Abstract.
We report a case of thelaziasis in a 26-year-old female, acquired in Oregon. A total of 14 worms were removed from the patient’s left eye and were morphologically identified as being Thelazia gulosa. Until now, only two species of Thelazia have been implicated in causing human disease, Thelazia callipaeda in Asia and Europe and occasional reports of Thelazia californiensis from the United States of America. Here, we describe a third, previously unreported parasite of humans, T. gulosa (the cattle eyeworm) as an agent of human thelaziasis and the first reported case of human thelaziasis in North America in over two decades.
INTRODUCTION
Thelaziasis is an ocular infection caused by nematodes of Thelazia spp., usually transmitted by flies that feed on lacrimal secretions. Although a common veterinary infection, human infection is considered to be a rare zoonotic event. Cases of human thelaziasis have been reported worldwide, predominantly in Europe and Asia.1 Cases occur predominantly in rural communities with close proximity to animals and poor living standards. Thelaziasis mainly affects the elderly and children, who may be less able to keep flies away from their faces.2 Most of the reported cases have been caused by Thelazia callipaeda, occurring in the old world, particularly in Japan.1 Thelazia callipaeda has also rarely been reported infecting humans in China, Japan, India, Burma, Korea, Taiwan, Thailand, Indonesia, Russia, Italy, and France.3 There have been 10 previously reported cases of human thelaziasis from the United States; nine from California and one in Utah, all were reported as Thelazia californiensis.4,5 Other species of Thelazia are reported as infecting animals in the United States, these being, Thelazia gulosa, Thelazia lacrymalis, Thelazia rhodesi, and Thelazia skrjabini.3
CASE REPORT
A 26-year-old avid outdoorswoman from Oregon reported left eye irritation accompanied by the sensation of a foreign object. The patient had, in previous weeks, been practicing horsemanship in Gold Beach, OR, a region where cattle farming occurs. The irritation worsened and on the eighth day of symptoms, the patient removed a small, translucent worm. She presented to a local physician who removed two additional worms. The worms were submitted to Northwest Pathology for analysis and identification where they were fixed in 10% buffered formalin and forwarded to the Centers for Disease Control and Prevention (CDC) Parasitic Diseases Reference Laboratory for identification. The following day, the patient presented to an optometrist where three additional worms were removed. The patient was arranged to see an infectious disease specialist who removed a partial worm, which was also sent to the CDC. The patient was diagnosed with parasitic infiltration of the left periocular tissues and a secondary bilateral papillary reaction of the upper and lower palpebral conjunctivae. The patient was advised to undergo manual extraction rather than topical or systemic antihelminthic therapy. Despite multiple washouts by ophtalmologists, no further worms were seen by providers; however, the patient continued to remove worms from her left eye. A total of 14 worms were removed from the patient’s left eye over 20 days. Since this time, the patient has been without symptoms and no further worms have been observed.
METHODS
A whole adult worm submitted to the CDC Parasitic Diseases Reference Laboratory Morphology Department was morphologically examined. The worm was preserved in 10% neutral buffered formalin, disallowing DNA extraction and molecular analysis. The worm was independently examined by microscopy by three experienced morphologists, followed by collation of their opinions to make a final identification. Further morphometric analysis was performed, in consultation with all major morphological references,3,6–8 to confirm the species identification.
Ethical approval.
Publication of this case report was granted institutional review board approval by the cdc center for global health office of the associate director for science, tracking number 2017-381.
