Abstract.
Burkholderia pseudomallei, the etiologic agent of melioidosis, is predicted to be ubiquitous in tropical regions of the world with areas of highest endemicity throughout Southeast Asia (SEA). Nevertheless, the distribution of B. pseudomallei and the burden of melioidosis in many SEA countries remain unclear. In Cambodia, only two human endemic cases of melioidosis were reported through 2008 and since then only a few hundred cases have been described in the literature. This is in sharp contrast to the annual burden of thousands of cases in surrounding areas. To further investigate the prevalence of melioidosis in Cambodia, we used a recently developed O-polysaccharide–based rapid enzyme-linked immunosorbent assay to detect B. pseudomallei–specific antibodies in serum samples obtained from 1,316 febrile illness or sepsis patients from 10 different provinces. Based on a cutoff value derived through culture-confirmed melioidosis cases, the proportion of positive samples in our cohort was approximately 12%. Regression analysis indicated that the odds of obtaining a positive result were 2.2 times higher for males than females controlling for age and province (95% confidence interval: 1.6–3.2, P < 0.001). Consistent with this, 9.2% of females were positive versus 18.2% of males (P < 0.001). Notably, 22.5% of grain or rice farmers were positive versus 10.1% of subjects with occupations not involving regular contact with soil. Positive results varied significantly by province. Collectively, the results of this study suggest that the true burden of melioidosis in Cambodia is greater than has previously been reported.
INTRODUCTION
Burkholderia pseudomallei is an environmental saprophyte that causes melioidosis, a potentially fatal disease in humans and animals. This gram-negative bacterium is frequently isolated from soil and water in endemic regions, and human infections are typically acquired via percutaneous inoculation, inhalation, or ingestion.1–6 Individuals with repeated exposure to contaminated soil, water, or environmental aerosols are at particular risk of developing B. pseudomallei infection.7 The clinical presentation of melioidosis is variable and ranges from asymptomatic infections to acute pneumonia and severe sepsis.7 Treatment of melioidosis patients involves prolonged antibiotic regimens, and mortality rates associated with acute forms of the disease remain high.8
Although predicted to be globally ubiquitous in tropical regions of the world,9 B. pseudomallei is known to be highly endemic in many areas of Southeast Asia (SEA) where it causes significant morbidity and mortality.10 After the first identification of melioidosis in SEA in 1912,11–13 the first case was reported in Cambodia in 1930.14 Although cases of animal melioidosis and nonendemic human cases of melioidosis were reported,15–17 the next endemic case of human melioidosis in Cambodia was not reported until 2008.18 With most of the reported cases worldwide occurring in neighboring Thailand, the scarcity of melioidosis reports in Cambodia indicates that melioidosis is drastically underrepresented.1,8,9 This is due in part to insufficient laboratory capabilities throughout the country. To date, only a few 100 cases of melioidosis in Cambodia have been described from a handful of studies since 2007, leaving the true distribution of B. pseudomallei and the burden of melioidosis unclear.19–26
Serology studies have proven useful in providing epidemiological characterization of B. pseudomallei distribution.27 The only recognized, clinically validated method of determining antibody titer is the indirect hemagglutination assay (IHA).28–30 Unfortunately, this method is burdensome and difficult to standardize, requires reagents that are often difficult to obtain in nonendemic areas, possesses poor sensitivity (56–70%), and is variably specific depending on the population being tested.31–33 As a result, some investigators have opted for more tractable assays such as the enzyme-linked immunosorbent assay (ELISA).34–36 Although evidence suggests that when properly validated, ELISAs can be a useful serological tool,37 experts urge caution in the use of unvalidated assays for drawing conclusions on the seroprevalence of B. pseudomallei in an endemic population.37,38
Recently, we developed a rapid O-polysaccharide (OPS)–based ELISA for the detection of antibodies to B. pseudomallei and validated it using serum from healthy and culture-confirmed melioidosis patients from Thailand.39 The OPS component of lipopolysaccharide is an ideal capture antigen for use in serologic assays because it has been shown to be the dominant antigen against which human immune responses are directed after infection with B. pseudomallei.39 We have previously used OPS as a target antigen in a rapid latex agglutination assay and demonstrated that it is a promising antigen for serodiagnosis of melioidosis, particularly in nonendemic areas.33 When used as the capture antigen in our rapid ELISA format, OPS outperformed the gold standard IHA by a significant margin when using serum samples from both nonendemic and endemic regions. In the present study, we sought to improve our understanding of the risk and distribution of B. pseudomallei in Cambodia. To this end, we conducted a retrospective study in which we used our rapid OPS-ELISA to detect B. pseudomallei–specific antibodies in serum samples obtained from 1,316 patients with fever or sepsis of unknown origin from 10 different provinces of Cambodia.
