Abstract.
It has been postulated that impaired host immunity due to HIV infection reduces parasite egg excretion. Schistosoma/HIV interactions have also been shown to differ by sex. We hypothesized that egg excretion would vary based on both HIV status and sex. We examined data from more than 1,700 participants in eight studies conducted in northwest Tanzania between 2010 and 2016. Schistosoma infection was defined by circulating anodic antigen (CAA) serum levels ≥ 30 pg/mL and/or egg positivity in either stool by Kato Katz method or urine by filtration. We used multivariable analyses to determine the impact of confounding factors such as sex, age, previous praziquantel treatment, and worm burden as measured by serum CAA level, on the relationship between egg excretion and HIV status. HIV-infected individuals were significantly less likely to excrete schistosome eggs than HIV-uninfected individuals, even after controlling for worm burden and sex (OR = 0.6 [0.4, 0.9], P = 0.005). Furthermore, after controlling for worm burden and HIV status, women had lower odds of egg excretion than men (OR = 0.4 [0.3, 0.5], P < 0.001). Sensitivity of egg microscopy was lower in HIV-infected women than HIV-uninfected men (41% versus 61%, P < 0.001), whereas sensitivity in women remained low in both groups (33% versus 37%, P = 0.664). Our study is the first to report that women with Schistosoma infection excrete fewer eggs than men for a given worm burden, regardless of HIV the status. These findings suggest that guidelines for use of microscopy to diagnose Schistosoma infections in HIV-infected individuals and in women merit reconsideration.
INTRODUCTION
Schistosomiasis is a zoonotic neglected tropical disease with a life cycle through fresh water snails that affects 218 million individuals worldwide.1 For endemic settings, the World Health Organization recommends microscopic examination of stool and urine for parasite eggs to detect Schistosoma infections.1 However, egg excretion is variable, depends on both the worm load and the host immunity, and can fluctuate on a daily basis.2–5 Microscopy is known to have a low sensitivity in areas of low endemicity and in individuals with light infections.6–8
With the development of an up-converting phosphor lateral flow assay to measure Schistosoma circulating anodic antigen (CAA), a newer technique has emerged with higher sensitivity and better specificity.9–11 Circulating anodic antigen is a glycosaminoglycan-like carbohydrate that is secreted into the bloodstream by adult worms of all Schistosoma species and can be used to estimate the burden of adult worms.4,7,12,13 Studies of Schistosoma infections that have used both CAA and microscopy have consistently revealed non-negligible numbers of patients who are CAA-positive while having a null egg count.14–20
Discordant findings of a CAA test positive for Schistosoma antigen but no eggs visualized microscopically may occur more commonly in the setting of HIV infection, which is co-endemic with Schistosoma infection in many regions of sub-Saharan Africa. Mouse models suggest that intact T-cell responses may be necessary for efficient parasite egg excretion,21 supporting field-based observations in humans.22,23 Several small studies have reported lower Schistosoma egg excretion in those with HIV infection as compared with those without,22,24–26 whereas larger studies have not been able to show an association.27–29 Only one study looked at CAA values in relation to HIV status and found no difference.30 None of these studies has investigated whether the sex of the infected host affects differential results between CAA and egg microscopy
We sought to determine the effect of HIV infection on Schistosoma egg excretion using CAA and microscopy data from a total of eight different studies and screening projects conducted by our team in northern Tanzania31,32 and to investigate this relationship in both Schistosoma mansoni and Schistosoma haematobium infection. We hypothesized that HIV-infected individuals with Schistosoma infection were significantly less likely to shed eggs than those without HIV infection. We also hypothesized that the ratio of egg to CAA values, reflecting eggs excreted for a given worm burden, would differ by HIV status and that egg excretion may differ by sex.
METHODS
We compiled data from eight cross-sectional studies conducted in northwest Tanzania from 2010 to 2016, two of which had their methods previously described.31,32 Altogether, the eight studies covered 20 villages of the Lake Zone. In all studies, individuals greater than 18 years old were enrolled after providing written informed consent. All included participants underwent testing for Schistosoma infection both by egg count in stool and urine and by serum CAA. HIV status was determined by a rapid test on site in accordance with the Tanzanian national algorithm for HIV testing at the time of the study, with all positive results confirmed by a second different rapid test. Those testing positive for HIV infection for the first time were referred to their local clinics for ongoing free care and treatment. All Schistosoma infections were treated with praziquantel. Basic demographic data was collected including age, sex, and treatment of schistosomiasis within the last 5 years. Details of the individual studies are presented in Table 1.
