Abstract
High dietary sodium intake can lead to hypertension and increased incidence of cardiovascular disease. We sought to determine the effect of short-term dietary sodium loading on central blood pressure and arterial stiffness in young (YG; 22–40 years) and middle-aged (MA; 41–60 years) normotensive adults. YG (n=49; age: 27±1 yrs) and MA (n=36; age: 52±1 yrs) subjects were randomized, in a cross-over design, to 7 days of low sodium (20mmol/day) or high sodium (300mmol/day) diet. On the last day of each diet, central pressures, forward and reflected wave amplitudes (via radial artery applanation tonometry) and carotid-femoral pulse wave velocity (CF-PWV) were assessed. Central systolic blood pressure (cSBP) was greater after HS in both YG (LS: 96±1 vs. HS: 99±1mmHg; p=0.012) and MA (LS: 106±2 vs. HS: 115±3mmHg; p<0.001). However, the increase in cSBP was greater in MA (YG: 4±1 vs. MA: 9±2; p=0.02). In MA subjects, HS elicited greater forward (LS: 25±1 vs. HS: 29±1mmHg; p<0.001) and reflected (LS: 19±1 vs. HS: 23±1mmHg; p<0.001) wave amplitudes. CF-PWV was also greater in MA on HS but after adjustment for MAP the difference was no longer significant. Our data indicate that HS intake leads to a greater increase in cSBP in MA adults, which may be the result of increased forward and reflected wave amplitudes.
Keywords: diet, wave reflections, central pressure
INTRODUCTION
Cardiovascular disease (CVD) continues to be the leading cause of death in the United States [1], therefore understanding factors that may predispose individuals to CVD remains important. The mechanical load imposed by the systemic circulation to the left ventricle is a significant determinant of cardiovascular function [2]. Left ventricular afterload, which is the impedance to flow from the left ventricle, is largely determined by properties of the arterial tree [3]. The central aortic pressure waveform is determined by the interaction between the left ventricle and load imposed by the arterial tree [3] thus assessing aortic waveforms can provide valuable information on central hemodynamics and cardiovascular function.
A central aortic pressure wave is composed of a forward traveling wave generated by left ventricular ejection and a later-arriving reflected wave from the periphery [4]. As arterial stiffness increases, elevations in central systolic blood pressure (cSBP) can occur due to increased forward and reflected wave amplitudes and earlier return of the reflected wave to the proximal aorta. Elevated cSBP can lead to increased left ventricular workload, left ventricular hypertrophy, and decreased diastolic perfusion pressure resulting in reduced coronary blood flow [5]. Previous studies have demonstrated aortic stiffness to be predictive of cardiovascular events and/or poor clinical outcomes in hypertension [6], chronic kidney disease [7–9] and heart failure [10]. Meta-analyses have shown aortic pulse wave velocity to be a strong predictor of future cardiovascular events and all-cause mortality [11] and may improve prediction when added to standard risk factors [12].
Previous work has demonstrated adverse effects of increased dietary sodium intake on cardiovascular health [13–15]. In a study of normotensive, healthy, young men, high sodium intake was associated increased cSBP, arterial stiffness, and reflected wave amplitude [16]. Additional studies have found arterial stiffness to be reduced with lower sodium intake in hypertensive individuals [17] and when compared to age and blood pressure matched individuals on a normal sodium diet [13].
The aim of this study was to determine the impact of short-term dietary sodium loading on central blood pressure and its components, forward and reflected wave amplitude, in young and middle-aged, normotensive adults. We hypothesized that sodium loading would elicit increases in central systolic pressure and reflected wave amplitude.
METHODS
This was a controlled feeding study in which 85 normotensive adults were randomized, in a cross-over design, to a low-sodium (LS; 20 mmol sodium/day) and a high-sodium (HS; 300 mmol sodium/day) diet.
Subjects
Study participants were apparently healthy, normotensive adults. Subjects were divided into two age groups: 49 Young (YG), defined as 22–40 years of age, and 36 Middle-Aged (MA), defined as 41–60 years of age. Informed consent was obtained from all subjects, and the study protocol and procedures were approved by the Institutional Review Board of the University of Delaware and conform to the provisions of the Declaration of Helsinki.
Subjects reported to the laboratory following a 12-hour fast. Additionally, subjects were instructed to abstain from caffeine, alcohol, and exercise during the 12 hours prior to testing. A complete medical history was provided by each subject. A resting 12-lead electrocardiogram (ECG), resting blood pressure, height and weight, and a venous blood sample were collected. Exclusion criteria included a history of hypertension, cardiovascular disease, malignancy, diabetes mellitus, and renal impairment. Subjects were also excluded if they were obese [body mass index (BMI) ≥ 30 kg/m2] or used tobacco products.