MATERIALS AND METHODS
Samples.
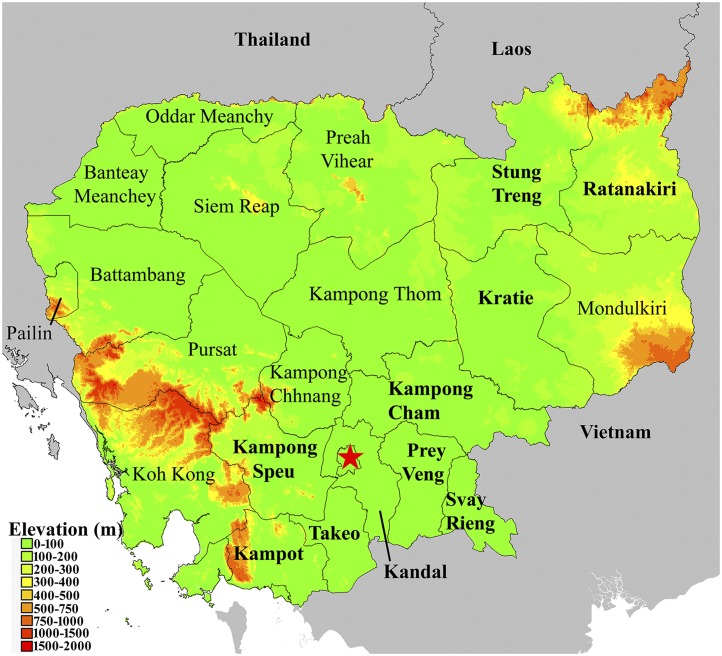
Serum samples were obtained from three studies conducted in 10 provinces throughout Cambodia (Figure 1). The study protocols were approved by the Naval Medical Research Center Institutional Review Board in compliance with all applicable federal regulations governing the protection of human subjects. Sera (N = 986) were derived from an acute febrile illness (AFI) study initiated in 2005 with collection sites established in suburban and rural areas throughout Cambodia. An additional 140 serum samples, including patients with culture-confirmed melioidosis, were derived from an observational study of sepsis in a single hospital in Takeo province, Cambodia, between 2014 and 2015.26 The confirmed melioidosis patients were experiencing symptoms between 4 and 15 days (median: 5 days) before serum collection and have been thoroughly described.26 Finally, 198 samples were also obtained from healthy volunteers with poultry contact who participated in a serosurvey of avian influenza A in Kampong Cham province. In all cases, patients or –volunteers or their legal authorized representatives provided written informed consent and granted permission to store samples for future studies. A de-identified sample database was created for the present study with specimen ID, age, gender, occupation, and home demographic information.
Figure 1.
Cambodia. An elevation map of Cambodia was generated using DIVA-GIS (www.diva-gis.org). Provinces where archived samples were originally obtained are shown in bold and surrounding areas are shown in grey and depicted as flat. Note: At the time samples were collected, Kampong Cham existed as a single, discrete province. However, Kampong Cham was recently divided along the Mekong River resulting in two provinces, Kampong Cham and Tboung Khmum. The map depicts provincial boarders at the time the samples were collected.
ELISA.
The rapid ELISA based on the detection of IgG antibodies against OPS antigen was performed as described previously.39 The concentration of OPS antigen at 1 μg/mL and serum dilution 1:2,000 were used. The absorbance value (optical density, OD) was determined at a wavelength 450 nm using a microtiter plate reader (BioTek, Winooski, VT).
Maps.
Seropositivity map and population maps were generated using ArcGIS software version 10.3.1 (www.arcgis.com) whereas the elevation map was generated using DIVA-GIS (www.diva-gis.org).
Statistical analysis.
