Abstract
Endogenous metabolites play essential roles in the regulation of cellular identity and activity. Here we have investigated the process of oligodendrocyte precursor cell (OPC) differentiation, a process that becomes limiting during progressive stages of demyelinating diseases, including multiple sclerosis, using mass-spectrometry-based metabolomics. Levels of taurine, an aminosulfonic acid possessing pleotropic biological activities and broad tissue distribution properties, were found to be significantly elevated (~20-fold) during the course of oligodendrocyte differentiation and maturation. When added exogenously at physiologically relevant concentrations, taurine was found to dramatically enhance the processes of drug-induced in vitro OPC differentiation and maturation. Mechanism of action studies suggest that the oligodendrocyte-differentiation-enhancing activities of taurine are driven primarily by its ability to directly increase available serine pools, which serve as the initial building block required for the synthesis of the glycosphingolipid components of myelin that define the functional oligodendrocyte cell state.
Multiple sclerosis is a debilitating autoimmune disease characterized by primary demyelination of axons and subsequent neuronal dysfunction1,2. Disease remission in multiple sclerosis is dependent on a regenerative process known as remyelination, which persists throughout adulthood and involves the migration and subsequent differentiation of OPCs, leading to the formation of newly formed oligodendrocytes2–7. OPCs are found to be abundantly present in chronic lesions of multiple sclerosis patients, and as such, local inhibition of OPC differentiation is thought to be causative in multiple sclerosis disease progression8,9. A promising complementary approach to the treatment of multiple sclerosis, as well as other demyelinating diseases, therefore, involves the identification of pharmacological agents that directly stimulate the process of remyelination by enhancing the process of OPC differentiation2,10–13. Recently, our lab and others have identified multiple drug candidates using high-throughput screening approaches that induce OPC differentiation in vitro and enhance remyelination in vivo in relevant rodent models14–18. However, additional potential treatment strategies, as well as a systems biology level of understanding of the mechanisms associated with OPC differentiation and function, are clearly still needed.
Metabolomic profiling can not only define cell state and function, but also can play a critical role in understanding processes associated with cellular differentiation and reprogramming19–24. Several recent studies have demonstrated the important role of small-molecule metabolites in mediating cellular reprogramming and differentiation. For example, the production of glycolytic end products is found to be elevated in induced pluripotent stem cells (iPSCs) following reprogramming, and somatic cell oxidative bio-energetic transitions have been shown to facilitate nuclear reprogramming20. Similarly, using a global metabolomics approach, we have previously found that cell-type-dependent metabolite oxidation plays a key role in regulating embryonic stem cell (ESC) differentiation19. In a very recent study, naive ESCs were found to maintain a high level of intracellular α-ketoglutarate, which is an important cofactor for demethylation reactions that help maintain the pluripotent state23. Compared to metabolomics-based studies of iPSCs and ESCs, the role of cellular metabolism, and notably central carbon metabolism, in the regulation of OPC differentiation and myelination remains poorly understood. Herein, with the goal of identifying mechanisms and molecules that could be used in the development of new treatment strategies for multiple sclerosis, we have used a global metabolomics approach to successfully identify endogenous metabolites that are significantly altered over the course of the OPC differentiation process and can serve to directly impact cell fate.
RESULTS
Global comparative metabolomic analysis
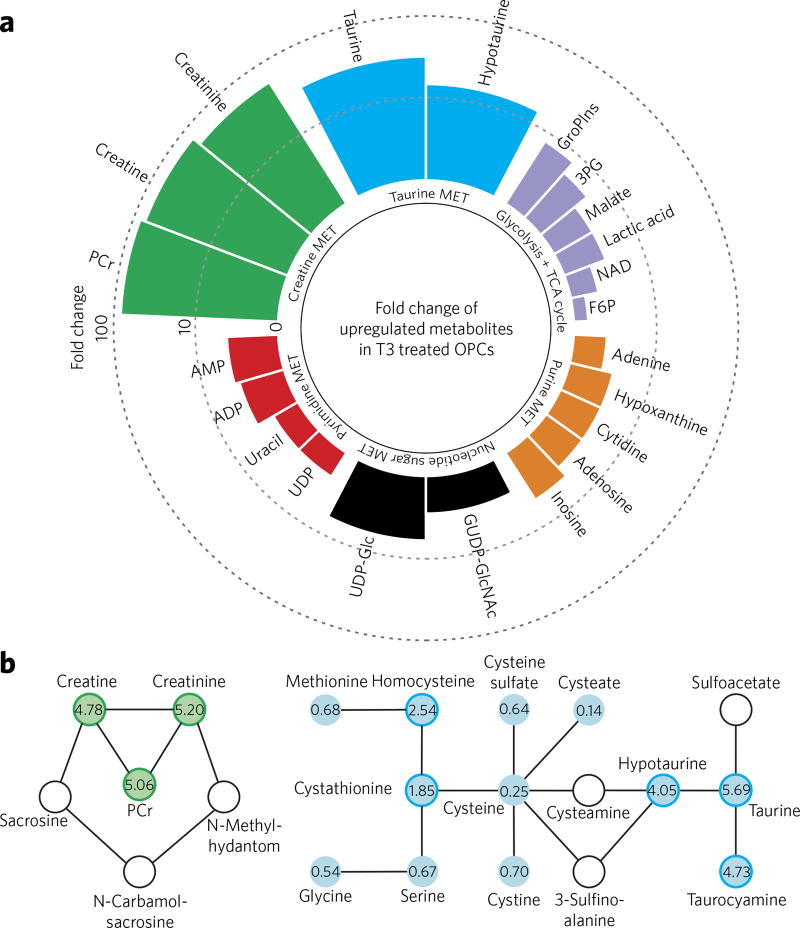
Differentially regulated metabolites from OPCs and mature oligodendrocyte (OL) cells were characterized by MS-based untargeted metabolomic and lipidomic analysis, involving hydrophilic interaction chromatography (HILIC) and reversed-phase liquid chromatography (RP), respectively, combined with electrospray ionization quadruple time-of-flight mass spectrometry (LC–ESI–QTOF–MS) (Supplementary Results, Supplementary Fig. 1). Using established conditions for the in vitro differentiation of purified primary OPC cultures generated by immunopanning of rat optic nerves17, we differentiated cells over the course of 6 d to a mature myelin basic protein positive (MBP+) fate using triiodothyronine (T3), a known inducer of OPC differentiation25. We performed metabolomics analysis on normalized lysates derived from OPCs cultured for 6 d in basal media (2 ng/mL PDGFαα) containing T3 or DMSO vehicle. Western-blot-based analysis of MBP in lysates derived from parallel cultures was used to confirm relative differentiation efficiency. Consistent with previous studies of ESC cultures19, RP lipidomics showed more altered lipids with a highly unsaturated degree in OPCs compared to those in the OLs (Supplementary Fig. 2). Using HILIC-based metabolomics analysis, we evaluated metabolites associated with cellular respiration, which, as described above, have previously been found to play essential roles in cellular differentiation processes20. Mature OLs were found to have many altered features in comparison to immature OPCs, with over 100 features identified following manual filtering. Most of these features were found to be upregulated in the mature OL population. Among 22 identified metabolites associated with respiration and redox processes, glycolysis, TCA cycle, nucleoside, pyrimidine and purine biosynthesis pathways were all significantly enriched (Fig. 1a). This observation is also consistent with previous studies investigating the differences between iPSCs and somatic cells20,26. Furthermore, levels of central carbon metabolites, including tricarboxylic acids as well as pyrimidine and purine nucleotides, decrease significantly when somatic cells are reprogrammed to iPSCs27, suggesting some overlap between the proliferating metabolomic states of OPCs and iPSCs.
Figure 1. Observed metabolic differences between T3- and DMSO-treated OPCs.
(a) Fold change of 22 upregulated metabolites (excluding lipids) in OPCs treated for 6 d with T3 (1 µM) compared to DMSO treatment, determined using accurate mass and MS/MS data, and significant pathway enrichment analyzed by Metaboanalyst (http://www.metaboanalyst.ca/). P < 0.05; n = 5 replicate cell cultures. MET, metabolic pathway. (b) Targeted analysis of creatine and taurine pathway metabolites using triple-quad mass spectrometry (QQQ–MS) in multiple reaction monitoring (MRM) mode (n = 5 replicate cell cultures). The numbers represent fold change of T3-treated compared to DMSO-treated OPCs. Values smaller than one suggest downregulation, whereas values larger than one (darker outline) suggest upregulation in T3-treated OPC. White indicates nondetected (i.e., below the limit of detection).
As mentioned, changes in oxidative status are associated with and play an important role in cellular reprogramming and differentiation19. When mammalian cells are exposed to increased oxidative stress, the glutathione/oxidized glutathione (GSH/GSSG) ratio decreases as GSH is converted to GSSG. Thus, we quantified GSH/GSSG to evaluate the change in oxidation status over the course of OPC differentiation. As shown in Supplementary Figure 3, a substantial decrease in the GSH/GSSG ratio was observed in OLs when compared to OPCs. These observations suggest that redox regulation could be an important factor in mediating OPC differentiation and/or OL maturation.
