Abstract.
Anaplasmosis, cat-scratch disease, and Lyme disease are emerging vector-borne infectious diseases in Korea. Although the prevalence of vector-borne pathogens (VBPs) in domestic animals and vector arthropods has been documented, there is limited information on the presence of VBPs in wild animals. The raccoon dog (Nyctereutes procyonoides), a wild canid found in East Asia and Europe, represents a potential wildlife reservoir for zoonotic diseases. To investigate the prevalence of VBPs in raccoon dogs, 142 carcasses and 51 blood samples from captured raccoon dogs were collected from 2003 to 2010 and from 2008 to 2009, respectively, in Korea. In addition, 105 Haemaphysalis flava (14 larvae, 43 nymphs, 32 males, and 16 females) and nine Haemaphysalis longicornis (all female) were collected from three raccoon dogs. Samples of the spleen and blood were tested for the presence of VBPs by using nested polymerase chain reaction. Among the samples collected from 193 raccoon dogs and 114 ticks, two samples were positive for Anaplasma phagocytophilum, four for Anaplasma bovis, two for Borrelia theileri, and two for Bartonella henselae. To the best of our knowledge, this study is the largest survey of raccoon dogs aimed at the analysis of VBPs in this species. Moreover, the present study represents the first identification of A. phagocytophilum, B. henselae, and B. theileri in raccoon dogs in their native habitat (East Asia).
INTRODUCTION
The raccoon dog (Nyctereutes procyonoides) is a species native to East Asia (East Siberia, East Mongolia, China, North Vietnam, Japan, and Korea), but was introduced into the European fauna in the twentieth century for fur production.1 The typical habitat of raccoon dogs includes forested river valleys, surrounding lakes, and reed beds. The particular behavioral characteristics of raccoon dogs, such as the habitation of burrows constructed by other animals or their own, exposes them to contact with ectoparasites infected with several pathogens.1 Recently, in Korea, the population of raccoon dogs has increased significantly owing to the absence of natural predators and their high adaptability to diverse environments.2 This is important as the raccoon dog plays a central role in the circulation of rabies in Korea.2,3 Moreover, the prevalence of tick-borne pathogens that originate from raccoon dogs has been reported in Europe, Japan, and Korea.4–7 Therefore, the increased population of raccoon dogs, and the resulting elevated zoonotic disease risk, represents a potential public health threat.
Anaplasma phagocytophilum is a zoonotic intracellular bacterium transmitted by ticks of the Ixodes and Haemaphysalis genera in the United States, Europe, and Asia.8–10 This bacterium infects various hosts worldwide, including humans, ruminants, horses, dogs, cats, birds, deer, and rodents.11 Recently, in Korea, A. phagocytophilum DNA has been detected in several mammals such as deer, rodents, goats, dogs, and cats.9,12–16 Previous reports have indicated that A. phagocytophilum is widely circulating among wildlife systems and may be transmitted to humans and companion animals. Accordingly, the first isolation of A. phagocytophilum from a Korean resident has been recently reported.17
Bartonella henselae, a gram-negative facultative intracellular bacillus transmitted by the cat flea (Ctenocephalides felis), causes cat-scratch disease.18 Although fleas are known to be the main vector of Bartonella spp., Bartonella DNA has recently been detected in ticks and other animals in Korea, including Korean water deer (KWD, Hydropotes inermis argyropus), dogs, leeches, and cats.9,13,19–23 A recent study showed that Ixodes spp. are capable of transmitting B. henselae via salivary contents,24 suggesting that the reservoir/host range of Bartonella spp. could include wildlife. In addition, the first isolation of B. henselae from humans was reported in Korea25 and the first detection of Bartonella spp. was reported in raccoon dogs in Japan.6
Borrelia spp. are generally classified into the Lyme disease group and relapsing fever group.26,27 The Lyme disease group is associated with many Borrelia spp. such as Borrelia burgdorferi sensu stricto, Borrelia afzelii, Borrelia garinii, etc., and are transmitted by hard ticks (Ixodidae).26,27 On the other hand, the relapsing fever group of Borrelia spp., such as Borrelia duttonii and Borrelia crocidurae, are transmitted by soft ticks (Argasidae).28 However, some members of the relapsing fever group, including Borrelia theileri, Borrelia miyamotoi, and B. lonestari are transmitted mainly by hard ticks (Ixodidae).29–31 In Korea, B. afzelii has been isolated from Ixodes spp. and the DNAs of Borrelia turdi and unidentified Borrelia spp. have been detected in Haemaphysalis ticks collected from migratory birds and goats.13,19,32 Moreover, the serological prevalence of Lyme disease in horses and dogs has also been reported.33,34 However, information detailing the prevalence of Borrelia spp. in animals is still lacking.
In Korea, Haemaphysalis, the predominant genus of ticks found in grasses/herbaceous vegetation habitats and wild/domestic animals, is infected with numerous vector-borne pathogens (VBPs).9,10,19,20 According to a previous study, VBPs are endemic, present in ticks collected from deer in Korea.12,20,22 These reports indicate that wild animals may represent additional reservoirs for VBPs. However, the prevalence of vector-borne agents has not been reported in populations of wild animals in Korea (other than in KWD). Here, we describe the prevalence of VBPs in raccoon dogs in Korea. This will have implications for the future control of such pathogens in East Asia.
MATERIALS AND METHODS
Ethical statement.
All samples were collected with consent from the Conservation Genome Resource Bank (CGRB) for Korean Wildlife. This study was approved by the Institutional Committee of Graduate studies and Research at Seoul National University.
