Abstract
Early-life adversity is a well-established risk factor for the development of depression later in life. Here we discuss the relationship between early-life adversity and depression, focusing specifically on effects of early-life caregiver deprivation on alterations in the neural and behavioral substrates of reward-processing. We also examine vulnerability to depression within the context of sensitive periods of neural development and the timing of adverse exposure. We further review the development of the ventral striatum, a limbic structure implicated in reward processing, and its role in depressive outcomes following early-life adversity. Finally, we suggest a potential neurobiological mechanism linking early-life adversity and altered ventral striatal development. Together these findings may help provide further insight into the role of reward circuitry dysfunction in psychopathological outcomes in both clinical and developmental populations.
Keywords: adolescence, caregiver deprivation, depression, early-life adversity, ventral striatum
Introduction
Early-life stress can be defined as exposure to adverse events during childhood that negatively impact emotional or physical well-being to an extent that exceeds an individual’s ability to cope.1 Considerable evidence suggests that such negative experiences are associated with the development of depressive disorders.2–4 Specifically, early life seems to be particularly sensitive to environmental hardships that increase depression risk.5 Previous research indicates that exposure to early-life adversity may alter neurobiological development, including those regions that regulate responsiveness to reward, which may in turn influence depressive outcomes later in life.
This review will examine the link between early-life adversity and depression. First, we will provide a brief overview of the epidemiology of early-life adversity and depression. Because timing of exposure to these stressors is critically important in depressive outcomes, we will also discuss the issue of sensitive periods, leading to a review of the main neurobiological indices of depression, including the critical role of reward-related neural circuitry and the development of this circuit. Thus, we will attempt to address the underlying neurobiological mechanisms relating early-life adversity to depressive outcomes, specifically as related to reward processing.
Early Adversity and Depression
Early-life adversity encompasses environmental exposure to abuse, neglect, distress, and negative family relations, among other negative experiences during the infancy/toddler period. These adverse exposures occur at every socioeconomic level, across ethnic and cultural lines, and at all levels of education. In the United States alone, more than 3,000,000 reports of child abuse involving more than 6,000,000 youth are reported each year.6 While the U.S. has one of the highest rates of child maltreatment/neglect among industrialized nations, these numbers only reflect a fraction of domestic early-life adversity exposures. Of note, more than 33% of confirmed cases of maltreatment affect children under 4 years old, while 24% of cases are 4–7 years old, 18% of cases are 8–11 years old, and 16% of cases are 12–15 years old.7 These statistics suggest that early-life adversity is not uncommon, particularly among young children—an important distinction, as early childhood may represent a period of heightened vulnerability to the negative effects of stress,1 and differential psychological outcomes may be dependent upon the specific timing of exposure.8
The scientific literature overwhelmingly demonstrates an association between early-life adversity and depression.9–14 Though genetic factors have been shown to influence vulnerability for depression following early-life adversity,15 twin studies demonstrate that the effects of adverse environments play a substantial role in depressive outcomes beyond the influence of genetics.16,17 Clinical evidence highlights a dose-response relationship between early-life adversity and mental health in adulthood,18 specifically with regard to the severity of early-life adversity and lifetime chronic depression.9 For example, the risk of depression in persons with multiple early-life adverse experiences is 4 times that of a person who has not experienced early-life trauma.19 The results from these clinical studies support outcomes that have been reported in epidemiological research. A 17-year longitudinal study examining more than 750 randomly selected children found that adolescents and young adults with a history of childhood maltreatment were 3 times more likely to become depressed compared with individuals without such a history.10 Thus, vulnerability for depression may increase linearly with both the quantity and severity of adverse experiences, suggesting a possible causal link between early-life adversity and depression.20
According to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), depression is characterized by the presence of the majority of 9 symptoms: depressed mood, loss of interest or pleasure, disturbed sleep, disturbed appetite, anxiety, low energy, feelings of guilt or low self-worth, poor concentration, and thoughts about death.21 While categorical diagnostic systems remain a valuable tool, recent advances in the understanding of psychopathology have given rise to a new multidimensional framework for conceptualizing mental health. The dimensional approach of the Research Domain Criteria (RDoC) matrix,22 an integrative diagnostic system, suggests that the anhedonic aspects of depression (eg, loss of pleasure), which are a central feature of depression,23–25 are consistent with dysfunction of the positive valence system, which includes measures of behavioral and neural responsiveness to reward. Thus, alterations in the neural circuitry that supports reward processing may underlie the emergence of depression following early-life adversity.
Of note, early-life adversity is not limited only to vulnerability for depressive outcomes, but instead constitutes a major risk factor for the development of numerous psychological disorders.26–30 However the onset for the majority of these disorders occurs during childhood, and precedes the emergence of depressive symptoms not seen until the adolescent period.31–33 Therefore we will argue that the protracted emergence of depression following early-life adversity may implicate those neural regions that also demonstrate protracted developmental trajectories—one of which is the reward circuit.
The Role of Timing
Because adversity has been identified as a key experiential factor that programs and modifies brain development,34,35 a comprehensive understanding of the mechanisms involved in depressive outcomes following early-life adversity requires that we evaluate sensitive periods in neural development. Sensitive periods are characterized by periods of neural plasticity during which neural development may be especially vulnerable to environmental influence.36 Environmental influence may in turn have profound effects on physical, social, and emotional development36–38; the brain may be particularly vulnerable to negative experiences, allowing for exaggerated effects of these experiences on neural development.38 Although childhood maltreatment encompasses a variety of behaviors, we will focus our discussion on caregiver deprivation (ie, maternal deprivation*)—an unfortunate, yet robust example of early-life adversity39 —which has been most studied in animal models to allow for translation across studies.