Targeted metabolomic pathway analysis
Intriguingly, taurine and creatine pathways were found to be the most highly altered events associated with OPC differentiation. Taurine and hypotaurine increased by over 20- and 10-fold, respectively, and metabolites in the creatine pathway were also upregulated by at least an order of magnitude (Fig. 1a). A targeted analysis of ~20 metabolites upstream and downstream of the taurine and creatine pathways was performed using triple-quadrupole MS (QQQ–MS) in multiple reaction monitoring (MRM) mode to increase the sensitivity and examine additional metabolites associated with these pathways (Fig. 1b). Metabolites from both pathways were found to be upregulated in differentiated OLs, consistent with global metabolomic analyses. Specifically, creatine, creatinine and phosphocreatine, as well as taurine, hypotaurine and taurocyamine, were all found to be ~4–5-fold upregulated in differentiated OLs (Fig. 1b) based on targeted quantitative analysis. The upstream metabolite cysteine was significantly (~4-fold) decreased in mature OLs, consistent with its role as a primary precursor of taurine (Fig. 1b). Overall, results derived from targeted metabolomic analysis were consistent with the findings of the global metabolomics study, with more metabolites along the relevant pathways being observed at differentiable levels.
Impact of exogenously supplemented metabolites
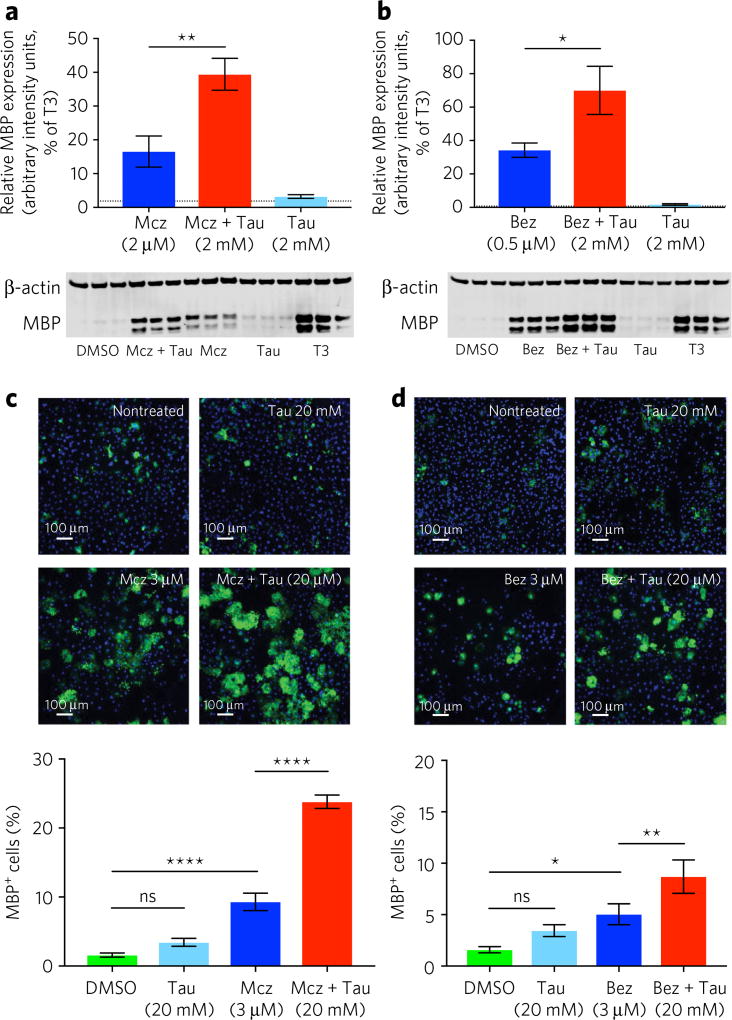
To examine the physiological function of these metabolites and determine their potential ability to impact the OPC differentiation process, we supplemented several of the most altered metabolites associated with OPC differentiation, including taurine, hypotaurine, creatine and phosphocreatine (PCr), to basal and drug-induced differentiation conditions at three physiologically relevant concentrations (0.2, 2 and 20 mM). Compounds were added alone or as mixtures in the presence or absence of optimal or suboptimal concentrations of the known OPC-differentiation-inducing drugs benztropine or miconazole. Notably, although both of these drugs have been shown to induce OPC differentiation in vitro and in vivo15,17, the observed maximal in vitro efficacy of both drugs is suboptimal in comparison to that observed for T3. When evaluated in the absence of benztropine or miconazole, no significant effect was observed for any combination or single metabolite. However, when added to either benztropine- or miconazole-containing media, taurine was found to dramatically increase the efficacy of drug-induced OPC differentiation, as determined by western blot (Fig. 2a,b) or automated immunofluorescence analysis (Fig. 2c,d). Specifically, expression levels of MBP in miconazole- or benztropine-induced differentiation cultures were increased by 2.3- and 2-fold, respectively (Fig. 2a,b; Supplementary Fig. 4a,b). Creatine and PCr also showed some additive effects; however, interpretation of results derived from creatine supplementation were confounded by its essential pre-existing presence in media used for the culture and differentiation of OPCs.
Figure 2. Impact of taurine treatment on the efficacy of drug-induced OPC differentiation.
(a) MBP expression following 6 d of differentiation with miconazole (Mcz, 2 µM), miconazole (2 µM) plus taurine (Tau, 2 mM) or taurine (2 mM) alone. Miconazole treatment initiated at day 0, and taurine treatment initiated at day 2. The data are mean ± s.d. (n = 3 replicate cell cultures) and normalized to MBP levels observed following induction with T3 (1 µM). Dotted line represents DMSO activity. β-actin was used as internal control. Full gel provided in Supplementary Figure 4a. (b) MBP expression following 6 d of differentiation with benztropine (Bez; 0.5 µM), benztropine (0.5 µM) plus taurine (2 mM) or taurine (2 mM) alone. Benztropine treatment initiated at day 0, and taurine treatment initiated at day 2. The data are mean ± s.d. (n = 3 replicate cell cultures) and are normalized to MBP levels observed following induction with T3 (1 µM). Dotted line represents DMSO activity. β-actin was used as an internal control. Full gel provided in Supplementary Figure 4b. (c,d) Automated immunofluorescence analysis (MBP, green; DAPI, blue) of DMSO, taurine (20 mM), miconazole (3 µM), miconazole (3 µM) plus taurine (20 mM), benztropine (3 µM), and benztropine (3 µM) plus taurine (20 mM). Drug and taurine treatment initiated at day 0. Data are mean ± s.d. for the percentage of MBP-positive cells per well (n = 4 replicate cell cultures). ns, no significance; *P < 0.05; **P < 0.01; ****P < 0.0001. Scale bars, 100 µm.
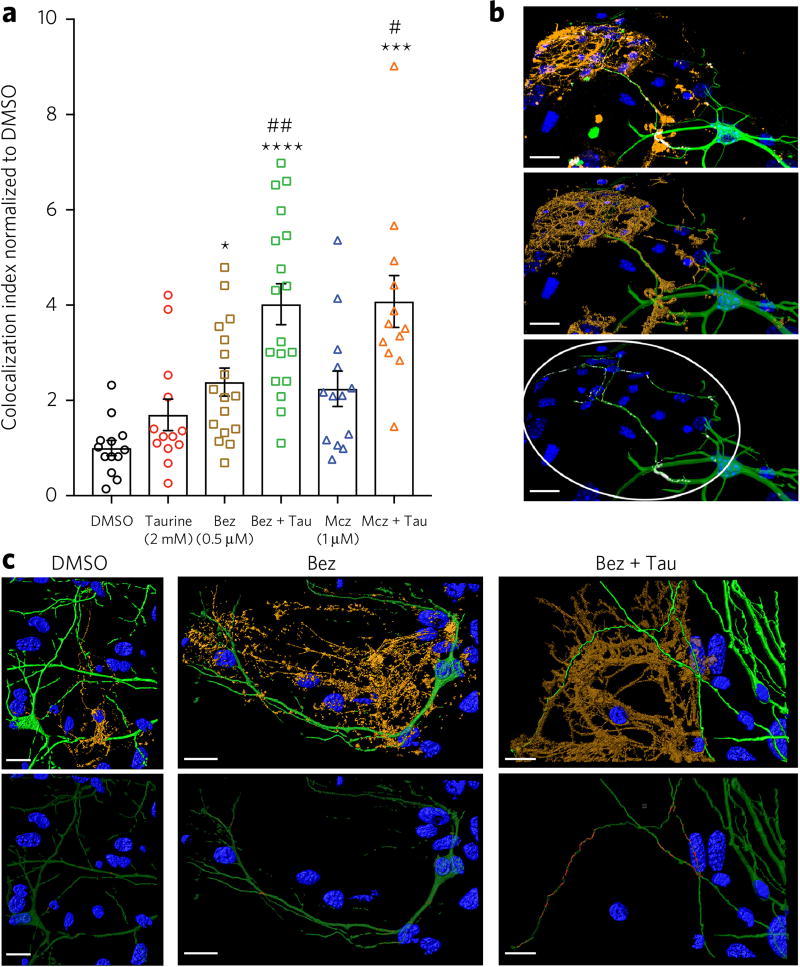
Using an established high-content imaging assay17, based on MBP expression, we evaluated matrices of 10 concentrations each of taurine and miconazole or benztropine to determine optimal combination treatment conditions. In addition to confirming the overall enhancement of induced differentiation that was observed by western-blot-based analysis, the results of the high-content imaging analysis indicate that addition of >2 mM taurine also enhances overall cell number by over 20% relative to drug treatment alone (Supplementary Fig. 4c,d). Furthermore, we assessed the impact of exogenous taurine addition on drug-induced OL maturation and function using an established confocal-microscopy-based imaging assay involving co-cultured primary mouse cortical neurons17. Following 14 d of neuron–OPC co-culture, 2 mM taurine was found to significantly increase the observed index of MBP colocalization with axons in cultures treated with either benzotropine or miconazole to induce OPC differentiation (Fig. 3a–c).