Sample collection.
The carcasses of raccoon dogs were collected from eight provinces in Korea, between 2003 and 2010, by the CGRB for Korean Wildlife. Carcasses were stored at −20°C until necropsy for harvesting splenic tissues. Small sections of splenic tissue were frozen at −80°C until DNA extraction.
Live raccoon dogs were captured using traps in three provinces for a rabies bait vaccine program in Korea between 2008 and 2009. Captured raccoon dogs were anesthetized by intramuscular injection of 2 mg/kg of xylazine (Bayer Korea Ltd., Seoul, Korea) and 10 mg/kg of ketamine (Yuhan Corporation, Seoul, Korea). After anesthesia, blood was harvested from the jugular vein and stored in ethylenediaminetetraacetic acid–containing tube. All samples were stored at 4°C until DNA extraction. Ticks were collected from the captured raccoon dogs by placing fine tweezers around the mouth part, slowly removing the attached tick, and then storing them in vials containing 70% ethanol. The ticks were transported to the laboratory and identified microscopically to assess the stage of development and species, according to previously reported guidelines.35
DNA extraction and polymerase chain reaction (PCR) amplification.
DNA was extracted from spleen and blood samples using DNeasy Blood and Tissue Kits (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. For DNA extraction, 10 mg of splenic tissue and 100 μL of blood were used. Conventional and nested PCRs were performed using primers specific for A. phagocytophilum (16S rRNA, groEL, msp2, and ankA genes), Anaplasma bovis (16S rRNA), Bartonella (16S-23S internal transcribed spacer [ITS], rpoB, and groEL), and Borrelia spp. (16S rRNA and groEL), as described in previous studies.12,13,36–38 A molecular identification of tick species was conducted to confirm the visual assessment of tick species, using ITS2 and 16S rRNA primer sets.39 In raccoon dogs, mitochondrial cytochrome b (mt cyt b) and c (mt cyt c) genes were selected as conserved genes to prevent false-negative results in the PCR. The primer set used to amplify mt cyt b was a universal primer set specific for mammalian hosts.40 The primer used to amplify mt cyt c was specific for raccoon dogs.41 Anaplasma phagocytophilum (genomic DNA from the Webster strain, provided by J. Stephen Dumler [Johns Hopkins University School of Medicine, Baltimore, MD]) was used as a positive control for detecting Anaplasma spp.; B. henselae (Houston-1 strain, ATCC® 49882™) and B. burgdorferi sensu stricto (297 strain, ATCC 53899™) isolates were purchased from the American Type Culture Collection and used as positive controls. The first and nested PCRs were performed in a total volume of 25 μL. Each PCR mixture consisted of 10 pmol of primers, 1 U of recombinant Taq DNA polymerase (Takara Bio, Inc., Koyto, Japan), 10× PCR buffer (Takara Bio, Inc.), 2.5 mM deoxynucleotide solution mixture (Takara Bio, Inc.), 1 µL samples of genomic DNA for the first PCR, and 1 μL of the first PCR product for the second PCR. Amplification of PCR products was performed using a SimpliAmp™ thermal cycler (Thermo Fisher Scientific Inc., Singapore), and PCR conditions, as previously described.12,13 The PCR amplicons were visualized by gel electrophoresis using a 1.2% agarose gel.
Nucleotide sequencing and phylogenetic analysis.
The PCR products were purified using QIAquick Gel Extraction kits (Qiagen). All positive PCR amplicons were directly sequenced on an ABI prism 3730 DNA sequence analyzer (Applied Biosystems, Foster City, CA). The obtained sequences were evaluated using Chromas software (Ver 2.33; Technelysium Pty Ltd, South Brisbane, Australia), aligned using the Clustal X algorithm (Ver 2.1; Conway Institute, University College Dublin, Dublin, Ireland), and then analyzed with a similarity matrix. Relationships between individuals were assessed using a maximum likelihood method with nucleotide distance (Kimura 2 parameter) with 1,000 replications for a bootstrap test. The phylogenetic trees of nucleotide sequences (547-bp 16S rRNA gene of Anaplasma, 507-bp 16S rRNA gene of Borrelia, 467-bp msp2 gene of Anaplasma (b), 460-bp ankA gene of Anaplasma, and 569-bp ITS gene of Bartonella) were constructed using the MEGA7 program (http://www.megasoftware.net/). GenBank accession numbers of the 16S rRNA, msp2, ankA, and ITS region sequences, and specific genospecies sequences related to pathogens for sequence comparisons are included in Figures 1–3.
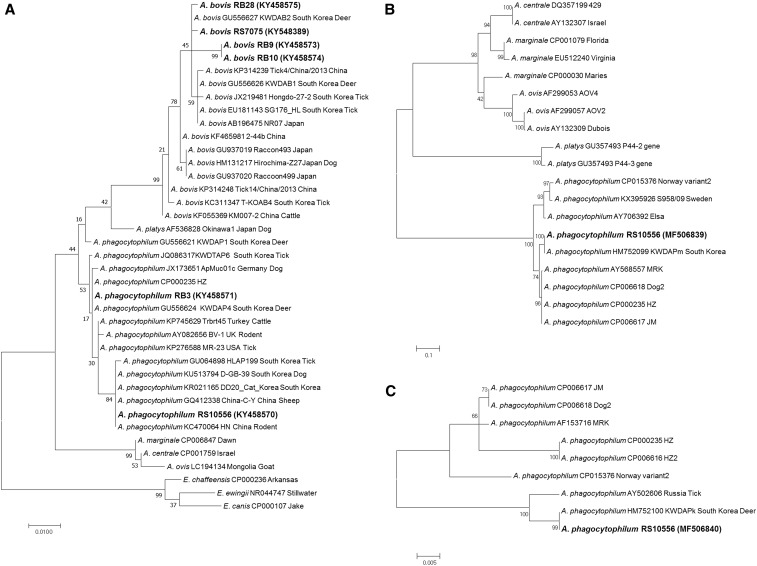
Figure 1.
