Abstract.
Malarial infections are uncommon in the United States and almost all reported cases stem from recent travelers coming from endemic countries. Cerebral malaria (CM) is a severe form of the disease usually affecting children and individuals with limited immunity. Despite proper management, mortality from CM can reach up to 25%, especially when it is associated with brain edema. Inefficient management of the edema may result in brain herniation and death. Uniform guidelines for management of CM-associated brain edema are lacking. In this report, we present a case of CM with associated severe brain edema that was successfully managed using a unique combination of therapeutic hypothermia, hypertonic saline, mannitol, and hyperventilation along with the antimalarial drugs quinidine and doxycycline. Our use of hypothermia was based on its proven benefit for improving neurological outcomes in post-cardiac arrest patients and previous in vitro research, suggesting its potential inhibitory role on malaria growth.
INTRODUCTION
Malaria is a serious parasitic disease affecting over 200 million people worldwide and accounting for about half a million deaths every year. Malarial infections are uncommon in the United States. According to the Centers for Disease Control and Prevention, about 1,500–2,000 malarial cases have been reported annually in the United States; almost all of them were among recent travelers coming from endemic countries.1,2
Cerebral malaria (CM) is a severe form of malarial infection usually caused by Plasmodium falciparum and mostly affecting children, pregnant women, or adults with malaria-limited immunity. Despite proper management and advancements in healthcare, mortality rates remain devastatingly high, ranging between 15% and 25%.3 Poor outcomes are especially common among patients who present late, those who develop signs of cerebral edema and/or the immunocompromised.4,5 We present a case of a young adult who suffered a severe form of CM and successfully recovered despite poor initial prognosis.
CASE REPORT
A 32-year-old Caucasian male presented to the hospital with a 3-day history of fever, headache, diarrhea, fatigue, and confusion. He had recently returned from Uganda where he used to work for the past 6 years. The patient had a history of two previous malarial episodes, which were successfully treated, 2 and 4 years before presentation, and he was not on any antimalarial prophylaxis thereafter. Other medical histories included previous schistosomiasis, attention deficit hyperactivity disorder, and depression. In the emergency room, he was disoriented and lethargic. He then developed a seizure episode, after which he became minimally responsive. His Glasgow coma score dropped from 12/15 initially to 7/15 after the seizure episode. He was subsequently intubated for airway protection.
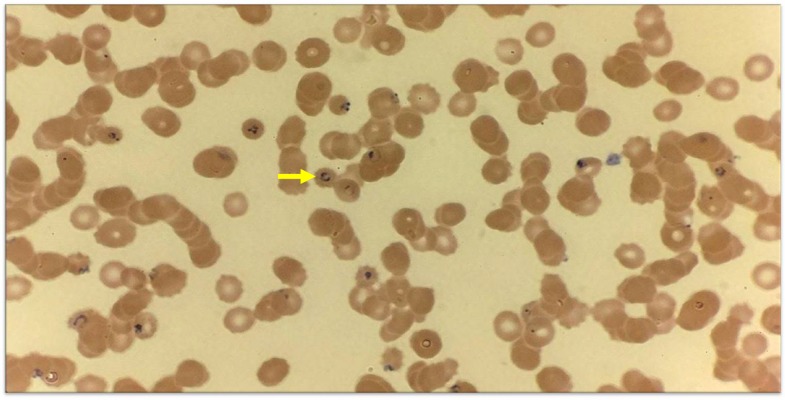
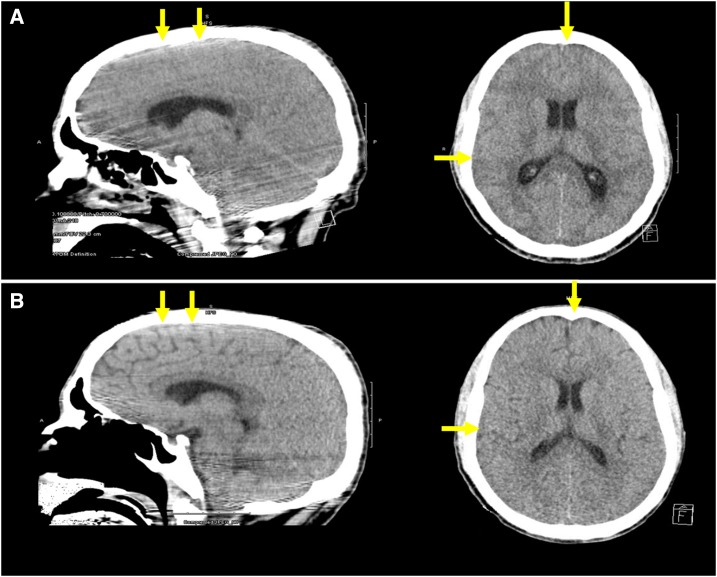
On physical examination, he was sedated, intubated, and mechanically ventilated. Vital signs showed a temperature of 97.9°F, blood pressure of 110/69 mm of Hg, pulse of 111 beats per minute, respiratory rate of 22 breaths per minute, and peripheral oxygen saturation of 100% on 35% FiO2. Pupils were equal and reactive. Fundoscopic examination revealed papilledema. Other physical examination findings were unremarkable. Laboratory work was significant for hemoglobin of 9.6 g/dL, white blood cell count of 8.6 (103/µL), platelet count of 30 (103/µL), lactate dehydrogenase of 477 (U/L), bilirubin of 4.8 (mg/dL), decreased haptoglobin, and mild metabolic acidosis. Peripheral blood smear was positive for P. falciparum malarial rings with 9.8% parasitemia (Figure 1). Computed tomography scan of the head showed severe cerebral edema with loss of sulci/gyri differentiation (Figure 2A).
