Abstract
Background
A nontoxic chemopreventive intervention efficacious against different subtypes of breast cancer is still a clinically unmet need. The present study was undertaken to determine the efficacy of an Ayurvedic medicine phytochemical (Withaferin A, [WA]) for chemoprevention of breast cancer and to elucidate its mode of action.
Methods
Chemopreventive efficacy of WA (4 and 8 mg/kg body weight) was determined using a rat model of breast cancer induced by N-methyl-N-nitrosourea (MNU; n = 14 for control group, n = 15 for 4 mg/kg group, and n = 18 for 8 mg/kg group). The mechanisms underlying breast cancer chemoprevention by WA were elucidated by immunoblotting, biochemical assays, immunohistochemistry, and cytokine profiling using plasma and tumors from the MNU-rat (n = 8–12 for control group, n = 7–11 for 4 mg/kg group, and n = 8–12 for 8 mg/kg group) and/or mouse mammary tumor virus-neu (MMTV-neu) models (n = 4–11 for control group and n = 4–21 for 4 mg/kg group). Inhibitory effect of WA on exit from mitosis and leptin-induced oncogenic signaling was determined using MCF-7 and/or MDA-MB-231 cells. All statistical tests were two-sided.
Results
Incidence, multiplicity, and burden of breast cancer in rats were decreased by WA administration. For example, the tumor weight in the 8 mg/kg group was lower by about 68% compared with controls (8 mg/kg vs control, mean = 2.76 vs 8.59, difference = –5.83, 95% confidence interval of difference = −9.89 to –1.76, P = .004). Mitotic arrest and apoptosis induction were some common determinants of breast cancer chemoprevention by WA in the MNU-rat and MMTV-neu models. Cytokine profiling showed suppression of plasma leptin levels by WA in rats. WA inhibited leptin-induced oncogenic signaling in cultured breast cancer cells.
Conclusion
WA is a promising chemopreventative phytochemical with the ability to inhibit at least two different subtypes of breast cancer.
Nearly 40 000 women succumb to breast cancer every year in the Unites States alone (1). Breast cancer is a heterogeneous disease broadly classified into different subtypes and each with a distinct gene expression signature, including luminal-type, basal-like, human epidermal growth factor receptor 2–positive, and normal-like (2,3). Chemoprevention represents a promising approach for diminishing incidence and mortality from breast cancer (4). Chemoprevention of a subset of breast cancer reliant on estrogen receptor (ER) and estrogen for growth is feasible with selective ER modulators and aromatase inhibitors (5–7). Even though the clinical evidence for efficacy of these interventions for reduction of ER+ luminal-type breast cancer incidence is persuasive, they also have some side effects (5–8). A nontoxic chemopreventive intervention efficacious against different subtypes of breast cancer is still lacking.
Naturally occurring small molecules isolated from edible and medicinal plants remain attractive for cancer chemoprevention (9–12). Withaferin A (WA) is one such promising phytochemical, present in the root and leaf of an Ayurvedic medicine plant (Withania somnifera), whose inhibitory effect on cancer cells was first realized in the late sixties (13). Among different naturally occurring withanolides, WA is a much more potent inhibitor of breast cancer cell growth in vitro (14). We were the first to provide evidence for in vitro and in vivo activity of WA against breast cancer, including prevention of ER- breast cancer in a mouse mammary tumor virus-neu (MMTV-neu) transgenic mouse model (15–17).
Building upon our previous in vitro observations of ER suppression, cell growth inhibition, and apoptosis induction by WA treatment in luminal-type human breast cancer cell lines (16,18–20), the primary objective of this study was to determine the in vivo efficacy of WA for chemoprevention of ER+ breast cancer using a rat model of chemically induced cancer with histological and genetic similarities with the human luminal-type disease (21,22). A secondary goal of the study was to identify common mechanistic biomarkers predictive of WA’s efficacy in ER+ breast cancer in rats and ER- breast cancer in the MMTV-neu mouse model (17).
Methods
Reagents and Cell Lines
Sources of the reagents and cell lines are provided in the Supplementary Methods (available online).
Power Calculation and Random Assignment
Use and care of rats for the chemoprevention study described herein was approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Power calculation was based on tumor incidence (primary outcome) data from the published literature (23). A power of 88% was estimated for detection of a group difference of 40% in cancer incidence at a P value of .05 with 20 rats per group. A total of 60 20-day-old female Sprague-Dawley rats were purchased from the Charles River Laboratories (Wilmington, MA) in three batches of 20 each and assigned to one of the three groups: control, 4 mg/kg body weight, and 8 mg/kg body weight. At 21 days of age, rats were injected intraperitoneally with 50 mg N-methyl-N-nitrosourea (MNU)/kg body weight in 0.9% normal saline. One week post-MNU injection, the rats either received vehicle (control group) or vehicle containing 4 mg/kg or 8 mg/kg WA by intraperitoneal injection five times per week for 10 weeks. The number of evaluable rats at the conclusion of the study was 14 for the control group, 15 for the 4 mg/kg group, and 18 for the 8 mg/kg group. Other details are provided in the Supplementary Methods (available online).
Determination of WA Levels
WA content in the rat plasma (n = 6 for each group) and rat tumor tissues (n = 6 for control and 4 mg/kg groups, and n = 5 for 8 mg/kg group) was determined by mass spectrometry as detailed in the Supplementary Methods (available online).
Determination of Mitotic Index in Tumor Sections
Mitotic cells in hematoxylin and eosin (H&E)–stained mammary tumor sections from the MNU-rat (n = 12 for control group, n = 11 for 4 mg/kg group, and n = 12 for 8 mg/kg group) and MMTV-neu models (n = 11 for control group and n = 21 for 4 mg/kg group) were counted as described in the Supplementary Methods (available online).
Cell Cycle Distribution in MCF-7 Cells In Vitro
Kinetics of the exit from mitosis in vitro was determined by flow cytometry using MCF-7 cells synchronized in the G2/M phase by treatment with nocodazole and then released either in drug-free medium or medium supplemented with 2 µM WA. The cells were collected at different time points (one, two, four, eight, and 24 hours) after release, and the percentage of G2/M and G1 populations was determined by flow cytometry as described in the Supplementary Methods (available online).
Immunoblotting
Fresh-frozen tumors from rats (n = 8 for control group, n = 7 for 4 mg/kg group, and n = 8 for 8 mg/kg group) and MMTV-neu mice (n = 4 for both groups) were used for immunoblotting. Preparations of tumor and cell lysates and the immunoblotting details are included in the Supplementary Methods (available online).
Determination of Metabolic Intermediates
Commercially available kits were used for measurement of plasma (n = 12 for control group and n = 9–10 for 8 mg/kg group) and tumor (n = 11 for control group and n = 8–11 for 8 mg/kg group) levels of lactate, malate, and acetyl-CoA as described in the Supplementary Methods (available online).
Measurement of Complex III Activity
Mitochondrial respiratory chain complex III (ubiquinol cytochrome c oxidoreductase) activity using rat tumors (n = 9 for control group, n = 8 for 4 mg/kg group, and n = 9 for 8 mg/kg group) was measured as described by us previously with minor modifications (17). Experimental details, including modifications, are included in the Supplementary Methods (available online).
Immunohistochemistry
Immunohistochemistry (IHC) was performed as described by us previously (17,24,25). Details of IHC are described in the Supplementary Methods (available online). Stained rat tumor sections (n = 12 for control group, n = 11 for 4 mg/kg group, and n = 12 for 8 mg/kg group) were analyzed using Aperio ImageScope software (Leica Biosystems Inc., Buffalo Grove, IL) (17).
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End Labeling Assay
The effect of WA treatment on tumor apoptosis in vivo was determined by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay (n = 12 for control group, n = 11 for 4 mg/kg group, and n = 12 for 8 mg/kg group) using a commercially available kit and by following the supplier’s instructions that are detailed in the Supplementary Methods (available online).
Determination of Aldehyde Dehydrogenase 1 (ALDH1) Activity
Fresh frozen tumor specimens from the control (n = 10) and 8 mg/kg WA treatment groups (n = 10) were used to measure aldehyde dehydrogenase 1 (ALDH1) activity by flow cytometry using a commercially available kit essentially as described by us previously (26) and detailed in the Supplementary Methods (available online).
Cytokine Profiling
A commercially available kit was used to profile cytokines in the rat plasma (n = 12 for control group, n = 11 for 4 mg/kg group, and n = 11 for 8 mg/kg group) and tumor lysates (n = 12 for control group, n = 10 for 4 mg/kg group, and n = 11 for 8 mg/kg group) of control and WA treatment groups. Analytical details are described in more detail in the Supplementary Methods (available online).
Colony Formation and Cell Migration Assays
Details of colony formation and cell migration assays are included in the Supplementary Methods (available online). Boyden chamber-based cell migration assay was done as previously described (27,28).