Phylogenetic relationships for Anaplasma phagocytophilum (bold letters) and Anaplasma bovis (bold letters) detected from raccoon dogs and Anaplasma and Ehrlichia species based on partial nucleotide sequences of 547-bp 16S rRNA gene (A), 467-bp msp2 gene (B), and 460-bp ankA gene (C) fragments. The maximum likelihood method was used for constructing the phylogenetic tree. The numbers at the nodes are the proportions of 1,000 bootstrap iterations that support the topology shown.
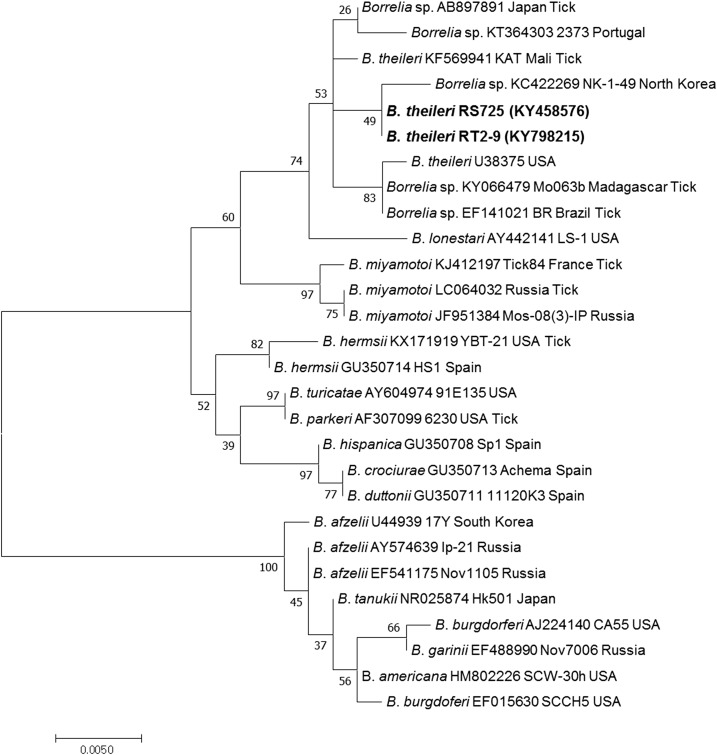
Figure 3.
Phylogenetic relationships for Borrelia theileri (bold letters) detected from raccoon dogs and Borrelia spp. based on partial nucleotide sequences of 16S rRNA gene fragments (507-bp). The maximum likelihood method was used for constructing the phylogenetic tree. The numbers at the nodes are the proportions of 1,000 bootstrap iterations that support the topology shown.
RESULTS
A total of 193 raccoon dogs and 114 ticks were used for this study. Splenic tissue samples were collected from 152 carcasses in eight provinces (20 from Seoul, 40 from Gyeonggi-do, 41 from Gwangwon-do, four from Chungcheongbuk-do, two from Chungcheongnam-do, 15 from Jeollanam-do, one from Gyeongsangbuk-do, 12 from Gyeongsangnam-do, and seven from unknown) (Table 1). Blood samples from 51 captured raccoon dogs were collected in three provinces (three from Seoul, 12 from Gyeonggi-do, 31 from Gwangwon-do, and five from unknown) (Table 1). A further 114 ticks, including 105 belonging to the Haemaphysalis flava species (14 larvae, 43 nymphs, 32 males, and 16 females) and nine of the Haemaphysalis longicornis species (all females), were collected from three captured raccoon dogs (from Gwangwon-do). However, blood samples were not collected from these raccoon dogs (Table 2).
Table 1.
Detection of vector-borne pathogens from the spleen and blood of raccoon dogs in Korea during 2003–2010
| Region | Spleen | Blood | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of samples | Number of PCR-positive samples (infection rate [%]) | Number of samples | Number of PCR-positive samples (infection rate [%]) | |||||
| Anaplasma phagocytophilum | Abovis bovis | Borrelia sp. | Bartonella henselae | A. phagocytophilum | A. bovis | |||
| Seoul | 20 | 0 | 0 | 0 | 0 | 3 | 1 (33.3) | 0 |
| Gyeonggi-do | 40 | 0 | 0 | 0 | 1 (2.5) | 12 | 0 | 0 |
| Gwangwon-do | 41 | 1 (2.4) | 1 (2.4) | 0 | 0 | 31 | 0 | 3 (9.7) |
| Chungcheongbuk-do | 4 | 0 | 0 | 0 | 0 | – | – | – |
| Chungcheongnam-do | 2 | 0 | 0 | 0 | 0 | – | – | – |
| Jeollanam-do | 15 | 0 | 0 | 1 (6.6) | 0 | – | – | – |
| Gyeongsangbuk-do | 1 | 0 | 0 | 0 | 0 | – | – | – |
| Gyeongsangnam-do | 12 | 0 | 0 | 0 | 0 | – | – | – |
| Unknown | 7 | 0 | 0 | 0 | 1 (14.3) | 5 | 0 | 0 |
| Total | 142 | 1 (0.7) | 1 (0.7) | 1 (0.7) | 2 (1.4) | 51 | 1 (2.0) | 3 (5.9) |
PCR = polymerase chain reaction.
