Abstract.
Negative results in serological routine screening of patients with microbiologically proven Paracoccidioidomycosis (PCM) are occasionally reported. Failure in detecting anti-Paracoccidioides antibodies has been ascribed to factors either related to serological techniques or to the status of the host immune reactivity. Recently, this issue has been renewed by the recognition that the Paracoccidioides genera comprises two species, lutzii and brasiliensis, which have distinct antigenic profiles and, therefore, may elicit different host antibody responses. We describe a patient with the acute form PCM due to Paracoccidioides brasiliensis with negative results on two reference centers’ routine screening for P. brasiliensis antibodies, but positive results with Paracoccidioides lutzii antigens. The present case report suggests that antibodies elicited during P. brasiliensis infection recognize antigenic fractions shared by both species, highlighting the difficulties in distinguishing the two infections by means of the currently available routine serological assays.
INTRODUCTION
Gold standard diagnosis of Paracoccidioidomycosis (PCM) is based on the observation of the characteristic yeast cells in patients’ specimens (sputum, biopsy specimens, or scrapings of lesions) or cultures. However, as these specimens are not always readily available, requiring invasive procedures, serological assays are considered an important tool and have been widely adopted for PCM diagnosis and for patient follow-up.1 On the other hand, patients with microbiologically proven PCM can occasionally present negative results on routine serological screening.2–5 Several factors were described that could account for the failure in detecting anti-Paracoccidioides antibodies in these cases. Immunoprecipitation assays such as double immunodiffusion (DID) or counterimmunoelectrophoresis (CIE), which are low-cost techniques most commonly used in reference centers, have been reported to exhibit limited sensitivity, missing the diagnosis in more than 10% of cases.3,6,7
Lack of standardization may contribute to this limitation; most reference centers use in-house techniques with different antigen preparations and lack external controls, resulting in significant interlaboratory variability.1 Another reason for false-negative results is the low anti-Paracoccidioides antibody levels generated by some patients, particularly those with a very localized chronic form disease.7,8 Other potential causes for false-negative reactions are the prozone effect,3 immune complex formation,3 impaired humoral immunity9,10 and production of low-avidity immunoglobulin (Ig) G2 antibodies directed against carbohydrate epitopes by patients with the chronic pulmonary form of the disease.4
Recently, this issue has been renewed by the recognition that the Paracoccidioides genera comprises two species, brasiliensis and lutzii, which have distinct antigenic profiles and, therefore, may elicit different host’s antibody responses.11 However, serological diagnosis of PCM due to Paracoccidioides lutzii has not yet been standardized in clinical practice.
We describe a boy with the acute form PCM (AF PCM) due to Paracoccidioides brasiliensis with negative results by two reference centers’ routine screening for P. brasiliensis antibodies, but positive results with P. lutzii antigens, highlighting the difficultly in identifying the infecting species using serological methods.
CASE REPORT
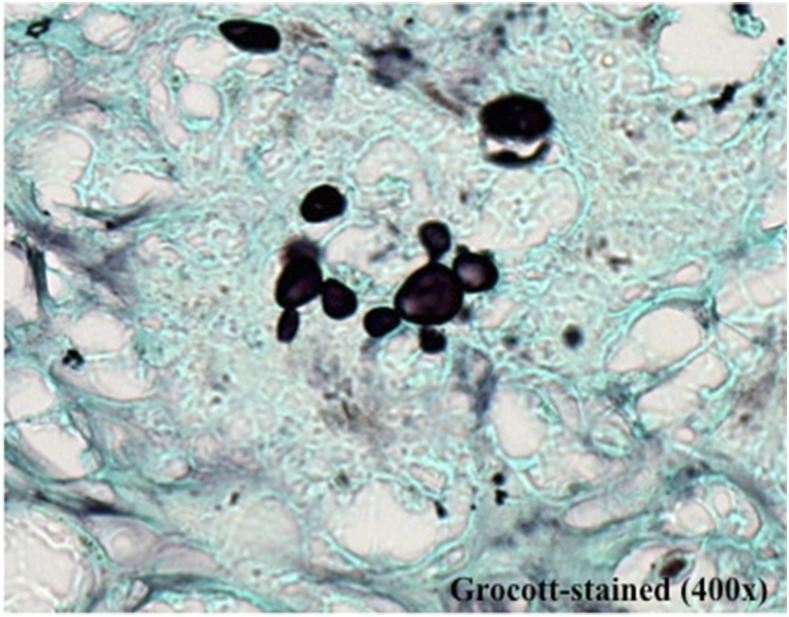
In February 2015, a previously healthy 16-year-old boy presented with a 4-month history of fever; cervical, axillary, and inguinal lymph node enlargements; asthenia;and a 10% weight loss. Physical examination disclosed generalized lymph node enlargement. Laboratory tests showed anemia (hemoglobin: 8.9 g/dL), leukocytosis (20,900 cells/mm3), and mild eosinophilia (418 cells/mm3), typical of the AF PCM. A Thoracic computed tomography (CT) scan showed normal pulmonary parenchyma but lymph node enlargements in several chains. An abdominal CT scan also revealed severe lymph node enlargement resulting in conglomerates with heterogenous enhancement delimiting central areas of necrosis. Histopathology of a cervical lymph node biopsy showed a granulomatous process with caseous necrosis and multiple budding yeast cells characteristic of Paracoccidioides spp. (Figure 1). Unfortunately, no microbiological cultures of the biopsy were done. The patient was treated for severe AF PCM with amphotericin B initially (21 days), followed by itraconazole with marked clinical improvement. The patient was still on itraconazole therapy on his last outpatient visit and was doing well.