RESULTS
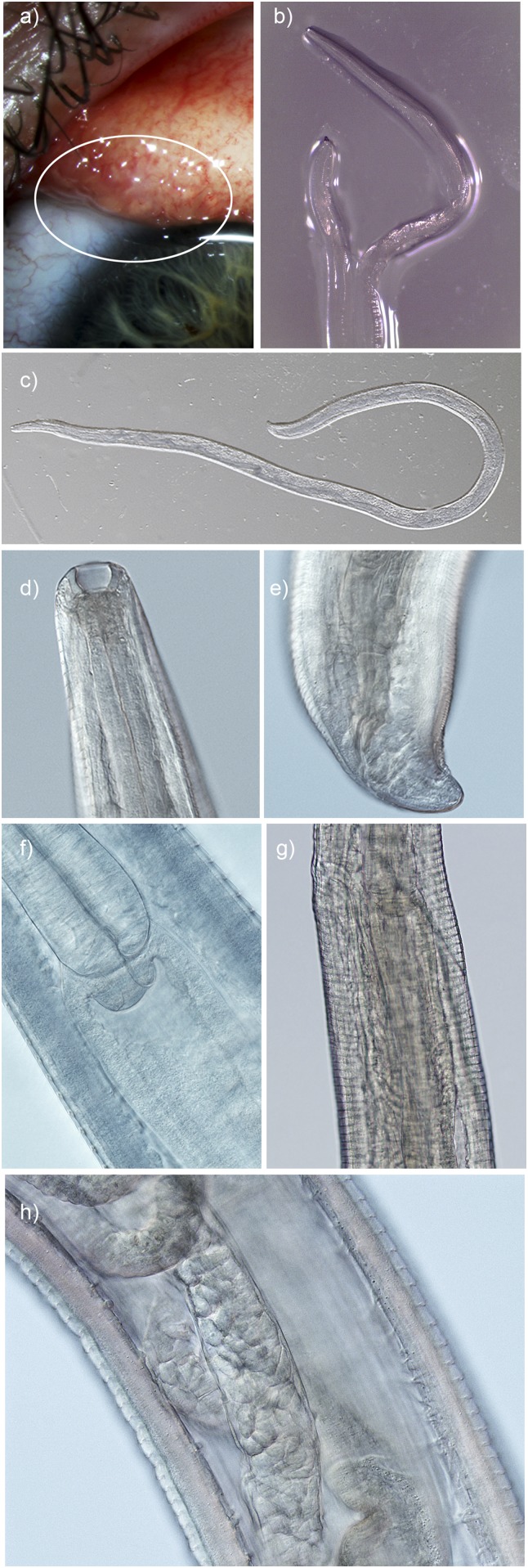
The worms submitted to the CDC were female and had acquired a degree of opacity in the formalin preservative (Figure 1). Minor shrinkage had occurred in the formalin fixative, one worm measured 11-mm long by 320 μm at the widest point. The anterior sharply tapered toward a deep, cup-shaped buccal cavity (width 35 μm, depth 25 μm). No lips were observed at the buccal cavity. The prominent esophagus met the intestinal junction 470 μm posterior to the anterior-most portion of the cephalic region of the worm (the anterior opening of the buccal cavity). The width of the widest point of the esophageal bulb was 180 μm. Oval, thin-shelled, spirurid-type eggs were observed within the ovaries. The vulva opened 20 μm anterior to the esophago-intestinal junction and 450 μm from the anterior-most portion of the cephalic region of the worm. The pattern of the cuticle at the vulval opening was not uniform and the vulval opening did not protrude. There were approximately 5.5 distinct transverse cuticular striations in each 10 μm of the cuticle at the widest point of the worm. These striations were rounded rather than serrated in appearance. The width at the anal opening was 100 μm. There was no protrusion of the anal opening, a unilateral postcloacal tapering and two postanal papillae were observed on the bluntly rounded tail. After analysis of the observed anatomical features and consultation with references describing the morphology of species of Thelazia reported from humans and animals,3,6–8 the worms were identified as belonging to the species T. gulosa, a cattle eyeworm. Several morphological features were used to differentiate this isolate from other Thelazia spp., including the two species previously recorded infecting humans, these are listed in Table 1. Features differentiating this isolate from other species found in North America and the two species previously identified as infecting humans were the following: the distance of the vulval opening from the anterior cephalic region that the vulval opening had no distinct features and was at the same level as the esophago-intestinal junction; possession of a deep and cup-like buccal cavity, rounded, tightly spaced, and relatively discrete cuticular ridges; a nonprotruding anus with unilateral tapering postanally and the presence of two postanal phasmids.
Figure 1.
Thelazia gulosa (A) in situ on the surface of the patient’s conjunctiva (circle); (B) adult female immediately after removal from the eye. Morphological identifying features of adult female worm submitted for analysis; (C) whole adult female (×40 magnification, cleared); (D) deep buccal cavity; (E) tail with nonprotruding anal opening and postanal papilla; (F) esophageal-intestinal junction; (G) nonprotruding vulval opening slightly anterior and to the left of the esophageal-intestinal junction; and (H) mid body with prominent cuticular striations, intestinal tube, and ovaries containing spirurid eggs (×200 magnification, cleared).
Table 1.