Table 1.
Baseline description of the population and studies included in the analysis
| Study | N | Females | Type of study* | Median age in years [IQR] | Species | Egg+ | CAA and/or Egg+ | HIV+ | Egg count median [IQR] | Previous treatment* | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 326 | 100.0% (326/326) | CB | 30 [25–37] | S. m. | 57.2% (95/166) | 12.0% (39/326) | 50.9% (166/326) | 6.1% (20/326) | Stool (/g feces) | 36 [24–60] | 17.0% (36/212) |
| S. h. | 4.8% (8/166) | Urine (/mL urine) | 9 [5.5–12] | |||||||||
| Un. | 38.0% (63/166) | – | – | |||||||||
| B | 82 | 81.7% (67/82) | OB | 36 [29–40] | S. m. | 100.0% (38/38) | 24.4% (20/82) | 46.3% (38/82) | 100.0% (82/82) | Stool (/g feces) | 21.6 [4.8–50.4] | Not available |
| S. h. | 0.0% (0/38) | Urine (/mL urine) | – | |||||||||
| Un. | 0.0% (0/38) | – | – | |||||||||
| C | 173 | 61.8% (107/173) | OB | 41 [36–46] | S. m. | 100% (84/84) | 11.6% (20/173) | 48.6% (84/173) | 100.0% (173/173) | Stool (/g feces) | 37.2 [14.4–50] | 2.3% (4/173) |
| S. h. | 0.0% (0/84) | Urine (/mL urine) | – | |||||||||
| Un. | 0.0% (0/84) | – | – | |||||||||
| D | 668 | 0.0% (0/668) | CB | 34 [25–42] | S. m. | 75.3% (321/425) | 40.0% (267/668) | 63.6% (425/668) | 5.7% (38/668) | Stool (/g feces) | 48 [14.4–231.6] | 27.6% (183/663) |
| S. h. | 17.4% (75/425) | Urine (/mL urine) | 6 [2–14] | |||||||||
| Un. | 7.8% (31/425) | – | – | |||||||||
| E | 109 | 60.6% (66/109) | CB and OB | 31 [25–40] | S. m. | 23.9% (11/46) | 27.5% (30/109) | 42.2% (46/109) | 44.0% (48/109) | Stool (/g feces) | 55.2 [18–85.2] | 7.2% (7/97) |
| S. h. | 80.4% (37/46) | Urine (/mL urine) | 4 [2–13.75] | |||||||||
| Un. | 0.0% (0/46) | – | – | |||||||||
| F | 108 | 100.0% (108/108) | CB | 25.5 [21–31] | S. m. | 2.4% (1/42) | 21.3% (23/108) | 38.9% (42/108) | 0.0% (0/108) | Stool (/g feces) | 72 [72–72] | Not available |
| S. h. | 97.6% (41/42) | Urine (/mL urine) | 6 [4–7.75] | |||||||||
| Un. | 0.0% (0/42) | – | – | |||||||||
| G | 199 | 100.0% (199/199) | CB | 29 [23–37] | S. m. | 63.0% (46/73) | 12.6% (25/199) | 36.7% (73/199) | 9.5% (19/199) | Stool (/g feces) | 14.4 [4.8–39] | 9.9% (19/191) |
| S. h. | 37.0% (27/73) | Urine (/mL urine) | 2 [1–3.75] | |||||||||
| Un. | 0.0% (0/73) | – | – | |||||||||
| H | 80 | 100.0% (80/80) | CB and OB | 30.5 [25–35] | S. m. | 89.8% (35/39) | 32.5% (26/80) | 48.8% (39/80) | 16.3% (13/80) | Stool (/g feces) | 14.4 [4.8–103.2] | 9.1% (7/77) |
| S. h. | 5.1% (2/39) | Urine (/mL urine) | 3.5 [3.25–3.75] | |||||||||
| Un. | 5.1% (2/39) | – | – | |||||||||
| Total | 1,745 | 54.6% (953/1,745) | – | 32 [25–40] | S. m. | 69.1% (631/913) | 25.8% (450/1,745) | 52.3% (913/1,745) | 22.5% (393/1,745) | Stool (/g feces) | 38.4 [14.4–129.6] | 18.1% (256/1,413) |
| S. h. | 20.8% (190/913) | Urine (/mL urine) | 5 [2–12] | |||||||||
| Un. | 10.5% (96/913) | – | – | |||||||||
CAA = circulating anodic antigen; CB = community-based; IQR = interquartile range; OB = outpatient clinic-based; S. h. = Schistosoma haematobium; S. m. = Schistosoma mansoni; Un. = unidentified. Only four individuals had known mixed infection. Individuals that were CAA+/Egg− in villages where both S. mansoni and S. haematobium coexist were classified as “species unidentified.”