Dietary Sodium Perturbation
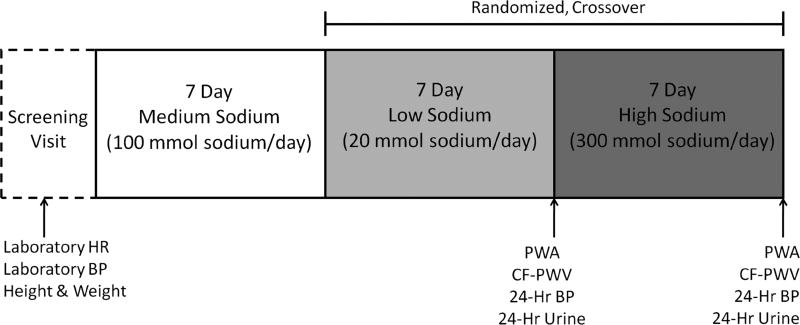
This was a controlled feeding study in which all food was prepared by a registered dietitian. Subjects consumed a 7-day run-in diet (100 mmol sodium/day), and were then randomized to a 7-day low sodium (LS) diet (20 mmol sodium/day) and 7-day high sodium (HS) diet (300 mmol sodium/day) (Figure 1). The diet was designed to be eucaloric and the percentage of carbohydrate, fat, and protein in the diet were 50%, 30%, and 20%, respectively and remained constant across sodium conditions. Fluid intake was monitored and recorded daily. Subjects were also instructed to maintain their typical activity levels throughout the duration of the study.
Figure 1.
Schematic representation of protocol. BP, Blood Pressure; CF-PWV, Carotid-Femoral Pulse Wave Velocity; HR, Heart Rate; PWA, Pulse Wave Analysis.
Twenty-four hour urine and BP collections
On the last day of the LS and HS diets, subjects collected their urine for twenty-four hours using a cool, dark container. Urine volume, collection time, electrolytes (Easy Electrolyte Analyzer; Medica, Bedford, MA), and osmolality (3D3 Osmometer; Advanced Instruments, Norwood, MA) were measured. Sodium excretion was determined. An ambulatory blood pressure monitor (Spacelabs Medical, Issaquah, WA) was worn over the non-dominant arm for the same twenty-four hour period. Blood pressure was measured every 20 minutes while the subject was awake and every 30 minutes while the subject was asleep.
Pulse wave analysis (PWA) and Pulse wave velocity (CF-PWV)
Central aortic pressure and carotid-femoral pulse wave velocity (CF-PWV) were assessed at the end of each diet phase using a SphygmoCor PVx system (AtCor Medical, SphygmoCor CvMS V9). Pressure waveforms were recorded at the radial artery via applanation tonometry using a high-fidelity strain-gauge transducer (Millar Instruments). The radial waveforms were calibrated from brachial systolic and diastolic pressures measured by an automated oscillometric sphygmomanometer (Dash 2000, GE Medical Systems). A central aortic pressure waveform was synthesized using a generalized transfer function. Central pressures and augmentation index (AIx) were derived from this method. AIx is defined as the ratio of augmented pressure to pulse pressure and is expressed as a percentage. Forward and reflected wave components were determined by wave separation analysis, performed by the SphygmoCor software (version 9), using a modified triangular flow wave [18]. Only waveforms of high-quality, defined as an operator’s index of ≥ 80, were selected for analysis.
Carotid-femoral PWV was determined by recording carotid and femoral waveforms using a high-fidelity strain gauge transducer (Millar Instruments) sequentially along with an ECG for reference and pulse transit time was determined by the time delay between the feet of carotid and femoral waveforms. The distance between the measurement sites was determined by subtracting the distance between the carotid recording site and the suprasternal notch, from the distance between the femoral recording site and the suprasternal notch [19]. CF-PWV was calculated by dividing the distance between the two recording sites (aortic distance) by the pulse transit time (PWV = Distance/Transit Time).
Statistical Analysis
Baseline characteristics were compared using a two-tailed, unpaired t-test. All other outcome variables were analyzed using 2×2 repeated measures ANOVA (age group × diet) to assess main effects of age and diet as well as age × diet interaction. Tukey's post-hoc testing was performed when appropriate. CF-PWV data were also analyzed with mean arterial pressure (MAP) as a covariate due to the known effect of blood pressure on arterial stiffness. The magnitude of change in cSBP from low to high salt was compared between age groups using an unpaired t-test. Exploratory analyses were also performed to examine potential sex differences. Statistical significance was defined as a 2-tailed p < 0.05. Data are presented as mean ± SEM.
RESULTS
Baseline subject characteristics for all eighty-five subjects are presented in Table 1. No differences were observed between the age groups (MA vs. YG) except for a higher BMI in the MA group (p < 0.05). All subjects completed the run in-diet followed by the two-week dietary salt perturbation.
Table 1.
Baseline Subject Characteristics
| Characteristic | Young | Middle-Aged |
|---|---|---|
| N | 49 | 36 |
| Males/Females | 30/19 | 13/23 |
| Age (yr) | 27 ± 1 | 52 ± 1# |
| Mass (kg) | 71.6 ± 1.5 | 73.0 ± 1.9 |
| Height (cm) | 173.8 ± 1.2 | 170.4 ± 1.3 |
| Body Mass Index (kg/m2) | 23.6 ± 0.4 | 25.1 ± 0.5# |
Values are mean ± SEM.
p < 0.05 vs Young.