Differentiating morphological features, geographic distribution, and known hosts of female Thelazia spp.3,6–8,14
| Differentiating morphological features of adult female Thelazia spp. nematodes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Thelazia species | Length of adult female (mm) | Diameter at widest point (μm) | Distance of vulval opening from anterior cephalic region (μm) | Position of vulval opening relative to esophago-intestinal junction | Morphological features of vulval opening | Morphological features of buccal cavity | Morphology of cuticular ridges in midsection | Morphological features of female posterior | Reported geographic range | Reported hosts |
| Thelazia bubalis | 6.5–7.8 | 250 | 900 | ND | ND | ND | ND | – | India | Water buffalo |
| Thelazia californiensis | 12–18.8 | 330–420 | 700–1,000 | Posterior | No distinctive features | Deep and cup like | Rounded, moderately prominent, and widely spaced | Anus protrudes, two phasmids protrude from tip of tail, and no unilateral tapering | USA and Brazil | Humans, canines, felines, bears, deer, and sheep |
| Thelazia callipaeda | 10.7–18.5 | 290–510 | 520–710 | Anterior | Short flap at opening | Wide and cup like | Serrated, very prominent, and tightly spaced | Anus protrudes, two phasmids protrude from tip of tail, and no unilateral tapering | Europe, Asia, and India | Humans, monkeys, canines, felines, and rabbits |
| Thelazia ershowi | 5.0–8.7 | 188–207 | 395–489 | ND | ND | ND | ND | ND | Former Union of Soviet Socialist Republics | Pig |
| Thelazia gulosa | 8.0–11.5 | 350–460 | 460–610 | Same level | No distinctive features | Deep and cup like | Rounded, very prominent, and widely spaced | Anus does not protrude, two postanal phasmids, and unilateral tapering postanally | North America, Europe, former USSR, and Australia | Human, Cattle |
| Thelazia lacrymalis | 10.5–12.5 | 279–289 | 493–593 | Posterior | No distinctive features | Deep and cup like | Rounded, not prominent, and tightly spaced | Anus does not protrude, two postanal phasmids, and unilateral tapering postanally | North America, South America, Europe, Asia, and North Africa | Buffalo, camel, dog, and equine species |
| Thelazia leesei | 14–21 | 400 | 425–440 | Same level | ND | ND | ND | ND | Europe, India, Central Asia, and Africa | Camels |
| Thelazia rhodesi | 12.5–20.5 | 300–500 | 505–536 | Posterior | Cuticular pattern differs, vulva protrudes | Deep and cup like | Serrated, very prominent, and widely spaced | Anus protrudes, one phasmids protrudes from tip of tail, and unilateral tapering postanally | North America, Europe, United Kingdom, Asia, Ghana, Zambia, Afghanistan, and Japan | Cattle, buffalo, zebu, bison horses, sheep, and goats |
| Thelazia skrjabini | 11–19 | 178–378 | 410–710 | Posterior | Protrudes | Short and indistinct | Rounded, scarcely visible, and widely spaced | Anus protrudes, two postanal phasmids and rounded with no unilateral tapering | North America, Europe, former USSR, India, and Australia | Cattle, buffalo, and horses |
ND = no data.
DISCUSSION
Thelazia gulosa: phylum Nematoda, order Spirurida, family Thelaziidae, and genus Thelazia are parasites affecting the conjunctiva of the definitive host, where they are ovoviparous. In North America, T. gulosa is distributed throughout the northern states of the United States and southern Canada.3,9 The intermediate host, Musca autumnalis (face flies) ingests L1 larvae passed in the definitive host lachrymal secretions.3,10 These larvae develop within the abdomen of the fly, then migrate to the hemocoel, where development to L3 larva requires a minimum of 9 days at 27°C.11 The L3 larvae migrate to the mouthparts and are inoculated into the conjunctiva of a new host with feeding.3,10 The L3 larvae then develop into the adult stage in the conjunctival sac and prebulbular tear film. This patient’s outdoor pastimes of riding horses and fishing during the summer months likely allowed exposure to vector face flies, and she may have delayed brushing away of these flies from her face. All recorded cases of human thelaziasis have been reported during the summer months.3
Thelazia gulosa is widely distributed throughout North America and Europe, Central Asia, and Australia, where it is commonly a parasite of cattle.3 The vector fly in North America, M. autumnalis, was introduced from the Palearctic region immediately after World War II and has spread across the continent, including the state of Oregon, since that time.11 This fly also transmits another cattle eyeworm, T. skrjabini, in North America.3 The prevalence of Thelazia spp. larva in face flies captured in Massachusetts, Iowa, and Alberta during the summer months varying between 1% and 37%, with an average of 2.5–4.2 larva per infected fly.11 Vector flies of T. gulosa in other regions of the world include M. autumnalis, Musca larvipara, and Musca osiris in Europe,12 Musca amica in the Russian Far East, Musca vitripennis in Crimea, and possibly also M. larvipara in Ukraine.9 Musca domestica has been suggested as a vector, but this remains controversial.13
The most common clinical findings are mild conjunctival inflammation, foreign body sensation, follicular hypertrophy of the conjunctiva, and excessive lacrimation. Occasionally, the worms migrate across the surface of the eye and cause corneal scarring, opacity, and blindness.3 Because the localization of Thelazia infestation is confined to the conjunctiva and removal of worms with a cotton swab or forceps is sufficient. The clinical signs usually resolve quickly after the removal of the parasites.3 Immediate postremoval irrigation with Lugol’s iodine or 2–3% boric acid may be considered.3 Injection of 2 mL levamisole into the conjunctival sac or administration of 5 mg/kg orally or parenterally has been used in the treatment of T. californiensis infection3 before the availability of ivermectin. A subcutaneous dose of 2.2 mg/kg of ivermectin has been used to cure human infections in Asia and Europe.3
Previously, only two Thelazia spp. have been implicated as causing human thelaziasis worldwide, these being T. californiensis (in the western United States) and T. callipaeda (in Europe and Asia).2,3 In this report, we add a third species, T. gulosa, an endemic eyeworm of cattle in North America, Europe, Central Asia, and Australia as a novel agent of human thelaziasis in the United States of America.