Figure 3. Impact of taurine treatment on observed indices of MBP colocalization with co-cultured axons.
(a) Quantification of MBP colocalization with the axons of primary mouse cortical neurons co-cultured with OPC s following 14 d of treatment. Cultures were treated with 0.5 µM benztropine (Bez) or 1 µM miconazole (Mcz) in the presence or absence of 2 mM taurine. The data are mean ± s.e.m.; * = significance of treatments vs. DMSO; # = significance of co-treatment vs. drug alone; one symbol: P < 0.05, two symbols: P < 0.01, four symbols: P < 0.0001; n = 13 technical replicates for DMSO, Tau, Mcz, Mcz + Tau; n = 17 technical replicates for Bez; n = 19 technical replicates for Bez + Tau. (b) Representative confocal microscopy fluorescence imaging of a co-culture following 14 d of treatment (DAPI, blue; Tuj1, green; MBP, orange). White indicates regions of colocalization (scale bars, 20 µm). Middle, 3D rendering of the image above used to quantify colocalization index (as shown on the bottom with the MBP channel removed to reveal sites of colocalization of Tuj1 and MBP). The colocalization index (see methods) is quantified on neuronal processes contained within the oligodendrocyte region of interest (ROI, circled) and normalized using DMSO control. (c) Example of rendered fluorescent confocal images of differentiated OPCs co-cultured with cortical neurons (labeled as above). The bottom copy of each pair illuminates the areas of colocalization overlap in between the two cell types in red to demonstrate proportions of MBP+ neuronal processes.
Differentiation-state-dependent effects of taurine
During the course of in vitro and in vivo OPC differentiation, cells transition though an immature MBP− ‘premyelinating’ oligodendrocyte state2,28. In our assay system, this occurs between day 2 and 3 of differentiation17. To determine the stage of OPC differentiation at which taurine enhances drug efficacy, we tested the effect of taurine at various time points by initiating dosing with taurine at days 0 to 5 and evaluating cell cultures at day 6. The most significant impact on MBP expression was observed when taurine was added between days 1 and 3, with addition on day 0 being significantly less efficacious (Supplementary Fig. 5a,b), suggesting that taurine facilitates the maturation of premyelinating OLs rather than inducing cell cycle exit and/or the differentiation of fully immature proliferating OPCs. Consistently, addition of taurine to basal (DMSO vehicle) or drug-induced differentiation conditions on day 0, 1 or 3 was found to have a minimal, albeit statistically significant, impact on the number of residual immature A2B5+ OPCs at day 3 or 6 (Supplementary Fig. 5c,d). This temporal trend also coincided with the decrease of the oxidative stress marker GSH/GSSG at day 3 (Supplementary Fig. 3).
Impact of taurine on mitochondrial function
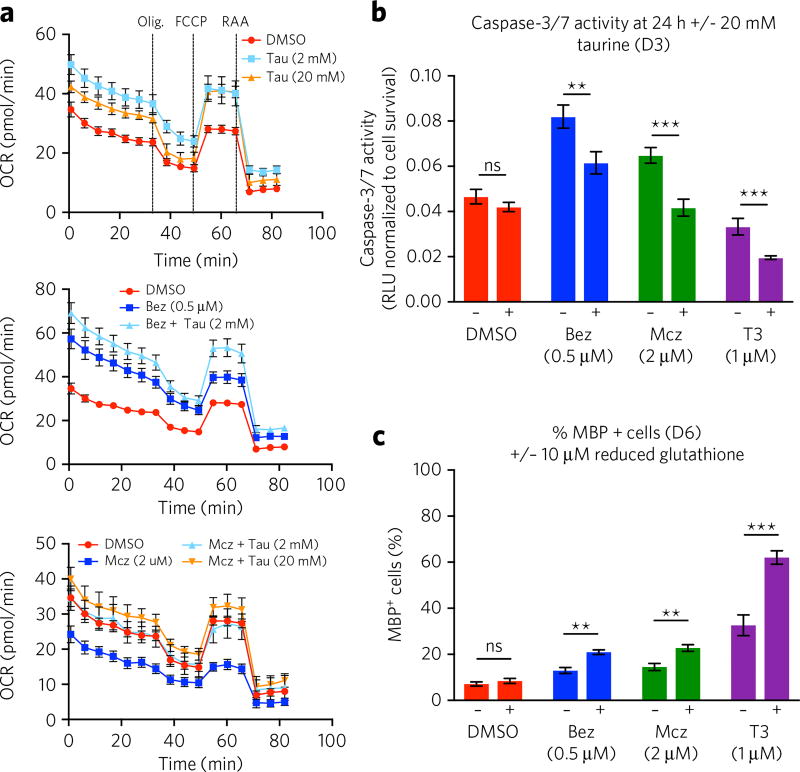
In addition to its ability to function in the maintenance of buffer capacity, redox potential, osmoregulation and membrane stabilization, taurine can modulate ion flux and act as a neuromodulator to control synaptic transmission and enhance or preserve mitochondrial function in response to increased secondary metabolite demand or excitotoxicity29,30. Specifically, taurine has been shown to be able to regulate intracellular calcium levels and preserve mitochondrial energy metabolism in glutamate-challenged cultures of cerebellar granule cells31. When proliferating OPCs differentiate to OLs that function to produce myelin, mitochondrial energy requirements are presumably challenged in response to the activation of phospholipid biosynthetic pathways. We therefore examined oxygen consumption rates (OCR) associated with mitochondrial respiratory activity using an XFe96 Seahorse instrument. Addition of 2 mM taurine to culture media significantly increased OCR values at day 6 under basal, as well as benztropine- or miconazole-induced, differentiation conditions (Fig. 4a). Interestingly, miconazole treatment alone was found to have a negative impact on OCR values compared to DMSO or benztropine treatment (Fig. 4a). These observations are in agreement with the ability of taurine to enhance mitochondrial function in both OPCs and OLs. Consistent with the critical dependence of OPCs and OLs on mitochondrial function, treatment with the mitochondrial function inhibitors oligomycin or rotenone induced cell death within 24 h at all concentrations tested, which precluded the ability to evaluate the impact of taurine rescue following treatment with these agents in the differentiation assay.
Figure 4. The impact of taurine treatment on OL mitochondrial function and the role of redox state on OPC differentiation.
(a) Oxygen consumption rate (OCR; pmol/min) of OPCs (40,000 cells/well) treated for 6 d with taurine (Tau; 2 or 20 mM) and DMSO, benztropine (Bez; 0.5 µM) with and without 2 mM taurine or miconazole (Mcz; 2 µM) with and without 2 and 20 mM taurine. Complex activity was measured at baseline, as well as after the successive addition of 1 µM oligomycin (Olig.), 2 µM carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP ) and 2 µM rotenone and antimycin A (RAA). The data are mean ± s.d. (n = 12 technical replicates). The dashed line in the top panel indicates the addition of the compound indicated. (b) Quantification of caspase-3/7 activation 24 h post-taurine addition on day 3 (D3), after treatment with DMSO, miconazole (2 µM), benztropine (0.5 µM), or T3 (1 µM) on day 0. Data are mean ± s.d. of the luminescence (RLU ) normalized to corresponding cell survival per well (n = 3 replicate cell cultures). (c) Quantification of MBP-positive OLs based on MBP immunofluorescent analysis on day 6 (D6) post-treatment with DMSO, miconazole (2 µM), benztropine (0.5 µM), or T3 (1 µM), following co-treatment with or without reduced glutathione (10 µM). Data are mean ± s.d. for the percentage of MBP-positive cells per well (n = 3 replicate cell cultures). ns, no significance; **P < 0.01; ***P < 0.001.