Table 2.
Prevalence of vector-borne pathogens in ticks collected from raccoon dogs in ROK during 2008–2009
| Species | Stage | Number of pools (number of ticks) | Number of PCR-positive samples (prevalence [%]*) against Borrelia sp. |
|---|---|---|---|
| Haemaphysalis flava | Larva | 2 (14) | 0 |
| Nymph | 9 (43) | 0 | |
| Male | 32 (32) | 0 | |
| Female | 16 (16) | 1 (6.3) | |
| Subtotal | 59 (105) | 1 (1.0) | |
| Haemaphysalis longicornis | Female | 9 (9) | 0 |
| Subtotal | 9 (9) | 0 | |
| Total | 68 (114) | 1 (0.9) |
PCR = polymerase chain reaction.
PCR-positive pathogens were calculated by minimum infection rate and number of positive pools/total number of individual ticks tested.
A molecular differentiation of tick species between H. flava and H. longicornis was conducted by PCR and sequencing to confirm the morphological identification of tick species. All raccoon dog samples were amplified using cyt b and cyt c primer sets. Amplified PCR products were randomly selected and sequenced, confirming they were raccoon dog sequences. The obtained cyt b and cyt c sequences were identical to the previously reported Nyctereutes procyonoides koreensis sequences, JX099864 and KF709435, respectively. In addition, all tick samples were amplified using ITS2 and 16S rRNA primers. The obtained H. flava and H. longicornis sequences were identical to a previously reported H. flava sequence (AB861941) and H. longicornis sequence (AB819210), respectively (data not shown). These results indicated that the morphological keys used for visual assessment were accurate.
Anaplasma phagocytophilum and A. bovis 16S rRNA genes, of product sizes 926 bp and 547-bp, respectively, were detected by species-specific nested PCR. Anaplasma phagocytophilum was detected in one (RS10556) of the 142 spleen samples and one (RB3) of the 51 blood samples (Table 1). The obtained sequences shared 99.6% identity with each other. The RB3 sequence (KY458571) was identical to the A. phagocytophilum HZ strain sequence (CP000235), Korean partial sequence (GU064899) detected in H. longicornis, and a partial sequence (JX173652) from Austria (Figure 1A). The RS10556 sequence (KY458570) was 99.9% identical to a Korean partial sequence (KU513794) detected in a sample from a dog. Anaplasma bovis 16 rRNA gene sequences were detected in one (RS7075) of the 142 spleen samples and in three (RB9, RB10, and RB28) of the 51 blood samples (Table 1). The gene sequences were compared with 16S rRNA gene sequence fragments to elucidate the genetic relationships between the Anaplasma spp. identified in raccoon dogs. The obtained A. bovis sequences were between 98.7% and 100.0% identical to each other and between 98.7% and 99.8% similar to a previously reported A. bovis sequence (GU556627) detected in a deer in Korea (Figure 1A). In comparison, the similarities between the obtained sequences and a previously reported A. bovis sequence (GU937020) collected from a raccoon, the most morphologically similar animal to the raccoon dog, were reported to be between 98.4% and 99.5%.
Using an msp2 and ankA gene-specific nested PCR, one A. phagocytophilum msp2 gene sequence and one A. phagocytophilum ankA gene sequence (MF506839) were obtained from the spleen samples. The A. phagocytophilum msp2 gene sequence (MF506840) was identical to an A. phagocytophilum sequence (HM752099) from Korea and 97.2% similar to several other A. phagocytophilum strains (MRK, Dog2, HZ, and JM) (Figure 1B). The A. phagocytophilum ankA gene sequence was identical to the Korean partial sequence (HM752100) detected from a KWD and was 98.7% similar to a previously reported A. phagocytophilum sequence (AY502606) from Russia (Figure 1C).
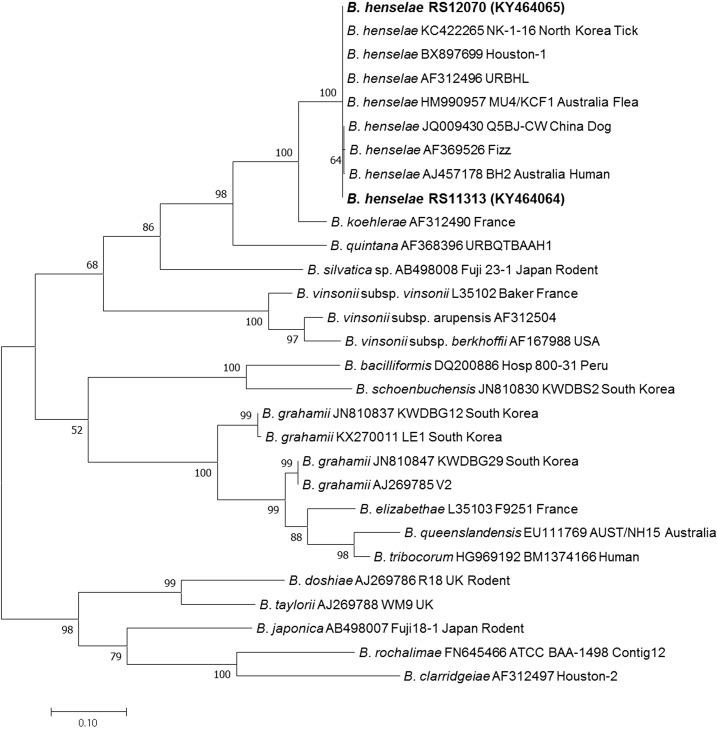
Two B. henselae sequences were detected in the spleen samples of raccoon dogs in Korea, via an ITS-specific nested PCR (Table 1). The B. henselae sequences were not detected in the blood samples. The size of the obtained product was 569-bp and contained a noncoding region. The two obtained B. henselae sequences (KY464064 and KY464065) were identical to each other and matched the B. henselae Houston-1 strain (BX897699), URBHLIE9 strain (AF312496), and a Korean partial sequence (KC422265). In addition, the sequences were 99.8% similar to a previously reported B. henselae sequence (JQ009430) detected in dogs in China (Figure 2). However, the nested PCR targeting rpoB and groEL genes that was performed for additional phylogenetic analysis did not yield any positive PCR products.