The potential negative impacts of caregiver deprivation are timing-specific. Each neural system has unique sensitive periods, and therefore, exposures to adversity at different ages should lead to differential neuro-behavioral phenotypes. Here we will address time-sensitive alterations in the hypothalamic–pituitary–adrenal (HPA) axis, one of the primary stress axes in mammals.40 The HPA axis displays altered function following early-life caregiver deprivation,41 and this dysfunction has been shown to increase vulnerability to depression.42 Furthermore, HPA axis dysfunction is particularly prevalent in individuals with anhedonic depression,43–45 and thus early-life alterations in HPA function may represent one mechanism by which long-term alterations in neural reward circuitry may occur.
Timing of adverse environmental exposures, in the form of caregiver deprivation, and the function of the HPA axis have been well studied in nonhuman animals. Rodent research has shown that the effects of caregiver deprivation on HPA axis function are dependent on the timing of exposure.46,47 Rodents separated on the third postnatal day (PND) demonstrated no immediate alterations in HPA function, whereas HPA responsiveness was markedly elevated in those separated on PND11. Additionally, examination of long-term alterations in stress reactivity indicated that rodents separated on PND3 and PND11 showed hyper and hypo HPA responsiveness, respectively, in adulthood.47 Related work in humans showed that separation from both parents during childhood was associated with increased HPA activation in adulthood, particularly if the separation occurred between ages 2 and 7.48 However, most human studies cannot conclude that their findings are specifically related to the timing of exposure to these adverse experiences. This may be due in part to the fact that most children exposed to early-life adversity continue to be exposed to adverse conditions throughout development. Therefore the unique contribution of early-life adversity to later mental health problems, after taking into account conditions such as family disruptions, persistent poverty, and broader patterns of social and emotional deprivation, remains unclear.49
* Note: Most animal models manipulate the presence/absence of the mother to examine caregiver deprivation. In humans, there is no evidence that this effect of the primary caregiver is specific to the mother, and therefore we will refer to caregiver deprivation when discussing the human literature.
One population that may better articulate the association between early-life adversity and depression is those who experience caregiving deprivation, by virtue of institutional care abroad, and then were adopted by families in the United States. Because previously institutionalized (PI) children often encounter numerous early adverse events followed by a supportive family environment, research on these children may provide information regarding the long-term psychosocial effects of a discrete period of early-life adversity. Youth exposed to this early institutional care exhibit a wide range of psychological outcomes with some children experiencing challenges and others not.50 Indeed, families of adopted post-institutionalized children have been shown to provide exceedingly high quality care, including optimal financial and educational resources, nurturing, and emotional support51 —all of which are important familial features associated with resilience following early childhood adversity. However, this type of early caregiving experience significantly raises the odds for difficulties in emotional development.
Timing of adoption has emerged as an important variable when considering these outcomes. Pollak et al52 found that PI children who were adopted into families after their first birthday had more psychological difficulties, including deficits in learning, memory, and inhibitory control, than children adopted at an earlier age. Similarly, research has shown that PI children who were adopted into families at later ages were more likely to develop behavioral problems, both internalizing and externalizing, when compared to children adopted into families at earlier ages.50,53–55 Later age of adoption has also been associated with atypical structural development of limbic regions involved in emotion regulation (eg, the amygdala).56,57 The timing of adversity is thus a critical variable when examining neurodevelopment, as outcomes can vary significantly depending on age.58–60
Ventral Striatum Development, Depression, and Early Adversity
Despite early environmental insults, depression typically does not emerge until the adolescent period,60–63 a relatively late-emerging phenotype as compared to others discussed above. Therefore, deciphering the causal relationship between early-life experiences and later developing depression can prove difficult. By examining the neurodevelopmental mechanisms involved in both early-life adversity and depression, we may begin to develop a framework by which the relationship between early-life adversity and depression is better understood.
Evidence suggests that varied depressive symptoms (ie, poor concentration, thoughts of death) and subtypes (ie, melancholic, seasonal) are likely mediated by different neurochemical mechanisms and may or may not be present in any particular individual with depression.64–66 Research has long focused on symptoms of negative affect in depressive outcomes, which implicates dysfunction in neural regions that include the amygdala, hippocampus, anterior cingulate cortex, and prefrontal cortex67–70 —structures commonly associated with emotion regulation.71,72 While increased negative affect is an established characteristic of depressed individuals,73 a greater emphasis is now being placed on the anhedonic aspects, or atypical positive reward-related functioning, as an important aspect of depression.74–79 Thus, depression may represent dysfunction in regions implicated in emotion regulation and regions responsible for reward processing, the combined effect of which may reflect the depressive characteristic of concurrent high negative affect and low positive affect.73
Evidence for the role of atypical positive, or reward-related, processing in depression could implicate dysfunction in the responsiveness of mesolimbic dopamine circuits,80 which are stress-sensitive,81,82 as a potential underlying neural mechanism of depression.83 The ventral striatum, a neural structure within the reward circuit, serves as a primary target of dopamine neuron projections,84–87 and, as will be discussed in the following sections, reaches its developmental peak during adolescence. This peak in ventral striatal development coincides with the typical age of onset for depression following early-life adversity,55,88,89 and previous research has shown that dysfunction in this region has been associated with depressive symptoms in this population.88,90 Indeed, research provides evidence for the role of the ventral striatum in reward learning and motivation,91–95 and ventral striatum dysfunction has been robustly associated with depression.80,96–99 Moreover the ventral striatum in healthy individuals has been shown to be highly modulated by the social environment100–102; notably, individuals with depression struggle with social behaviors.103 Though the neural reward system is a highly complex and interconnected circuit involving a network of cortical and subcortical structures,86 given this evidence, we will focus our discussion on the role of the ventral striatum (comprising the nucleus accumbens, ventral caudate, and ventral putamen) within this circuit.