Figure 1.
Peripheral blood smear showing classic rings of Plasmodium falciparum (arrow). This figure appears in color at www.ajtmh.org.
Figure 2.
Computed tomography scan of the head showing severe brain edema at presentation with loss of sulci/gyri differentiation (A) and the complete resolution of brain edema after treatment with restoration of normal radiologic brain anatomy (B). This figure appears in color at www.ajtmh.org.
The patient was admitted to the intensive care unit under the care of our critical care team. Because of the severity of the brain edema, in addition to the accepted standard of care treatment of CM, decision was made to place the patient under therapeutic hypothermia (TH) as a trial to minimize neurological damage. We used cooling blankets to achieve a target temperature of 32–34°C with an esophageal probe to continuously monitor the core body temperature. We also initiated aggressive management of the brain edema with 3% saline, mannitol and hyperventilation. We continued TH for 2 days until complete resolution of the brain edema, as evidenced by repeat CT scan (Figure 2B) and fundoscopic examination. The patient was then rewarmed and subsequently extubated. He received a 3-day course of quinidine and a 7-day course of doxycycline, which reduced the level parasitemia from 9.8% initially to 1.3% within 48 hours and undetectable by day 8 (Table 1). He had no neurological deficits, tolerated diet, and was transferred to the medical floor where he gradually recuperated.
Table 1.
Timeline for daily neurologic status, parasitic load, and implemented treatment modalities
| Hospital day | Neurologic status | Parasitemia level (%) | Antimalarial drugs | Adjunct treatment modalities |
|---|---|---|---|---|
| Day 0 | Disoriented, lethargic (Glasgow coma score 12/15) then after seizures obtunded (Glasgow coma score 7/15), intubated, papilledema | 9.8 | Quinidine 24 mg/kg loading then 12 mg/kg every 8 hours for 3 days + doxycycline 100 mg every 12 hours for 7 days | Initiated therapeutic hypothermia 32–34°C, hyperventilation (target PCO2 30–35 mm of Hg), 3% saline for target serum Na increase of 6–8 meq/day and mannitol 1 mg/kg every 6 hours |
| Day 1 | Sedated, intubated, papilledema | 3.8 | ||
| Day 2 | Sedated, intubated, improved papilledema | 1.3 | ||
| Day 3 | No papilledema, off sedation, extubated, full neurological recovery | 0.4 | Rewarming, 3% saline and mannitol stopped | |
| Day 4 | No neurological deficits, recuperating, transferred to the medical floor | 0.25 | ||
| Day 5 | 0.2 | |||
| Day 6 | 0.05 | |||
| Day 7 | 0.05 | |||
| Day 8 | 0 |
DISCUSSION
Our case is a presentation of a young immunocompetent adult who developed a severe form of CM with acute brain edema. The pathophysiology of CM and associated brain edema is still not well understood. Many researchers suggest that the sequestration of infected red blood cells inside small cerebral blood vessels would cause their occlusion resulting in brain ischemia. Parasite-infected red blood cells are believed to induce endothelial cell dysfunction and subsequent release of tumor necrosis factor-α and nitric oxide, which are believed to be the culprit behind the development of brain edema.6,7
Specific guidelines addressing CM-associated brain edema management are lacking. Delayed or inadequate management of the edema can result in brain herniation and subsequent death.