A receiver operator characteristic (ROC) curve was constructed by GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA) as described previously.39 The Mann–Whitney test was used to determine the difference between serum groups. The analyses of demographic characteristics were conducted using Stata version 14 (StataCorp, College Station, TX). Subjects seropositive for B. pseudomallei and seronegative subjects were compared using χ2 or Student’s t test. Odds ratios were estimated using logistic regression models. Information on occupation was missing for all subjects from Kampong Cham province. Tests of statistical significance were two-tailed, and in all cases, significance was defined as P < 0.05. Samples (N = 19) obtained in Phnom Penh (Figure 1, star) were included in data for Kandal province.
RESULTS AND DISCUSSION
In total, 1,316 samples from 10 provinces were used in this study. The gender was roughly evenly divided (52/48) between male and female. Thirty-seven percent were rice or grain farmers, 60% of whom (282) were male whereas 20% did not specify an occupation. The median age was 23 years (range 2–83). Included with these samples were controls collected from 198 healthy volunteers in Kampong Cham province (Figure 1). Thirty percent of these individuals were male, the median age was 41 (range 23–75), and they were enrolled between March 15 and November 1, 2011.
ROC analysis of OPS-ELISA.
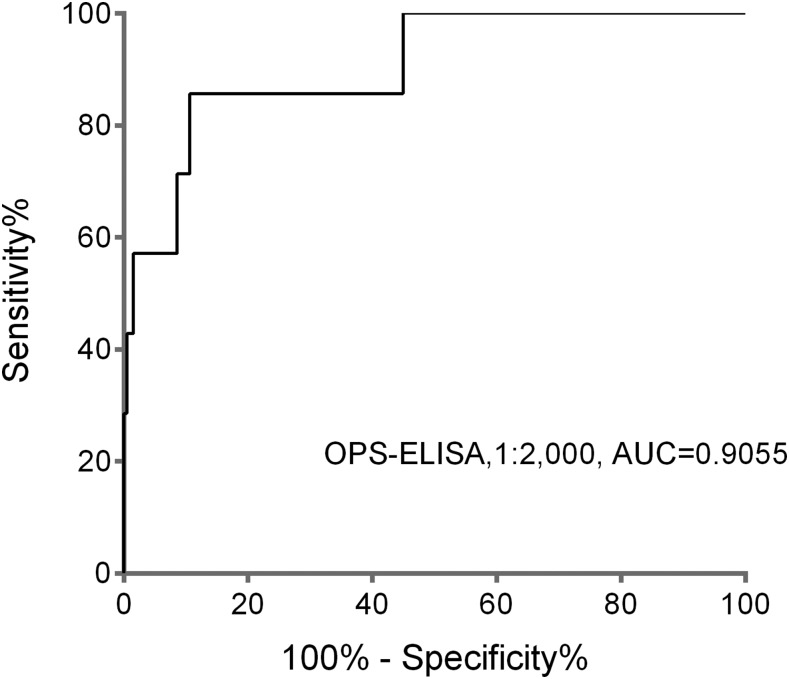
ROC curves were plotted by calculating the sensitivity and specificity of increasing numbers of the true-positive rate and false-positive rate (1 – specificity) from the results of OPS-ELISA using the culture-confirmed melioidosis group (N = 7, including one non-bacteremic case) and healthy Cambodian donors (N = 198). The area under the ROC curves for predicting past or present B. pseudomallei infection was used to determine our cutoff value (Figure 3). Cutoff ODs used were set at 0.72, 0.97, and 1.35 to obtain 85%, 90%, and 95% specificity, respectively (Table 1).
Figure 3.
Area under the receiver operating characteristic curve of the O-polysaccharide–based enzyme-linked immunosorbent assay (OPS-ELISA) results of serum samples from the culture-confirmed melioidosis group and healthy Cambodian donors. The serum samples were diluted at 1:2,000 for OPS-ELISA.
Table 1.
Sensitivity and specificity of OPS-ELISA
| Cutoff (OD450) | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Melioidosis patients (N = 7) | Healthy donors (N = 198) | |
| 0.72 | 85.7 | 85.9 |
| 0.97 | 71.4 | 90.9 |
| 1.35 | 57.1 | 95.5 |
OD = optical density; OPS-ELISA = O-polysaccharide–based enzyme linked immunosorbent assay. Three cutoff values were calculated from patients who had melioidosis and from healthy donors.