Statistical Analyses
Results are expressed as mean with corresponding 95% confidence interval (CI). The generalized linear mixed models were performed to evaluate treatment effects. The Poisson regression model was used to evaluate difference in tumor multiplicity (number of tumors per rat). Analysis of variance (ANOVA) was used to examine the treatment effects on cumulative tumor weight. Two sample comparisons were analyzed by unpaired Student’s t test. Dunnett’s method was used for multiple comparisons to a control alone. The P values for multiple group comparisons were adjusted using Bonferroni’s method. All statistical tests were two-sided, and a statistical significance level was set at .05. Statistical analyses were performed using SAS v. 9.4 (SAS Institute, Cary, NC) or GraphPad Prism 6.07 (La Jolla, CA).
Results
Effect of WA on MNU-Induced Breast Cancer in Rats
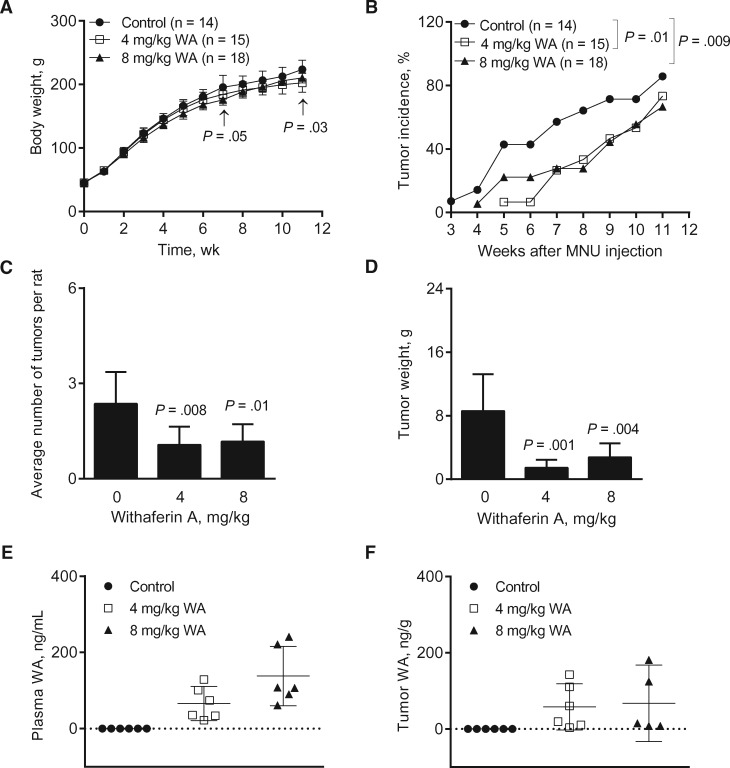
The MNU rat model is an excellent choice for preclinical chemoprevention trials because 1) a single MNU injection produces highly reproducible ER+ breast tumors in rats with histological and genetic similarities to human luminal-type breast cancer (21,22,29) and 2) this model has been used to demonstrate chemopreventive efficacy of agents targeting hormonal axis, including tamoxifen and aromatase inhibitors (30,31). Except for a modest weight loss in the 4 mg/kg and 8 mg/kg groups on days 78 and 50, respectively, the body weights of the WA-treated rats did not differ from controls (Figure 1A). The tumor incidence was significantly lower in the 4 mg/kg (4 mg/kg vs control, odds ratio = 0.27, 95% CI = 0.09 to 0.75, P = .01 by generalized linear mixed model) and 8 mg/kg groups (8 mg/kg vs control, odds ratio = 0.27, 95% CI = 0.10 to 0.72, P = .009 by generalized linear mixed model) compared with controls (Figure 1B). Chemoprevention of breast cancer after WA treatment was also reflected by a decrease in average number of tumors per rat (Figure 1C) and tumor weight (Figure 1D). For instance, tumor weight in the 8 mg/kg group was lower by about 68% compared with controls (8 mg/kg vs control, mean = 2.76 vs 8.59, difference = −5.83, 95% confidence interval of difference = −9.89 to −1.76, P = .004). WA was detectable in the plasma (Figure 1E) and tumors (Figure 1F) of WA treatment groups. These results showed bioavailability of WA in the tumor and its inhibitory effects on incidence and burden of mammary tumors in rats.
Figure 1.
Effect of Withaferin A (WA) administration on N-methyl-N-nitrosourea (MNU)-induced mammary tumor development in female Sprague-Dawley rats. A) Body weight of the rats over time of the control and the WA treatment groups. Results shown are mean body weights with their 95% confidence intervals (error bars, n = 14 for control; n = 15 for 4 mg/kg; n = 18 for 8 mg/kg, except on week 11 where n = 11 for control; n = 12 for 4 mg/kg; n = 15 for 8 mg/kg). Statistical significance of difference was analyzed by Dunnett’s test. B) Tumor incidence over time (palpable tumors) in rats administered with either vehicle or WA (4 or 8 mg/kg). Results shown are percentage of tumor incidence over time in control and WA treatment groups (n = 14 for control; n = 15 for 4 mg/kg; n = 18 for 8 mg/kg except on week 11, where n = 11 for control; n = 12 for 4 mg/kg; n = 15 for 8 mg/kg). The generalized linear mixed model was performed to examine the difference of the percentage or probability of tumor presence between the groups. C) Average number of tumors per rat (tumor multiplicity) in control and WA treatment groups. Results shown are the average number of tumors per rat with their 95% confidence intervals (error bars, n = 14 for control; n = 15 for 4 mg/kg; n = 18 for 8 mg/kg). Statistical significance was determined by Poisson regression model. D) Tumor weight in rats of control and WA treatment groups. Results shown are mean tumor weight with their 95% confidence intervals (error bars, n = 14 for control; n = 15 for 4 mg/kg; n = 18 for 8 mg/kg). Statistical significance was determined by Dunnett’s test. E) WA concentration in the plasma of control- and WA-treated rats. The lines in the scatter dot plot indicate mean WA levels and their 95% confidence intervals (error bars, n = 6 for all groups). Plasma specimens from different rats of each group were used for determination of WA levels. F) WA concentration in the tumor of control- and WA-treated rats. The lines in the scatter dot plot indicate mean WA levels and their 95% confidence intervals (error bars, n = 6 for control and 4 mg/kg; n = 5 for 8 mg/kg). Tumor tissues from different rats of each group were used for determination of WA levels. All P values were two-sided.
Effect of WA Administration on Mitotic Index
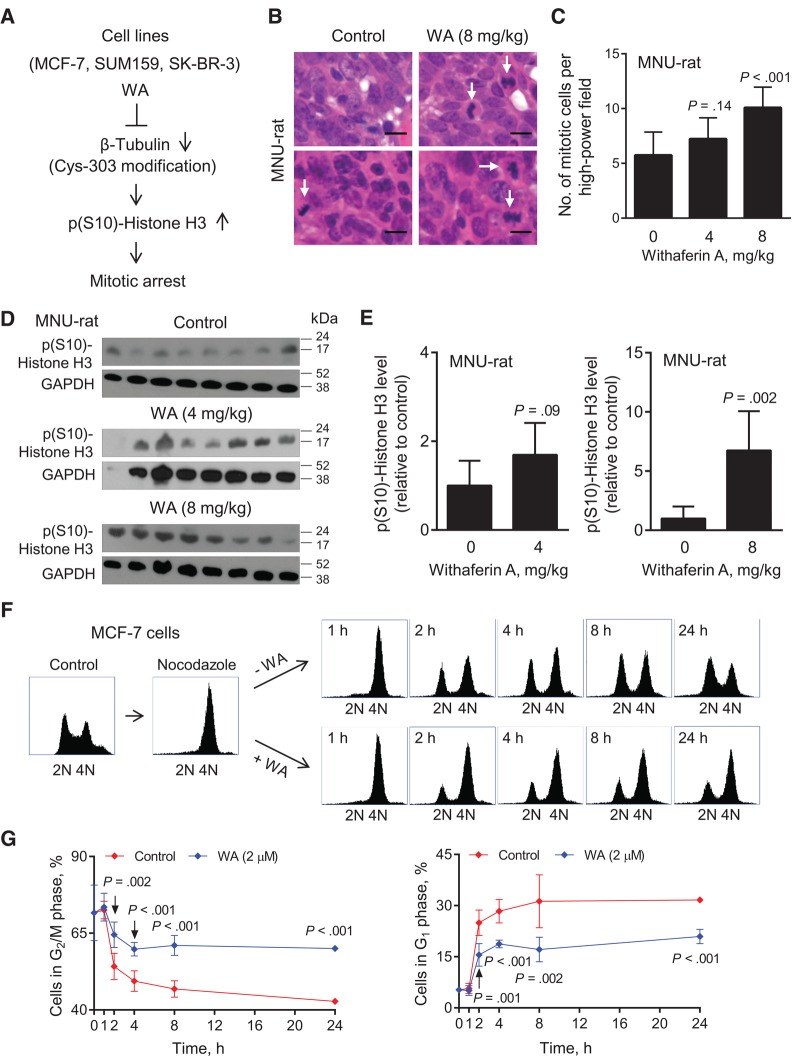
Previous in vitro studies from our laboratory have revealed accumulation of mitotic cells in association with downregulation of β-Tubulin and its covalent modification at cysteine-303 by WA treatment in human breast cancer cells (Figure 2A) (14). Representative H&E-stained sections from tumors of two different rats of the control and the 8 mg/kg WA treatment groups are shown in Figure 2B (mitotic cells are identified by arrows). The number of mitotic cells per high-power field was significantly higher in the tumors of 8 mg/kg group compared with controls (8 mg/kg vs control, mean = 10.08 vs 5.74, difference = 4.34, 95% CI of difference = 2.47 to 6.21, P < .001 by Dunnett’s test) (Figure 2C). Figure 2D shows immunoblots for p(S10)-Histone H3 using tumor lysates from different rats of each group. The level of p(S10)-Histone H3 was significantly higher in the tumors of the 8 mg/kg group compared with controls (8 mg/kg vs control, mean = 6.75 vs 1.00, difference = 5.75, 95% CI of difference = 2.60 to 8.89, P = .002 by unpaired Student’s t test) (Figure 2E). A trend for a decrease in the tumor levels of β-Tubulin in the WA treatment groups was discernible, but the difference was not significant from controls (Supplementary Figure 1, available online).