Figure 2.
Phylogenetic relationships for Bartonella henselae (bold letters) detected from raccoon dogs and Bartonella spp. based on partial nucleotide sequences of internal transcribed spacer gene fragments (569-bp). The maximum likelihood method was used for constructing the phylogenetic tree. The numbers at the nodes are the proportions of 1,000 bootstrap iterations that support the topology shown.
The B. theileri 16S rRNA gene was also detected by nested PCR, with an amplicon size of 507-bp. Two B. theileri sequences were identified from the spleen sample from a raccoon dog and a female H. flava (Tables 1 and 2). The obtained B. theileri sequences (KY458576 and KY798215) were identical to each other and showed 99.8% homology to a Borrelia sp. sequence (AB897891) from Japan, 99.6% homology to a B. theileri sequence (KF569941) from Mali, and 99.6% homology to a Borrelia sp. sequence (KC422269) from Korea. However, phylogenetic analysis indicated that KC422269 and KF569941 were more closely related to the obtained sequence than AB897891 (Figure 3). In addition, KC422269 (the obtained sequence from H. longicornis collected from goats in North Korea) appeared to be B. theileri, based on phylogenetic analysis (Figure 3). However, no PCR amplicons were obtained from the groEL gene-specific nested PCR.
In summary, two A. phagocytophilum, four A. bovis, two B. theileri, and two B. henselae sequences were detected by nested PCR in 193 raccoon dogs and 114 ticks (Tables 1 and 2). None of the raccoon dogs or ticks analyzed showed dual-pathogen co-infection.
DISCUSSION
Over the last decade, the investigation of VBPs in Korea has mainly focused on domestic animals, rodents, and vectors.9,10,14–16,42,43 However, recent studies examining wild animals have indicated that VBPs are prevalent in wild/domestic animals and vectors, and that the occurrence of human vector-borne diseases in Korea may, therefore, be more likely.12,22 Consistent with these findings, the first isolation of B. henselae and A. phagocytophilum from humans has been reported, and the reemergence of Lyme disease in Korea represents a potential public health concern.17,25,44
The aim of the present study was to investigate the role that raccoon dogs may play in the enzootic maintenance of VBPs in Korea. To the best of our knowledge, this study is the largest survey of such pathogens in raccoon dogs. The sampling period was over 8 years and 193 raccoon dogs were studied in total. Importantly, the present work constitutes the first report of the prevalence of A. phagocytophilum, B. henselae, and B. theileri in raccoon dogs in their East Asian native habitat.
To date, studies of the occurrence of A. phagocytophilum in wild animals in Asia have focused mainly on deer. For example, A. phagocytophilum and A. bovis have been detected in KWD and sika deer (Cervus nippon) in Korea and Japan.12,45 Very recently, A. bovis was detected in raccoon dogs in Korea.6 In Europe, the molecular detection of A. phagocytophilum in raccoon dogs by real-time PCR based on the msp2 gene has been attempted, although gene sequences matching those of A. phagocytophilum were not obtained.4 In this study, the 16S rRNA gene sequences of A. phagocytophilum (1.0%, 2/193) and A. bovis (2.1%, 4/193) were successfully detected in both spleen and blood samples from raccoon dogs sampled in Koreas. This study is, therefore, the first to report the molecular detection of A. phagocytophilum DNA in raccoon dogs. The acquired 16S rRNA sequences of A. phagocytophilum and A. bovis were either identical or similar to previously reported sequences detected in wild rodents, deer, and ticks in Korea (Figure 1A). Additional phylogenetic analysis of ankA, msp2, and groEL genes via PCR was also performed. However, only one ankA (MF506839) and one msp2 (MF506840) of A. phagocytophilum sequence were amplified from a single splenic tissue (Figure 1B and C). These data suggest that the prevalence of A. phagocytophilum in raccoon dogs is lower than that in KWD (although only limited studies on the prevalence of Anaplasma spp. in raccoon dogs are available) and indicate that raccoon dogs may play a role in the dispersal or maintenance of A. phagocytophilum in Korea. Further studies are needed to clarify the role that species plays in the ecology of A. phagocytophilum.