Dietary Salt Perturbation
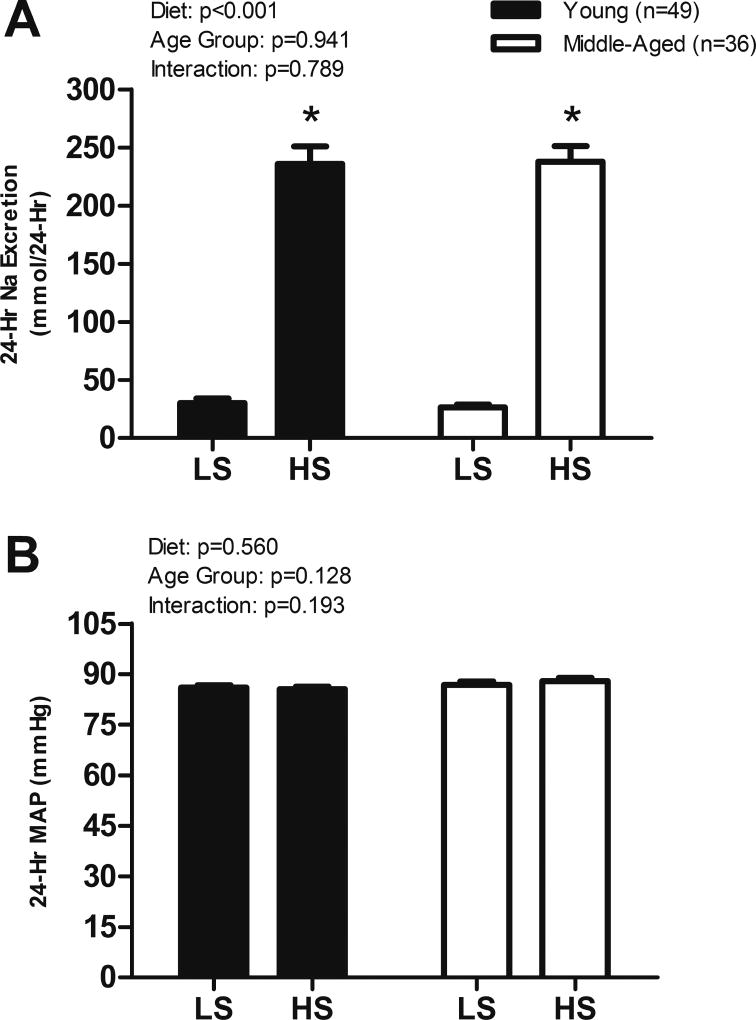
As expected, 24-hour sodium excretion was significantly greater on the HS diet compared to LS in both age groups (Figure 2A). However, no difference was observed in 24-hour MAP between the two dietary conditions in either age group (Figure 2B). Significant increases in brachial blood pressure (average of 3 measurements in laboratory) were observed for SBP, DBP, and MAP in the YG group and SBP, MAP, and PP in the MA group on the HS diet (Table 2).
Figure 2.
(A) 24-hour sodium (Na) excretion and (B) 24-hour mean arterial pressure (MAP) on LS and HS diets. Values are mean ± SEM. HS, High Sodium; LS, Low Sodium. *p < 0.05 vs LS.
Table 2.
Hemodynamic Measures
| Young | Middle-Aged | |||
|---|---|---|---|---|
| LS | HS | LS | HS | |
| Brachial SBP (mmHg) | 114 ± 2 | 117 ± 2* | 114 ± 2 | 122 ± 2*# |
| Brachial DBP (mmHg) | 64 ± 1 | 67 ± 1* | 69 ± 1# | 70 ± 2 |
| Brachial MAP (mmHg) | 79 ± 1 | 82 ± 1* | 86 ± 2# | 90 ± 2*# |
| Brachial PP (mmHg) | 50 ± 1 | 50 ± 1 | 46 ± 2 | 53 ± 2* |
| Central SBP (mmHg) | 96 ± 1 | 99 ± 1* | 106 ± 2# | 115 ± 3*# |
| Central PP (mmHg) | 31 ± 1 | 32 ± 1 | 37 ± 2# | 44 ± 2*# |
| HR (bpm) | 60 ± 1 | 57 ± 1* | 59 ± 2 | 55 ± 2* |
| CF-PWV (m.s−1) | 6.1 ± 0.2 | 5.9 ± 0.2 | 7.1 ± 0.3# | 7.7 ± 0.5*# |
| Augmentation Index (%) | 3.6 ± 1.7 | 5.5 ± 1.8* | 28.4 ± 1.6# | 31.1 ± 1.5*# |
Values are mean ± SEM. CF-PWV, Carotid-Femoral Pulse Wave Velocity; DBP, Diastolic Blood Pressure; HS, High Sodium; LS, Low Sodium; MAP, Mean Arterial Pressure; PP, Pulse Pressure; SBP, Systolic Blood Pressure.
p < 0.05 vs LS.
p < 0.05 vs Young.