Ventral Striatal Functional Development
Though much of the brain develops before birth and during early childhood,104 the ventral striatum has been shown to develop in an inverted “U”-shaped pattern, such that the functional development of the striatum peaks in adolescence and then decreases into early adulthood.105–109 This inverted “U”-shaped pattern seen in the development of the striatum is paralleled by behavioral patterns of increased reward sensitivity during adolescence.108 Importantly, depressive symptoms most commonly emerge during the adolescent period.60,62,110,111
As depression is being increasingly understood as arising from atypical maturational changes in the brain,62 adolescence may reflect one period during which neural regions implicated in depression are vulnerable to dysregulation as a consequence of their remodeling.62,76 Thus alterations in the development of reward-related circuitry may provide one explanation for the emergence of depression in adolescence.
Altered Ventral Striatum Function Following Early Adversity
Given the strong associations found between depression and hypofunctioning in the ventral striatum,112 we turn our focus to the ventral striatum’s role in depression following early adversity. In our own laboratory, we have observed that PI youth are at significantly higher risk for depressive behaviors; the risk increases between childhood and adolescence,88 and this finding is highly consistent with other laboratories.55,89 This increase in depressive behaviors was associated with hypoactivity in the ventral striatum in the PI adolescents88 —a neural characteristic that has been demonstrated in other PI groups90.
There is strong evidence for the role of atypically low ventral striatum activity in depressive outcomes following early-life adversity exposure. Rodents exposed to early adverse experiences have demonstrated altered function of dopaminergic pathways,113–115 including alterations in the function of the ventral striatum115,116 and reduced responsiveness to reward.117 Corroborating studies in humans have found that individuals with a history of early-life maltreatment displayed dampened behavioral responsiveness to reward and reduced activation in striatal structures,118 and that children who experienced early-life adversity in the form of caregiver deprivation exhibit hyporesponsivity in the ventral striatum in response to reward during adolescence.90 We have similarly found reduced striatal activation to rewarding stimuli during adolescence in PI youth, which was associated with higher levels of depression.88
Potential Mechanisms Linking Early-Life Adversity and Altered Ventral Striatal Development
While the relationship between deficits in reward-related processing and depression is generally well understood, we know less about the precise neurobiological mechanism that underlies this relationship. One hypothesis suggests that the reduced ability to experience pleasure (anhedonia) is driven by reduced dopaminergic transmission, resulting in hypoactivation of reward-related neural circuits, which include the ventral striatum.119 Indeed, there is considerable evidence that dopamine plays a core role in the neural reward system.120 Research in rodents and humans suggests that suppression of dopaminergic neurotransmission mediates the anhedonia of drug withdrawal in addiction,121–125 while other research has suggested that blunted dopamine transmission may serve as a unique biological marker for anhedonia.126,127 Dopamine may also specifically mediate the hedonic properties of food, drugs, and other rewards.120,128,129 Importantly, there is also evidence suggesting that early-life caregiver deprivation alters dopamine function in the ventral striatum.130–132 Therefore it may be that alterations in dopamine transmission early in life influence later ventral striatum functioning, particularly during the sensitive adolescent period, which serves as the underlying mechanism in adolescent-emergent depression following early-life adversity.
Though the association between early-life adversity and altered function of the ventral striatum is well established,55,88,89 the neurochemical pathways by which early adversity alters ventral striatal development have not yet been well characterized. Nonetheless, there is some evidence that, for those who have experienced early-life adversity, striatal alterations may be the consequence of dysfunction in the HPA axis. Indeed emerging research has linked variation in HPA axis activity with functional and structural differences in striatal regions central to reward processing.133–135 As discussed earlier, much attention has focused on the ability of early-life adversity to atypically program the HPA axis.42,136 Following early adversity, dysregulation of the HPA axis results in persistent dysregulation of glucocorticoid secretion, which has been causally linked to depression.13,137,138 Of note, the psychosocial effects of HPA dysfunction differ across development. Research in rodents has demonstrated that early-life neglect–induced HPA alterations may result in numerous social behavior deficits, though specific depressive-like behaviors do not emerge until the adolescent period,63 which may reflect the delayed impact of these alterations on reward-processing. That is, given the adolescent emergence of ventral striatum reactivity, we would anticipate the cascading effect of HPA axis dysregulation to be observed at that time.