Figure 2.
Effect of Withaferin A (WA) treatment on mitotic fraction in vivo in rat tumors and in MCF-7 cells in vitro. A) A schematic showing the mechanism underlying WA-mediated mitotic arrest in human breast cancer cell lines. B) Representative hematoxylin and eosin–stained mammary tumor sections from two different rats of each group (control and 8 mg/kg WA) showing mitotic cells (identified by arrows; ×400 magnification, scale bar = 10 μm). C) Quantitation of mitotic cells in mammary tumor sections of control- and WA-treated rats. Results shown are mean number of mitotic cells per high-power field with their 95% confidence intervals (error bars, n = 12 for control; n = 11 for 4 mg/kg; n = 12 for 8 mg/kg). Tumor sections from different rats of each group were used for quantitation of mitotic cells. Statistical significance of difference was analyzed by Dunnett’s test. D) Immunoblotting for p(S10)-Histone H3 and GAPDH using tumor lysates from control- and WA-treated rats. Each lane represents tumor lysate from a different rat of each group. E) Quantitation of p(S10)-Histone H3 protein in tumor lysates from control- and WA-treated rats. Results shown are p(S10)-Histone H3 level relative to control with their 95% confidence intervals (error bars, n = 8 for control; n = 7 for 4 mg/kg; n = 8 for 8 mg/kg). Statistical significance of difference was analyzed by unpaired Student’s t test. F) Representative flow histograms showing synchronization of MCF-7 cells in the G2/M phase (4N) by treatment with nocodazole, and subsequent exit from mitosis (evidenced by a decrease in 4N G2/M population) and resumption of cell cycle progression (evidenced by an increase in 2N G1 population) at different time points after release of the synchronized cells in drug-free medium or medium containing 2 μM WA. G) Percentage of cells in G2/M phase (left panel) or G1 phase (right panel) for data shown in (F). The experiment was done in triplicate three times independently, and the results were consistent. Data shown are mean percentage of cells in respective phase with their 95% confidence intervals (error bars, n = 3 for both groups). Statistical significance of difference was analyzed by unpaired Student’s t test. All P values were two-sided.
An increase in the number of mitotic cells in the tumors of the 8 mg/kg WA group compared with control (Figure 2C) could be due to increased mitosis and/or inhibition of exit from mitosis. To experimentally probe these possibilities, in vitro experiments were carried out using MCF-7 cells. The MCF-7 cells synchronized in the G2/M phase (4N) by treatment with nocodazole (Figure 2F) quickly resumed cell cycle progression when released in drug-free medium, as evidenced by a time-dependent decrease in the G2/M population with a concomitant increase in G1 phase cells (Figure 2G). A relatively higher proportion of the cells remained in the G2/M phase when the synchronized cells were released in WA-containing medium, indicating inhibition of exit from mitosis (Figure 2G).
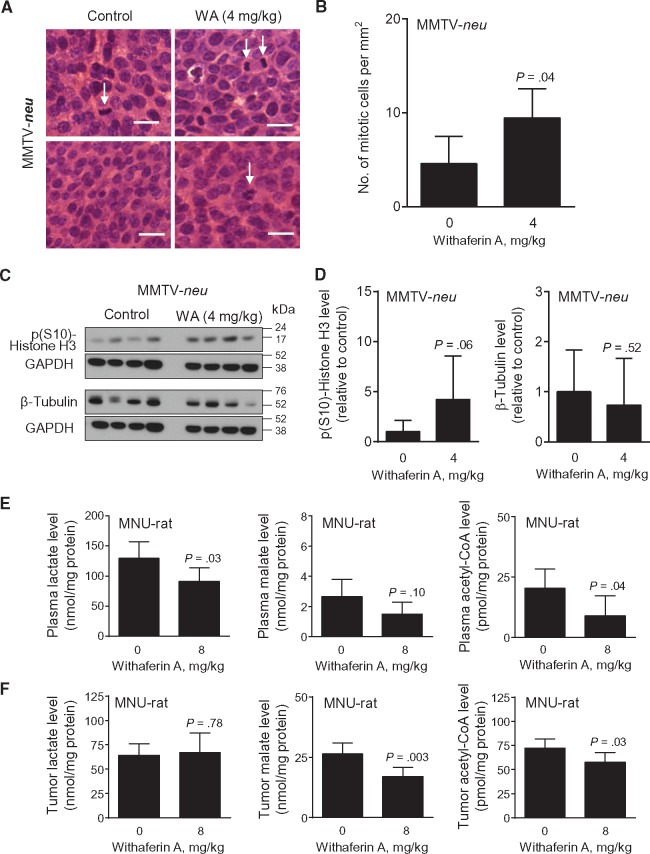
We used archived H&E-stained tumor sections from control- and WA-treated MMTV-neu mice (17) to test whether accumulation of mitotic cells was a disease subtype–independent effect of this agent. The number of mitotic cells per mm2 (mitotic cells identified by arrows) (Figure 3A) was about 2.1-fold higher in the tumor sections of WA-treated mice than those of control (4 mg/kg vs control, mean = 9.46 vs 4.59, difference = 4.87, 95% CI of difference = 0.26 to 9.47, P = .04 by unpaired Student’s t test) (Figure 3B). Immunoblotting for p(S10)-Histone H3 revealed a relatively higher level in the tumors of WA-treated mice compared with controls (Figure 3, C and D). The β-Tubulin protein level was lower in the tumors of WA-treated MMTV-neu mice compared with controls, but, similar to rat tumors, the difference was not significant (Figure 3D). Based on these in vitro and in vivo findings, we conclude that WA treatment causes mitotic arrest at least in breast cancer cells.
Figure 3.
Effect of Withaferin A (WA) administration on mitotic fraction in mammary tumors of MMTV-neu mice. A) Representative hematoxylin and eosin–stained breast tumor sections from two different MMTV-neu mice of each group (control and 4 mg/kg) showing mitotic cells (identified by arrows; ×400 magnification, scale bar = 10 μm). B) Quantitation of mitotic cells in the tumors from MMTV-neu mice administered either vehicle or WA. Tumor sections from different mice of each group were analyzed. Results shown are mean number of mitotic cells per mm2 with their 95% confidence intervals (error bars, n = 11 for control; n = 21 for 4 mg/kg). Statistical significance of difference was analyzed by unpaired Student’s t test. C) Immunoblotting for p(S10)-Histone H3, β-Tubulin, and GAPDH using tumor lysates from MMTV-neu mice administered with either vehicle or 4 mg/kg. Each lane represents tumor lysate from a different mouse of each group. D) Quantitation of proteins shown in (C). Results shown are corresponding protein level relative to control with their 95% confidence intervals (error bars, n = 4 for both groups). Statistical significance of difference was analyzed by unpaired Student’s t test. E) Plasma levels of lactate, malate, and acetyl-CoA in rats of control and WA treatment groups. Results shown are mean levels of metabolites and their 95% confidence intervals (error bars, n = 12 for control ; n = 9–10 for 8 mg/kg). Plasma samples from different rats of each group were used for determination of metabolite levels. Statistical significance of difference was analyzed by unpaired Student’s t test. F) Tumor levels of lactate, malate, and acetyl-CoA in rats of control and WA treatment groups. Results shown are mean levels of metabolites and their 95% confidence intervals (error bars, n = 11 for control; n = 8–11 for 8 mg/kg). Tumor tissues from different rats of each group were used for determination of the levels of the specified metabolites. Statistical significance of difference was analyzed by unpaired Student’s t test. All P values were two-sided.