Figure 1.
Methamine silver staining of a section of a cervical lymph node biopsy showing multiple budding yeast cells characteristic of Paracoccidioides spp. This figure appears in color at www.ajtmh.org.
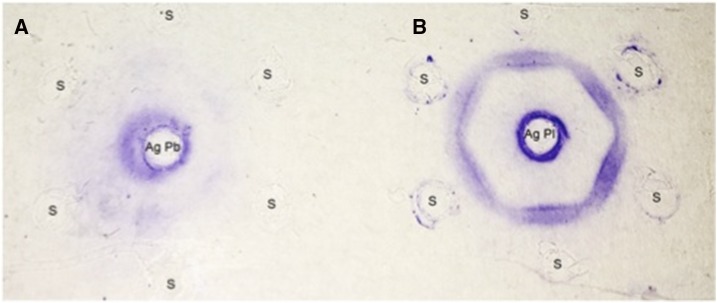
However, serological diagnosis of the patient done in parallel was inconclusive. Routine anti–P. brasiliensis serology testing carried out at the Immunology Center of the Institute Adolfo Lutz, the reference laboratory of the Health Department of the State of São Paulo, before starting antifungal treatment was negative. This test was repeated with a new serum sample collected 2 weeks later and confirmed the lack of reactivity against Pb113-exoantigen (AgPb113); however, the DID carried out at the same time with cell-free antigens from P. lutzii-208 (AgPl208),11 was strongly positive (up to 1:256 dilution) (Figure 2). The patient had no epidemiologic history suggestive of P. lutzii infection; he was born and lived in a rural area close to São Paulo city (Itapecerica da Serra) except for the last year when he moved to the city of São Paulo. He had traveled only once in his life, to Saquarema, a nonendemic city in the state of Rio de Janeiro.
Figure 2.
Immunodiffusion test showing reactivity of the patient’s serum with antigens from Paracoccidioides. The center well contains AgPb113 (A) and AgPl208 (B). This figure appears in color at www.ajtmh.org.