Impact of taurine on OPC and OL survival
Related to a beneficial impact on the process of mitochondrial function, the observed overall differentiation-enhancing effect of taurine could be derived from a protective effect associated with inhibition of apoptosis. Substantial apoptosis would be expected to occur as OPCs exit the cell cycle in response to growth-factor withdrawal, as well as during the process of pre-oligodendrocyte maturation. As such, we evaluated the impact of taurine addition on cell survival and apoptosis over the course of the oligodendrocyte differentiation process using CellTiter Glo and Caspase-Glo 3/7 assays. When added at day 0 (Supplementary Fig. 6a,c) or day 3 (Fig. 4b; Supplementary Fig. 6b,d), taurine was found to decrease caspase-3/7 activation 24 h following supplementation under drug-induced differentiation conditions (i.e., 1 µM T3, 0.5 µM benztropine or 2 µM miconazole). This finding is consistent with the observed beneficial impact on overall survival at day 6, as described above (Supplementary Fig. 4c,d). Taurine was also found to significantly inhibit apoptosis in OPCs treated with staurosporine, which served as a positive control for apoptosis induction (Supplementary Fig. 6a,b). Taurine did not display any impact on apoptosis or survival under basal (DMSO vehicle) differentiation conditions (Fig. 4b; Supplementary Fig. 6a,b).
Impact of taurine on oxidative stress in OPCs and OLs
Oxidative stress has been demonstrated to disrupt oligodendrocyte differentiation by directly decreasing the expression of key pro-differentiation genes and increasing the expression of genes known to inhibit differentiation32. Furthermore, changes in intracellular redox state have been reported to be sufficient to drive the balance between self-renewal and differentiation during the course of OL differentiation33. We evaluated the potential impact of taurine-mediated redox state modulation on OL differentiation efficiency by evaluating the impact of alternative reducing agents on basal (DMSO vehicle) or drug-induced OL differentiation. Although reduced glutathione (10 µM GSH; Fig. 4c) had no significant effect on differentiation efficiency under basal conditions (DMSO), it was found to significantly enhance the overall efficiency of T3-induced differentiation (Fig. 4c; Supplementary Fig. 7a), as well as the observed total nuclei count (Supplementary Fig. 7b). Reduced glutathione was also found to enhance the overall efficiency of benztropine- or miconazole-induced differentiation, albeit to a lesser extent than what is observed following taurine addition (Fig. 4c; Supplementary Fig. 7a), and the impact on total cell number was not found to be significant (Supplementary Fig. 7b). As such, modulation of redox state could be a beneficial contributing mechanism that serves to enhance the process of drug-induced OL differentiation. However, the inability to fully recapitulate the activity of taurine with alternative biological reducing agents indicates that this activity is not likely the sole mechanism by which taurine enhances the process of OL differentiation.
Taurine-induced metabolomic changes
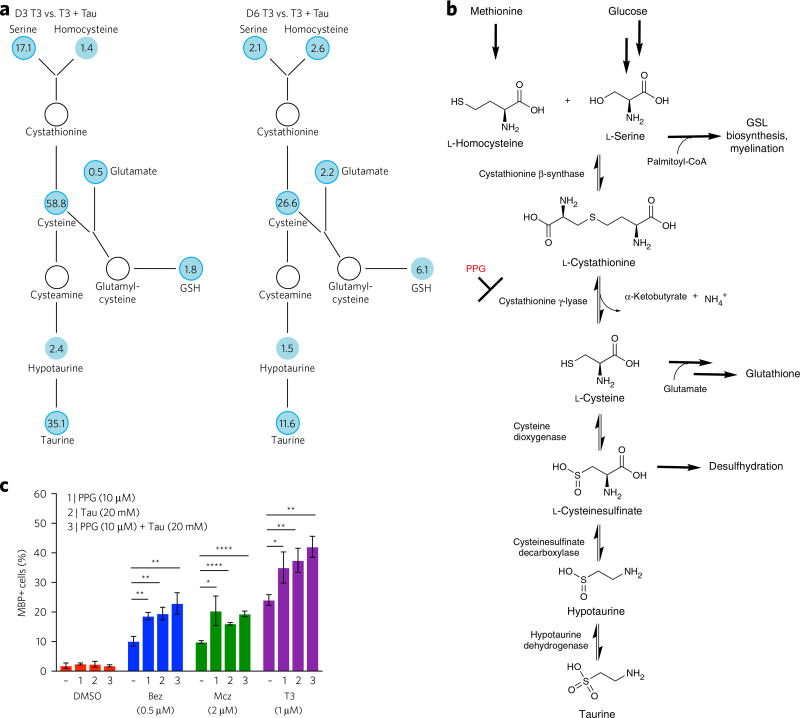
To identify metabolic changes that are directly impacted by exogenous taurine supplementation in OPCs and OLs, binary global metabolomics analysis was performed between cell lysates following treatment with a given differentiation-inducing agent (i.e., T3, benztropine or miconazole) in the presence or absence of 20 mM taurine for 3 or 6 d. This pair-wise analysis allowed drug-specific and differentiation-state-dependent effects to be filtered out, thereby enabling the identification of metabolomic changes that are specific to taurine supplementation. This analysis revealed that relatively few metabolite pools are impacted by taurine supplementation. However, we observed significantly increased serine and cysteine pools in lysates derived from taurine-treated OPCs following 3 d of drug-induced differentiation using T3 (1 µM, 17.1- and 58.8-fold, respectively; Fig. 5a), benztropine (0.5 µM, 10.0- and 154.4-fold, respectively; Supplementary Fig. 8a) or miconazole (2 µM, 19.4- and 76.5-fold, respectively; Supplementary Fig. 8b). Similarly, at day 6, we observed that serine and cysteine levels were significantly higher in lysates derived from taurine-treated OPCs differentiated using T3 (1 µM, 2.1- and 26.6-fold, respectively; Fig. 5a), benztropine (0.5 µM, 13.4- and 44.7-fold, respectively; Supplementary Fig. 8a) or miconazole (2 µM, 92.5- and 47.2-fold, respectively; Supplementary Fig. 8b). We found slightly higher GSH levels in lysates derived from taurine-treated OPCs differentiated using T3 (1 µM) at day 3 (1.8-fold; Fig. 5a) and day 6 (6.1-fold; Fig. 5a). Changes in GSH and glutamate were found be only minimally altered in benztropine- (0.5 µM) or miconazole (2 µM)-treated cultures at both time points (Supplementary Fig. 8a,b). Collectively, the observed impacts on metabolite pools at different stages of the differentiation process imply that the addition of taurine reduces the need for serine and cysteine biosynthesis and/or increases available pools of these metabolites for alternative biosynthetic functions that are potentially required for OL maturation and/or function. Indeed, under optimal conditions of T3-induced differentiation, lower observed serine levels at day 6 following taurine supplementation as compared to those observed at day 3 (Fig. 5a) suggests that serine pools derived from taurine are consumed by other biosynthetic pathways in mature OLs.
Figure 5. Binary global metabolomics analysis of taurine co-treatment and evaluation of the impact of inhibition of taurine biosynthesis on OL differentiation.
(a) Binary global metabolomics analysis of the impact of taurine (Tau, 20 mM) supplementation on pre-myelinating OLs (day 3, D3) and OLs (day 6, D6) differentiated using T3 (1 µM). Numbers represent fold changes of metabolites, with values smaller than one suggesting downregulation and values larger than one suggesting upregulation. White indicates nondetected (i.e., below the limit of detection). Darker circles represent P < 0.05. (b) Taurine biosynthesis pathway highlighting the relationship to glycosphingolipid (GSL) biosynthesis and the impact of propargylglycine (PPG)-mediated inhibition of cystathionine γ-lyase on taurine and GSH production. (c) Quantification of MBP-positive OLs based on MBP immunofluorescence analysis on day 6 post-treatment with DMSO, miconazole (2 µM), benztropine (0.5 µM), or T3 (1 µM), following addition of PPG (10 µM), taurine (20 mM) or PPG (10 µM) and taurine (20 mM). Data are mean ± s.d. for the percentage of MBP-positive cells per well (n = 3 replicate cell cultures). ns, no significance; *P < 0.05; **P < 0.01; ****P < 0.0001.