This column reports treatment of schistosomiasis with praziquantel within the past 5 years.
Microscopic testing was performed on 10 mL of urine (for S. haematobium) by the filtration technique and on feces (for S. mansoni) following the Kato Katz method. Testing was performed on site by the same experienced parasitologists from the National Institute of Medical Research in Mwanza, Tanzania, for all studies. For study A, two Kato Katz slides were prepared from each stool sample using 41.7 mg of stool per slide, whereas five Kato Katz slides using 41.7 mg of stool per slide were used for all other studies. Serum CAA testing was performed at Leiden University Medical Center for Study A, and the remaining CAA testing was performed at the National Institute for Medical Research in Mwanza as previously described, using a positivity threshold of 30 pg/mL (dry reagent SCAA20 assay format)10,33 for all studies. Species were determined by egg morphology. In the case of CAA-positive, egg-negative cases, Schistosoma species cannot be identified, but as the epidemiological distribution of both species of Schistosoma was known for all villages, the most likely species was assigned in monospecies villages.
Informed consent was obtained from all participants. All studies received ethical approval from Bugando Medical Center, the National Institute for Medical Research in Dar es Salaam, Tanzania, and Weill Cornell Medical College, New York.
Statistical analysis was performed using Stata version 13 (College Station, TX). Individuals were defined as Schistosoma-infected if they had a serum CAA concentration of ≥ 30 pg/mL and/or Schistosoma eggs detected by microscopy. Binary variables were described as proportions and continuous variables were described using median and interquartile range and compared using χ2 tests. We investigated the association between HIV status and egg excretion, regardless of Schistosoma species, by running univariate logistic regressions for the subgroup “Schistosoma-infected” with the outcome being the presence of eggs in urine and/or feces and input being HIV status.
We further ran multivariable analyses to explore the impact of other competing factors such as sex, age, previous praziquantel treatment of schistosomiasis, and worm load, on the relationship between egg excretion and HIV status. HIV status did not take into account the duration of HIV infection or the use of antiretroviral therapy (ART). The natural logarithm of CAA values was used as a proxy for worm load.4,12 Variables included in the final model were determined by backward selection procedure with the standard threshold of 0.1 as well as exploration of interaction terms.
In addition, we explored CAA values by sex and HIV status. Finally, we examined the relationship between the CAA and egg load, by Schistosoma species because of large differences in excretion numbers, with regard to sex and HIV status by running rank-sum tests on egg/CAA ratios.
RESULTS
In total, results were available from 1,745 participants tested in 20 villages near Lake Victoria. Among them, 54.6% (953/1,745) were female, 52.3% (913/1,745) were positive for Schistosoma infection, 22.5% (393/1,745) were HIV-infected, and 18.1% (256/1,413) reported treatment of schistosomiasis in the past 5 years (Table 1).