Lactate, Malate, and Acetyl-CoA Levels in Rat Plasma and Tumors
Chemoprevention of ER- breast cancer in MMTV-neu mice following WA administration is associated with inhibition of glycolysis and tricarboxylic acid cycle (17). The levels of key intermediates of glycolysis (lactate) and tricarboxylic acid cycle (malate), as well as acetyl-CoA, were lower in the plasma (Figure 3E) and/or tumors (Figure 3F) of the 8 mg/kg group rats compared with controls. These results indicate that inhibition of glycolysis and tricarboxylic acid cycle are common mechanisms of WA-mediated chemoprevention in ER+ (present study) and ER- breast cancers (17).
Apoptotic Cell Death Biomarkers in Rat Tumors
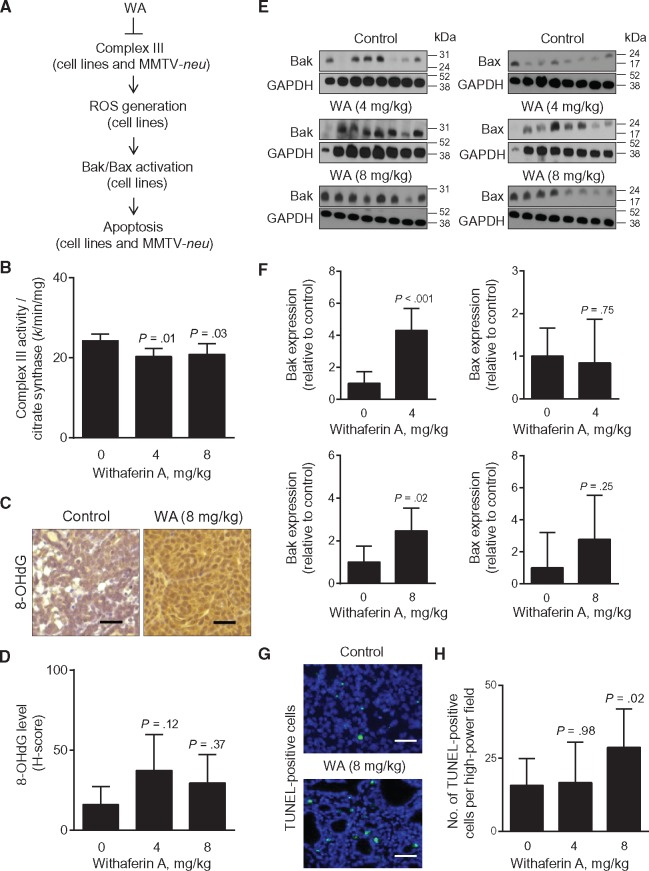
Figure 4A summarizes a mechanistic model for WA-induced apoptosis in breast cancer from our own in vitro and in vivo findings (16,17). Rat tumors from the present study revealed a modest but statistically significant decrease in complex III activity in vivo in the 4 mg/kg (4 mg/kg vs control, mean = 20.35 vs 24.30, difference = –3.95, 95% CI of difference = –7.13 to –0.77, P = .01 by Dunnett’s test) and the 8 mg/kg (8 mg/kg vs control, mean = 20.85 vs 24.30, difference = –3.45, 95% CI of difference = –6.53 to –0.36, P = .03 by Dunnett’s test) groups compared with controls (Figure 4B). Immunohistochemical staining for 8-OHdG, a biochemical marker of reactive oxygen species, in a representative tumor of the control group and the 8 mg/kg group is shown in Figure 4C. The H-score for 8-OHdG level was higher by 1.85- to 2.35-fold in the tumors of WA-treated rats compared with controls, but the difference was not significant (Figure 4D).
Figure 4.
The role of Bak in Withaferin A (WA)–mediated inhibition of rat mammary tumor development. A) A scheme showing the mechanism underlying WA-induced apoptosis in human breast cancer cells in vitro and in mammary tumors of MMTV-neu mice in vivo. B) Complex III activity in tumors of control- and WA-treated rats. Results shown are mean complex III activity/citrate synthase with their corresponding 95% confidence intervals (error bars, n = 9 for control; n = 8 for 4 mg/kg; n = 9 for 8 mg/kg). Tumor tissues from different rats of each group were used for complex III activity determination. Statistical significance of difference was analyzed by Dunnett’s test. C) Representative images for 8-OHdG immunohistochemistry (×200 magnification, scale bar = 50 μm). D) Quantitation of 8-OHdG levels in tumors of rats administered with either vehicle or WA (4 or 8 mg/kg). Results shown are mean H-score with their corresponding 95% confidence intervals (error bars, n = 12 for control; n = 11 for 4 mg/kg; n = 12 for 8 mg/kg). Tumor tissue sections from different rats of each group were used for immunohistochemistry of 8-OHdG. Statistical significance of difference was analyzed by Dunnett’s test. E) Immunoblotting for Bak, Bax, and GAPDH using tumor lysates from control- and WA-treated rats. Each lane represents tumor lysate from a different rat of each group. F) Quantitation of proteins shown in (E). Results shown are protein expression relative to control with their 95% confidence intervals (error bars, n = 8 for control; n = 7 for 4 mg/kg; n = 8 for 8 mg/kg). Statistical significance of difference was determined by unpaired Student’s t test. G) Representative images showing TUNEL-positive apoptotic cells (×400 magnification, scale bar = 10 μm) in a control tumor and a tumor of the 8 mg/kg group. H) Quantitation of TUNEL-positive cells per high-power field in rat tumor sections of the control, 4 mg/kg, and 8 mg/kg groups. Results shown are mean number of TUNEL-positive cells and their 95% confidence intervals (error bars, n = 12 for control; n = 11 for 4 mg/kg; n = 12 for 8 mg/kg). Tumor sections from different rats of each group were used for TUNEL assay. Statistical significance was determined by Dunnett’s test. All P values were two-sided.
We have shown previously that WA-induced apoptosis in cultured breast cancer cell lines is attenuated by knockdown of multidomain proapoptotic proteins Bax and Bak (16). Generally, the protein level of Bak, but not Bax, was higher in the tumors of WA-treated rats compared with controls (Figure 4, E and F). Cleavage of poly-(ADP-ribose) polymerase (cleaved PARP), another well-accepted marker of apoptosis, was higher in the tumors of WA-treated rats compared with controls (Supplementary Figure 2, available online). The number of TUNEL-positive cells per high-power field (green fluorescence in Figure 4G) was significantly higher in rat tumors of the 8 mg/kg group compared with controls (8 mg/kg vs control, mean = 28.79 vs 15.72, difference = 13.07, 95% CI of difference = 1.54 to 24.61, P = .02 by Dunnett’s test) (Figure 4H). Collectively, these results provided in vivo evidence for WA-induced apoptosis in rat mammary tumors.
Cell Proliferation and Neoangiogenesis in Rat Tumors
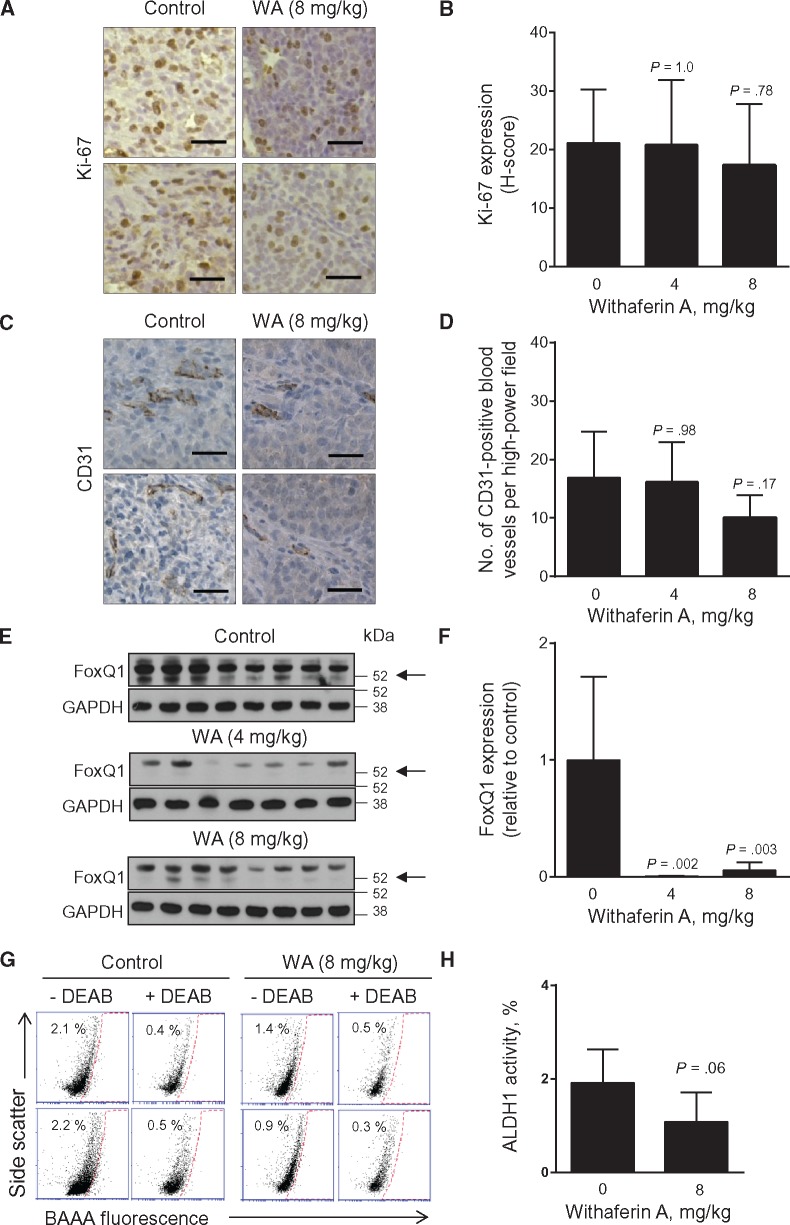
Even though WA is highly effective in suppressing in vitro proliferation of ER+ and ER- human breast cancer cells (16), the H-score for proliferation marker Ki-67 (Figure 5A) was not different between the tumors of control and WA treatment groups (Figure 5B), consistent with data in MMTV-neu tumors (17). The number of CD31-positive blood vessels per high-power field (Figure 5C) was lower in the tumors of WA-treated rats compared with controls, but the difference was not significant (Figure 5D).