Direct effect of taurine on OPC differentiation
An alternative mechanism by which exogenously added taurine could enhance the process of drug-induced differentiation is by directly modulating the activity of a receptor or ion transporter (as described above), which serves to either stimulate or inhibit OL differentiation. Indeed, M1/M3 muscarinic receptor antagonism, and the associated altered intracellular calcium levels, is an essential component of the mechanism of benztropine- and clemastine-induced OPC differentiation17,18. To determine whether taurine itself contributes to the process of OPC differentiation, as opposed to having indirect effects on redox state or mitochondrial health and cell viability, we inhibited endogenous taurine biosynthesis using propargylglycine (PPG). PPG is an irreversible inhibitor of cystathionine γ-lyase, which can be used to inhibit the conversion of l-cystathionine to l-cysteine and downstream taurine biosynthesis in cells34. Importantly, this also results in the inhibition of glutathione biosynthesis (Fig. 5b). The relative contribution of these two potential mechanisms could therefore be elucidated by adding either exogenous taurine or glutathione in combination with PPG. If taurine acts by a mechanism involving direct modulation of the function of an essential protein, PPG treatment would inhibit oligodendrocyte differentiation, and this effect could be rescued by exogenously adding back taurine. Similarly, if the OPC differentiation-enhancing activity of taurine is derived exclusively from a redox effect that results from the combined activities of increased levels taurine and glutathione, PPG treatment would inhibit oligodendrocyte differentiation, and this effect could be rescued by exogenously adding back reduced glutathione and/or other alternative biological reducing agents. However, paradoxically, PPG (10 µM) was found to significantly enhance the observed efficacy of all drug-induced differentiation conditions when added at day 0 (Fig. 5c; Supplementary Fig. 9a). Furthermore, the magnitude of this effect was found to be similar to the effect of adding taurine itself to any given drug-induced differentiation condition, including T3 (Fig. 5c). Consistent with the effect of taurine addition, PPG had virtually no impact on differentiation efficiency under basal vehicle-treated conditions. (Fig. 5c). Importantly, PPG was not found to impact overall survival in OPC cultures treated with DMSO, benztropine (0.5 µM) or miconazole (2 µM) (Supplementary Fig. 9b). In the case of T3 (1 µM) treatment, PPG (10 µM) was actually found to enhance overall cell number (Supplementary Fig. 9b). These observations make it unlikely that taurine itself stimulates a pro-differentiation process by directly interacting with a relevant receptor or alternative protein target, but rather that exogenous taurine enhances OL differentiation by serving as a feedstock to produce a limiting metabolite pool. Furthermore, as PPG negatively impacts GSH biosynthesis, and, as GSH and glutamate levels were found to be only minimally impacted by taurine supplementation (Fig. 5a; Supplementary Fig. 8a,b), it is unlikely that a redox state effect is the primary mechanism contributing to the overall beneficial impact of taurine supplementation on OL differentiation and maturation.
DISCUSSION
This study used unbiased global metabolomics together with targeted analyses to identify altered metabolite pools associated with the process of OL differentiation and maturation. In addition to the significant observed changes that were anticipated in lipid metabolite profiles, many hydrophilic small-molecule metabolites were identified as being highly altered. Several of these metabolites were found to dramatically improve the processes of OPC differentiation when co-treated with various mechanistically unrelated remyelination-enhancing drugs. These findings provide support for the notion that cell state and/or cell fate can be effectively modulated using strategies that involve supplementation with endogenously produced upregulated metabolites that are associated with the desired phenotype. Specifically, several exogenously supplemented metabolites that were found to increase significantly in mature OLs when compared to immature OPCs—most notably taurine—were found to significantly enhance the processes of OL differentiation.
Mechanistic studies suggest that although taurine supplementation can impact the processes of mitochondrial function and oxidative stress in a beneficial manner in this system, neither of these effects alone fully account for the observed differentiation- and maturation-enhancing activities of taurine. Surprisingly, the effect of taurine supplementation was found to be fully recapitulated when endogenous taurine biosynthesis, as well as GSH production, was blocked via the inhibition of an upstream enzyme. This seemingly paradoxical finding suggests that the primary mechanism by which exogenous taurine supplementation enhances OL differentiation is by reversing pathway flux. This notion is consistent with the results of binary global metabolomics analysis, which indicated that serine and cysteine are the most altered metabolite pools impacted by exogenous taurine addition. A reversal in flux, and/or taurine’s ability to serve as an alternative feedstock, results in dramatically increased pools of serine, which serves as a primary building block for the glycosphingolipid (GSL) biosynthesis that is required to produce myelin.
GSL biosynthesis begins with the condensation of l-serine and palmitoyl-CoA to form 3-ketosphinganine and, subsequently, ceramide, which is the common precursor of sphingomyelin, gangliosides and galactolipids35. Increased flux through the GSL biosynthetic pathway would impact OL maturation both by directly increasing the production of functional biomolecules that define the OL cell state and by modulating lipid signaling pathways that are known to impact differentiation at the transcriptional level. OL differentiation proceeds through a series of intermediate cell states that are not only defined by the presence of GSL surface antigens, but also directly impacted by the relative levels of specific GSLs35. It has been demonstrated, for example, that GSL levels serve as sensors and transmitters of environmental information that can directly impact the expression of myelin genes and cause the arrest of terminal oligodendrocyte differentiation36,37. Perhaps more importantly, enhanced flux through GSL biosynthetic pathways would lead to the direct production of myelin components and potentially the enhanced functional myelination of axons. When one considers that an essential function of an oligodendrocyte is the production of GSLs, which along with cholesterol make up the majority of the lipid components of myelin (~31% and ~27%, respectively35), it is internally consistent that taurine supplementation can enhance OL maturation by directly enabling GSL biosynthesis.
The actual clinical relevance of our findings will require extensive preclinical evaluation involving quantitative analysis of the impact of taurine supplementation on in vivo drug-induced remyelination in the context of relevant rodent models of demyelinating disease. Nonetheless, the potential utility is appealing. Taurine is found in the brain at millimolar concentrations and it is a very well-tolerated agent that is actively transported across the blood-brain barrier38. As such, taurine supplementation, in combination with existing standard of care drugs, could be a feasible treatment strategy to improve remyelination by enhancing GSL biosynthesis in OLs. A similar strategy involving oral formulation of high dose biotin, which serves as a cofactor for essential carboxylase enzymes required for fatty acid synthesis, has recently been demonstrated to provide benefit in patients with not-active progressive multiple sclerosis39. Intriguingly, taurine has previously been identified as a dysregulated metabolite in animal models of multiple sclerosis disease, including nonhuman primate models40, and its plasma levels can be used to distinguish PLP-induced experimental autoimmune encephalomyelitis (EAE) mice from naive controls41–44. At a minimum, these findings demonstrate the utility of global metabolomic analyses in the identification of endogenous metabolites that can serve to modulate cellular phenotypes.
ONLINE METHODS
Metabolites and small molecules
All the endogenous metabolites used in this study were from Sigma-Aldrich. Benztropine, miconazole and triiodothyronine (T3) were purchased from Sigma-Aldrich (Saint Louis, MO). All the chemicals used in this study have a purity ≥ 95%.
Rat primary optic nerve OPCs were isolated by panning (>99% A2B5+) and cultured in poly-d-lysine (10 µg/ml)-coated TC dishes in OPC culture media (Neurobasal Media, Invitrogen) supplemented with B27 without vitamin A (Invitrogen), 1× nonessential amino acids, 1× Glutamax, 1× anti-anti, β-mercaptoethanol and PDGFαα (50 ng/ml; Peprotech) at 37 °C with 5% CO2. The culture medium was replaced every 48 h, and cells were collected before the confluency reached 60% to maintain a naive state. For differentiation, OPCs were plated at 1 million cells in one 10-cm Petri dish filled with differentiation media, which is identical to the culture media but with PDGF-AA at 2 ng/ml. T3 and DMSO were used as the positive control and negative control, respectively. Five replicates of cells were collected at different times (0, 3 and 6 d) for metabolomics studies.
Global metabolomics and lipidomics
Cells incubated in differentiation medium were collected at day 0, 3 and 6 for both the T3- and DMSO-treated OPCs. The cells were first rinsed with PBS twice to completely remove the culture medium and then scraped into a 1.5-mL Eppendorf vial using 500–1,000 µL PBS. Subsequently, cells were collected by aspirating the supernatant after centrifugation at 1,000 r.p.m. at 4 °C for 3 min. The global metabolites were extracted from cell pellets by a methanol:acetonitrile:water (2:2:1; v/v) solvent mixture. A volume of 600 µL cold solvent was added to each pellet, vortexed for 30 s, and soaked in liquid nitrogen for 1 min. Samples were then allowed to thaw at room temperature and then sonicated for 10 min. This freeze-thaw process was repeated twice. To further precipitate proteins, samples were incubated for 1 h at −20 °C and then centrifuged at 16,000g at 4 °C for 15 min. The protein concentration of the cells was measured in the final pellet after centrifugation using BCA assay. The resulting supernatant was removed and evaporated to dryness in a vacuum concentrator (Labconco CentriVap Benchtop). The dry extracts were then reconstituted in the appropriate volume of acetonitrile/water (1:1; v/v), normalized by the protein concentration with the lowest concentration approximately 50 µL, sonicated for 10 min, and centrifuged for 15 min at 16, 000g and 4 °C to remove insoluble debris. The supernatants were transferred to HPLC vials with inserts and stored at −20 °C before LC–MS analysis. The Folch method was used to extract the lipids from OPCs treated with T3 and DMSO for 6 d. A mixture of 600 µL chloroform/MeOH (2:1; v/v) was used in the freeze-thaw extraction method as described above, and a subsequent addition of 600 µL of water was performed. Using this liquid–liquid extraction method, the organic phase was collected, dried and finally reconstituted in IPA/MeOH/H2O (5:1:4; v/v/v) before analysis.