Among the 913 Schistosoma sp.–infected individuals, by univariate analysis, HIV infection was associated with lower odds of egg excretion (OR = 0.5 [0.4, 0.7], P < 0.001) as was female sex (OR = 0.4 [0.3, 0.5], P < 0.001). Past treatment with praziquantel was associated with higher odds of egg excretion (OR = 1.5 [1.1, 2.2], P = 0.025), whereas age was not significantly associated with egg excretion (OR = 1.0 [0.98, 1.01], P = 0.25). The final model determined by backward selection procedure included HIV status, sex, and worm load as measured by serum CAA level. HIV remained associated with egg excretion after adjustment for sex and worm load (OR = 0.6 [0.4, 0.8], P = 0.004). Of note, when adding an interaction term between HIV and sex, HIV was still significantly associated with egg positivity (OR = 0.4 [0.2, 0.7], P = 0.001 for male) and the interaction between sex and HIV status was marginally significant (OR = 1.9 [0.95, 3.83], P = 0.069, with male sex as the baseline), indicating that the effect of HIV infection in decreasing egg excretion was greater in males than in females.
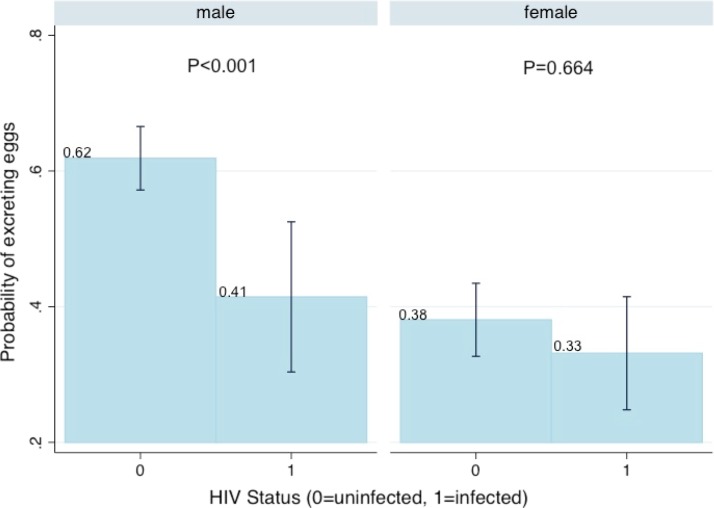
Among Schistosoma-infected individuals, regardless of the infecting species and of HIV status, women were overall less likely than men to excrete eggs. In HIV-noninfected women, egg microscopy had a sensitivity of 38% and in HIV-noninfected men, egg microscopy had a sensitivity of 62% for Schistosoma infection (38% versus 62% difference, P < 0.001). In HIV-infected women, egg microscopy had a sensitivity of 33% and in HIV-infected men, egg microscopy had a sensitivity of 41% (33% versus 41% difference, P = 0.23). These results are shown in Table 2 and the interaction margins are shown in Figure 1.
Table 2.
Results of the multivariable logistic regression for factors associated with Schistosoma sp. egg positivity
| Variables | Odds ratio | 95% confidence interval | P value | |
|---|---|---|---|---|
| Main effects on egg positivity | HIV infection | 0.6 | [0.4, 0.8] | 0.004 |
| Female sex | 0.4 | [0.3, 0.5] | < 0.001 | |
| Serum CAA level (natural log) | 1.2 | [1.1, 1.3] | < 0.001 | |
| With interaction allowed | HIV infection for male | 0.4 | [0.2, 0.7] | 0.001 |
| Female sex for HIV-uninfected | 0.4 | [0.3, 0.5] | < 0.001 | |
| Serum CAA level (natural log) | 1.2 | [1.1, 1.3] | 0.003 | |
| HIV*sex (ref = male) | 1.9 | [1.0, 3.8] | 0.069 |
CAA = circulating anodic antigen.
Figure 1.
Predictive margins with 95% confidence intervals for the probability of schistosome egg excretion in Schistosoma sp.–infected individuals, by HIV status and sex. The predictive margins probability of excreting eggs based on sex and HIV status interaction show that women are overall less likely than men to excrete eggs regardless of HIV status. Men secrete significantly fewer eggs when HIV-infected, as compared with men who are HIV-noninfected. This figure appears in color at www.ajtmh.org.