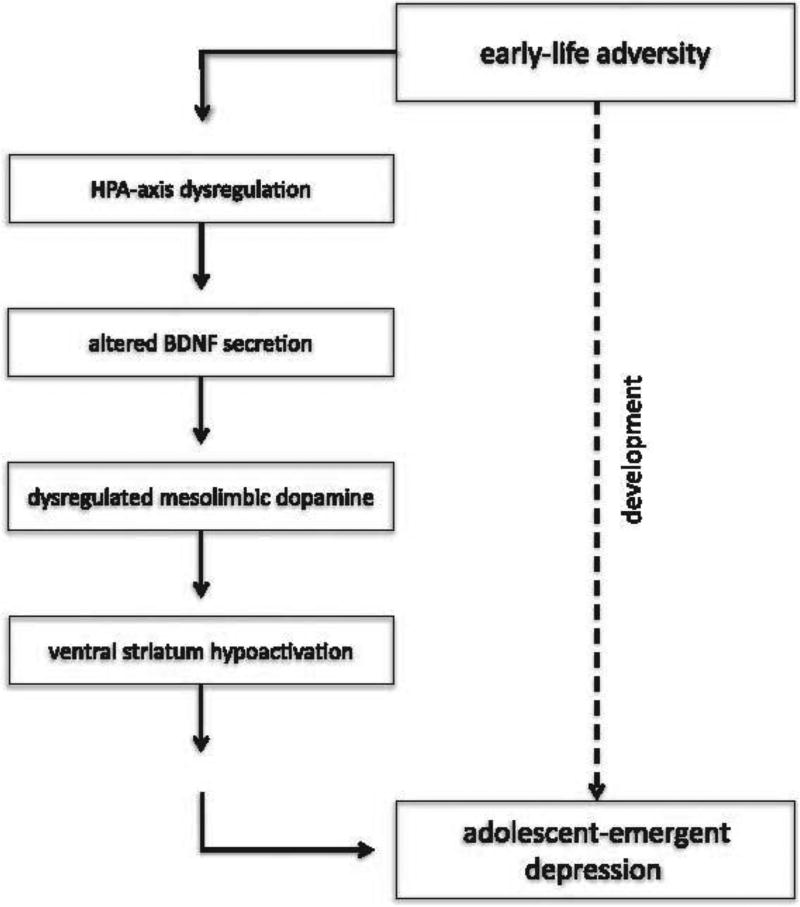
Here we propose a potential mechanism by which HPA axis dysfunction following early-life adversity may in turn alter ventral striatum function in adolescence. In rodents, the effects of HPA dysfunction specific to striatal development have been investigated via exposure to both early-life adversity and direct glucocorticoid injection. Results showed that increased glucocorticoid exposure early in life resulted in volumetric reductions of the nucleus accumbens,139,140 asubstructure of the ventral striatum, as well as decreased density in mesolimbic dopamine receptors141,142 in the nucleus accumbens. Additionally, rodents exposed to early caregiver deprivation showed reduced dopamine function in the striatum during adulthood.143 The relationship between glucocorticoids and dysfunction of the striatum may also involve the activity of brain-derived neurotrophic factor (BDNF), the neurotrophin that serves as the key regulator of the mesolimbic dopamine pathway.144–146 It has been hypothesized that specific adverse effects of glucocorticoids may involve attenuation of BDNF expression or signaling.147 Indeed, caregiver deprivation has been shown to induce long-term changes in BDNF expression in the striatum,148 and reductions in BDNF have been strongly associated with depression in adulthood.149–154 Thus, it may be that early-life adversity results in HPA axis–induced glucocortiocoid secretion, which influences reductions in BNDF. These alterations in turn compromise the function of the mesolimbic dopamine system early in life, which results in hypoactivation of the ventral striatum to reward—a hallmark of anhedonic depression—during the adolescent period (Figure 1).
Figure 1.
Illustration of the proposed model demonstrating a neurobiological mechanism by which early-life adversity may result in adolescent depression.
Conclusions
Previous research has established the role of stress, anhedonia, and dopamine on depressive outcomes,82,155 and has laid the groundwork for characterization of potential mechanistic links between early-life adversity and ventral striatal hypofunction during adolescence, which will be an important step toward understanding how early experiences increase the risk for later depression. While the antecedents of depression are complex and not fully understood, there is increasing evidence to suggest that the association between early-life adversity and depression in later life is largely mediated by stress-induced alterations to the ventral striatum. Due in part to sensitive periods in neural plasticity, evidence suggests that early life is a time of particular vulnerability, and the timing of adverse environmental experiences is critical for depressive outcomes. Neurobiological research indicates that changes in the development of neural mechanisms that influence reward processing may impact depression risk later in life. In adults, typical development of the reward circuit results in activation of the ventral striatum, serving to mediate balanced reward-seeking behaviors. However, in cases of atypical development of neural reward circuitry, imbalances in striatal activation may result in psychopathological outcomes, such as anhedonia, a common symptom seen in depressed individuals. These findings may help to further elucidate the mechanisms underlying dysfunction in this circuitry that may result in psychopathological outcomes in both clinical and developmental populations.
Acknowledgments
This work was supported by NIMH R01MH091864 (NT) and The Dana Foundation.
Footnotes
Disclosures
Nim Tottenham and Bonnie Goff have nothing to disclose.
References
- 1.Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 2.Kendler K. Toward a comprehensive developmental model for major depression in women. Am J Psychiatry. 2002;159(7):1133–1145. doi: 10.1176/appi.ajp.159.7.1133. [DOI] [PubMed] [Google Scholar]
- 3.Maier W. Genetic epidemiology of psychiatric disorders. Eur Arch Psychiatry Clin Neurosci. 1993;243(3–4):119–120. doi: 10.1007/BF02190717. [DOI] [PubMed] [Google Scholar]
- 4.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 5.Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52(7):671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- 6.Child Welfare Information Gateway. Determining the best interests of the child. Washington, DC: Child Welfare Information Gateway; 2012. Available at: https://www.childwelfare.gov/systemwide/laws_policies/statutes/best_interest.pdf. [Google Scholar]
- 7.Cornell University, College of Human Ecology. National Data Archive on Child Abuse and Neglect. http://www.ndacan.cornell.edu.