Figure 5.
Effect of Withaferin A (WA) administration on cell proliferation, angiogenesis, and bCSC markers. A) Representative Ki-67 immunohistochemical images from tumors of two different rats of control and 8 mg/kg groups (×200 magnification, scale bar = 50 μm). B) Quantitation of Ki-67 expression (H-score). Results shown are mean H-score for Ki-67 expression with their corresponding 95% confidence intervals (error bars, n = 12 for control; n = 11 for 4 mg/kg; n = 12 for 8 mg/kg). Tumor tissue sections from different rats of each group were used for immunohistochemistry of Ki-67. Statistical significance of difference was determined by Dunnett’s test. C) Representative images showing CD31-positive blood vessels in tumors of two different rats of control and 8 mg/kg groups (×200 magnification, scale bar = 50 μm). Tumor tissue sections from different rats of each group were used for immunohistochemistry of CD31. D) Quantitation of CD31-positive blood vessels in tumors of control- and WA-treated rats. Results shown are mean number of CD31-positive blood vessels per high-power field with their corresponding 95% confidence intervals (error bars, n = 12 for control; n = 11 for 4 mg/kg; n = 12 for 8 mg/kg). Statistical significance was analyzed by Dunnett’s test. E) Immunoblotting for FoxQ1 and GAPDH using tumor lysates from control- and WA-treated rats. Each lane represents tumor lysate from a different rat of each group. Arrow indicates FoxQ1 band. F) Quantitation of FoxQ1 immunoblots shown in (E). Results shown are FoxQ1 expression relative to control with their 95% confidence intervals (error bars, n = 8 for control; n = 7 for 4 mg/kg; n = 8 for 8 mg/kg). Statistical significance was determined by Dunnett’s test. G) Representative flow histograms showing ALDH1 activity in dissociated cells from two different rat tumors of each group. The ALDH inhibitor diethylaminobenzaldehyde was used as a control. H) Quantitation of ALDH1 activity for data shown in (G). Results shown are mean percentage of ALDH1 activity with their 95% confidence intervals (error bars, n = 10 for both groups). Tumor tissues from different rats of each group were used for determination of ALDH1 activity. Statistical significance was determined by unpaired Student’s t test. All P values were two-sided. DEAB = diethylaminobenzaldehyde; BAAA = BODIPY-aminoacetaldehyde.
Breast Cancer Stem Cell Biomarkers in Rat Tumors
With the growing evidence for a role of breast cancer stem cells (bCSC) in tumorigenesis and therapy resistance (32), it is equally important to assess the efficacy of promising chemopreventive agents on this population. WA is a potent inhibitor of the bCSC population in vitro and in MMTV-neu tumors in vivo (26). Forkhead box Q1 (FoxQ1) is one of the proteins implicated in bCSC maintenance (33,34). For example, we have shown recently that overexpression of FoxQ1 in MCF-7 and SUM159 cells increases ALDH1 activity and mammosphere number (34). FoxQ1 protein level was visually lower in the tumors of WA treatment groups compared with controls (Figure 5, E and F). Figure 5G shows flow histograms for ALDH1 activity in dissociated cells from representative tumors of two different rats of the control and the 8 mg/kg groups. ALDH1 activity was lower in dissociated cells from the tumors of the 8 mg/kg group compared with controls with P = .06 by two-sided Student’s t test (Figure 5H).
Cytokine Profiling in Rat Plasma and Tumors
The circulating leptin level was lower in the rat plasma of the WA treatment groups compared with controls (Table 1). Levels of tumor cytokines were not different between the control and the WA treatment groups (Table 2).
Table 1.
Cytokine levels in the plasma of control- and WA-treated rats
| Cytokine | Cytokine levels by group, pg/mg |
||
|---|---|---|---|
| Control (n = 12) Mean (95% CI) | 4 mg/kg WA (n = 11) Mean (95% CI) | 8 mg/kg WA (n = 11) Mean (95% CI) | |
| EGF | 0.06 (−0.02 to 0.14) | 0.18 (−0.05 to 0.40) | 0.02 (−0.03 to 0.07) |
| Eotaxin | 1.24 (0.58 to 1.89) | 1.38 (0.94 to 1.82) | 1.09 (0.50 to 1.67) |
| Fractalkine | 16.77 (14.49 to 19.05) | 14.58 (12.05 to 17.11) | 15.28 (11.30 to 19.27) |
| G-CSF | 3.40 (−1.05 to 7.84) | 3.89 (−0.17 to 7.96) | 1.32 (−0.54 to 3.17) |
| GM-CSF | 3.37 (−4.05 to 10.79) | 2.83 (−3.47 to 9.12) | 0.29 (−0.35 to 0.93) |
| GRO | ND | ND | 6.16 (−7.57 to 19.89) |
| IFN-γ | 176.0 (−117.0 to 468.9) | 351.9 (38.35 to 665.5) | 51.23 (−62.92 to 165.4) |
| IL-1α | 1.94 (−2.32 to 6.20) | 2.03 (−2.49 to 6.54) | 1.27 (−1.55 to 4.09) |
| IL-1β | 1.90 (−0.38 to 4.18) | 2.52 (−0.21 to 5.25) | 9.36 (−11.11 to 29.82) |
| IL-2 | 10.93 (3.33 to 18.54) | 5.67 (1.69 to 9.65) | 9.10 (2.83 to 15.37) |
| IL-4 | 0.87 (−1.04 to 2.78) | 0.98 (−1.21 to 3.17) | ND |
| IL-5 | 45.40 (−4.11 to 94.90) | 55.88 (−2.06 to 113.8) | 35.98 (−3.06 to 75.02) |
| IL-6 | 372.7 (−447.6 to 1193) | 220.2 (−133.5 to 573.9) | ND |
| IL-10 | 1.82 (0.20 to 3.44) | 3.18 (0.57 to 5.79) | 1.45 (−0.35 to 3.25) |
| IL-12p70 | 607.4 (239.1 to 975.8) | 499.5 (89.03 to 910.0) | 213.8 (−85.19 to 512.9) |
| IL-13 | 16.58 (−10.56 to 43.71) | 22.95 (−9.20 to 55.09) | 10.44 (−11.46 to 32.34) |
| IL-17 | 1.16 (−0.07 to 2.39) | 1.44 (0.52 to 2.36) | 0.85 (0.002 to 1.69) |
| IL-18 | 194.6 (102.3 to 287.0) | 318.2 (98.65 to 537.7) | 257.6 (107.2 to 407.9) |
| IP-10 | 27.27 (21.32 to 33.22) | 21.63 (15.60 to 27.66) | 39.38 (4.36 to 74.40) |
| Leptin | 1093 (691.3 to 1496) | 829.7 (672.8 to 986.7)* | 606.2 (417.8 to 794.6)* |
| LIX | 27.96 (13.07 to 42.85) | 92.76 (25.01 to 160.5)* | 41.62 (21.25 to 62.00)* |
| MCP-1 | 158.5 (104.5 to 212.5) | 142.8 (87.73 to 197.8) | 209.5 (108.6 to 310.5) |
| MIP-1α | 0.45 (0.12 to 0.78) | 0.45 (0.18 to 0.73) | 0.47 (0.06 to 0.88) |
| MIP2 | 1.08 (−1.30 to 3.46) | 1.20 (−0.59 to 3.00) | 1.89 (−1.18 to 4.96) |
| RANTES | 473.9 (370.9 to 576.9) | 652.3 (500.4 to 804.1) | 537.9 (365.8 to 710.1) |
| TNF-α | 22.43 (−1.43 to 46.29) | 13.33 (−5.00 to 31.66) | 5.90 (−7.25 to 19.04) |
| VEGF | 75.62 (55.70 to 95.53) | 54.37 (35.64 to 73.10) | 61.12 (42.77 to 79.46) |
Statistically significant by one-way analysis of variance followed by Dunnett’s test. The statistical test was two-sided. CI = confidence interval; ND = not detectable; WA = Withaferin A.