The median of the logarithm of CAA value did not differ by HIV status (P = 0.84) but differed by sex, with women having significantly lower CAA values (median in male = 6.6 pg/mL, median in female = 6.0 pg/mL, P < 0.001). Moreover, the ratio of eggs to CAA, reflective of the number of eggs excreted for a given worm burden, varied significantly between men and women and between HIV-infected and HIV-noninfected individuals with S. mansoni co-infection (P = 0.014 and P = 0.0077 respectively). Men and HIV-noninfected individuals shed more eggs for a given CAA value than did women and HIV-infected individuals. In S. haematobium infection, for which the sample size was much smaller, similar trends were observed but the difference did not reach significance. These results are shown in Table 3.
Table 3.
Median ratios and interquartile range for eggs excreted per natural log of serum CAA value, by Schistosoma species, sex, and HIV status
| Schistosoma mansoni | Schistosoma haematobium | ||||
|---|---|---|---|---|---|
| Eggs per gram stool/ln (CAA) | P value | Eggs per 10 mL urine/ln (CAA) | P value | ||
| By sex | Male | 6.0 [2.3–26.5] | 0.014 | 1.4 [0.5–2.2] | 0.26 |
| Female | 4.4 [1.6–8.6] | 0.7 [0.4–1.9] | |||
| By HIV status | Negative | 6.2 [2.1–20.6] | 0.0077 | 0.9 [0.4–2.3] | 0.73 |
| Positive | 4.1 [1.7–7.4] | 1.3 [0.6–2.2] | |||
CAA = circulating anodic antigen.
To determine the effect of the use of two Kato Katz slides instead of five in study A, we conducted a sensitivity analysis that included only the results from the first two Kato Katz slides of each study. All findings remained statistically significant.
DISCUSSION
This is the first study, to our knowledge, to investigate the impact of sex on egg excretion in adults in an HIV-endemic area. Examination of serum samples from more than 1,700 people in Tanzania showed that both women and HIV-infected individuals were significantly less likely to excrete Schistosoma sp. eggs when infected, even after controlling for a given worm antigen level. The sensitivity of egg microscopy, regardless of species, was much lower in HIV-infected men than in HIV-noninfected men (41% versus 62%), whereas the sensitivity in HIV-infected versus HIV-noninfected women remained low in both groups (33% versus 38%). Given the marked geographical overlap of Schistosoma sp. and HIV infections, our work suggests that guidelines for use of microscopy to determine Schistosoma sp. infection status in HIV-infected individuals and in women merit reconsideration.
To our knowledge, our study is the first to report overall lower odds of egg excretion for Schistosoma sp. infection in women compared with men. We additionally report the novel finding that HIV infection impacts egg excretion in men but not in women.24,25 Only one study conducted in children reports higher S. haematobium egg excretion in boys than girls, although this study did not control for worm burden.34 Our finding that the ratios of egg excretion to worm burden were lower in women infected with S. mansoni implies that the sex difference cannot be attributed to worm burden alone for this species. It is possible that CD4+ T-cell counts could have been lower in men35,36 and that this could have impacted egg excretion via an effect on T cells.22,23 It is also possible that anatomical pelvic differences between men and women could lead to higher numbers of migrating parasite eggs trapped in female pelvic tissues than those in males, or that worm fecundity could be affected by disparate immunological responses to S. mansoni worms in men versus women.37,38 We had very few data points of participants both infected with HIV and S. haematobium, and additional studies are needed to understand the effects of HIV infection on egg excretion in individuals with S. haematobium infection.
Our finding that HIV infection status did not significantly affect Schistosoma sp. egg excretion in women could explain why several larger studies, which included mostly or entirely women,27,28 failed to demonstrate an effect of HIV infection on egg excretion. Our study confirms the reduction in egg excretion in HIV-infected individuals that was previously observed in smaller studies,22,24–26 even after controlling for other confounding variables. Further studies are required to look at the potential difference of old versus new HIV infection and possible impact of ART on egg excretion.
The fact that age was not associated with egg excretion seems somewhat surprising.2 It seems likely that this finding could be a consequence of the relatively tight age range (20–47 years) of adults included in our study. We did not enroll children younger than age 18 and included only few adults greater than 40.