Therapeutic hypothermia has been well known to improve neurological outcome and decrease mortality in post-cardiac arrest patients.8,9 The role of TH in management of CM has not yet been documented. A recent in vitro trial suggested that P. falciparum growth was inhibited by medical hypothermia.10 This was in concordance with other in vitro studies, suggesting similar effects with lower temperatures.11,12 During that trial, hypothermia had no negative impact on the activity of the antimalarial drugs, namely, chloroquine, mefloquine, and artemisinin derivatives. On the other hand, other reports suggest that hyperthermia would also inhibit plasmodial growth,13 yet the use of hyperthermia would worsen brain edema and accelerate neurological damage.
Although we cannot know for sure which of our treatment modalities helped our patient the most, we believe that our treatment approach was successful. Early intensive management of the patient’s severe brain edema with the combination of hypertonic saline, mannitol, and hyperventilation was crucial in preventing brain herniation. Moreover, we believe that TH may have played a protective role in delaying neurological damage and decreasing intracranial pressure. Yet, we cannot prove that effect without controlled trials which would be difficult to implement, given the low incidence of CM cases in developed countries.
Our treatment bundle entailed various modalities including mechanical ventilation, controlled temperature management, and osmotic diuresis, which would necessitate ICU-level care including frequent vitals and laboratory monitoring. Those settings are readily available in developed countries and may constitute a potential adjunct neuroprotective regimen. Nevertheless, the widespread application of this treatment bundle would be challenging in many poor-resource malaria-endemic regions.
Acknowledgments:
We thank Dr. Yasir Ahmed and Dr. William Davis from Department of Internal Medicine at Texas Tech University Health Sciences Center, Odessa, TX, for their valuable help in reviewing this case report.
REFERENCES
- 1.Cullen KA, Mace KE, Arguin PM, 2016. Malaria surveillance—United States, 2013. MMWR Surveill Summ 65: 1–22. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization, 2016. Fact Sheet: World Malaria Report 2016 Available at: http://www.who.int/malaria/media/world-malaria-report-2016/en/. Accessed August 24, 2017.
- 3.Mishra SK, Newton CR, 2009. Diagnosis and management of the neurological complications of falciparum malaria. Nat Rev Neurol 5: 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seydel KB, et al. 2015. Brain swelling and death in children with cerebral malaria. N Engl J Med 372: 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker M, Kublin JG, Zunt JR, 2006. Parasitic central nervous system infections in immunocompromised hosts: malaria, microsporidiosis, leishmaniasis, and African trypanosomiasis. Clin Infect Dis 42: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahu PK, Satpathi S, Behera PK, Mishra SK, Mohanty S, Wassmer SC, 2015. Pathogenesis of cerebral malaria: new diagnostic tools, biomarkers, and therapeutic approaches. Front Cell Infect Microbiol 5: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wassmer SC, Moxon CA, Taylor T, Grau GE, Molyneux ME, Craig AG, 2011. Vascular endothelial cells cultured from patients with cerebral or uncomplicated malaria exhibit differential reactivity to TNF. Cell Microbiol 13: 198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K, 2002. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 346: 557–563. [DOI] [PubMed] [Google Scholar]
- 9.Hypothermia after Cardiac Arrest Study Group , 2002. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 346: 549–556. Erratum in N Engl J Med 2002;346:22. [DOI] [PubMed] [Google Scholar]
- 10.Rehman K, Sauerzopf U, Veletzky L, Lötsch F, Groger M, Ramharter M, 2016. Effect of mild medical hypothermia on in vitro growth of Plasmodium falciparum and the activity of anti-malarial drugs. Malar J 15: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan L, et al. 2014. Refrigeration provides a simple means to synchronize in vitro cultures of Plasmodium falciparum. Exp Parasitol 140: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rojas MO, Wasserman M, 1993. Effect of low temperature on the in vitro growth of Plasmodium falciparum. J Eukaryot Microbiol 40: 149–152. [DOI] [PubMed] [Google Scholar]
- 13.Long HY, Lell B, Dietz K, Kremsner PG, 2001. Plasmodium falciparum: in vitro growth inhibition by febrile temperatures. Parasitol Res 87: 553–555. [DOI] [PubMed] [Google Scholar]