- 8.Maercker A, Michael T, Fehm L, Becker ES, Margraf J. Age of traumatisation as a predictor of post-traumatic stress disorder or major depression in young women. Br J Psychiatry. 2004;184(6):482–487. doi: 10.1192/bjp.184.6.482. [DOI] [PubMed] [Google Scholar]
- 9.Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. 2004;82(2):217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Brown J, Cohen P, Johnson JG, Smailes EM. Childhood abuse and neglect: specificity of effects on adolescent and young adult depression and suicidality. J Am Acad Child Adolesc Psychiatry. 1999;38(12):1490–1496. doi: 10.1097/00004583-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 11.McCauley J, Kern DE, Kolodner K, et al. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA. 1997;277(17):1362–1368. [PubMed] [Google Scholar]
- 12.Mullen PE, Martin JL, Anderson JC, Romans SE, Herbison GP. The long-term impact of the physical, emotional, and sexual abuse of children: a community study. Child Abuse Negl. 1996;20(1):7–21. doi: 10.1016/0145-2134(95)00112-3. [DOI] [PubMed] [Google Scholar]
- 13.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 14.Agid O, Shapira B, Zislin J, et al. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4(2):163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- 15.Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. The role of corticotropin-releasing factor–norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol Psychiatry. 1999;46(9):1153–1166. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- 16.Kendler KS, Neale MC, Kessler RC, Heath aC, Eaves LJ. A population-based twin study of major depression in women: the impact of varying definitions of illness. Arch Gen Psychiatry. 1992;49(4):257–266. doi: 10.1001/archpsyc.1992.01820040009001. [DOI] [PubMed] [Google Scholar]
- 17.Romanov K, Varjonen J, Kaprio J, Koskenvuo M. Life events and depressiveness—the effect of adjustment for psychosocial factors, somatic health and genetic liability. Acta Psychiatr Scand. 2003;107(1):25–33. doi: 10.1034/j.1600-0447.2003.01419.x. [DOI] [PubMed] [Google Scholar]
- 18.Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. 2003;160(8):1453–1460. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- 19.Felitti VJ, Anda RF, Nordenberg D, et al. Household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) study. Am J Prev Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 20.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156(6):837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4 text rev. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 22.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 23.Chiu PH, Deldin PJ. Neural evidence for enhanced error detection in major depressive disorder. Am J Psychiatry. 2007;164(4):608–616. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- 24.Loas G, Boyer P. Anhedonia in endogenomorphic depression. Psychiatry Res. 1996;60(1):57–65. [Google Scholar]
- 25.Snaith P. Anhedonia: a neglected symptom of psychopathology. Psychol Med. 1993;23(4):957–966. doi: 10.1017/s0033291700026428. [DOI] [PubMed] [Google Scholar]
- 26.Cicchetti D, Toth SL. Child maltreatment. Annu Rev Clin Psychol. 2005;1:409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029. [DOI] [PubMed] [Google Scholar]
- 27.Gunnar MR. Integrating neuroscience and psychological approaches in the study of early experiences. Ann N Y Acad Sci. 2003;1008:238–247. doi: 10.1196/annals.1301.024. [DOI] [PubMed] [Google Scholar]
- 28.Heim C, Bradley B, Mletzko TC, et al. Effect of childhood trauma on adult depression and neuroendocrine function: sex-specific moderation by CRH receptor 1 gene. Front Behav Neurosci. 2009;3:41. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stovall-McClough KC, Cloitre M. Unresolved attachment, PTSD, and dissociation in women with childhood abuse histories. J Consult Clin Psychol. 2006;74(2):219–228. doi: 10.1037/0022-006X.74.2.219. [DOI] [PubMed] [Google Scholar]
- 30.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27(1–2):33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 31.Letcher P, Smart D, Sanson A, Toumbourou JW. Psychosocial precursors and correlates of differing internalizing trajectories from 3 to 15 years. Social Development. 2009;18(3):618–646. [Google Scholar]
- 32.Mason WA, Kosterman R, Hawkins JD, Herrenkohl TI, Lengua LJ, McCauley E. Predicting depression, social phobia, and violence in early adulthood from childhood behavior problems. J Am Acad Child Adolesc Psychiatry. 2004;43(3):307–315. doi: 10.1097/00004583-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Mazza JJ, Abbott RD, Fleming CB, et al. Early predictors of adolescent depression: a 7-year longitudinal study. Journal of Early Adolescence. 2009;29(5):664–692. [Google Scholar]
- 34.Teicher MH, Tomoda A, Andersen SE. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann N Y Acad Sci. 2006;1071:313–323. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- 35.Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on brain structure and function: clinical implications. Biol Psychiatry. 2000;48(8):778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- 36.Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss MJS, Wagner SH. What explains the negative consequences of adverse childhood experiences on adult health? Insights from cognitive and neuroscience research. Am J Prev Med. 1998;14(4):356–360. doi: 10.1016/s0749-3797(98)00011-7. [DOI] [PubMed] [Google Scholar]
- 39.Gunnar MR, Fisher PA. Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Dev Psychopathol. 2006;18(3):651–677. [PubMed] [Google Scholar]
- 40.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886(1–2):172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 41.Loman MM, Gunnar MR Early Experience, Stress, and Neurobehavioral Development Center. Early experience and the development of stress reactivity and regulation in children. Neurosci Biobehav Rev. 2010;34(6):867–876. doi: 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Gold PW, Gabry KE, Yasuda MR, Chrousos GP. Divergent endocrine abnormalities in melancholic and atypical depression: clinical and pathophysiologic implications. Endocrinol Metab Clin North Am. 2002;31(1):37–62. doi: 10.1016/s0889-8529(01)00022-6. [DOI] [PubMed] [Google Scholar]