Finally, as could be expected, we found some CAA+/Egg− patients, which is likely because the CAA test is more sensitive than egg count. We did also identify a small number of CAA−/Egg+ patients. It is likely that CAA testing with increased sample volume, which has an even higher sensitivity, would have identified CAA infection in some of these patients.10 In addition, some individuals could have been recently treated with praziquantel and, because of the rapid clearance of CAA, would test negative for CAA while continuing to excrete eggs.39 Our finding that HIV status was not associated with a difference in CAA values, as shown in previous studies,30,40 further strengthens support for the use of CAA as a superior diagnostic tool for Schistosoma sp. infection in HIV-endemic settings and suggests that efforts to expand CAA testing are warranted.
In conclusion, our study demonstrates for the first time the effect of sex on Schistosoma sp. egg excretion and clarifies past studies on the relationship between HIV and egg excretion. Our work indicates that decreased egg excretion in the setting of HIV infection is limited to men. Our finding that HIV does not impact the relationship between CAA values and egg load suggests that the more sensitive CAA assay for diagnosis of Schistosoma infections in HIV-endemic settings, particularly for women, should be the preferred test.
Acknowledgments:
We thank Daniel Fitzgerald (Weill Cornell Medicine) for his helpful comments as well as Philbert Kashangaki, Honest Nagai, Mnyeshi Petro, Ndalloh Paul, Jane Mlingi, Inobena Tosiri, and Ester Zanzibar for their tireless and outstanding work in the field.
REFERENCES
- 1.WHO , 2017. Schistosomiasis Factsheet. Available at: http://www.who.int/mediacentre/factsheets/fs115/en/. Accessed April 15, 2017.
- 2.Doehring E, Feldmeier H, Daffalla AA, 1983. Day-to-day variation and circadian rhythm of egg excretion in urinary schistosomiasis in the Sudan. Ann Trop Med Parasitol 77: 587–594. [DOI] [PubMed] [Google Scholar]
- 3.Birrie H, Medhin G, Erko B, 1994. Variability of egg excretion in Schistosoma mansoni infection in Ethiopia: a case report. East Afr Med J 71: 545–547. [PubMed] [Google Scholar]
- 4.Agnew A, et al. 1996. Age-dependent reduction of schistosome fecundity in Schistosoma haematobium but not Schistosoma mansoni infections in humans. Am J Trop Med Hyg 55: 338–343. [DOI] [PubMed] [Google Scholar]
- 5.Bethony J, et al. 2002. Additive host genetic factors influence fecal egg excretion rates during Schistosoma mansoni infection in a rural area in Brazil. Am J Trop Med Hyg 67: 336–343. [DOI] [PubMed] [Google Scholar]
- 6.De Vlas SJ, Gryseels B, Van Oortmarssen GJ, Polderman AM, Habbema JDF, 1993. A pocket chart to estimate true Schistosoma mansoni prevalences. Parasitol Today 9: 306–307. [PubMed] [Google Scholar]
- 7.Van Lieshout L, Polderman AM, Deelder AM, 2000. Immunodiagnosis of schistosomiasis by determination of the circulating antigens CAA and CCA, in particular in individuals with recent or light infections. Acta Trop 77: 69–80. [DOI] [PubMed] [Google Scholar]
- 8.Doenhoff MJ, Chiodini PL, Hamilton JV, 2004. Specific and sensitive diagnosis of schistosome infection: can it be done with antibodies? Trends Parasitol 20: 35–39. [DOI] [PubMed] [Google Scholar]
- 9.Corstjens PL, van Lieshout L, Zuiderwijk M, Kornelis D, Tanke HJ, Deelder AM, van Dam GJ, 2008. Up-converting phosphor technology-based lateral flow assay for detection of Schistosoma circulating anodic antigen in serum. J Clin Microbiol 46: 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corstjens PL, et al. 2014. Tools for diagnosis, monitoring and screening of Schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitol 141: 1841–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corstjens PL, Nyakundi RK, de Dood CJ, Kariuki TM, Ochola EA, Karanja DM, Mwinzi PNM, van Dam GJ, 2015. Improved sensitivity of the urine CAA lateral-flow assay for diagnosing active Schistosoma infections by using larger sample volumes. Parasit Vectors 8: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson AR, van Dam GJ, Kariuki TM, Farah IO, Deelder AM, Coulson PS, 2006. The detection limits for estimates of infection intensity in schistosomiasis mansoni established by a study in non-human primates. Int J Parasitol 36: 1241–1244. [DOI] [PubMed] [Google Scholar]