The molecular detection of Bartonella spp. in raccoon dogs has also been reported in Japan and their sequences were found to be most closely related to those of Bartonella rochalimae.7 In addition, Bartonella washonensis and other Bartonella spp. were detected in carnivores of the suborder Caniformia in Japan.7 In the present study, the obtained ITS gene sequences from raccoon dogs were identified as similar to those of B. henselae, a species transmitted by the cat flea (C. felis).18 Although B. rochalimae (genetically similar to Bartonella clarridgeiae and lineage three of the Bartonella genus) was detected in raccoon dogs, the detection of B. henselae (lineage four of the Bartonella genus) in this study is a novel discovery.46
To our knowledge, B. theileri has primarily been detected in ungulate mammals and hard ticks.31,47–49 However, B. theileri DNA was detected in raccoon dogs in this study, which is the first identification of this species in a carnivore rather than in an ungulate species. Although this result indicates that B. theileri can infect raccoon dogs, it also suggests that further studies are needed to clarify the role that the species may have as a potential reservoir of B. theileri. In addition, one B. theileri sequence was detected from a H. flava tick. We found 105 H. flava and nine H. longicornis sequences collected from three raccoon dogs demonstrating that H. flava is the dominant species found on raccoon dogs. Although the AB897891 sequence (detected from a Haemaphysalis japonica tick in Japan) and KC422269 sequence (detected from a H. longicornis tick in North Korea) were not categorically identified as belonging to B. theileri, they were included in the same sub-clade with KF569941, KY458576, KY798215, and U38375, based on phylogenetic analysis (Figure 3). We, therefore, suggest that the AB897891 and KC422269 sequences could potentially belong to B. theileri. Although Rhipicephalus spp. are known to be the main vector for B. theileri, our studies indicate that Haemaphysalis ticks may be additional vectors of B. theileri in East Asia.17
In one previous study, 117 H. flava and 18 Ixodes tanuki were collected from one raccoon dog.50 Moreover, in another previous study, 105 H. flava and nine H. longicornis were collected from three raccoon dogs.51 Although these previous studies, and our own results, are not representative of the total tick population in raccoon dogs in Korea, they indicate that H. flava is the dominant tick species found on raccoon dogs in the country. It has in addition been shown that H. longicornis represents the dominant tick species found on domestic/wild animals that live in grasses in Korea.9,10,13,19,20 To fully understand the differences in the tick populations between raccoon dogs and other animals, further study is required.
Ixodes spp. are the main vectors for both anaplasmosis and Lyme borreliosis.8,27 However, Haemaphysalis spp. in addition act as a vector for the transmission of Anaplasma spp., and possibly Bartonella and Borrelia spp., in Korea.9,10,13,19,20 In particular, although Bartonella spp. are mainly transmitted by fleas, recent evidence demonstrating transmission by ticks has been reported.11,17,18,22 Moreover, the rapid expansion of reservoir-adapted pathogens, such as Anaplasma, Bartonella, and Borrelia spp., has been described.4,5,12,13,22,23,52 These previous studies and our present work suggest that raccoon dogs may serve as potential reservoirs for the transmission of VBPs. Furthermore, the increased population of wild animals may contribute to the transmission of VBPs between wildlife, humans, and companion animals in Korea. These findings indicate that further investigation is required to fully understand the tick populations present within the raccoon dog population and elucidate the distribution of ectoparasites and VBPs in wild animals in Korea.
REFERENCES
- 1.Ward OG, Wurster-Hill DH, 1990. Mammalian Species: Nyctereutes procyonoides Oxford, United Kingdom: Oxford University Press. [Google Scholar]
- 2.Hyun BH, Lee KK, Kim IJ, Lee KW, Park HJ, Lee OS, An SH, Lee JB, 2005. Molecular epidemiology of rabies virus isolates from South Korea. Virus Res 114: 113–125. [DOI] [PubMed] [Google Scholar]
- 3.Oh SY, Kim SA, Kim JY, Yoo HS, Lee KK, Shin NS, 2012. Detection of antibodies against the rabies virus in Korean raccoon dogs (Nyctereutes procyonoides koreensis). J Zoo Wildl Med 43: 174–176. [DOI] [PubMed] [Google Scholar]
- 4.Härtwig V, von Loewenich FD, Schulze C, Straubinger RK, Daugschies A, Dyachenko V, 2014. Detection of Anaplasma phagocytophilum in red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) from Brandenburg, Germany. Ticks Tick Borne Dis 5: 277–280. [DOI] [PubMed] [Google Scholar]
- 5.Wodecka B, Michalik J, Lane RS, Nowak-Chmura M, Wierzbicka A, 2016. Differential associations of Borrelia species with European badgers (Meles meles) and raccoon dogs (Nyctereutes procyonoides) in western Poland. Ticks Tick Borne Dis 7: 1010–1016. [DOI] [PubMed] [Google Scholar]