- 44.Gold PW, Chrousos GP. The endocrinology of melancholic and atypical depression: relation to neurocircuitry and somatic consequences. Proc Assoc Am Physicians. 1999;111(1):22–34. doi: 10.1046/j.1525-1381.1999.09423.x. [DOI] [PubMed] [Google Scholar]
- 45.Pintor L, Torres X, Navarro V, Martinez de Osaba MJ, Matrai S, Gasto C. Corticotropin-releasing factor test in melancholic patients in depressed state versus recovery: a comparative study. Prog Neuropsychopharmacology Biol Psychiatry. 2007;31(5):1027–1033. doi: 10.1016/j.pnpbp.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Coplan JD, Smith ELP, Altemus M, et al. Maternal-infant response to variable foraging demand in nonhuman primates: effects of timing of stressor on cerebrospinal fluid corticotropin-releasing factor and circulating glucocorticoid concentrations. Ann N Y Acad Sci. 2006;1071:525–533. doi: 10.1196/annals.1364.057. [DOI] [PubMed] [Google Scholar]
- 47.Van Oers HJ, de Kloet ER, Levine S. Early vs. late maternal deprivation differentially alters the endocrine and hypothalamic responses to stress. Brain Res Dev Brain Res. 1998;111(2):245–252. doi: 10.1016/s0165-3806(98)00143-6. [DOI] [PubMed] [Google Scholar]
- 48.Pesonen AK, Räikkönen K, Feldt K, et al. Childhood separation experience predicts HPA axis hormonal responses in late adulthood: a natural experiment of World War II. Psychoneuroendocrinology. 2010;35(5):758–767. doi: 10.1016/j.psyneuen.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 49.Mullen PE, Martin JL, Anderson JC, Romans SE, Herbison GP. Childhood sexual abuse and mental health in adult life. Br J Psychiatry. 1993;163(6):721–732. doi: 10.1192/bjp.163.6.721. [DOI] [PubMed] [Google Scholar]
- 50.MacLean K. The impact of institutionalization on child development. Dev Psychopathol. 2003;15(4):853–884. doi: 10.1017/s0954579403000415. [DOI] [PubMed] [Google Scholar]
- 51.Gunnar MR, Bruce J, Grotevant HD. International adoption of institutionally reared children : research and policy. Dev Psychopathol. 2000;12(4):677–693. doi: 10.1017/s0954579400004077. [DOI] [PubMed] [Google Scholar]
- 52.Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka SS, Wiik KL. Neurodevelopmental effects of early deprivation in post-institutionalized children. Child Dev. 2010;81(1):224–236. doi: 10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gunnar MR, van Dulmen MHM International Adoption Project Team. Behavior problems in postinstitutionalized internationally adopted children. Dev Psychopathol. 2007;19(1):129–148. doi: 10.1017/S0954579407070071. [DOI] [PubMed] [Google Scholar]
- 54.Rutter ML, Kreppner JM, O’Connor TG English and Romanian Adoptees (ERA) Study Team. Specificity and heterogeneity in children’s responses to profound institutional privation. Br J Psychiatry. 2001;179(2):97–103. doi: 10.1192/bjp.179.2.97. [DOI] [PubMed] [Google Scholar]
- 55.Zeanah CH, Egger HL, Smyke AT, et al. Institutional rearing and psychiatric disorders in Romanian preschool children. Am J Psychiatry. 2009;166(7):777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- 56.Mehta MA, Golembo NI, Nosarti C, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J Child Psychol Psychiatry. 2009;50(8):943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 57.Tottenham N, Hare TA, Quinn BT, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage. 2014;97:236–244. doi: 10.1016/j.neuroimage.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31(4):183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Angold A, Costello EJ. Puberty and depression. Child Adolesc Psychiatr Clin N Am. 2006;15(4):919–937. ix. doi: 10.1016/j.chc.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 62.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raineki C, Cortés MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. 2012;32(22):7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 65.Stahl S, Zhang L, Damatarca C, Grady M. Brain Circuits Determine Destiny in Depression : A Novel Approach to the Psychopharmacology of wakefulness, fatigue, and executive dysfunction in major depressive disorder. J Clin Psychiatry. 2003;64(Suppl 14):6–17. [PubMed] [Google Scholar]
- 66.Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 67.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48(8):813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 69.Anand A, Li Y, Wang Y, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57(10):1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 70.Soares JC, Mann JJ. The anatomy of mood disorders—review of structural neuroimaging studies. Biol Psychiatry. 1997;41(1):86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 71.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol Bull. 2000;126(6):890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- 73.Roberts JE, Kassel JD. Mood-state dependence in cognitive vulnerability to depression: the roles of positive and negative affect. Cognitive Therapy and Research. 1996;20(1):1–12. [Google Scholar]
- 74.Elliott R, Sahakian BJ, Michael A, Paykel ES, Dolan RJ. Abnormal neural response to feedback on planning and guessing tasks in patients with unipolar depression. Psychol Med. 1998;28(3):559–571. doi: 10.1017/s0033291798006709. [DOI] [PubMed] [Google Scholar]
- 75.Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry. 2002;59(7):597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- 76.Forbes EE, Dahl RE. Neural systems of positive affect: relevance to understanding child and adolescent depression? Dev Psychopathol. 2005;17(3):827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitterschiffthaler MT, Kumari V, Malhi GS, et al. Neural response to pleasant stimuli in anhedonia: an fMRI study. Neuroreport. 2003;14(2):177–182. doi: 10.1097/00001756-200302100-00003. [DOI] [PubMed] [Google Scholar]
- 78.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 79.Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51(9):693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- 80.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64(3):327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 81.Cabib S, Puglisi-Allegra S. Stress, depression and the mesolimbic dopamine system. Psychopharmacology (Berl) 1996;128(4):331–342. doi: 10.1007/s002130050142. [DOI] [PubMed] [Google Scholar]