- 13.Utzinger J, Becker SL, van Lieshout L, van Dam GJ, Knopp S, 2015. New diagnostic tools in schistosomiasis. Clin Microbiol Infect 21: 529–542. [DOI] [PubMed] [Google Scholar]
- 14.De Clercq D, Sacko M, Vercruysse J, Diarra A, Landoure A, vanden Bussche V, Gryseels B, Deelder A, 1995. Comparison of the circulating anodic antigen detection assay and urine filtration to diagnose Schistosoma haematobium infections in Mali. Trans R Soc Trop Med Hyg 89: 395–397. [DOI] [PubMed] [Google Scholar]
- 15.Van Lieshout L, Polman K, Gryseels B, Deelder AM, 1998. Circulating anodic antigen levels in two areas endemic for schistosomiasis mansoni indicate differences in worm fecundity. Trans R Soc Trop Med Hyg 92: 115–119. [DOI] [PubMed] [Google Scholar]
- 16.Cai YC, et al. 2014. Field comparison of circulating antibody assays versus circulating antigen assays for the detection of schistosomiasis japonica in endemic areas of China. Parasit Vectors 7: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knopp S, et al. 2015. Sensitivity and specificity of a urine circulating anodic antigen test for the diagnosis of Schistosoma haematobium in low endemic settings. PLoS Negl Trop Dis 9: e0003752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Dam GJ, et al. 2015. An ultra-sensitive assay targeting the circulating anodic antigen for the diagnosis of Schistosoma japonicum in a low-endemic area, People’s Republic of China. Acta Trop 141: 190–197. [DOI] [PubMed] [Google Scholar]
- 19.Balahbib A, et al. 2017. Selecting accurate post-elimination monitoring tools to prevent reemergence of urogenital schistosomiasis in Morocco: a pilot study. Infect Dis Poverty 6: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vonghachack Y, et al. 2017. Comparison of novel and standarddiagnostic tools for the detection of Schistosoma mekongi infection in Lao People’s Democratic Republic and Cambodia. Infect Dis Poverty 6: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doenhoff MJ, Hassounah OA, Lucas SB, 1985. Does the immunopathology induced by schistosome eggs potentiate parasite survival? Immunol Today 6: 203–206. [DOI] [PubMed] [Google Scholar]
- 22.Karanja DM, Colley DG, Nahlen BL, Ouma JH, Secor WE, 1997. Studies on schistosomiasis in western Kenya: I. Evidence for immune-facilitated excretion of schistosome eggs from patients with Schistosoma mansoni and human immunodeficiency virus coinfections. Am J Trop Med Hyg 56: 515–521. [DOI] [PubMed] [Google Scholar]
- 23.Muok EM, Simiyu EW, Ochola EA, Ng’ang’a ZW, Secor WE, Karanja DM, Mwinzi PM, 2013. Association between CD4+ T-lymphocyte counts and fecal excretion of Schistosoma mansoni eggs in patients coinfected with S. mansoni and human immunodeficiency virus before and after initiation of antiretroviral therapy. Am J Trop Med Hyg 89: 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mwanakasale V, Vounatsou P, Sukwa TY, Ziba M, Ernest A, Tanner M, 2003. Interactions between Schistosoma haematobium and human immunodeficiency virus type 1: the effects of coinfection on treatment outcomes in rural Zambia. Am J Trop Med Hyg 69: 420–428. [PubMed] [Google Scholar]
- 25.Sanya RE, Muhangi L, Nampijja M, Nannozi V, Nakawungu PK, Abayo E, Webb EL, Elliott AM, 2015. Schistosoma mansoni and HIV infection in a Ugandan population with high HIV and helminth prevalence. Trop Med Int Health 20: 1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontanet AL, Woldemichael T, Sahlu T, van Dam GJ, Messele T, Rinke de Wit T, Masho W, Yeneneh H, Coutinho RA, van Lieshout L, 2000. Epidemiology of HIV and Schistosoma mansoni infections among sugar-estate residents in Ethiopia. Ann Trop Med Parasitol 94: 145–155. [PubMed] [Google Scholar]