PWV not different between diets when corrected for MAP. Augmentation Index not different between diets when corrected for HR.
Central Pressure Measures
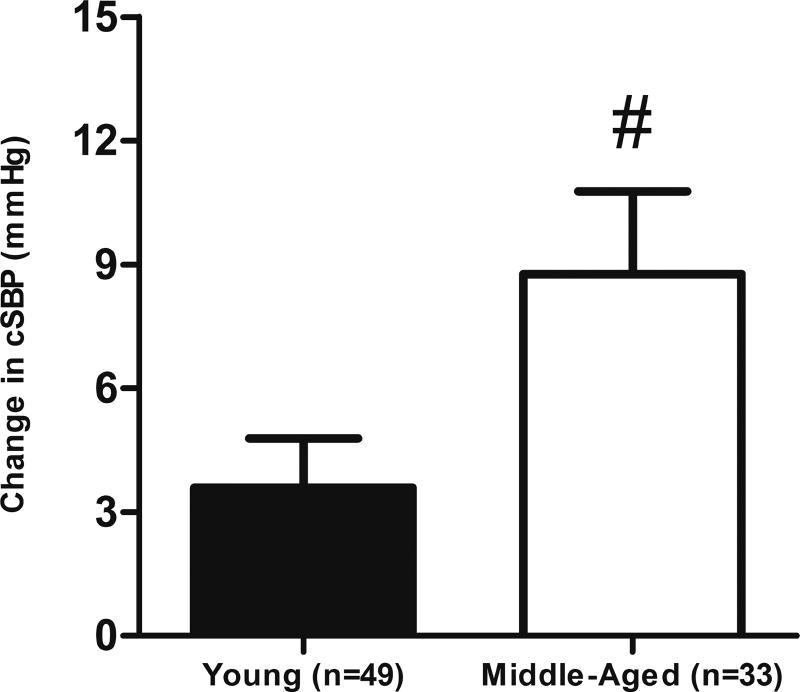
cSBP was significantly greater on the HS diet in both YG (LS: 96 ± 1 vs. HS: 99 ± 1 mmHg, p = 0.012) and MA groups (LS: 106 ± 2 vs. HS: 115 ± 3 mmHg, p < 0.001). A greater increase in cSBP was observed in the MA group compared to the YG group (YG: 4 ± 1 vs. MA: 9 ± 2 mmHg, p = 0.02, Figure 3). Additionally, the HS diet resulted in a greater central PP in only the MA group (LS: 37 ± 2 vs. HS: 44 ± 2 mmHg, p < 0.001).
Figure 3.
Change in central systolic pressure in all subjects. Values are mean ± SEM. cSBP, central systolic pressure; HS, High Sodium; LS, Low Sodium. #p < 0.05 vs Young.
cSBP was greater on high salt in women (W, LS: 99 ± 2 vs. HS: 108 ± 3 mmHg; p < 0.001) and approached significance in men (M, LS: 101 ± 2 vs. HS: 104 ± 2 mmHg; p = 0.06). The change in cSBP in response to the HS diet was greater in women compared to men (8.4 ± 1.5 vs. 3.3 ± 1.7 mmHg; p = 0.027) however there was no effect of age (p=0.105) or a sex by age interaction (p=0.5).
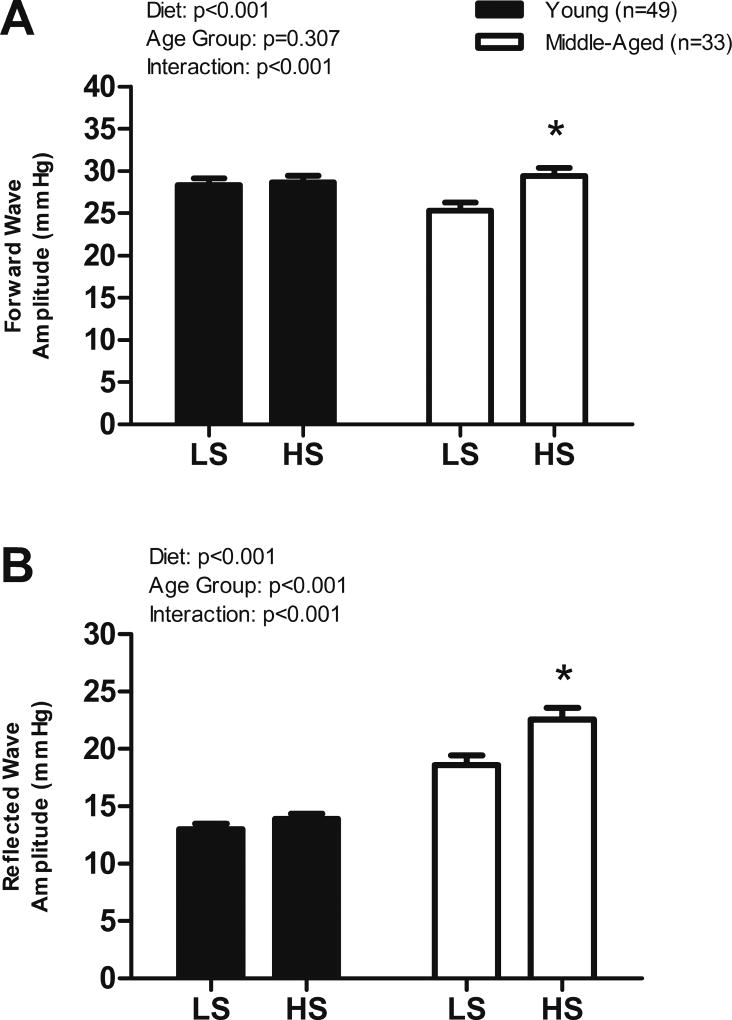
Forward and Reflected Wave Amplitudes and Arterial Stiffness
The HS diet elicited greater forward (LS: 25 ± 1 vs. HS: 29 ± 1 mmHg, p < 0.001, Figure 4A) and reflected (LS: 19 ± 1 vs. HS: 23 ± 1 mmHg, p < 0.001, Figure 4B) wave amplitudes in the MA group but no differences were observed in the YG group. Forward pressure wave amplitude was greater in men compared to women on both diets (p<0.05) but was increased by HS only in the women (W, LS: 24.5 ± 0.7 vs. HS: 27.5 ± 0.9, p<0.001; M, LS: 29.7 ± 0.8 vs HS: 30.4 ± 0.8 mmHg, p=0.37). The forward pressure wave was greater on HS in MA women but not YG women (MA, LS: 24.4 ± 1.2 vs. HS: 29 ± 1.3, p<0.001; YG, LS: 24.6 ± 0.8 vs. HS: 25.8 ± 1.1 mmHg, p=0.171). Reflected pressure wave amplitude was greater in both men and women on the HS diet (W, LS: 16.2 ± 0.8 vs. HS: 19.1 ± 1.1, p<0.001; M, LS: 14.3 ± 0.6 vs HS: 15.7 ± 0.7, p=0.017) and was greater in women compared to men on the HS diet (p = 0.006). The reflected wave amplitude was greater on the HS diet in MA women but not YG women (MA, LS: 19.4 ± 1.0 vs. HS: 23.7± 1.2, p<0.001; YG, LS: 12.5 ± 0.7 vs. HS: 13.8 ± 0.9, p=0.125). Likewise, the reflected wave amplitude was greater on the high salt diet in MA men but not YG men (MA, LS: 17 ± 1.5 vs. HS: 20.4 ± 1.8, p=0.001; YG, LS: 13.4 ± 0.6 vs. HS: 14 ± 0.5, p=0.277).
Figure 4.