Cell extracts were analyzed on a 6550 iFunnel QTOF mass spectrometer (Agilent Technologies) coupled with a 1290 UPLC system (Agilent Technologies). For global metabolomics, HPLC was carried out on a Luna Aminopropyl, 3 µm, 150 mm × 1.0 mm I.D. HILIC column (Phenomenex). The mobile phase was composed of A = 20 mM ammonium acetate and 40 mM ammonium hydroxide in 95% water (v/v) and B = 95% acetonitrile; the remaining 5% components were either acetonitrile or water, respectively. A linear gradient from 100% B (0–5 min) to 100% A (50–55 min) was applied. A 10-min re-equilibration time was applied to the HILIC column for re-equilibration and maintenance of reproducibility. The flow rate was 50 µL/min, and the sample injection volume was 5 µL. ESI source conditions were set as follows: dry gas temperature, 200 °C; flow, 11 L/min; fragmentor, 380 V; sheath gas temperature, 300 °C; flow, 9 L/min; nozzle voltage, 500 V; capillary voltage, −500 V in ESI negative mode. The instrument was set to acquire data over the m/z range of 50–1,000, with the MS acquisition rate of 1 spectra/s. The sample sequence was randomized to avoid systematic decreases in signals over sample sets. For the MS/MS of selected precursors, the default isolation width was set as narrow (~1.3 m/z), with a MS acquisition rate at 2 spectra/s and MS/MS acquisition at 2 spectra/s to acquire over the m/z range 50–1,000 and 25–1,000, respectively. MS/MS data were acquired at the collision energy of 20 V and 40 V.
For the lipidomics profiling, the separation was conducted using an Agilent ZORBAX Eclipse Plus RRHD C18 column (2.1 × 100 mm, 1.8 µm). The mobile phase contains A (5:1:4 IPA/MeOH/H2O with 5 mM NH4OAc and 0.1% CH3COOH) and B (99:1 IPA/H2O with 5 mM NH4OAc and 0.1% CH3COOH). The mobile phase starts with 0% B for 3 min and increases to 20% B in the following two minutes. The gradient gradually increases to 30% B until 25 min and further to 95% B in next 10 min, which is maintained for 2 min. Subsequently, the mobile phase is set to the original 0% B (37–38 min) and another 10 min re-equilibration time was applied to for re-equilibration. The flow rate is 0.35 mL/min and the temperature keeps at 50 °C.
LC–MS data were converted to mzXML files using Masshunter Acquisition Software (Agilent Masshunter 6.0B). The mzXML files were uploaded to XCMS Online web platform for data processing (https://xcmsonline.scripps.edu)45 including peak detection, retention time correction, profile alignment, and isotope annotation. Data were processed using both pair-wise and multigroup comparison, and the parameter settings were as follows: centWave for feature detection (Δm/z = 15 p.p.m.; minimum peak width = 10 s; and maximum peak width = 60 s); obiwarp settings for retention time correction (profStep = 0.5); and parameters for chromatogram alignment, including mzwid = 0.015, minfrac = 0.5, and bw = 5. The relative quantification of metabolite features was based on extracted ion chromatogram (EIC) areas. Paired parametric t-test and one-way ANOVA (post-hoc Tukey test) were used to test the variation pattern of metabolite features between and across cell samples collected at different times after being treated with T3 and DMSO. Multiple group analysis and pairwise comparisons between DMSO and T3 at individual incubation time were conducted. The results output, including EICs, pairwise/multigroup cloud plot, multidimensional scaling plots, and principle components were exported directly from XCMS Online. Generally, the numbers of total pairwise comparison features and significantly altered features (statistically defined as P < 0.01, including both upregulated and downregulated features) were reported in this study.
Targeted multiple pathway analysis
The metabolites in the upstream and downstream of taurine and creatine pathways are of great interest in this study. Due to the poor stability of several sulfur-containing compounds including cysteine, GSH and GSSG, a different extraction method from the global metabolomics was developed and validated. Briefly, cell pellets were extracted with 150 µL 75% ACN in water modified with 0.1% formic acid using the freeze-thaw method mentioned above. Samples were centrifuged at 16,000g and 4 °C for 15 min, and the supernatant was directly injected into the Agilent triple-quad (QQQ, Agilent 6495). It was operated in multiple reaction monitoring mode (MRM), in which the collision energies and product ions (MS2 or quantifier and qualifier ion transitions) were pre-optimized for each metabolite of interest (Supplementary Table 1). Cycle time was 50 ms for each transition. ESI source conditions were set as the following: gas temperature, 250 °C; gas flow, 14 L/min; nebulizer, 20 psi; sheath gas, 250 °C; sheath gas flow, 11 L/min; capillary voltage, 3,000 V; nozzle voltage, 1,500 V; and EMV, 1,000 V in ESI positive mode. The analyses were performed on the Imtakt Amino Acid column (length 150 × i.d. 2 mm, particle size 3 µm). The mobile phase was composed of A = 50 mM ammonium formate in water (v/v) and B = 100% acetonitrile in 0.1% formic acid. A linear gradient from 70% B (0–1 min) to 70% A (1–12 min, maintaining for 2 min) was applied. A 12-min re-equilibration time was applied to the column for re-equilibration. The flow rate was 200 µL/min, and the sample injection volume was 20 µL.
High-content imaging
For dose–response co-treatment experiments, black, clear-bottom 384-well plates (Greiner) were coated with poly-d-lysine (10 µg/mL in PBS). Miconazole and benztropine were added at 8 doses in differentiation media; three-fold dilutions down from 10 µM. Taurine was then added at 8 concentrations; three-fold dilutions down from 20 mM. This 384-well format was also used for experiments combining benztropine (0.5 µM), miconazole (2 µM) or T3 (1 µM) with propargylglycine (0.1, 3, 10 or 30 µM), reduced glutathione (1, 10, 30 or 100 µM) or ascorbic acid (1, 10, 30 or 100 mM). OPCs were then seeded at a density of 1,000 cells per well, and the plates were incubated at 37 °C with 5% CO2 for 6 d. For time-course experiments, cells were seeded at a density of 5,000 cells per well in poly-d-lysine-coated 96-well plates. Cells were treated with DMSO (0.1%), benztropine (0.5 µM), miconazole (2 µM) or T3 (1 µM) on day 0, and taurine (20 mM) was added at 0, 1 or 3 d post-drug treatment. The plates were incubated at 37 °C with 5% CO2 for 6 d. On day 6, cells were then fixed for 20 min with 4% formaldehyde solution and stained with anti-MBP antibody (cat. #MAB382, Millipore) or anti-A2B5 antibody (cat. #MAB312, Millipore) in 3% BSA, 0.3% Triton X-100 with overnight incubation at 4 °C. The cells were washed and incubated with secondary antibody (Alexa Fluor 488 goat anti-mouse IgG, Life Technologies) and DAPI (Invitrogen) for 1 h at room temperature. The cells were washed and plates were sealed and imaged using an Opera confocal imaging reader (PerkinElmer) or a Cellomics Cell Insight imaging reader (Thermo). An air ×10 lens was used to capture nine images per well at both wavelengths (488 and 365 nm), with each image representing a different unique locations field in a single well. For image analysis, DAPI-stained nuclei and MBP-positive or A2B5-positive cell bodies were detected using an algorithm that selects for positive cell bodies and nuclei within a range of fluorescent emission values and sizes as determined by fitting parameters to positive (T3, 1 µM) and negative (DMSO, 0.1%) controls. The number of DAPI-positive objects were enumerated in experiments where cell counts were required. Numerical results from the analyzed images were later exported for analysis using Excel (Microsoft) and/or Genedata software.
Cell viability analysis
In poly-d-lysine-coated white 384-well plates (Greiner), benztropine (0.5 µM), miconazole (2 µM) or T3 (1 µM) were added in combination with rotenone (0.1, 1, 3 or 10 µM) or oligomycin (0.1, 1, 3 or 10 µM). Cells were then seeded at a density of 1,000 cells per well, and the plates were incubated at 37 °C with 5% CO2 for 1, 3 or 6 d. At these three time points, the CellTiter-Glo Luminescent Cell Viability Assay (Promega) was used to analyze cytotoxicity.