Table 2.
Cytokine levels in tumors of control- and WA-treated rats*
| Cytokine | Cytokine levels by group, pg/mg |
||
|---|---|---|---|
| Control (n = 12) Mean (95% CI) | 4 mg/kg WA (n = 10) Mean (95% CI) | 8 mg/kg WA (n = 11) Mean (95% CI) | |
| EGF | 3.32 (0.92 to 5.72) | 1.60 (0.21 to 2.99) | 1.10 (0.29 to 1.91) |
| Eotaxin | 3.44 (2.85 to 4.04) | 2.89 (2.17 to 3.61) | 2.83 (1.69 to 3.97) |
| Fractalkine | 110.5 (65.57 to 155.5) | 75.76 (45.52 to 106.0) | 81.00 (57.55 to 104.4) |
| G-CSF | 0.13 (−0.09 to 0.35) | 0.12 (−0.15 to 0.38) | 0.99 (−0.59 to 2.56) |
| GM-CSF | 3.79 (−1.86 to 9.44) | 3.24 (−4.09 to 10.57) | 0.62 (−0.51 to 1.76) |
| GRO | 63.66 (15.93 to 111.4) | 67.72 (32.82 to 102.6) | 57.52 (18.51 to 96.54) |
| IFN-γ | 305.0 (202.5 to 407.6) | 287.6 (208.7 to 366.5) | 318.9 (258.3 to 379.5) |
| IL-1α | 26.41 (9.98 to 42.85) | 20.77 (3.62 to 37.92) | 18.11 (13.30 to 22.92) |
| IL-1β | 16.71 (10.87 to 22.55) | 14.09 (7.48 to 20.70) | 15.80 (7.85 to 23.75) |
| IL-2 | 11.60 (5.98 to 17.22) | 10.03 (5.06 to 15.00) | 10.88 (5.39 to 16.38) |
| IL-4 | 13.17 (4.68 to 21.65) | 13.00 (5.54 to 20.46) | 15.36 (7.01 to 23.70) |
| IL-5 | 12.28 (6.05 to 18.52) | 13.65 (6.31 to 20.98) | 15.45 (8.13 to 22.77) |
| IL-6 | 48.27 (−9.19 to 105.7) | 48.40 (−18.48 to 115.3) | 46.09 (−9.48 to 101.7) |
| IL-10 | 13.29 (9.21 to 17.38) | 11.15 (5.91 to 16.39) | 12.98 (8.69 to 17.27) |
| IL-12p70 | 1.95 (0.09 to 3.80) | 2.37 (0.62 to 4.12) | 3.01 (0.01 to 6.02) |
| IL-13 | 20.33 (7.99 to 32.68) | 14.32 (0.94 to 27.70) | 23.78 (7.80 to 39.76) |
| IL-17 | 0.32 (−0.15 to 0.80) | ND | 0.14 (−0.17 to 0.44) |
| IL-18 | 4507 (3391 to 5623) | 3542 (2216 to 4868) | 4200 (3141 to 5259) |
| IP-10 | 56.31 (35.92 to 76.70) | 79.33 (−8.76 to 167.40) | 63.99 (29.97 to 98.02) |
| Leptin | 265.0 (135.8 to 394.2) | 202.0 (38.70 to 365.4) | 242.2 (−65.65 to 550.0) |
| LIX | 26.88 (19.23 to 34.53) | 30.90 (21.54 to 40.25) | 35.04 (23.52 to 46.56) |
| MCP-1 | 57.40 (29.83 to 84.96) | 52.67 (22.83 to 82.51) | 63.58 (33.22 to 93.93) |
| MIP-1α | 4.52 (2.29 to 6.74) | 4.45 (1.22 to 7.68) | 4.75 (2.04 to 7.45) |
| MIP2 | 10.63 (0.68 to 20.57) | 9.29 (−1.35 to 19.92) | 6.02 (−1.21 to 13.24) |
| RANTES | 133.0 (84.29 to 181.6) | 122.6 (−20.95 to 266.1) | 107.9 (52.74 to 163.1) |
| TNF-α | 0.06 (−0.07 to 0.18) | 0.27 (−0.35 to 0.89) | 0.49 (−0.26 to 1.25) |
| VEGF | 474.2 (288.8 to 659.7) | 369.9 (118.9 to 621.0) | 605.2 (417.8 to 792.6) |
CI = confidence interval; ND = not detectable; WA = Withaferin A.
In Vitro Effects of WA on Leptin-Induced Signaling
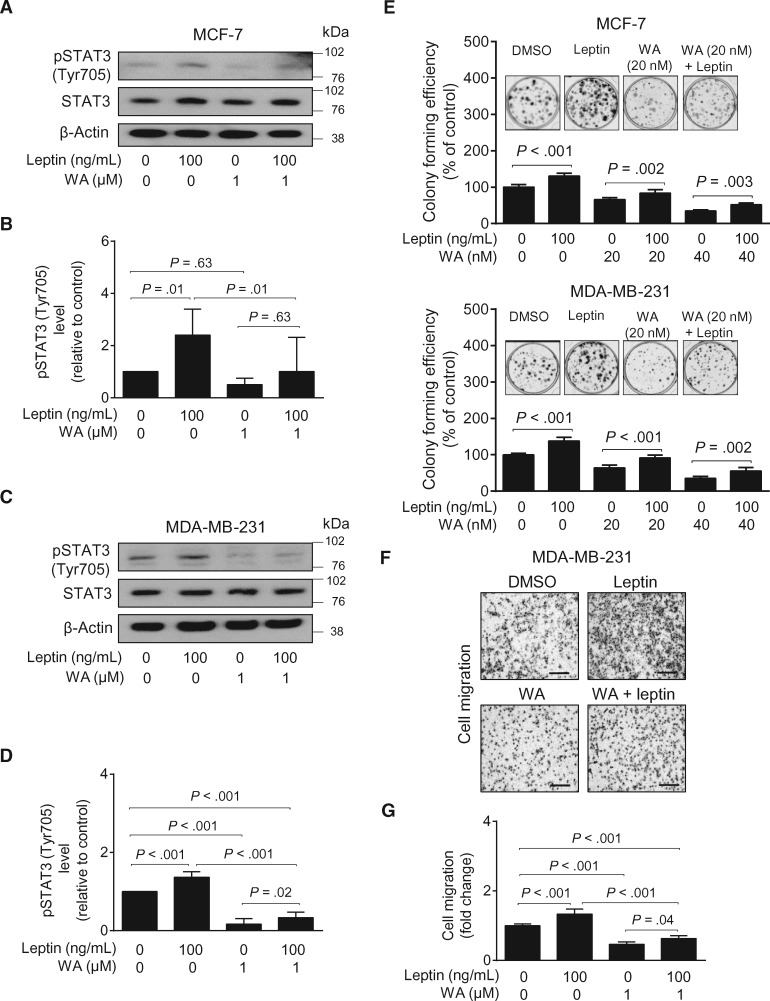
Because of the well-known association of leptin levels with breast cancer risk (35–37), we determined the in vitro effect of WA on leptin-induced oncogenic signaling. Exposure of serum-starved MCF-7 (Figure 6, A and B) and MDA-MB-231 cells (Figure 6, C and D) to physiological levels of leptin resulted in a marked increase in Tyr705-phosphorylated signal transducer and activator of transcription 3 (STAT3; indicative of STAT3 activation), which is a known target of leptin (38). The leptin-induced activation of STAT3 was partly attenuated in the presence of a pharmacological dose of WA (39) in both MCF-7 and MDA-MB-231 cells. Leptin-induced colony formation (Figure 6E) and cell migration (Figure 6, F and G) were also suppressed in the presence of WA. These results indicated inhibition of leptin-induced oncogenic signaling by WA treatment in vitro.
Figure 6.