(A) Central forward and (B) central reflected wave amplitude on LS and HS diets in all subjects. Values are mean ± SEM. HS, High Sodium; LS, Low Sodium. *p < 0.05 vs LS.
CF-PWV was also greater following the HS diet in the MA group (LS: 7.1 ± 0.3 vs HS: 7.7 ± 0.5 ms, p = 0.009). However, when CF-PWV was corrected for MAP (24 hour and laboratory) the difference was no longer present indicating the changes in CF-PWV were due to changes in MAP. AIx was significantly increased in both YG (LS: 3.6 ± 1.7 vs HS: 5.5 ± 1.8%, p = 0.01) and MA (LS: 28.4 ± 1.6 vs HS: 31.1 ± 1.5%, p = 0.01) on HS. These diet differences were no longer present when AIx was corrected for HR indicating an influence of HR on AIx.
DISCUSSION
The novel findings of the present study were that a 7-day HS diet resulted in increases in cSBP in both YG and MA adults when compared to a LS diet. Additionally, the increase observed in cSBP was larger in the MA group and was accompanied by increases in both forward and reflected wave amplitudes. These findings provide evidence that cSBP can be impacted by increased levels of sodium consumption, particularly in middle age adults.
The negative effects of increased sodium intake on cardiovascular health in humans has been well documented [13–15, 20]. As CVD disease continues to be the leading cause of death in the US [1], it is important to identify measures that can provide information about an individual's risk for future cardiovascular events and if lifestyle choices impact these measures. Evidence from previous studies on multiple populations suggest that central pressure is a better predictor of cardiovascular events than brachial pressure [21–23]. The heart, kidneys, and major arteries supplying the brain are exposed to central rather than brachial pressure [24]. When referring to the heart specifically, increased cSBP can result in increased left ventricular workload and left ventricular hypertrophy [5], which could contribute to future cardiovascular events.
The increase in cSBP observed in our YG group is in agreement with Starmans-Kool et al. [16], who also reported an increase in cSBP in normotensive, healthy young men after a high sodium diet. The present study also reports data on a MA group, providing information on a larger age range. While both groups exhibited increases in cSBP, the increase was significantly greater in the MA group. The larger increase in the MA group suggests a potential link between dietary salt and increased risk for future cardiovascular events in middle age.