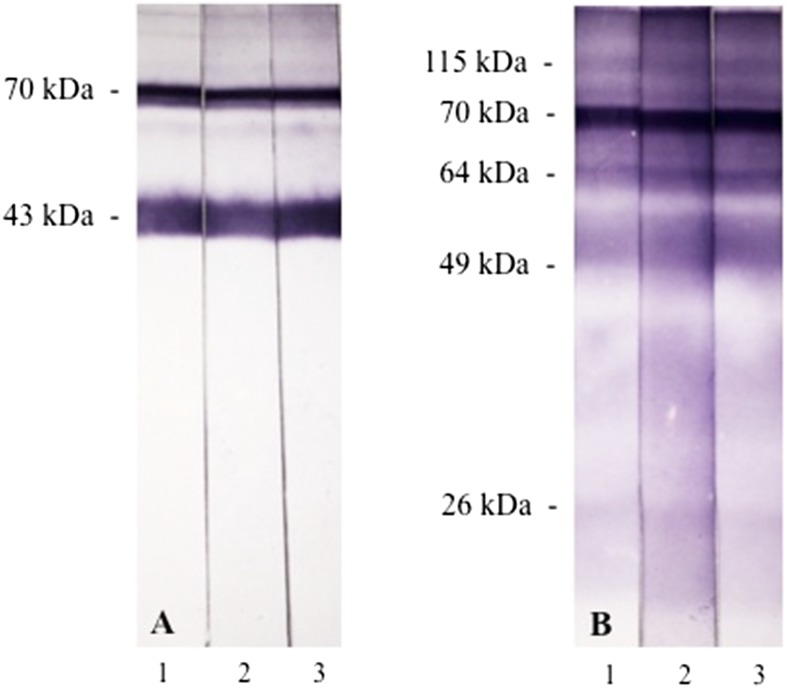
With these conflicting results, additional serological assays were performed at the Institute Adolfo Lutz laboratory and at another reference center, the Medical Mycology Laboratory of the University of São Paulo, to clarify whether the PCM was due to P. brasiliensis or P. lutzii. First, immunoblotting (IB) of all three serum samples using the Pb113-exoantigen (AgPb113) showed the presence of anti-gp43 and anti-70 kDa antibodies, as expected for P. brasiliensis–infected patients (Figure 3A).12–14 Two additional exoantigens were used, from P. brasiliensis339 (AgPb339) and from P. lutzii66 (AgPl66) for assaying immunoprecipitating antibodies using CIE. Consistent with the previous DID assays, the patient’s sample #2 was negative with AgPb339 but strongly positive (1:64 dilution) with AgPl66. Third, IB of the three serum samples against AgPl208 used in the DID showed reactivity against several fractions with molecular masses of approximately 115, 70, 64, 49, and 26 kDa (Figure 3B).
Figure 3.
Immunoblotting of the patient’s serum response to antigens from Paracoccidioides. (A) AgPb113 and (B) AgPl208. Antigens were transferred electrophoretically to nitrocellulose membranes and incubated with sequential patient’s serum samples (1, 2, and 3) and peroxidase-conjugated antihuman immunoglobulin G. Reactions were developed with 4-chloro-1-naphthol. This figure appears in color at www.ajtmh.org.
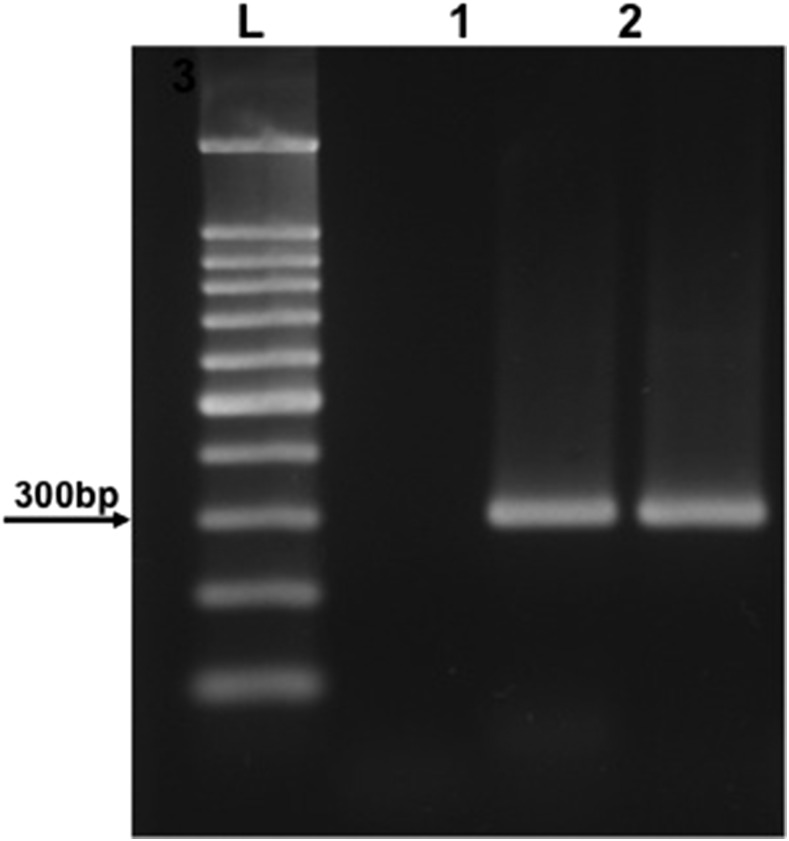
As the serological survey was not able to discriminate the Paracoccidioides species, molecular studies were carried out. DNA from the lymph node biopsy was extracted the using QIAamp DNA tissue kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. A seminested polymerase chain reaction (PCR) assay was performed using primers previously designed from the P. brasiliensis gp43 sequence.15 DNA samples from both P. brasiliensis 339 (Pb339) and P. lutzii 66 (Pl66) isolates were amplified in parallel. The PCR products of approximately 300 bp were obtained from both the patient’s and the Pb339 DNA samples, although the amplification was negative for the Pl66 DNA sample, as expected for P. lutzii isolates (Figure 4).16 Identification of the species as brasiliensis was further confirmed by sequencing the products of amplification (ABI 3500 DNA Analyzer; Thermo Fisher, Carlsbad, CA). The patient’s sequence showed 98% identity when compared with the gp43 sequences of P. brasiliensis available in GenBank.