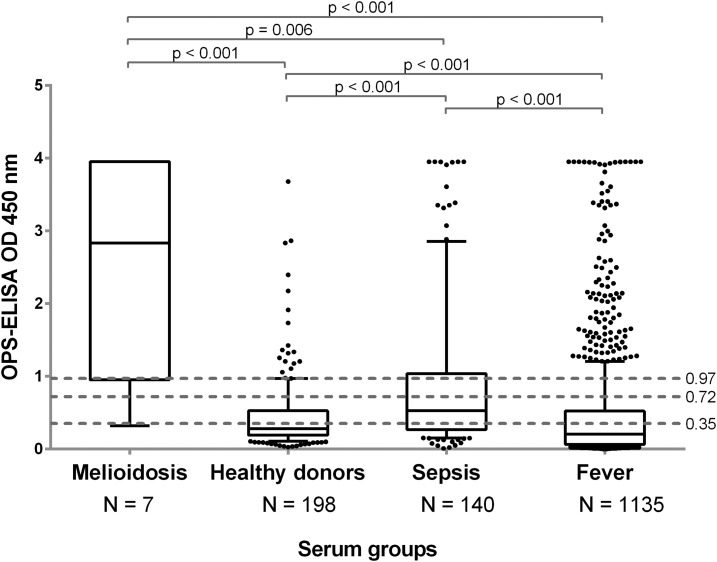
The ability of these cutoffs to discriminate between the different serum groups (i.e., confirmed melioidosis, suspected sepsis, AFI, and healthy donors) was then evaluated at each of the potential cutoff values. The results depicted in Figure 2 demonstrate that a statistically significant difference could be observed when comparing each illness group to healthy donors. Furthermore, the results from the AFI and sepsis patients also demonstrated a statistically significant difference from the melioidosis group. Finally, the cutoff value with the highest sensitivity and specificity was determined to be 85.71% and 89.39%, respectively, and OD450 of 0.905 based on its discriminating power at 1:2,000 dilution (Figure 3).
Figure 2.
Enzyme-linked immunosorbent assay results of four serum groups. The cutoff optical densities (ODs) used were set at 85% (OD 0.72), 90% (OD 0.97), and 95% (OD 1.35) specificity. The median line is presented within the boxes which encompass the 25th and 75th percentiles whereas the whiskers extend to the 10th and 90th percentiles.
Although these results suggest cutoff values of a highly sensitive and specific assay capable of identifying melioidosis patients in a cohort of unknown illnesses, the number of confirmed melioidosis patients used to generate these cutoffs was relatively low (N = 7). However, our previous work using Thai sera used a cutoff OD450 of 0.87, which demonstrated a sensitivity and specificity of 71.6% and 96.7%, respectively.39 Because our cutoff OD450 of 0.905 is more stringent than the results generated in neighboring Thailand, we sought to estimate the prevalence of antibodies to B. pseudomallei as an indicator of potentially undiagnosed B. pseudomallei infections in Cambodia using this value.
Seropositivity in Cambodia.
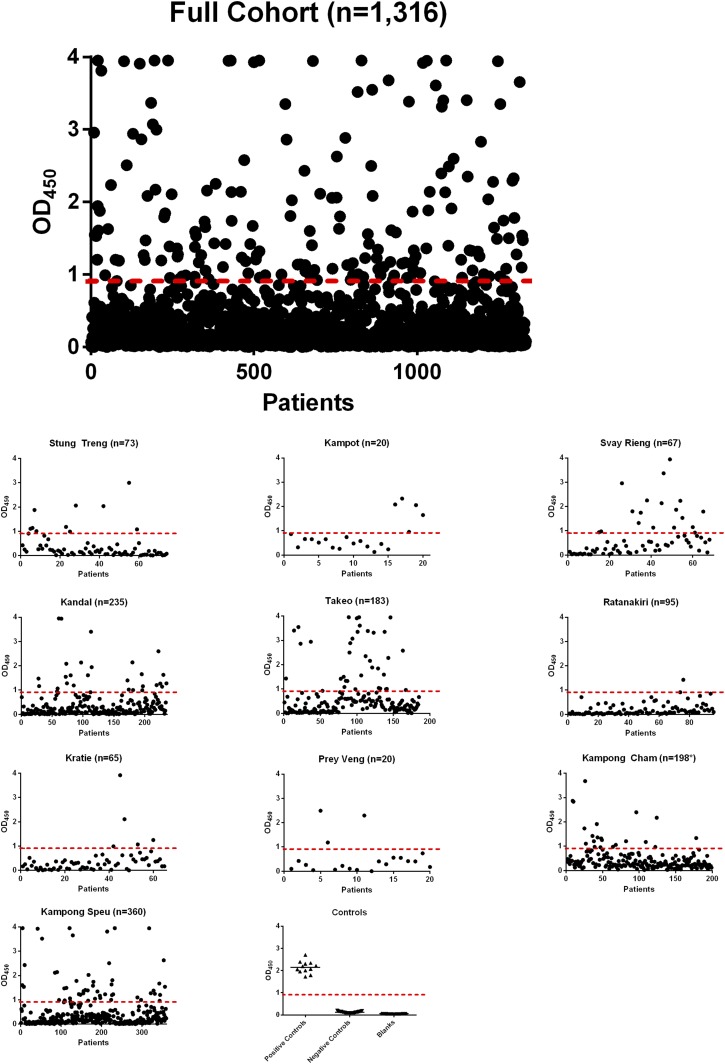
Overall, 163 of 1,316 (12%) samples tested fell above the cutoff value (OD450 ≥ 0.905) indicating a high likelihood of B. pseudomallei infection (Figure 4). Regression analysis (Table 2) indicated the odds of B. pseudomallei seropositivity were 2.2 times higher for males than for females controlling for age and province (95% confidence interval [CI]: 1.6–3.2, P < 0.001). Consistent with that, 9.2% of females were seropositive versus 18.2% of males (P < 0.001). Notably, 22.5% of grain or rice farmers were seropositive versus 10.1% of subjects with other occupations. The median age was 21 for seronegative subjects and 33 for seropositive subjects (P < 0.001).