A central aortic pressure wave is comprised of two separate components, a forward traveling wave from the heart and a reflected wave from sites of impedance mismatch in the periphery [4]. Augmentation index (AIx), a surrogate measure of wave reflection, has been widely used but can be influenced by wave reflection timing, heart rate, body height, and other factors [25–28]. Wave separation analysis allows for evaluation of the forward and reflected waves individually. The reflected wave can influence both central pressure and flow waveforms [29]. In young individuals, reflected waves arising from multiple points in the arterial tree predominantly enhance diastolic perfusion pressure in the coronary circulation [28]. The net effect of these reflected waves on systole versus diastole is determined by the pulse wave velocity of the aorta and its interaction with muscular arterial segments. When reflected waves arrive during systole, they can augment pressure and impact left ventricular afterload [30]. Furthermore, wave reflections have been found to be predictive of cardiovascular events in hypertensive individuals [31] and of incident heart failure in an adult, multiethnic population free of cardiovascular disease [30]. The forward wave amplitude has been shown to be an important predictor of incident cardiovascular disease and has been proposed to be due to a mismatch between aortic diameter and pulsatile flow [32]. However, Phan et al. [33] more recently demonstrated that forward wave amplitude is also strongly influenced by wave reflections that are re-reflected/rectified at the heart, highlighting the importance of assessing both forward and reflected wave amplitudes.
In the present study, significant increases in both the forward and reflected wave amplitudes following the HS diet were found only in the MA group. To our knowledge, this is the first study to demonstrate the negative impact of a HS diet on forward and reflected wave amplitudes in MA adults. It appears the increases in forward and reflected wave amplitudes are contributing to the larger augmentation of cSBP observed in the MA group. It should be noted that Starmans-Kool et al. [16] reported increased reflected wave amplitudes following a high sodium diet in young, healthy males. We have also demonstrated that the CSBP response to dietary sodium appears to be greater in women. More work is needed to fully elucidate the potential sex differences in the aging response to dietary sodium.
The increases in reflected wave amplitude demonstrated in the MA group are of clinical importance as this can have adverse consequences on the heart. As age increases, wave reflection returns to the aorta during mid-to-late systole resulting in increased left ventricular afterload [2]. Increases in the wave amplitude can further contribute to this augmentation. This increased wave reflection can have deleterious effects on left ventricular structure and function [34]. Specifically, left ventricular hypertrophy and fibrosis have been linked to this increased systolic load [35].
The mechanism(s) responsible for the increases in cSBP and wave amplitudes seen in this study are yet to be defined. Rodent [36, 37] and human [38, 39] studies have indicated that increased reactive oxygen species (ROS) leading to reduced nitric oxide (NO) bioavailability can be attributed to sodium loading. Impaired NO release could result in increased wave reflection due to its action as a regulator of arterial diameter [40] as high dietary sodium has been shown to impair both conduit artery and microvascular function [38, 41–43]. Further investigation into the specific mechanisms is warranted.
The results of our study should be interpreted in the context of its strengths and limitations. Our assessment of forward and reflected wave amplitude was based on pressure measurements. Future studies with both pressure and flow measurements will allow us to better assess the impact of dietary salt on forward and backward pressure, as well as specific determinants of arterial hemodynamics such as aortic characteristic impedance, stroke volume and systemic vascular resistance. In addition, our sample consisted of normotensive young and middle-aged adults and may not be generalizable to older or hypertensive adults. The effects of salt on central hemodynamics are likely to be greater in hypertensive subjects (a population with a high proportion of salt-sensitive individuals), and this should be the focus of future studies. Lastly, the sex comparisons made in the present study should be interpreted with caution give the smaller sample sizes when making these comparisons.
In conclusion, data from the current study provide new information regarding the effects of dietary salt on central blood pressure. We demonstrated that a HS diet leads to increases in cSBP in both YG and MA adults. The increase in cSBP was larger in the MA group which may be attributed to increases in forward and reflected wave amplitudes. Taken together, these new findings provide additional evidence that high dietary salt intake negatively impacts cardiovascular health.
Highlights.
High dietary sodium intake augments central systolic blood pressure.
The increase in central systolic BP in response to sodium is larger in middle-aged adults.
Greater forward and reflected wave amplitudes may contribute to the larger increase central systolic blood pressure.
