN. Anticipatory reward processing in addicted populations: a focus on the monetary incentive delay task. Biol Psychiatry. 2015;77(5):434–44. doi: 10.1016/j.biopsych.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rose EJ, et al. Acute nicotine differentially impacts anticipatory valence- and magnitude-related striatal activity. Biol Psychiatry. 2013;73(3):280–8. doi: 10.1016/j.biopsych.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sweitzer MM, et al. Dissociated effects of anticipating smoking versus monetary reward in the caudate as a function of smoking abstinence. Biol Psychiatry. 2014;76(9):681–8. doi: 10.1016/j.biopsych.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rose EJ, et al. Chronic exposure to nicotine is associated with reduced reward-related activity in the striatum but not the midbrain. Biol Psychiatry. 2012;71(3):206–13. doi: 10.1016/j.biopsych.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sweitzer MM, et al. Blunted striatal response to monetary reward anticipation during smoking abstinence predicts lapse during a contingency-managed quit attempt. Psychopharmacology (Berl) 2016;233(5):751–60. doi: 10.1007/s00213-015-4152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson SJ, et al. Weak ventral striatal responses to monetary outcomes predict an unwillingness to resist cigarette smoking. Cogn Affect Behav Neurosci. 2014;14(4):1196–207. doi: 10.3758/s13415-014-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters J, et al. Lower ventral striatal activation during reward anticipation in adolescent smokers. Am J Psychiatry. 2011;168(5):540–9. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- 61.Karoly HC, et al. Does incentive-elicited nucleus accumbens activation differ by substance of abuse? An examination with adolescents. Dev Cogn Neurosci. 2015;16:5–15. doi: 10.1016/j.dcn.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Addicott MA, et al. Smoking withdrawal is associated with increases in brain activation during decision making and reward anticipation: a preliminary study. Psychopharmacology (Berl) 2012;219(2):563–73. doi: 10.1007/s00213-011-2404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nestor LJ, et al. Smokers and ex-smokers have shared differences in the neural substrates for potential monetary gains and losses. Addict Biol. 2016 doi: 10.1111/adb.12484. [DOI] [PubMed] [Google Scholar]
- 64.Peechatka AL, Janes AC. Association Between Reward Reactivity and Drug Use Severity is Substance Dependent: Preliminary Evidence From the Human Connectome Project. Nicotine Tob Res. 2017;19(6):710–715. doi: 10.1093/ntr/ntw252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hong LE, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(30):13509–13514. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grucza RA, et al. A risk allele for nicotine dependence in CHRNA5 is a protective allele for cocaine dependence. Biol Psychiatry. 2008;64(11):922–9. doi: 10.1016/j.biopsych.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moran LV, et al. Insular and anterior cingulate circuits in smokers with schizophrenia. Schizophr Res. 2012;142(1–3):223–9. doi: 10.1016/j.schres.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moran LV, et al. Brain circuits that link schizophrenia to high risk of cigarette smoking. Schizophr Bull. 2013;39(6):1373–81. doi: 10.1093/schbul/sbs149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li S, et al. CYP2A6 Genetic Variation Alters Striatal-Cingulate Circuits, Network Hubs, and Executive Processing in Smokers. Biol Psychiatry. 2017;81(7):554–563. doi: 10.1016/j.biopsych.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Loughlin J, et al. Genetically decreased CYP2A6 and the risk of tobacco dependence: a prospective study of novice smokers. Tob Control. 2004;13(4):422–8. doi: 10.1136/tc.2003.007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schoedel KA, et al. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14(9):615–26. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 72.Styn MA, et al. CYP2A6 Genotype and Smoking Behavior in Current Smokers Screened for Lung Cancer. Substance use & misuse. 2013;48(7):490–494. doi: 10.3109/10826084.2013.778280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huettel SA, et al. Functional Magnetic Mesonance Imaging. 2nd. Sinauer Associates, Inc; 2008. [Google Scholar]
- 74.Biswal B, et al. Functional Connectivity in the Motor Cortex of Resting Human Brain Using Echo-Planar Mri. Mag Resonance Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 75.Kelly AMC, et al. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 76.Baldassarre A, et al. Individual variability in functional connectivity predicts performance of a perceptual task. Proc Natl Acad Sci U S A. 2012;109(9):3516–21. doi: 10.1073/pnas.1113148109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gorelick DA, et al. Transcranial magnetic stimulation in the treatment of substance addiction. Ann N Y Acad Sci. 2014;1327:79–93. doi: 10.1111/nyas.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dunlop K, et al. Noninvasive brain stimulation treatments for addiction and major depression. Ann N Y Acad Sci. 2017;1394(1):31–54. doi: 10.1111/nyas.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diana M, et al. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nature Reviews Neuroscience. 2017;18:685. doi: 10.1038/nrn.2017.113. [DOI] [PubMed] [Google Scholar]
- 80.Dinur-Klein L, et al. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol Psychiatry. 2014;76(9):742–9. doi: 10.1016/j.biopsych.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 81.Pripfl J, et al. Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power. Brain Stimul. 2014;7(2):226–33. doi: 10.1016/j.brs.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 82.Hanlon CA, et al. Individual variability in the locus of prefrontal craving for nicotine: implications for brain stimulation studies and treatments. Drug Alcohol Depend. 2012;125(3):239–43. doi: 10.1016/j.drugalcdep.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fox MD, et al. Efficacy of Transcranial Magnetic Stimulation Targets for Depression Is Related to Intrinsic Functional Connectivity with the Subgenual Cingulate. Biological Psychiatry. 2012;72(7):595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hanlon CA, et al. Chapter Six - Biomarkers for Success: Using Neuroimaging to Predict Relapse and Develop Brain Stimulation Treatments for Cocaine-Dependent Individuals. In: Zahr NM, Peterson ET, editors. International Review of Neurobiology. Academic Press; 2016. pp. 125–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. PNAS. 2005;102(27):9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sridharan D, et al. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. PNAS. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hahn B, et al. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci. 2007;27(13):3477–89. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Loughead J, et al. Working Memory-Related Neural Activity Predicts Future Smoking Relapse. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2014.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weissman DH, et al. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971–8. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 90.Froeliger B, et al. The effects of N-Acetylcysteine on frontostriatal resting-state functional connectivity, withdrawal symptoms and smoking abstinence: A double-blind, placebo-controlled fMRI pilot study. Drug Alcohol Depend. 2015;156:234–42. doi: 10.1016/j.drugalcdep.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kalivas BC, Kalivas PW. Corticostriatal circuitry in regulating diseases characterized by intrusive thinking. Dialogues Clin Neurosci. 2016;18(1):65–76. doi: 10.31887/DCNS.2016.18.1/pkalivas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmaal L, et al. Efficacy of N-acetylcysteine in the treatment of nicotine dependence: a double-blind placebo-controlled pilot study. Eur Addict Res. 2011;17(4):211–6. doi: 10.1159/000327682. [DOI] [PubMed] [Google Scholar]
- 93.Knackstedt LA, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65(10):841–5. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prado E, et al. N-acetylcysteine for therapy-resistant tobacco use disorder: a pilot study. Redox Rep. 2015;20(5):215–22. doi: 10.1179/1351000215Y.0000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.CDC. Quitting smoking among adults: United States, 2000–2015. Morbidity and Mortality Weekly Report. 2017;65(52):1457–1464. doi: 10.15585/mmwr.mm6552a1. [DOI] [PubMed] [Google Scholar]