Acknowledgments:
We would like to thank Ms. Sarah Sapp, of the University of Georgia for providing a helpful critique of this manuscript before submission. We would like to acknowledge the input of Ms. Leslie Wheeless, Nurse Practitioner, who was the primary care provider for this case.
REFERENCES
- 1.Koyama Y, Ohira A, Kono T, Yoneyama Y, Shiwaku K, 2000. Five cases of thelaziasis. Br J Ophthalmol 84: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen J, Gasser RB, Chu D, Wang Z, Yuan X, Cantacessi C, Otranto D, 2006. Human thelaziosis—a neglected parasitic disease of the eye. J Parasitol 92: 872–875. [DOI] [PubMed] [Google Scholar]
- 3.Naem S, 2011. Thelazia species and conjunctivitis. Pelikan Z, ed. Conjunctivitis—A Complex and Multifaceted Disorder Rijeka, Croatia: Intech Open Access Press, 201–232. [Google Scholar]
- 4.Weinmann CJ, Anderson JR, Roubtzoff P, Connolly G, Longhurst WM, 1974. Eyeworms and face flies in California. Calif Agric 28: 4–5. [Google Scholar]
- 5.Doezie AM, Lucius RW, Aldeen W, Hale DV, Smith DR, Mamalis N, 1996. Thelazia californiensis conjunctival infestation. Ophthalmic Surg Lasers 27: 716–719. [PubMed] [Google Scholar]
- 6.Erschow WS, 1928. Drei Thelaziaarten aus der conjunctiva des rindes in der U.S.S.R. [in German]. Dtsch Tierarztl Wochenschr 33: 553–556. [Google Scholar]
- 7.Naem S, 2005. Ultrastructural observations on the surface of Thelazia lacrymalis (Nematoda: Spirurida, Thelaziidae). Acta Vet Hung 53: 205–212. [DOI] [PubMed] [Google Scholar]
- 8.Naem S, 2007. Morphological differentiation among three Thelazia species (Nematoda: Thelaziidae) by scanning electron microscopy. Parasitol Res 101: 145–151. [DOI] [PubMed] [Google Scholar]
- 9.Anderson RC, 2000. Nematode Parasites of Vertebrates: Their Development and Transmission, 2nd edition. Wallingford, United Kingdom: CABI Publishing. [Google Scholar]
- 10.Otranto D, Cantacessi C, Testini G, Lia RP, 2006. Phortica variegata as an intermediate host of Thelazia callipaeda under natural conditions: evidence for pathogen transmission by a male arthropod vector. Int J Parasitol 36: 1167–1173. [DOI] [PubMed] [Google Scholar]
- 11.Krafsur ES, Moon RD, 1997. Bionomics of the face fly, Musca autumnalis. Annu Rev Entomol 42: 503–523. [DOI] [PubMed] [Google Scholar]
- 12.Otranto D, Tarsitano E, Traversa D, De Luca F, Giangaspero A, 2003. Molecular epidemiological survey on the vectors of Thelazia gulosa, Thelazia rhodesi and Thelazia skrjabini (Spirurida: Thelaziidae). Parasitol 127: 365–373. [DOI] [PubMed] [Google Scholar]
- 13.Otranto D, Traversa D, 2005. Thelazia eyeworm: an original endo-and ecto-parasitic nematode. Trends Parasitol 21: 1–4. [DOI] [PubMed] [Google Scholar]
- 14.Pinto RM, Vicente JJ, Rodrigues HDO, 1998. First report of Thelazia californiensis price (Nematoda, Thelazioidea) in South America from the eyes of a Brazilian deer, mazama gouazoupira (Fischer) (Mammalia, Cervidae). Rev Bras Zool 15: 1121–1124. [Google Scholar]