Acknowledgments
We would like to thank the Eugene DuPont Preventative Medicine and Rehabilitation Institute for preparing the diets used in our study as well as the research participants. This work was funded by National Institute of Health Grant R01 HL104106.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Authors have no conflict of interest to disclose.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackley RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu J, Alger HM, Wong SS, Muntner P. Heart disease and stroke Statistics—2017 update: A report from the american heart association. Circulation. 2017:1350. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: Part 2: Arterial pressure-flow and pressure-volume relations in humans. Hypertension. 2010;56(4):563–70. doi: 10.1161/HYPERTENSIONAHA.110.157339. [DOI] [PubMed] [Google Scholar]
- 3.Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. American Journal of Hypertension. 2005;18(1):3–10. doi: 10.1016/j.amjhyper.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 4.O'Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. American Journal of Hypertension. 2002;15(5):426–44. doi: 10.1016/s0895-7061(01)02319-6. [DOI] [PubMed] [Google Scholar]
- 5.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(5):932–43. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 6.Laurent S, Katsahian S, Fassot C, Tropeano A, Gautier I, Laloux B, Boutouyrie P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34(5):1203–6. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 7.Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney International. 2003;63(5):1852–60. doi: 10.1046/j.1523-1755.2003.00932.x. [DOI] [PubMed] [Google Scholar]
- 8.Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Carotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal disease. Hypertension. 1998;32(3):570–4. doi: 10.1161/01.HYP.32.3.570. [DOI] [PubMed] [Google Scholar]
- 9.Chirinos JA, Khan A, Bansal N, Dries DL, Feldman HI, Ford V, Anderson AH, Kallem R, Lash JP, Ojo A, Schreiber M, Sheridan A, Strelsin J, Teal V, Go AS, Townsend RR. Arterial stiffness, central pressures and incident hospitalized heart failure in the chronic renal insufficiency cohort (CRIC) study. Circulation: Heart Failure. 2014;7(5):709–16. doi: 10.1161/CIRCHEARTFAILURE.113.001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonapace S, Rossi A, Cicoira M, Targher G, Valbusa F, Benetos A, Vassanelli C. Increased aortic pulse wave velocity as measured by echocardiography is strongly associated with poor prognosis in patients with heart failure. Journal of the American Society of Echocardiography : Official Publication of the American Society of Echocardiography. 2013;26(7):714–20. doi: 10.1016/j.echo.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. Journal of the American College of Cardiology. 2010;55(13):1318–27. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen C, Cruickshank JK, Hwang S, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang K, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. Journal of the American College of Cardiology. 2014;63(7):636–46. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He J, Ogden LG, Vupputuri S, Bazzano LA, Loria C, Whelton PK. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. Jama. 1999;282(21):2027–34. doi: 10.1001/jama.282.21.2027. [DOI] [PubMed] [Google Scholar]
- 14.Strazzullo P, D'Elia L, Kandala N, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. British Medical Journal. 2009:339b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuomilehto J, Jousilahti P, Rastenyte D, Moltchanov V, Tanskanen A, Pietinen P, Nissinen A. Urinary sodium excretion and cardiovascular mortality in finland: A prospective study. Lancet. 2001;357(9259):848–51. doi: 10.1016/S0140-6736(00)04199-4. [DOI] [PubMed] [Google Scholar]
- 16.Starmans-Kool MJ, Stanton AV, Xu YY, McG Thom SA, Parker KH, Hughes AD. High dietary salt intake increases carotid blood pressure and wave reflection in normotensive healthy young men. Journal of Applied Physiology. 2011;110(2):468–71. doi: 10.1152/japplphysiol.00917.2010. [DOI] [PubMed] [Google Scholar]
- 17.Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004;44(1):35–41. doi: 10.1161/01.HYP.0000132767.74476.64. [DOI] [PubMed] [Google Scholar]
- 18.Westerhof BE, Guelen I, Westerhof N, Karemaker JM, Avolio A. Quantification of wave reflection in the human aorta from pressure alone: A proof of principle. Hypertension. 2006;48(4):595–601. doi: 10.1161/01.HYP.0000238330.08894.17. [DOI] [PubMed] [Google Scholar]
- 19.Dzeko M, Peters CD, Kjaergaard KD, Jensen JD, Jespersen B. Aortic pulse wave velocity results depend on which carotid artery is used for the measurements. Journal of Hypertension. 2013;31(1):117–22. doi: 10.1097/HJH.0b013e32835b051b. [DOI] [PubMed] [Google Scholar]