Figure 4.
Agarose gel showing the seminested polymerase chain reaction products obtained from DNA samples after amplification with primers gp43.15 L = mass ladder 100 bp; 1 = DNA sample from Paracoccidioides lutzii 66; 2 = DNA sample from the patient’s biopsy; 3 = DNA sample from Paracoccidioides brasiliensis 339.
DISCUSSION
Negative results in serological routine screening of patients with microbiologically proven PCM is occasionally reported.2–5 Failure in detecting anti-Paracoccidioides antibodies has been ascribed to factors either related to serological techniques or to the status of the host immune reactivity.3,4,9,10,17,18 Recently, this issue has been renewed by the recognition that the Paracoccidioides genus comprises two species with distinct antigenic profiles and geographical distribution. Whereas PCM due to P. brasiliensis appears widespread in Brazil and other South American countries, PCM due to P. lutzii appears restricted to central areas of Brazil.19
The immunodominant antigen of P. brasiliensis is a 43 kDa glycoprotein that, although with a yet unknown biological function in the fungus physiology/metabolism, is highly expressed by yeast cells. Previous studies showed that removal of gp43 from P. brasiliensis antigenic preparation strongly reduced patients’ serological reactivity.20 Paracoccidioides lutzii, on the other hand, expresses small amounts of a 43 kDa glycoprotein that has glucanase activity and antigenic properties distinct from P. brasiliensis’ gp43.21,22 In fact, several mutations distinguish the gp43 gene of the two species.16,22 This and other antigenic differences may explain the early observations made by some clinicians about the high rate of negative results with gp43-based routine serological screening of PCM patients from the central areas of Brazil (C. J. Fontes, personal communication), as well as the studies reporting unexpected negative serological results in patients from these areas.5,23–25
The unexpectedly very high titers in the DID and CIE techniques against P. lutzii antigen preparations supported the suspicion that the patient was indeed infected by P. lutzii. On the other hand, the IB showed marked reactivity against both gp43 and gp70, characteristic of P. brasiliensis infection. It remains to be determined why the anti-gp43 and anti-70 reactivity did not translate into positive reactions in the DID and CIE assays carried out at two reference centers with serum samples collected during the first 2 months of follow-up. Some hypotheses can be raised based on previous studies of both the host antibody response to P. brasiliensis and the differing characteristics of immunoprecipitating (DID and CIE) and IB techniques. One hypothesis is that, because the DID reactivity is based mainly on recognition of gp43 and because different isolates can produce different gp43 isoforms, these isoforms may not be recognized in serological assays that use soluble antigens, such as the DID, but they do not interfere in assays that use membrane-bound antigens, such as the IB.17 Other hypothesis is that in patients, especially those with the acute form of the disease, the specific antibody response comprises mainly antibodies of the IgM class, which can fail to react or react weakly in DID/CIE but not in IB.26
On the other hand, IB of the three consecutive serum samples of the patient carried out with a P. lutzii antigen preparation consistently recognized four main fractions and other minor fractions. Paradoxically to the fact that the patient was infected with P. brasiliensis, as defined by its molecular characterization from a lymph node biopsy rich in fungal organisms, IB with P. lutzii antigen is consistent with the strong reactivity in the DID and CIE assays using antigen preparations from this species. Unfortunately, little is still known about the characteristics of the antibody response of patients with PCM due to P. lutzii. No immunodominant P. lutzii-specific fraction has yet emerged in preliminary studies performed with five different isolates of this species (A. P. Vicentini, V. Morais, C. P.Taborda, unpublished data).