- 6.Han YJ, Park JH, Lee YS, Chae JS, Yu DH, Park BK, Kim HC, Choi KS, 2017. Molecular identification of selected tick-borne pathogens in wild deer and raccoon dogs from the Republic of Korea. Vet Parasitol Reg Stud Rep 7: 25–31. [DOI] [PubMed] [Google Scholar]
- 7.Sato S, Kabeya H, Miura T, Suzuki K, Bai Y, Kosoy M, Sentsui H, Kariwa H, Maruyama S, 2012. Isolation and phylogenetic analysis of Bartonella species from wild carnivores of the suborder Caniformia in Japan. Vet Microbiol 161: 130–136. [DOI] [PubMed] [Google Scholar]
- 8.Chen SM, Dumler JS, Bakken JS, Walker DH, 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol 32: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim CM, et al. 2006. Tick-borne rickettsial pathogens in ticks and small mammals in Korea. Appl Environ Microbiol 72: 5766–5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh JY, Moon BC, Bae BK, Shin EH, Ko YH, Kim YJ, Park YH, Chae JS, 2009. Genetic identification and phylogenetic analysis of Anaplasma and Ehrlichia species in Haemaphysalis longicornis collected from Jeju Island, Korea. J Bacteriol Virol 39: 1–11. [Google Scholar]
- 11.Dugat T, Lagrée AC, Maillard R, Boulouis HJ, Haddad N, 2015. Opening the black box of Anaplasma phagocytophilum diversity: current situation and future perspectives. Front Cell Infect Microbiol 5: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang JG, Ko S, Kim YJ, Yang HJ, Lee H, Shin NS, Choi KS, Chae JS, 2011. New genetic variants of Anaplasma phagocytophilum and Anaplasma bovis from Korean water deer (Hydropotes inermis argyropus). Vector Borne Zoonotic Dis 11: 929–938. [DOI] [PubMed] [Google Scholar]
- 13.Kang JG, Kim HC, Choi CY, Nam HY, Chae HY, Chong ST, Klein TA, Ko S, Chae JS, 2013. Molecular detection of Anaplasma, Bartonella, and Borrelia species in ticks collected from migratory birds from Hong-do Island, Republic of Korea. Vector Borne Zoonotic Dis 13: 215–225. [DOI] [PubMed] [Google Scholar]
- 14.Seong G, Han YJ, Chae JB, Chae JS, Yu DH, Lee YS, Park J, Park BK, Yoo JG, Choi KS, 2015. Detection of Anaplasma sp. in Korean native goats (Capra aegagrus hircus) on Jeju Island, Korea. Korean J Parasitol 53: 765–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SH, VanBik D, Kim NH, Park SJ, Kwon OD, Kim TH, Kwak D, 2016. First molecular detection and genetic analysis of Anaplasma phagocytophilum in shelter cats in Seoul, Korea. Infect Genet Evol 46: 71–73. [DOI] [PubMed] [Google Scholar]
- 16.Lee S, Lee SH, VanBik D, Kim NH, Kim KT, Goo YK, Rhee MH, Kwon OD, Kwak D, 2016. First molecular detection and phylogenetic analysis of Anaplasma phagocytophilum in shelter dogs in Seoul, Korea. Ticks Tick Borne Dis 7: 945–950. [DOI] [PubMed] [Google Scholar]
- 17.Kim KH, Yi J, Oh WS, Kim NH, Choi SJ, Choe PG, Kim NJ, Lee JK, Oh MD, 2014. Human granulocytic anaplasmosis, South Korea, 2013. Emerg Infect Dis 20: 1708–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chomel BB, Boulouis HJ, Maruyama S, Breitschwerdt EB, 2006. Bartonella spp. in pets and effect on human health. Emerg Infect Dis 12: 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang JG, Ko S, Smith WB, Kim HC, Lee IY, Chae JS, 2016. Prevalence of Anaplasma, Bartonella and Borrelia species in Haemaphysalis longicornis collected from goats in North Korea. J Vet Sci 17: 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang JG, et al. 2016. Prevalence of Anaplasma and Bartonella spp. in ticks collected from Korean water deer (Hydropotes inermis argyropus). Korean J Parasitol 54: 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YS, Seo KW, Lee JH, Choi EW, Lee HW, Hwang CY, Shin NS, Youn HJ, Youn HY, 2009. Prevalence of Bartonella henselae and Bartonella clarridgeiae in cats and dogs in Korea. J Vet Sci 10: 85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko S, Kim SJ, Kang JG, Won S, Lee H, Shin NS, Choi KS, Youn HY, Chae JS, 2013. Molecular detection of Bartonella grahamii and B. schoenbuchensis-related species in Korean water deer (Hydropotes inermis argyropus). Vector Borne Zoonotic Dis 13: 415–418. [DOI] [PubMed] [Google Scholar]
- 23.Kang JG, Won S, Kim HW, Kim BJ, Park BK, Park TS, Seo HY, Chae JS, 2016. Molecular detection of Bartonella spp. in terrestrial leeches (Haemadipsa rjukjuana) feeding on human and animal blood in Gageo-do, Republic of Korea. Parasit Vectors 9: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cotté V, Bonnet S, Le Rhun D, Le Naour E, Chauvin A, Boulouis HJ, Lecuelle B, Lilin T, Vayssier-Taussat M, 2008. Transmission of Bartonella henselae by Ixodes ricinus. Emerg Infect Dis 14: 1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Im JH, Baek JH, Lee HJ, Lee JS, Chung MH, Kim M, Lee SM, Kang JS, 2013. First case of Bartonella henselae bacteremia in Korea. Infect Chemother 45: 446–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP, 1982. Lyme disease-a tick-borne spirochetosis? Science 216: 1317–1319. [DOI] [PubMed] [Google Scholar]