- 82.Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hardin MG, Schroth E, Pine DS, Ernst M. Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents: development and psychopathology related differences. J Child Psychol Psychiatry. 2007;48(5):446–454. doi: 10.1111/j.1469-7610.2006.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35(1):27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20(6):2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6(2):228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 88.Goff B, Gee DG, Telzer EH, et al. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–138. doi: 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bos K, Zeanah CH, Fox NA, Drury SS, McLaughlin KA, Nelson CA. Psychiatric outcomes in young children with a history of institutionalization. Harv Rev Psychiatry. 2011;19(1):15–24. doi: 10.3109/10673229.2011.549773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SCR, Sonuga-Barke E. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. JCogn Neurosci. 2010;22(10):2316–2325. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- 91.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304(5669):452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 93.Pagnoni G, Zink CF, Montague PR, Berns GS. Activity in human ventral striatum locked to errors of reward prediction. Nat Neurosci. 2002;5(2):97–98. doi: 10.1038/nn802. [DOI] [PubMed] [Google Scholar]
- 94.Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat Neurosci. 2004;7(8):887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- 95.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31(1):6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 96.Epstein J, Pan H, Kocsis JH, et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163(10):1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- 97.Lawrence NS, Williams AM, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55(6):578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 98.McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology (Berl) 2009;205(4):667–677. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Steele JD, Kumar P, Ebmeier KP. Blunted response to feedback information in depressive illness. Brain. 2007;130(Pt 9):2367–2374. doi: 10.1093/brain/awm150. [DOI] [PubMed] [Google Scholar]
- 100.Izuma K, Saito DN, Sadato N. Processing of the incentive for social approval in the ventral striatum during charitable donation. J Cogn Neurosci. 2010;22(4):621–631. doi: 10.1162/jocn.2009.21228. [DOI] [PubMed] [Google Scholar]
- 101.Fareri DS, Delgado MR. The Importance of Social Rewards and Social Networks in the Human Brain. The Neuroscientist. 2014;20(4):387–402. doi: 10.1177/1073858414521869. [DOI] [PubMed] [Google Scholar]
- 102.Fliessbach K, Weber B, Trautner P, et al. Social comparison affects reward-related brain activity in the human ventral striatum. Science. 2007;318(5854):1305–1308. doi: 10.1126/science.1145876. [DOI] [PubMed] [Google Scholar]
- 103.Hirschfeld RM, Montgomery SA, Keller MB, et al. Social functioning in depression: a review. J Clin Psychiatry. 2000;61(4):268–275. doi: 10.4088/jcp.v61n0405. [DOI] [PubMed] [Google Scholar]
- 104.Giedd JN, Snell JW, Lange N, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6(4):551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 105.Ernst M, Nelson EE, Jazbec S, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 106.Galvan A, Hare TA, Parra CE, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Geier C, Luna B. The maturation of incentive processing and cognitive control. Pharmacol Biochem Behav. 2009;93(3):212–221. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Urošević S, Collins P, Lim K, Muetzel R, Luciana M. Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Dev Psychol. 2012;48(5):1488–1500. doi: 10.1037/a0027502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb Cortex. 2010;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- 110.Costello EJ, Pine DS, Hammen C, et al. Development and natural history of mood disorders. Biol Psychiatry. 2002;52(6):529–542. doi: 10.1016/s0006-3223(02)01372-0. [DOI] [PubMed] [Google Scholar]
- 111.Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: gender and psychopathology. Annu Rev Clin Psychol. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]
- 112.Bogdan R, Nikolova YS, Pizzagalli DA. Neurogenetics of depression: a focus on reward processing and stress sensitivity. Neurobiol Dis. 2013;52:12–23. doi: 10.1016/j.nbd.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Powell SB, Geyer MA, Preece MA, Pitcher LK, Reynolds GP, Swerdlow NR. Dopamine depletion of the nucleus accumbens reverses isolation-induced deficits in prepulse inhibition in rats. Neuroscience. 2003;119(1):233–240. doi: 10.1016/s0306-4522(03)00122-2. [DOI] [PubMed] [Google Scholar]
- 114.Hall FS, Wilkinson LS, Humby T, et al. Isolation rearing in rats: Pre-and postsynaptic changes in striatal dopaminergic systems. Pharmacol Biochem Behav. 1998;59(4):859–872. doi: 10.1016/s0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- 115.Jones GH, Hernandez TD, Kendall DA, Marsden CA, Robbins TW. Dopaminergic and serotonergic function following isolation rearing in rats: study of behavioural responses and postmortem and in vivo neurochemistry. Pharmacol Biochem Behav. 1992;43(1):17–35. doi: 10.1016/0091-3057(92)90635-s. [DOI] [PubMed] [Google Scholar]
- 116.Fulford AJ, Marsden CA. Effect of isolation-rearing on conditioned dopamine release in vivo in the nucleus accumbens of the rat. J Neurochem. 1998;70(1):384–390. doi: 10.1046/j.1471-4159.1998.70010384.x. [DOI] [PubMed] [Google Scholar]
- 117.Lapiz MDS, Mateo Y, Parker T, Marsden C. Effects of noradrenaline depletion in the brain on response to novelty in isolation-reared rats. Psychopharmacology (Berl) 2000;152(3):312–320. doi: 10.1007/s002130000534. [DOI] [PubMed] [Google Scholar]
- 118.Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal in adulthood. Biol Psychiatry. 2009;66(3):206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wise RA. Neuroleptics and operant behavior: the anhedonia hypothesis. Behav Brain Sci. 1982;5(1):39–53. [Google Scholar]