Effect of Withaferin A (WA) treatment on leptin-induced signaling in breast cancer cells. A) Immunoblotting for pSTAT3 (Tyr705), total STAT3, and β-Actin using lysates from MCF-7 cells pretreated with vehicle (DMSO) or WA (1 µM) for two hours and then treated with leptin (100 ng/mL) for one hour in the absence or presence of WA. B) Quantitation of pSTAT3 (Tyr705) level for data shown in (A). Combined results from three independent experiments are shown as mean pSTAT3 (Tyr705) level relative to control with their 95% confidence intervals (error bars, n = 3 for all groups). C) Immunoblotting for pSTAT3 (Tyr705), total STAT3, and β-Actin using lysates from MDA-MB-231 cells treated with vehicle (DMSO), leptin, and/or WA as described in (A). D) Quantitation of pSTAT3 (Tyr705) level for data shown in (C). Combined results from three independent experiments are shown as mean pSTAT3 (Tyr705) level relative to control with their 95% confidence intervals (error bars, n = 3 for all groups). E) Quantitation of colony formation in MCF-7 and MDA-MB-231 cells treated with vehicle (DMSO), leptin (100 ng/mL), WA (20 or 40 nM), or leptin and WA combination for 10 days. Results shown are mean percentage of colony-forming efficiency (% of control) from three independent experiments with their corresponding 95% confidence intervals (error bars, n = 9 for all groups). Inset shows representative images for colony formation for MCF-7 and MDA-MB-231 cells. F) Representative images for migration of MDA-MB-231 cells treated with vehicle (DMSO) or leptin (100 ng/mL), WA (1 µM), or WA and leptin combination for 24 hours (×100 magnification, scale bar = 500 µm). G) Quantitation of cell migration data shown in (F). Results shown are mean cell migration (fold change) of three independent experiments with their corresponding 95% confidence intervals (error bars, n = 9 for all groups). Statistical significance of difference was analyzed by Bonferroni’s multiple comparison test. All P values were two-sided.
Discussion
We have shown previously that the burden, but not the overall incidence, of ER- breast cancer in MMTV-neu mice is inhibited significantly by three times per week treatment with 0.1 mg WA per mouse (equates to ∼4 mg/kg body weight) for 28 weeks (17). We reasoned that a higher dose and/or more frequent administration of WA might be necessary to achieve inhibition of breast cancer incidence. Generally, the rat equivalent of a mouse dose is approximately 50% lower because of metabolic differences (40). Indeed, the WA regimen intensification with five times per week treatment in the present study (4 mg/kg and 8 mg/kg rat doses correspond to ∼8 and 16 mg/kg body weight for mice, respectively) resulted in inhibition of cancer incidence. Similar to the MMTV-neu study, the WA regimen was well tolerated by the rats.
Because breast cancer is a heterogeneous disease, it is clinically attractive to identify chemopreventive interventions that are efficacious against different subtypes of the disease. Identification of a mechanistic biomarker is especially important for clinical development of a chemopreventive intervention. The present study identifies several disease subtype–independent biomarkers of WA potentially useful in future clinical investigations, including increased mitotic index in the tumor, decreased plasma and/or tumor levels of lactate, malate, and acetyl-CoA (noninvasive biomarkers in plasma), inhibition of tumor complex III activity, inhibition of bCSC fraction (tumor ALDH1 activity), Bak induction, and increased tumor cell apoptosis. Most of these biomarkers were similarly modulated by WA treatment in preclinical models of ER+ (present study) and ER- (MMTV-neu model; 17) breast cancers.
The present study shows that the protein level of FoxQ1 is significantly decreased in vivo in the mammary tumors of WA-treated rats. Thus, FoxQ1 appears to be a critical mechanistic target of bCSC inhibition by WA. Overexpression of FoxQ1 has been reported in high-grade basal-like breast cancers and associated with poor clinical outcome (33). FoxQ1 is also implicated in promotion of epithelial-mesenchymal transition and cancer metastasis (33,41,42). In this context, we have shown previously inhibition of epithelial-mesenchymal transition by WA in breast cancer cells (43).
It is equally exciting to note that WA administration not only decreases circulating levels of leptin in vivo in the rat but also inhibits oncogenic signaling (STAT3 activation) induced by this adipokine in cultured human breast cancer cells. A role for leptin in breast carcinogenesis is substantiated by the epidemiological data, as well as by experimental studies (35,36,44,45). Leptin level is positively correlated with obesity (46). Blood leptin levels were found to be greater than twofold higher in obese women than those with normal weight and body mass index (47). In addition, both serum leptin levels and leptin and body mass index ratio were significantly greater in patients with breast cancer (36). The mechanism underlying mammary tumor growth promotion by leptin is complex and involves activation/induction of multiple oncogenic signaling pathways, including STAT3 (38,46). The present study reveals in vitro inhibition of leptin-induced STAT3 activation, cell proliferation, and cell migration following WA exposure.
The intraperitoneal route of WA administration is a potential limitation of this study because only the oral administration is practical for chemopreventive intervention for chronic administration in high-risk subjects. Based on the results of a study published since submission of this article (48), it is reasonable to postulate that chemoprevention of breast cancer may be plausible with oral WA administration. Specifically, oral administration of WA (3–5 mg/kg body weight) resulted in chemoprevention in a rather aggressive transgenic mouse model of prostate cancer (48). A human equivalent of the oral 5 mg/kg body weight mouse dose is about 0.42 mg/kg (about 25 mg WA for a 60 kg body weight subject) based on guidance from the United States Food and Drug Administration Center for Drug Evaluation and Research. While the maximally tolerated dose for WA in humans is yet to be established, three times daily administration of 400 mg Withania somnifera extract (WSE; the plant from which WA is derived) for one month (equates to about 18 mg WA in each 400 mg WSE capsule) was not only well tolerated but also resulted in a statistically significant decrease in levels of serum triglycerides and fasting blood glucose (49). Moreover, the no observed adverse effect level (NOAEL) of the same WSE preparation was 2000 mg/kg in a toxicity study in Wistar rats after daily oral administration for 28 days (50). The LD50 of oral WSE in Wistar rats was greater than 2000 mg/kg body weight (50). We are encouraged by these findings, which would be helpful in the design of future clinical trials.
Funding
This investigation was supported by the National Cancer Institute at the National Institutes of Health grant award RO1 CA142604-07 (to SVS). This research project used the Animal Facility, the Flow Cytometry Facility, the Cancer Pharmacokinetics and Pharmacodynamics Facility, the Proteomics Facility, the Biostatistics Facility, and the Tissue and Research Pathology Facility supported in part by a grant from the National Cancer Institute at the National Institutes of Health (P30 CA047904). The funder had no role in the design or execution of the experiments or in the preparation of the manuscript.
Notes
Suman K. Samanta, Anuradha Sehrawat, and Eun-Ryeong Hahm were responsible for the treatment of rats, determination of body weights, necropsy, collection of tissues, and data collection. Power calculation and statistical analyses were performed by Yongli Shuai. Susan M. Christner and Jan H. Beumer were responsible for determination of WA levels in the rat plasma and tumors. Experiments related to leptin signaling and ALDH1 activity were conducted by Su-Hyeong Kim. Immunoblotting, immunohistochemical analyses, determination of in vitro kinetics of mitotic exit, and lactate, malate, and acetyl-CoA quantitations were performed by Suman K. Samanta, Anuradha Sehrawat, Ruchi Roy, Subrata K. Pore, Eun-Ryeong Hahm, and Krishna B. Singh. Each listed author, including Nancy E. Davidson, contributed to preparation of the manuscript. Shivendra V. Singh was responsible for the overall experimental design and supervision of the experiments, data interpretation, and preparation of the final manuscript.
The authors thank Shveta Hooda for assistance with histopathology.
None of the authors declare any conflict of interest.