- 27.Margos G, Vollmer SA, Ogden NH, Fish D, 2011. Population genetics, taxonomy, phylogeny and evolution of Borrelia burgdorferi sensu lato. Infect Genet Evol 11: 1545–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elbir H, FotsoFotso A, Diatta G, Trape JF, Arnathau C, Renaud F, Durand P, 2015. Ubiquitous bacteria Borrelia crocidurae in western African ticks Ornithodoros sonrai. Parasit Vectors 8: 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbour AG, Maupin GO, Teltow GJ, Carter CJ, Piesman J, 1996. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J Infect Dis 173: 403–409. [DOI] [PubMed] [Google Scholar]
- 30.Fukunaga M, Takahashi Y, Tsuruta Y, Matsushita O, Ralph D, McClelland M, Nakao M, 1995. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int J Syst Bacteriol 45: 804–810. [DOI] [PubMed] [Google Scholar]
- 31.Theiler A, 1904. Spirillosis of cattle. J Comp Pathol Ther 17: 47–55. [Google Scholar]
- 32.Park KH, Lee SH, Won WJ, Jang WJ, Chang WH, 1992. Isolation of Borrelia burgdorferi, the causative agent of Lyme disease, from Ixodes ticks in Korea. J Korean Soc Microbiol 27: 307–312. [Google Scholar]
- 33.Jung BY, Gebeyehu EB, Seo MG, Byun JW, Kim HY, Kwak D, 2012. Prevalence of vector-borne diseases in shelter dogs in Korea. Vet Rec 171: 249. [DOI] [PubMed] [Google Scholar]
- 34.Lee SH, Yun SH, Choi E, Park YS, Lee SE, Cho GJ, Kwon OD, Kwak D, 2016. Serological detection of Borrelia burgdorferi among horses in Korea. Korean J Parasitol 54: 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaguti N, Tipton VJ, Keegan HL, Toshioka S, 1971. Ticks of Japan, Korea and the Ryukyu Islands. Sci Bull Biol Ser 15: 1–227. [Google Scholar]
- 36.Renesto P, Gouvernet J, Drancourt M, Roux V, Raoult D, 2001. Use of rpoB gene analysis for detection and identification of Bartonella species. J Clin Microbiol 39: 430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeaiter Z, Fournier PE, Ogata H, Raoult D, 2002. Phylogenetic classification of Bartonella species by comparing groEL sequences. Int J Syst Evol Microbiol 52: 165–171. [DOI] [PubMed] [Google Scholar]
- 38.Park HS, Lee JH, Jeong EJ, Koh SE, Park TK, Jang WJ, Park KH, Kim BJ, Kook YH, Lee SH, 2004. Evaluation of groEL gene analysis for identification of Borrelia burgdorferi sensu lato. J Clin Microbiol 42: 1270–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng WY, Zhao GH, Jia YQ, Bian QQ, Du SZ, Fang YQ, Qi MZ, Yu SK, 2013. Characterization of Haemaphysalis flava (Acari: Ixodidae) from Qingling subspecies of giant panda (Ailuropoda melanoleuca qinlingensis) in Qinling Mountains (central China) by morphology and molecular markers. PLoS One 8: e69793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R, 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3: 294–299. [PubMed] [Google Scholar]
- 41.Slaska B, Grzybowska-Szatkowska L, 2011. Analysis of the mitochondrial haplogroups of farm and wild-living raccoon dogs in Poland. Mitochondrial DNA 22: 105–110. [DOI] [PubMed] [Google Scholar]
- 42.Lee M, Yu D, Yoon J, Li Y, Lee J, Park J, 2009. Natural co-infection of Ehrlichia chaffeensis and Anaplasma bovis in a deer in South Korea. J Vet Med Sci 71: 101–103. [DOI] [PubMed] [Google Scholar]
- 43.Kim BJ, et al. 2013. First report for the seasonal and annual prevalence of flea-borne Bartonella from rodents and soricomorphs in the Republic of Korea. Vector Borne Zoonotic Dis 13: 457–467. [DOI] [PubMed] [Google Scholar]
- 44.Moon S, Hong Y, Hwang KJ, Kim S, Eom J, Kwon D, Park JH, Youn SK, Sohn A, 2015. Epidemiological features and clinical manifestations of Lyme borreliosis in Korea during the period 2005–2012. Jpn J Infect Dis 68: 1–4. [DOI] [PubMed] [Google Scholar]
- 45.Jilintai, Seino N, Hayakawa D, Suzuki M, Hata H, Kondo S, Matsumoto K, Yokoyama N, Inokuma H, 2009. Molecular survey for Anaplasma bovis and Anaplasma phagocytophilum infection in cattle in a pastureland where sika deer appear in Hokkaido, Japan. Jpn J Infect Dis 62: 73–75. [PubMed] [Google Scholar]
- 46.Engel P, et al. 2011. Parallel evolution of a type IV secretion system in radiating lineages of the host-restricted bacterial pathogen Bartonella. PLoS Genet 7: e1001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Callow LL, 1967. Observations on tick-transmitted spirochaetes of cattle in Australia and South Africa. Br Vet J 123: 492–497. [DOI] [PubMed] [Google Scholar]
- 48.Khoo JJ, Lim FS, Tan KK, Chen FS, Phoon WH, Khor CS, Pike BL, Chang LY, AbuBakar S, 2017. Detection in Malaysia of a Borrelia sp. from Haemaphysalis hystricis (Ixodida: Ixodidae). J Med Entomol 54: 1444–1448. [DOI] [PubMed] [Google Scholar]
- 49.Smith RD, Miranpuri GS, Adams JH, Ahrens EH, 1985. Borrelia theileri: isolation from ticks (Boophilus microplus) and tick-borne transmission between splenectomized calves. Am J Vet Res 46: 1396–1398. [PubMed] [Google Scholar]
- 50.Lee WK, Lim JW, Lee SY, Lee IY, 1997. Redescription of Haemaphysalis flava and Ixodes tanuki collected from a raccoon dog in Korea. Korean J Parasitol 35: 1–8. [DOI] [PubMed] [Google Scholar]
- 51.Kim HC, et al. 2011. Ticks collected from selected mammalian hosts surveyed in the Republic of Korea during 2008–2009. Korean J Parasitol 49: 331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Angelakis E, Billeter SA, Breitschwerdt EB, Chomel BB, Raoult D, 2010. Potential for tick-borne Bartonellosis. Emerg Infect Dis 16: 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]