- 120.Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- 121.Dackis CA, Gold MS. New concepts in cocaine addiction: the dopamine depletion hypothesis. Neurosci Biobehav Rev. 1985;9(3):469–477. doi: 10.1016/0149-7634(85)90022-3. [DOI] [PubMed] [Google Scholar]
- 122.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 123.Markou A, Koob GF. Postcocaine anhedonia: an animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4(1):17–26. [PubMed] [Google Scholar]
- 124.Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992;221(2–3):227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- 125.Volkow ND, Wang GJ, Fowler JS, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386(6627):830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 126.Di Chiara G, Tanda G. Blunting of reactivity of dopamine transmission to palatable food: a biochemical marker of anhedonia in the CMS model? Psychopharmacology (Berl) 1997;134(4):351–353. doi: 10.1007/s002130050465. discussion 371–377. [DOI] [PubMed] [Google Scholar]
- 127.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52(2):90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 128.Bechara A, Harrington F, Nader K, van der Kooy D. Neurobiology of motivation: double dissociation of two motivational mechanisms mediating opiate reward in drug-naive versus drug-dependent animals. Behav Neurosci. 1992;106(5):798–807. doi: 10.1037//0735-7044.106.5.798. [DOI] [PubMed] [Google Scholar]
- 129.Nader K, Bechara A, van der Kooy D. Neurobiological constraints on behavioral models of motivation. Annu Rev Psychol. 1997;48:85–114. doi: 10.1146/annurev.psych.48.1.85. [DOI] [PubMed] [Google Scholar]
- 130.Kehoe P, Shoemaker WJ, Triano L, Hoffman J, Arons C. Repeated isolation in the neonatal rat produces alterations in behavior and ventral striatal dopamine release in the juvenile after amphetamine challenge. Behav Neurosci. 1996;110(6):1435–1444. doi: 10.1037//0735-7044.110.6.1435. [DOI] [PubMed] [Google Scholar]
- 131.Hall FS, Wilkinson LS, Humby T, Robbins TW. Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse. 1999;32(1):37–43. doi: 10.1002/(SICI)1098-2396(199904)32:1<37::AID-SYN5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 132.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24(11):2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jahn AL, Fox AS, Abercrombie HC, et al. Subgenual prefrontal cortex activity predicts individual differences in hypothalamic-pituitary-adrenal activity across different contexts. Biol Psychiatry. 2010;67(2):175–181. doi: 10.1016/j.biopsych.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pruessner JC, Dedovic K, Pruessner M, et al. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations—2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35(1):179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 136.Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Horm Behav. 2006;50(4):632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 137.Carroll BJ, Curtis GC, Mendels J. Cerebrospinal fluid and plasma free cortisol concentrations in depression. Psychol Med. 1976;6(2):235–244. doi: 10.1017/s0033291700013775. [DOI] [PubMed] [Google Scholar]
- 138.Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: Implicatons for therapy. J Affect Disord. 2001;62(1–2):77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- 139.Martínez-Téllez RI, Hernández-Torres E, Gamboa C, Flores G. Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse. 2009;63(9):794–804. doi: 10.1002/syn.20664. [DOI] [PubMed] [Google Scholar]
- 140.Leão P, Sousa JC, Oliveira M, Silva R, Almeida OF, Sousa N. Programming effects of antenatal dexamethasone in the developing mesolimbic pathways. Synapse. 2007;61(1):40–49. doi: 10.1002/syn.20341. [DOI] [PubMed] [Google Scholar]
- 141.Biron D, Dauphin C, Di Paolo T. Effects of adrenalectomy and glucocorticoids on rat brain dopamine receptors. Neuroendocrinology. 1992;55(4):468–476. doi: 10.1159/000126158. [DOI] [PubMed] [Google Scholar]
- 142.Barrot M, Marinelli M, Abrous DN, Rougé-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12(3):973–979. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- 143.Andersen SL, Lyss PJ, Dumont NL, Teicher MH. Enduring neurochemical effects of early maternal separation on limbic structures. Ann N Y Acad Sci. 1999;877:756–759. doi: 10.1111/j.1749-6632.1999.tb09317.x. [DOI] [PubMed] [Google Scholar]
- 144.Küppers E, Beyer C. Dopamine regulates brain-derived neurotrophic factor (BDNF) expression in cultured embryonic mouse striatal cells. Neuroreport. 2001;12(6):1175–1179. doi: 10.1097/00001756-200105080-00025. [DOI] [PubMed] [Google Scholar]
- 145.Berton O, McClung CA, Dileone RJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 146.Cordeira JW, Frank L, Sena-Esteves M, Pothos EN, Rios M. Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J Neurosci. 2010;30(7):2533–2541. doi: 10.1523/JNEUROSCI.5768-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Smith MA. Hippocampal vulnerability to stress and aging: possible role of neurotrophic factors. Behav Brain Res. 1996;78(1):25–36. doi: 10.1016/0166-4328(95)00220-0. [DOI] [PubMed] [Google Scholar]
- 148.Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25(10):3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- 149.Lee B-H, Kim Y-K. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig. 2010;7(4):231–235. doi: 10.4306/pi.2010.7.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G. Low Brain-Derived Neurotrophic Factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry. 2005;57(9):1068–1072. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 151.Shimizu E, Hashimoto K, Okamura N, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54(1):70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 152.Dwivedi Y. Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr Dis Treat. 2009;5:433–449. doi: 10.2147/ndt.s5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 154.Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109(2):143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 155.Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl) 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]