Previous studies reported that sera from patients with PCM due to P. brasiliensis do not recognize antigen fractions contained in the cell-free preparations of P. lutzii, unlike sera from patients with PCM due to P. lutzii, which were able to recognize antigenic fractions from both species.11 However, other authors showed that 60% of a cohort of PCM patients living in the state of Paraná, in the southern region of Brazil and endemic for P. brasiliensis, yielded positive results in a DID assay with a P. lutzii antigen preparation.27
Altogether, our case reinforces the idea of an antigenic variability in the P. brasiliensis complex, which can in part explain some false-negative results in patients with active infection and the difficulty in adopting one single strain or molecule in the antigen preparation to diagnose a disease caused by two different species comprising isolates that may present distinctly unique antigen profiles. Our case report also suggests that antibodies elicited during P. brasiliensis infection recognize antigenic fractions shared by both species, highlighting the difficulties in distinguishing the two infections using the currently available routine serological assays.
Acknowledgments:
We thank Prof. Zoilo P. Camargo for the kind donation of the CFA from Paracoccidioides lutzii-208 and Anna S. Shafferman-Levin for the careful editing of the English.
Disclaimer: The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Vidal MS, Del Negro GM, Vicentini AP, Svidzinski TI, Mendes-Giannini MJ, Almeida AM, Martinez R, de Camargo ZP, Taborda CP, Benard G, 2014. Serological diagnosis of paracoccidioidomycosis: high rate of inter-laboratorial variability among medical mycology reference centers. PLoS Negl Trop Dis 8: e3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.del Negro GM, Benard G, de Assis CM, Vidal MS, Garcia NM, Otani C, Shikanai-Yasuda MA, da S Lacaz C, 1995. Lack of reactivity of paracoccidioidomycosis sera in the double immunodiffusion test with the gp43 antigen: report of two cases. J Med Vet Mycol 33: 113–116. [DOI] [PubMed] [Google Scholar]
- 3.Do Valle AC, Costa RL, Fialho Monteiro PC, Von Helder J, Muniz MM, Zancopé-Oliveira RM, 2011. Interpretation and clinical correlation of serological tests in paracoccidioidomycosis. Med Mycol 39: 373–377. [DOI] [PubMed] [Google Scholar]
- 4.Neves AR, Mamoni RL, Rossi CL, de Camargo ZP, Blotta MH, 2003. Negative immunodiffusion test results obtained with sera of paracoccidioidomycosis patients may be related to low-avidity immunoglobulin G2 antibodies directed against carbohydrate epitopes. Clin Diagn Lab Immunol 10: 802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidal MS, Benard G, de Brito T, Dantas KC, Pereira CN, França FO, da Silva AM, Martins JE, 2005. Atypical serological response marked by a lack of detectable anti-gp43 antibodies in a patient with disseminated paracoccidioidomycosis. J Clin Microbiol 43: 3014–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Restrepo A, 1992. Report of activities of the committee on paracoccidioidomycosis serodiagnosis. ISHAM Mycoses Newsletter 59: 4. [Google Scholar]
- 7.Shikanai-Yasuda MA, et al. 2017. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev Soc Bras Med Trop 50: 715–740. Erratum in Rev Soc Bras Med Trop 2017;50(5). [DOI] [PubMed] [Google Scholar]
- 8.Benard G, Costa AN, Leopércio AP, Vicentini AP, Kono A, Shikanai-Yasuda MA, 2013. Chronic paracoccidioidomycosis of the intestine as single organ involvement points to an alternative pathogenesis of the mycosis. Mycopathologia 176: 353–357. [DOI] [PubMed] [Google Scholar]
- 9.Paniago AM, de Freitas AC, Aguiar ES, Aguiar JI, da Cunha RV, Castro AR, Wanke B, 2005. Paracoccidioidomycosis in patients with human immunodeficiency virus: review of 12 cases observed in an endemic region in Brazil. J Infect 51: 248–252. [DOI] [PubMed] [Google Scholar]
- 10.Bellissimo-Rodrigues F, Vitali LH, Martinez R, 2010. Serological diagnosis of paracoccidioidomycosis in HIV-coinfected patients. Mem Inst Oswaldo Cruz 105: 904–907. [DOI] [PubMed] [Google Scholar]