Supplementary Material
References
- 1. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2016. CA Cancer J Clin. 2016;661:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Perou CM, Sørlie T, Eisen MB et al. , Molecular portraits of human breast tumours. Nature. 2000;4066797:747–752. [DOI] [PubMed] [Google Scholar]
- 3. Sotiriou C, Neo SY, McShane LM et al. , Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003;10018:10393–10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Umar A, Dunn BK, Greenwald P.. Future directions in cancer prevention. Nat Rev Cancer. 2012;1212:835–848. [DOI] [PubMed] [Google Scholar]
- 5. Fisher B, Costantino JP, Wickerham DL et al. , Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;9018:1371–1388. [DOI] [PubMed] [Google Scholar]
- 6. Cauley JA, Norton L, Lippman ME et al. , Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Breast Cancer Res Treat. 2001;652:125–134. [DOI] [PubMed] [Google Scholar]
- 7. Goss PE, Ingle JN, Alés-Martínez JE et al. , Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;36425:2381–2391. [DOI] [PubMed] [Google Scholar]
- 8. Mallick S, Benson R, Julka PK.. Breast cancer prevention with anti-estrogens: Review of the current evidence and future directions. Breast Cancer. 2016;232:170–177. [DOI] [PubMed] [Google Scholar]
- 9. Stan SD, Kar S, Stoner GD, Singh SV.. Bioactive food components and cancer risk reduction. J Cell Biochem. 2008;1041:339–356. [DOI] [PubMed] [Google Scholar]
- 10. Gullett NP, Rahul Amin ARM, Bayraktar S et al. , Cancer prevention with natural compounds. Semin Oncol. 2010;373:258–281. [DOI] [PubMed] [Google Scholar]
- 11. Lee KW, Bode AM, Dong Z.. Molecular targets of phytochemicals for cancer prevention. Nat Rev Cancer. 2011;113:211–218. [DOI] [PubMed] [Google Scholar]
- 12. Vyas AR, Singh SV.. Molecular targets and mechanisms of cancer prevention and treatment by withaferin A, a naturally occurring steroidal lactone. AAPS J. 2014;161:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shohat B, Gitter S, Abraham A, Lavie D.. Antitumor activity of withaferin A (NSC-101088). Cancer Chemother Rep.1967;515:271–276. [PubMed] [Google Scholar]
- 14. Antony ML, Lee J, Hahm ER et al. , Growth arrest by the antitumor steroidal lactone withaferin A in human breast cancer cells is associated with down-regulation and covalent binding at cysteine 303 of β-tubulin. J Biol Chem. 2014;2893:1852–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stan SD, Hahm ER, Warin R, Singh SV.. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 2008;6818:7661–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hahm ER, Moura MB, Kelley EE, Van Houten B, Shiva S, Singh SV.. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS One. 2011;68:e23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hahm ER, Lee J, Kim SH et al. , Metabolic alterations in mammary cancer prevention by withaferin A in a clinically relevant mouse model. J Natl Cancer Inst. 2013;10515:1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hahm ER, Lee J, Huang Y, Singh SV.. Withaferin A suppresses estrogen receptor-α expression in human breast cancer cells. Mol Carcinog. 2011;508:614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hahm ER, Singh SV.. Withaferin A-induced apoptosis in human breast cancer cells is associated with suppression of inhibitor of apoptosis family protein expression. Cancer Lett. 2013;3341:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hahm ER, Lee J, Singh SV.. Role of mitogen-activated protein kinases and Mcl-1 in apoptosis induction by withaferin A in human breast cancer cells. Mol Carcinog. 2014;5311:907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Russo J, Gusterson BA, Rogers AE, Russo IH, Wellings SR, van Zwieten MJ.. Comparative study of human and rat mammary tumorigenesis. Lab Invest. 1990;623:244–278. [PubMed] [Google Scholar]
- 22. Chan MM, Lu X, Merchant FM, Iglehart JD, Miron PL.. Gene expression profiling of NMU-induced rat mammary tumors: Cross species comparison with human breast cancer. Carcinogenesis. 2005;268:1343–1353. [DOI] [PubMed] [Google Scholar]
- 23. Smolarek AK, So JY, Burgess B et al. , Dietary administration of δ- and γ-tocopherol inhibits tumorigenesis in the animal model of estrogen receptor-positive, but not HER-2 breast cancer. Cancer Prev Res (Phila). 2012;511:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh SV, Kim SH, Sehrawat A et al. , Biomarkers of phenethyl isothiocyanate-mediated mammary cancer chemoprevention in a clinically relevant mouse model. J Natl Cancer Inst. 2012;10416:1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Powolny AA, Bommareddy A, Hahm ER et al. , Chemopreventative potential of the cruciferous vegetable constituent phenethyl isothiocyanate in a mouse model of prostate cancer. J Natl Cancer Inst. 2011;1037:571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim SH, Singh SV.. Mammary cancer chemoprevention by withaferin A is accompanied by in vivo suppression of self-renewal of cancer stem cells. Cancer Prev Res (Phila). 2014;77:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee J, Hahm ER, Singh SV.. Withaferin A inhibits activation of signal transducer and activator of transcription 3 in human breast cancer cells. Carcinogenesis. 2010;3111:1991–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee J, Sehrawat A, Singh SV.. Withaferin A causes activation of Notch2 and Notch4 in human breast cancer cells. Breast Cancer Res Treat. 2012;1361:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thompson HJ, Adlakha H.. Dose-responsive induction of mammary gland carcinomas by the intraperitoneal injection of 1-methyl-1-nitrosourea. Cancer Res. 1991;5113:3411–3415. [PubMed] [Google Scholar]
- 30. Gottardis MM, Jordan VC.. Antitumor actions of keoxifene and tamoxifen in the N-nitrosomethylurea-induced rat mammary carcinoma model. Cancer Res. 1987;4715:4020–4024. [PubMed] [Google Scholar]
- 31. Lubet RA, Steele VE, Casebolt TL, Eto I, Kelloff GJ, Grubbs CJ.. Chemopreventive effects of the aromatase inhibitors vorozole (R-83842) and 4-hydroxyandrostenedione in the methylnitrosourea (MNU)-induced mammary tumor model in Sprague-Dawley rats. Carcinogenesis. 1994;1512:2775–2780. [DOI] [PubMed] [Google Scholar]
- 32. O’Brien CS, Farnie G, Howell SJ, Clarke RB.. Breast cancer stem cells and their role in resistance to endocrine therapy. Horm Cancer. 2011;22:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qiao Y, Jiang X, Lee ST, Karuturi RK, Hooi SC, Yu Q.. FOXQ1 regulates epithelial-mesenchymal transition in human cancers. Cancer Res. 2011;718:3076–3086. [DOI] [PubMed] [Google Scholar]
- 34. Kim SH, Kaschula CH, Priedigkeit N, Lee AV, Singh SV.. Forkhead box Q1 is a novel target of breast cancer stem cell inhibition by diallyl trisulfide. J Biol Chem. 2016;29126:13495–13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gross AL, Newschaffer CJ, Hoffman-Bolton J, Rifai N, Visvanathan K.. Adipocytokines, inflammation, and breast cancer risk in postmenopausal women: A prospective study. Cancer Epidemiol Biomarkers Prev. 2013;227:1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Romero-Figueroa Mdel S, Garduño-García Jde J, Duarte-Mote J, Matute-González G, Gómez-Villanueva A, De la Cruz-Vargas J.. Insulin and leptin levels in obese patients with and without breast cancer. Clin Breast Cancer. 2013;136:482–485. [DOI] [PubMed] [Google Scholar]
- 37. Delort L, Rossary A, Farges MC, Vasson MP, Caldefie-Chézet F.. Leptin, adipocytes and breast cancer: Focus on inflammation and anti-tumor immunity. Life Sci. 2015;140:37–48. [DOI] [PubMed] [Google Scholar]
- 38. Dieudonne MN, Machinal-Quelin F, Serazin-Leroy V, Leneveu MC, Pecquery R, Giudicelli Y.. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2002;2931:622–628. [DOI] [PubMed] [Google Scholar]
- 39. Thaiparambil JT, Bender L, Ganesh T et al. , Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int J Cancer. 2011;12911:2744–2755. [DOI] [PubMed] [Google Scholar]
- 40. Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE.. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;504:219–244. [PubMed] [Google Scholar]
- 41. Zhang H, Meng F, Liu G et al. , Forkhead transcription factor Foxq1 promotes epithelial-mesenchymal transition and breast cancer metastasis. Cancer Res. 2011;714:1292–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ross JB, Huh D, Noble LB, Tavazoie SF.. Identification of molecular determinants of primary and metastatic tumour re-initiation in breast cancer. Nat Cell Biol. 2015;175:651–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee J, Hahm ER, Marcus AI, Singh SV.. Withaferin A inhibits experimental epithelial-mesenchymal transition in MCF-10A cells and suppresses vimentin protein level in vivo in breast tumors. Mol Carcinog. 2015;546:417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zheng Q, Dunlap SM, Zhu J et al. , Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr Relat Cancer. 2011;184:491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mauro L, Catalano S, Bossi G et al. , Evidences that leptin up-regulates E-cadherin expression in breast cancer: Effects on tumor growth and progression. Cancer Res. 2007;677:3412–3421. [DOI] [PubMed] [Google Scholar]
- 46. Andò S, Catalano S.. The multifactorial role of leptin in driving the breast cancer microenvironment. Nat Rev Endocrinol. 2011;85:263–275. [DOI] [PubMed] [Google Scholar]
- 47. Carroll PA, Healy L, Lysaght J et al. , Influence of the metabolic syndrome on leptin and leptin receptor in breast cancer. Mol Carcinog. 2011;508:643–651. [DOI] [PubMed] [Google Scholar]
- 48. Suman S, Das TP, Moselhy J et al. , Oral administration of withaferin A inhibits carcinogenesis of prostate in TRAMP model. Oncotarget. 2016;733:53751–53761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Agnihotri AP, Sontakke SD, Thawani VR, Saoji A, Goswami VS.. Effects of Withania somnifera in patients of schizophrenia: A randomized, double blind, placebo controlled pilot trial study. Indian J Pharmacol. 2013;454:417–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Patel SB, Rao NJ, Hingorani LL.. Safety assessment of Withania somnifera extract standardized for withaferin A: Acute and sub-acute toxicity study. J Ayurveda Integr Med. 2016;71:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.