Detection of caspase 3/7
In poly-d-lysine-coated white 96-well plates (Greiner), cells were seeded at 5,000 cells per well. Taurine (20 mM) was added on days 0 or 3, alone and in combination with benztropine (0.5 µM), miconazole (2 µM), T3 (1 µM) or staurosporine (1 µM) added on day 0. The Caspase-Glo 3/7 Assay (Promega) was used to quantify caspase-3/7 activity 6, 24 and 48 h after drug and taurine addition on day 0. Caspase-3/7 activity was also measured 24, 48 and 72 h (days 4, 5 and 6) after taurine addition on day 3.
In vitro OL maturation assay
Cortical and hippocampal tissue was dissected from C57BL/6 mice at embryonic day 14 and dissociated in 0.5% trypsin with 100 U/mL DNase I diluted in HBSS (+/+) for 10 min at 37 °C. The reaction was stopped by supplementing to a final concentration of 10% v/v FBS, 100 µg/mL Soybean Trypsin Inhibitor and 10 U/mL DNase I. Cells were resuspended in plating media consisting of equal volumes of FCS, HBSS (+/+), high-glucose DMEM with l-glutamine, and F-12 with l-glutamine, and then plated onto acid prewashed German glass coverslips coated with 50 µg/mL poly-d-lysine and 20 µg/mL laminin at a density of 50,000 cells/cm2. Cells were allowed to attach for 2 h and then coverslips were rinsed with PBS before being transferred to a 24-well plate containing 0.5 mL growth media consisting of: Neurobasal medium, B27 supplement (Gibco), 0.5× Glutamax (Thermo), 5 µg/mL gentamycin and 5 µM glutamic acid. Half of the cell media was replaced after 3 days in culture with supplemental growth media not containing glutamic acid46. After 5 days in culture, rat-derived cortical OPCs were added to the neuronal culture at a density of 5,000 cells/cm2. Co-culture media was used to resuspend and plate OPCs consisting of a 1:3 mixture of neuronal growth supplement (as above) and oligodendrocyte differentiation media, containing DMEM/F-12 (with l-glutamine), insulin-free N2 supplement, 1× Glutamax, 1× MEM nonessential amino acids, 1×β-mercaptoethanol, 2 ng/mL PDGF and 5 µg/mL gentamycin. All drugs and taurine co-treatment were added once to the culture upon plating of OPCs at the following concentrations: 0.5 µM benztropine and 1.0 µM miconazole, each with or without 2 mM taurine. Cultures were maintained by replacing half of the media every 3 d, maintaining an equivalent concentration of taurine throughout in appropriate wells. Fourteen days after addition of OPC and drug treatments co-cultures were fixed for 15 min in 4% PFA and stained sequentially overnight with TUJ1 (cat. #MMS-435P, Covance) and MBP (cat. #ab134018, Abcam) antibodies, each immediately followed by associated secondary antibodies (Alexa Fluor 488 goat anti-mouse IgG, Life Technologies; Cy3 goat anti-chicken, Jackson ImmunoResearch) (Thermo) and Cy3-Goat anti-Chicken (Jackson ImmunoResearch) and 1 µg/mL DAPI for nuclear counterstain. Immunostained coverslips were then S7 invert mounted on glass slides using Fluoromount G (Southern Biotech). Randomly selected regions of 25 fields or greater were imaged at 63× magnification using a Zeiss 780 confocal microsope. Z-projection images were then rendered in Imaris (Bitplane) to identify apposition of oligodendrocyte and neuronal processes. Colocalization was quantified within regions of interest delineated as the periphery of individual oligodendrocytes. The colocalization index (adapted from a previously described method47) was calculated as the total proportion of colocalized 3D surface area between Tuj1 (neuron) and oligodendrocyte (MBP) over total Tuj1 (neuronal process) area enclosed within each oligodendrocyte process tree as the selected region of interest (ROI; see Fig. 3b). Mean colocalization values for each condition were normalized to the DMSO condition mean analyzed statistically using Prism software (GraphPad, v7) for two-way ANOVA comparisons (drug treatment × taurine supplement) with Tukey’s post-tests for statistical significance.
Statistical comparison of myelination in drug-treated co-cultures as well as drug-treated co-cultures with taurine supplement showed a significant main effect for benztropine (F(1,56) = 27.82; P < 0.0001) and taurine supplement (F(1,56) = 10.8; P = 0.0018), with significant Tukey post-hoc results for benztropine alone vs. DMSO (q = 3.19; P = 0.0442), benztropine + taurine vs. DMSO (q = 8.48; P < 0.0001) and benztropine + taurine vs. benztropine alone (q = 5.09; P = 0.0037). Additionally, there was a significant main effect for miconazole treatment (F(1,46) = 24.9; P < 0.0001) and a main effect for taurine (F(1,46) = 10.55; P < 0.0022). Tukey’s post-hoc tests showed no significant difference for miconazole alone vs. DMSO (q = 3.61; P = 0.065); however, there was a significant difference for miconazole + taurine vs. DMSO (q = 8.24; P < 0.0001) and miconazole + taurine vs. miconazole alone (q = 4.54; P = 0.0125). Taurine treatment alone showed no significant difference vs. DMSO in either benztropine and miconazole experiments (P = 0.58 and P = 0.54, respectively).
Western blot analysis
OPCs were plated in basal differentiation medium at 1.7 × 105 cells/well and treated for 6 d with benztropine (0.5 µM), miconazole (2 µM) with and without the tested endogenous metabolites, T3 (1 µM) or DMSO. After washing with PBS, cells were collected in ice-cold RIPA buffer containing protease and phosphatase inhibitors. Following incubation on ice for >20 min and sonication, lysed cells were centrifuged (16,000g for 15 min at 4 °C). Total protein was quantified using a BCA analysis, and 25 µg of protein from each sample was denatured by boiling with Bolt LDS Sample Buffer (4×) and Sample Reducing Agent (10×). Proteins were electrophoresed using Bolt 4–12% Bis–Tris gels (Life Technologies) and transferred to a PVDF membrane (Bio-Rad). The membrane was blocked with Odyssey PBS Blocking Buffer (LI-COR) and incubated overnight at 4 °C with anti-MBP antibody (cat. #MAB382, Millipore) and anti-actin anti-β-actin antibody (cat. #sc-47778). Blots were incubated with HRP-conjugated secondary donkey anti-mouse antibody (IRDye800CW, LI-COR) and imaged using Odyssey CLx and Image Studio (LI-COR).
Mitochondrial respiration assay
Cellular oxygen consumption rate (OCR) was measured using an XFe96 extracellular flux analyzer (Seahorse Biosciences). OPCs were seeded at 40,000 cells/well in poly-D-lysine-coated XFe96 plates and cultured for 6 d with taurine (2 and 20 mM) alone, or together with miconazole and benztropine. Cells were then incubated in assay medium (DMEM with 20 mM glucose, 5 mM pyruvate and 5 mM sodium bicarbonate, pH ~ 7.4) for at least 30 min before the assay. Media to deliver a final well concentration corresponding to 1 µM oligomycin, 2 µM carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) and 2 µM rotenone + 2 µM antimycin A were preloaded into the compound delivery ports of the system. The assay starts with the recording of the basal OCR, which is followed by recording of the OCR in response to different compounds that constitute the Mitochondria Stress Test (Seahorse Biosciences, Agilent). Once the basal OCR was measured, the compounds were added sequentially and the effects on OCR measured 3 times every 5 min. Oligomycin blocks ATP synthase; FCCP dissipates the inner membrane potential enabling maximum electron flux through the electron transport chain (ETC); rotenone + antimycin A inhibit complexes I and III, respectively, thereby blocking the entry of electrons in the ETC and shutting down mitochondrial activity.
Statistical analysis
Unless otherwise stated, data represent mean ± s.d. of representative experiments. Unless otherwise stated, statistical analysis was performed using Prism software (GraphPad, v7) for one-way ANOVA comparisons with Tukey’s post-tests for statistical significance.
Life sciences reporting summary
Further information on experimental design and reagents is available in the Life Sciences Reporting Summary.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Material
Acknowledgments
We gratefully acknowledge financial support from the National Institutes of Health (Grants R01 GM114368-02, R24 EY017540-04, P30 MH062261-10, P01 DA026146-02, and 1S10OD16357).
Footnotes
Author contributions
B.A.B., M.F., G.S. and L.L.L. initiated the project, developed the strategy and generated experimental design. B.A.B., W.C.P. and B.S. performed molecular biology and cell-based experiments. M.F. and J.R.M.-B. performed mass spectrometry and metabolomics experiments. B.P.C.K., B.A.B. and M.F. performed OCR experiments. B.A.B., M.F., W.C.P., B.S., J.R.M.-B., G.S. and L.L.L. interpreted data. E.S. and T.K. contributed essential ideas and comments. B.A.B., M.F., G.S. and L.L.L. wrote the manuscript.
Competing financial interests
The authors declare no competing financial interests.
Any supplementary information, chemical compound information and source data are available in the online version of the paper.