- 11.Gegembauer G, Araujo LM, Pereira EF, Rodrigues AM, Paniago AM, Hahn RC, de Camargo ZP, 2014. Serology of paracoccidioidomycosis due to Paracoccidioides lutzii. PLoS Negl Trop Dis 8: e2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camargo ZP, Unterkircher C, Travassos LR, 1989. Identification of antigenic polypeptides of Paracoccidioides brasiliensis by immunoblotting. J Med Vet Mycol 27: 407–412. [PubMed] [Google Scholar]
- 13.Puccia R, Travassos LR, 1991. 43-kilodalton glycoprotein from Paracoccidioides brasiliensis: immunochemical reactions with sera from patients with paracoccidioidomycosis, histoplasmosis, or Jorge Lobo’s disease. J Clin Microbiol 29: 1610–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Mattos Grosso D, de Almeida SR, Mariano M, Lopes JD, 2003. Characterization of gp70 and anti-gp70 monoclonal antibodies in Paracoccidioides brasiliensis pathogenesis. Infect Immun 71: 6534–6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bialek R, Ibricevic A, Aepinus C, Najvar LK, Fothergill AW, Knobloch J, Graybill JR, 2000. Detection of Paracoccidioides brasiliensis in tissue samples by a nested PCR assay. J Clin Microbiol 38: 2940–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teixeira Mde M, Theodoro RC, Oliveira FF, Machado GC, Hahn RC, Bagagli E, San-Blas G, Soares Felipe MS, 2014. Paracoccidioides lutzii sp. nov.: biological and clinical implications. Med Mycol 52: 19–28. [DOI] [PubMed] [Google Scholar]
- 17.Campos MC, Gesztesi JL, Vincentini AP, Lopes JD, Camargo ZP, 1995. Expression and isoforms of gp43 in different strains of Paracoccidioides brasiliensis. J Med Vet Mycol 33: 223–227. [DOI] [PubMed] [Google Scholar]
- 18.Souza MC, Gesztesi JL, Souza AR, Moraes JZ, Lopes JD, Camargo ZP, 1997. Differences in reactivity of paracoccidioidomycosis sera with gp43 isoforms. J Med Vet Mycol 35: 13–18. [DOI] [PubMed] [Google Scholar]
- 19.Theodoro RC, Teixeira MM, Felipe MSS, Paduan KS, Ribolla PM, San-Blas G, Bagagli E, 2012. Genus Paracoccidioides: species recognition and biogeographic aspects. PLoS One 7: e37694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigues EG, Travassos LR, 1994. Nature of the reactive epitopes in Paracoccidioides brasiliensis polysaccharide antigen. J Med Vet Mycol 32: 77–81. [PubMed] [Google Scholar]
- 21.Machado GC, Moris DV, Arantes TD, Silva LR, Theodoro RC, Mendes RP, Vicentini AP, Bagagli E, 2013. Cryptic species of Paracoccidioides brasiliensis: impact on paracoccidioidomycosis immunodiagnosis. Mem Inst Oswaldo Cruz 108: 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leitão NP, Jr, Vallejo MC, Conceição PM, Camargo ZP, Hahn R, Puccia R, 2014. Paracoccidioides lutzii Plp43 is an active glucanase with partial antigenic identity with P. brasiliensis gp43. PLoS Negl Trop Dis 8: e3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahn RC, Rodrigues AM, Fontes CJ, Nery AF, Tadano T, Queiroz Lde P, Jr, de Camargo ZP, 2014. Fatal fungemia due to Paracoccidioides lutzii. Am J Trop Med Hyg 91: 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Queiroz Júnior Lde P, de Camargo ZP, Tadano T, Rodrigues AM, Takarara DT, Gegembauer G, Araujo LM, Hahn RC, 2014. Serological and antigenic profiles of clinical isolates of Paracoccidioides spp. from central western Brazil. Mycoses 57: 466–472. [DOI] [PubMed] [Google Scholar]
- 25.Batista J, De Camargo ZP, Fernandes GF, Vicentini AP, Fontes CJF, Hahn RC, 2010. Is the geographical origin of a Paracoccidioides brasiliensis isolate important for antigen production for regional diagnosis of paracoccidioidomycosis? Mycoses 53: 176–180. [DOI] [PubMed] [Google Scholar]
- 26.Negroni R, 1968. New studies on antigens for serological testing in South American blastomycosis [in Spanish]. Dermatol Iber Lat Am 4: 409–416. [Google Scholar]
- 27.Lenhard-Vidal A, Assolini JP, Ono MA, Bredt CS, Sano A, Itano EN, 2013. Paracoccidioides brasiliensis and P. lutzii antigens elicit different serum IgG responses in chronic paracoccidioidomycosis. Mycopathologia 176: 345–352. [DOI] [PubMed] [Google Scholar]