- 27.Kleppa E, Klinge KF, Galaphaththi-Arachchige HN, Holmen SD, Lillebo K, Onsrud M, Gundersen SG, Taylor M, Ndhlovu P, Kjetland EF, 2015. Schistosoma haematobium infection and CD4+ T-cell levels: a cross-sectional study of young South African women. PLoS One 10: e0119326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kallestrup P, Zinyama R, Gomo E, Butterworth AE, van Dam GJ, Erikstrup C, Ullum H, 2005. Schistosomiasis and HIV-1 infection in rural Zimbabwe: implications of coinfection for excretion of eggs. J Infect Dis 191: 1311–1320. [DOI] [PubMed] [Google Scholar]
- 29.Mazigo HD, Dunne DW, Wilson S, Kinung’hi SM, Pinot de Moira A, Jones FM, Morona D, Nuwaha F, 2014. Co-infection with Schistosoma mansoni and human immunodeficiency virus-1 (HIV-1) among residents of fishing villages of north-western Tanzania. Parasit Vectors 7: 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ssetaala A, et al. 2015. Schistosoma mansoni and HIV acquisition in fishing communities of Lake Victoria, Uganda: a nested case–control study. Trop Med Int Health 20: 1190–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Downs JA, et al. 2012. Association of schistosomiasis and HIV infection in Tanzania. Am J Trop Med Hyg 87: 868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Downs JA, et al. 2017. Schistosomiasis and human immunodeficiency virus in men in Tanzania. Am J Trop Med Hyg 96: 856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Dam GJ, Claudia J, Lewis M, Deelder AM, van Lieshout L, Tanke HJ, van Rooyen LH, Corstjens PL, 2013. A robust dry reagent lateral flow assay for diagnosis of active schistosomiasis by detection of Schistosoma circulating anodic antigen. Exp Parasitol 135: 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ndyomugyenyi R, Minjas JN, 2001. Urinary schistosomiasis in schoolchildren in Dar-es-Salaam, Tanzania, and the factors influencing its transmission. Ann Trop Med Parasitol 95: 697–706. [DOI] [PubMed] [Google Scholar]
- 35.Delmas M-C, Jadand C, De Vincenzi I, Deveau C, Persoz A, Sobel A, Kazatchkine M, Brunet JB, Meyer L, 1997. Gender differences in CD4+ cell counts persist after HIV-1 infection. AIDS 11: 1071–1073. [PubMed] [Google Scholar]
- 36.Loupa CV, Rodriguez B, McComsey G, Gripshover B, Salata RA, Valdez H, Lisgaris MV, Fulton SA, Lederman MM, 2006. Gender differences in human immunodeficiency virus (HIV) RNA and CD4 cell counts among new entrants to HIV care. Clin Microbiol Infect 12: 389–391. [DOI] [PubMed] [Google Scholar]
- 37.Cheever AW, Kamel IA, Elwi AM, Mosimann JE, Danner R, 1977. Schistosoma mansoni and S. haematobium infections in Egypt. Am J Trop Med Hyg 26: 702–716. [DOI] [PubMed] [Google Scholar]
- 38.Wilson S, Jones FM, van Dam GJ, Corstjens PL, Riveau G, Fitzsimmons CM, Sacko MBJ, Vennervald BJ, Dunne DW, 2014. Human Schistosoma haematobium antifecundity immunity is dependent on transmission intensity and associated with immunoglobulin G1 to worm-derived antigens. J Infect Dis 210: 2009–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stete K, Krauth SJ, Coulibaly JT, Knopp S, Hattendorf J, Müller I, Lohourignon LK, Kern WV, N’Goran EK, Utzinger J, 2012. Dynamics of Schistosoma haematobium egg output and associated infection parameters following treatment with praziquantel in school-aged children. Parasit Vectors 5: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kallestrup P, Zinyama R, Gomo E, Butterworth AE, van Dam GJ, Gerstoft J, Erikstrup C, Ullum H, 2006. Schistosomiasis and HIV in rural Zimbabwe: efficacy of treatment of schistosomiasis in individuals with HIV coinfection. Clin Infect Dis 42: 1781–1789. [DOI] [PubMed] [Google Scholar]