- 20.Farquhar WB, Edwards DG, Jurkovitz CT, Weintraub WS. Dietary sodium and health: More than just blood pressure. Journal of the American College of Cardiology. 2015;65(10):1042–50. doi: 10.1016/j.jacc.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pini R, Cavallini MC, Palmieri V, Marchionni N, Di Bari M, Devereux RB, Masotti G, Roman MJ. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: The ICARe dicomano study. Journal of the American College of Cardiology. 2008;51(25):2432–9. doi: 10.1016/j.jacc.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 22.Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: The strong heart study. Hypertension. 2007;50(1):197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- 23.Wang K, Cheng H, Chuang S, Spurgeon HA, Ting C, Lakatta EG, Chou P, Chen C. Central or peripheral systolic or pulse pressure: Which best relates to target-organs and future mortality? Journal of Hypertension. 2009;27(3):461–7. doi: 10.1097/HJH.0b013e3283220ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McEniery CM, Cockcroft JR, Roman MJ, Franklin SS, Wilkinson IB. Central blood pressure: Current evidence and clinical importance. European Heart Journal. 2014;35(26):1719–25. doi: 10.1093/eurheartj/eht565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hope SA, Tay DB, Meredith IT, Cameron JD. Waveform dispersion, not reflection, may be the major determinant of aortic pressure wave morphology. The American Journal of Physiology: Heart and Circulatory Physiology. 2005;289(6):H2502. doi: 10.1152/ajpheart.00411.2005. [DOI] [PubMed] [Google Scholar]
- 26.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: Differential effects on wave reflection and aortic pulse wave velocity: The anglo-cardiff collaborative trial (ACCT) Journal of the American College of Cardiology. 2005;46(9):1753–60. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell GF. Triangulating the peaks of arterial pressure. Hypertension. 2006;48(4):543–5. doi: 10.1161/01.HYP.0000238325.41764.41. [DOI] [PubMed] [Google Scholar]
- 28.Nichols WW, O'Rourke MF. McDonald's Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. London, UK: 1998. pp. 201–22. [Google Scholar]
- 29.Westerhof N, Sipkema P, Van Den Bos GC, Elzinga G. Forward and backward waves in the arterial system. Cardiovascular Research. 1972;6(6):648–56. doi: 10.1093/cvr/6.6.648. [DOI] [PubMed] [Google Scholar]
- 30.Chirinos JA, Kips JG, Jacobs DR, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P. Arterial wave reflections and incident cardiovascular events and heart failure: The multiethnic study of atherosclerosis. Journal of the American College of Cardiology. 2012;60(21):2170–7. doi: 10.1016/j.jacc.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manisty C, Mayet J, Tapp RJ, Parker KH, Sever P, Poulter NH, Thom SAM, Hughes AD. Wave reflection predicts cardiovascular events in hypertensive individuals independent of blood pressure and other cardiovascular risk factors: An ASCOT (anglo-scandinavian cardiac outcome trial) substudy. Journal of the American College of Cardiology. 2010;56(1):24–30. doi: 10.1016/j.jacc.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 32.Cooper LL, Rong J, Benjamin EJ, Larson MG, Levy D, Vita JA, Hamburg NM, Vasan RS, Mitchell GF. Components of hemodynamic load and cardiovascular events: The framingham heart study. Circulation. 2015;131(4):354–61. doi: 10.1161/CIRCULATIONAHA.114.011357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phan TS, Li JK, Segers P, Chirinos JA. Misinterpretation of the determinants of elevated forward wave amplitude inflates the role of the proximal aorta. Journal of the American Heart Association. 2016;5(2):e003069. doi: 10.1161/JAHA.115.003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashimoto J, Westerhof B, Westerhof N, Imai Y, O’Rourke M. Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. Journal of Hypertension. 2008;26(5):1017–24. doi: 10.1097/HJH.0b013e3282f62a9b. [DOI] [PubMed] [Google Scholar]
- 35.Zamani P, Bluemke D, Jacobs J, David, Duprez D, Kronmal R, Lilly S, Ferrari V, Townsend R, Lima J, Budoff M, Segers P, Hannan P, Chirinos J. Resistive and pulsatile arterial load as predictors of left ventricular mass and geometry: The multiethnic study of atherosclerosis. Hypertension. 2015;65(1):85–92. doi: 10.1161/HYPERTENSIONAHA.114.04333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. American Journal of Physiology - Heart and Circulatory Physiology. 2000;279(1):H14. doi: 10.1152/ajpheart.2000.279.1.H7. [DOI] [PubMed] [Google Scholar]
- 37.Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. Journal of Vascular Research. 2007;44(5):382–90. doi: 10.1159/000102955. [DOI] [PubMed] [Google Scholar]
- 38.Greaney JL, DuPont JJ, Lennon-Edwards SL, Sanders PW, Edwards DG, Farquhar WB. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: Role of oxidative stress. The Journal of Physiology. 2012;590(21):5519–28. doi: 10.1113/jphysiol.2012.236992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. Journal of the American College of Cardiology. 2013;61(3):335–43. doi: 10.1016/j.jacc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chowienczyk PJ, Kelly RP, MacCallum H, Millasseau SC, Andersson TLG, Gosling RG, Ritter JM, Anggard EE. Photoplethysmographic assessment of pulse wave reflection: Blunted response to endothelium-dependent beta2-adrenergic vasodilation in type II diabetes mellitus. Journal of the American College of Cardiology. 1999;34(7):2007–14. doi: 10.1016/S0735-1097(99)00441-6. [DOI] [PubMed] [Google Scholar]
- 41.Boegehold MA. Effect of high salt intake on endothelial function: Reduced vascular nitric oxide in the absence of hypertension. Journal of Vascular Research. 2013;50(6):458–67. doi: 10.1159/000355270. [DOI] [PubMed] [Google Scholar]
- 42.DuPont JJ, Greaney JL, Wenner MM, Lennon-Edwards SL, Sanders PW, Farquhar WB, Edwards DG. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. Journal of Hypertension. 2013;31(3):530–6. doi: 10.1097/HJH.0b013e32835c6ca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lennon-Edwards S, Ramick MG, Matthews EL, Brian MS, Farquhar WB, Edwards DG. Salt loading has a more deleterious effect on flow-mediated dilation in salt-resistant men than women. Nutrition, Metabolism, and Cardiovascular Diseases : NMCD. 2014;24(9):990–5. doi: 10.1016/j.numecd.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]