References
- 1.Pouly S, Antel JP. Multiple sclerosis and central nervous system demyelination. J. Autoimmun. 1999;13:297–306. doi: 10.1006/jaut.1999.0321. [DOI] [PubMed] [Google Scholar]
- 2.Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 3.Gallo V, Armstrong RC. Myelin repair strategies: a cellular view. Curr. Opin. Neurol. 2008;21:278–283. doi: 10.1097/WCO.0b013e3282fd1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubois-Dalcq M, et al. From fish to man: understanding endogenous remyelination in central nervous system demyelinating diseases. Brain. 2008;131:1686–1700. doi: 10.1093/brain/awn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franklin RJ, Hinks GL. Understanding CNS remyelination: clues from developmental and regeneration biology. J. Neurosci. Res. 1999;58:207–213. [PubMed] [Google Scholar]
- 6.Chamberlain KA, Nanescu SE, Psachoulia K, Huang JK. Oligodendrocyte regeneration: Its significance in myelin replacement and neuroprotection in multiple sclerosis. Neuropharmacology. 2016;110(Pt B):633–643. doi: 10.1016/j.neuropharm.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franklin RJM. Why does remyelination fail in multiple sclerosis? Nat. Rev. Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- 8.Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J. Neurosci. 1998;18:601–609. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N. Engl. J. Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- 10.Franklin RJ, Gallo V. The translational biology of remyelination: past, present, and future. Glia. 2014;62:1905–1915. doi: 10.1002/glia.22622. [DOI] [PubMed] [Google Scholar]
- 11.Huang JK, et al. Myelin regeneration in multiple sclerosis: targeting endogenous stem cells. Neurotherapeutics. 2011;8:650–658. doi: 10.1007/s13311-011-0065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang JK, et al. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat. Neurosci. 2011;14:45–53. doi: 10.1038/nn.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucchinetti CF, Noseworthy JH, Rodriguez M. Promotion of endogenous remyelination in multiple sclerosis. Mult. Scler. 1997;3:71–75. doi: 10.1177/135245859700300202. [DOI] [PubMed] [Google Scholar]
- 14.Mei F, et al. Identification of the kappa-opioid receptor as a therapeutic target for oligodendrocyte remyelination. J. Neurosci. 2016;36:7925–7935. doi: 10.1523/JNEUROSCI.1493-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najm FJ, et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature. 2015;522:216–220. doi: 10.1038/nature14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckley CE, et al. Drug reprofiling using zebrafish identifies novel compounds with potential pro-myelination effects. Neuropharmacology. 2010;59:149–159. doi: 10.1016/j.neuropharm.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Deshmukh VA, et al. A regenerative approach to the treatment of multiple sclerosis. Nature. 2013;502:327–332. doi: 10.1038/nature12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mei F, et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med. 2014;20:954–960. doi: 10.1038/nm.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanes O, et al. Metabolic oxidation regulates embryonic stem cell differentiation. Nat. Chem. Biol. 2010;6:411–417. doi: 10.1038/nchembio.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folmes CDL, et al. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011;14:264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shyh-Chang N, Daley GQ, Cantley LC. Stem cell metabolism in tissue development and aging. Development. 2013;140:2535–2547. doi: 10.1242/dev.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat. Rev. Mol. Cell Biol. 2014;15:243–256. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.TeSlaa T, et al. α-Ketoglutarate accelerates the initial differentiation of primed human pluripotent stem cells. Cell Metab. 2016;24:485–493. doi: 10.1016/j.cmet.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huan T, et al. Systems biology guided by XCMS Online metabolomics. Nat. Methods. 2017;14:461–462. doi: 10.1038/nmeth.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez M, et al. Thyroid hormone administration enhances remyelination in chronic demyelinating inflammatory disease. Proc. Natl. Acad. Sci. USA. 2004;101:16363–16368. doi: 10.1073/pnas.0407262101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meissen JK, et al. Induced pluripotent stem cells show metabolomic differences to embryonic stem cells in polyunsaturated phosphatidylcholines and primary metabolism. PLoS One. 2012;7:e46770. doi: 10.1371/journal.pone.0046770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panopoulos AD, et al. The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res. 2012;22:168–177. doi: 10.1038/cr.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fields RD. A new mechanism of nervous system plasticity: activity-dependent myelination. Nat. Rev. Neurosci. 2015;16:756–767. doi: 10.1038/nrn4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramos-Mandujano G, Hernandez-Benitez R, Pasantes-Morales H. Multiple mechanisms mediate the taurine-induced proliferation of neural stem/progenitor cells from the subventricular zone of the adult mouse. Stem Cell Res. 2014;12:690–702. doi: 10.1016/j.scr.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Shivaraj MC, et al. Taurine induces proliferation of neural stem cells and synapse development in the developing mouse brain. PLoS One. 2012;7:e42935. doi: 10.1371/journal.pone.0042935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Idrissi A, Trenkner E. Growth factors and taurine protect against excitotoxicity by stabilizing calcium homeostasis and energy metabolism. J. Neurosci. 1999;19:9459–9468. doi: 10.1523/JNEUROSCI.19-21-09459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.French HM, Reid M, Mamontov P, Simmons RA, Grinspan JB. Oxidative stress disrupts oligodendrocyte maturation. J. Neurosci. Res. 2009;87:3076–3087. doi: 10.1002/jnr.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith J, Ladi E, Mayer-Proschel M, Noble M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc. Natl. Acad. Sci. USA. 2000;97:10032–10037. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jurkowska H, Stipanuk MH, Hirschberger LL, Roman HB. Propargylglycine inhibits hypotaurine/taurine synthesis and elevates cystathionine and homocysteine concentrations in primary mouse hepatocytes. Amino Acids. 2015;47:1215–1223. doi: 10.1007/s00726-015-1948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackman N, Ishii A, Bansal R. Oligodendrocyte development and myelin biogenesis: parsing out the roles of glycosphingolipids. Physiology (Bethesda) 2009;24:290–297. doi: 10.1152/physiol.00016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bansal R, Pfeiffer SE. Reversible inhibition of oligodendrocyte progenitor differentiation by a monoclonal antibody against surface galactolipids. Proc. Natl. Acad. Sci. USA. 1989;86:6181–6185. doi: 10.1073/pnas.86.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bansal R, Pfeiffer SE. Regulation of gene expression in mature oligodendrocytes by the specialized myelin-like membrane environment: antibody perturbation in culture with the monoclonal antibody R-mAb. Glia. 1994;12:173–179. doi: 10.1002/glia.440120303. [DOI] [PubMed] [Google Scholar]
- 38.Albrecht J, Schousboe A. Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem. Res. 2005;30:1615–1621. doi: 10.1007/s11064-005-8986-6. [DOI] [PubMed] [Google Scholar]
- 39.Tourbah A, et al. MD1003 (high-dose biotin) for the treatment of progressive multiple sclerosis: A randomised, double-blind, placebo-controlled study. Mult. Scler. 2016;22:1719–1731. doi: 10.1177/1352458516667568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.t Hart BA, et al. 1H-NMR spectroscopy combined with pattern recognition analysis reveals characteristic chemical patterns in urines of MS patients and non-human primates with MS-like disease. J. Neurol. Sci. 2003;212:21–30. doi: 10.1016/s0022-510x(03)00080-7. [DOI] [PubMed] [Google Scholar]
- 41.Gebregiworgis T, et al. Potential of urinary metabolites for diagnosing multiple sclerosis. ACS Chem. Biol. 2013;8:684–690. doi: 10.1021/cb300673e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mangalam A, et al. Profile of circulatory metabolites in a relapsing-remitting animal model of multiple sclerosis using global metabolomics. J. Clin. Cell. Immunol. 2013;4 doi: 10.4172/2155-9899.1000150. http://dx.doi.org/10.4172/2155-9899.1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Preece NE, et al. Experimental encephalomyelitis modulates inositol and taurine in the spinal cord of Biozzi mice. Magn. Reson. Med. 1994;32:692–697. doi: 10.1002/mrm.1910320603. [DOI] [PubMed] [Google Scholar]
- 44.Noga MJ, et al. Metabolomics of cerebrospinal fluid reveals changes in the central nervous system metabolism in a rat model of multiple sclerosis. Metabolomics. 2012;8:253–263. doi: 10.1007/s11306-011-0306-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tautenhahn R, et al. XCMS online: A web-based platform to process untargeted metabolomic data. Anal. Chem. 2012;84:5035–5039. doi: 10.1021/ac300698c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pang Y, et al. Neuron-oligodendrocyte myelination co-culture derived from embryonic rat spinal cord and cerebral cortex. Brain Behav. 2012;2:53–67. doi: 10.1002/brb3.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diemel LT, Wolswijk G, Jackson SJ, Cuzner ML. Remyelination of cytokine- or antibody-demyelinated CNS aggregate cultures is inhibited by macrophage supplementation. Glia. 2004;45:278–286. doi: 10.1002/glia.10335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.