Figure 4.
Seropositivity by province. The overall and provincial incidences of seropositivity are presented using scatter plots. The cutoff value of OD450 of 0.905 is indicated with a dashed line. Samples that fall above that cutoff value were considered positive. The values for positive controls, negative controls, and blanks for each enzyme-linked immunosorbent assay plate are also presented. This figure appears in color at www.ajtmh.org.
Table 2.
Burkholderia pseudomallei seropositivity in 10 Cambodian provinces
| Province | Number | Farmer/other | Sex (M/F) | Median age (range) | Positive (%) | Odds ratio | P value | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Kampong Cham† | 198 | UNK | 60/138 | 42 (23–75) | 11 | 2.75 | 0.185 | 0.62–12.31 |
| Kampong Speu | 360 | 147/213 | 232/128 | 19 (2–66) | 14 | 6.76 | 0.009 | 1.60–28.57 |
| Kampot | 20 | 10/10 | 16/4 | 53 (31–80) | 25 | 3.76 | 0.143 | 0.64–22.15 |
| Kandal | 235 | 51/184 | 116/119 | 22 (2–78) | 12 | 5.36 | 0.025 | 1.24–23.28 |
| Kratie | 65 | 29/36 | 20/45 | 25 (2–68) | 8 | 3.55 | 0.141 | 0.66–19.29 |
| Prey Veng | 20 | 5/15 | 10/10 | 19 (5–60) | 15 | 7.04 | 0.045 | 1.04–47.65 |
| Ratanakiri | 95 | 40/55 | 49/46 | 16 (2–61) | 2 | * | * | * |
| Stung Treng | 73 | 31/42 | 35/38 | 22 (3–62) | 15 | 7.17 | 0.013 | 1.51–33.99 |
| Svay Rieng | 67 | 17/50 | 36/31 | 25 (4–67) | 27 | 13.94 | 0.001 | 3.05–63.66 |
| Takeo | 183 | 74/109 | 114/69 | 33 (2–83) | 21 | 6.01 | 0.017 | 1.38–26.20 |
| Total | 1,316 | 402/712 | 698/635 | 23 (2–83) | 12.3 | N/A | N/A | N/A |
| Age | N/A | N/A | N/A | N/A | N/A | 1.04 | < 0.001 | 1.03–1.05 |
| Gender | N/A | N/A | N/A | N/A | N/A | 2.24 | < 0.001 | 1.56–3.22 |
CI = confidence interval; N/A = not applicable; UNK = unknown. The details and results of the samples tested in each province are presented. In addition, the logistic regression analysis shows the odds of having antibodies against Burkholderia pseudomallei vary significantly by province, increase with age, and are more than two times higher for males compared with females.
Ratanakiri is the comparison province.
Samples collected in Kampong Cham were collected from healthy volunteers.
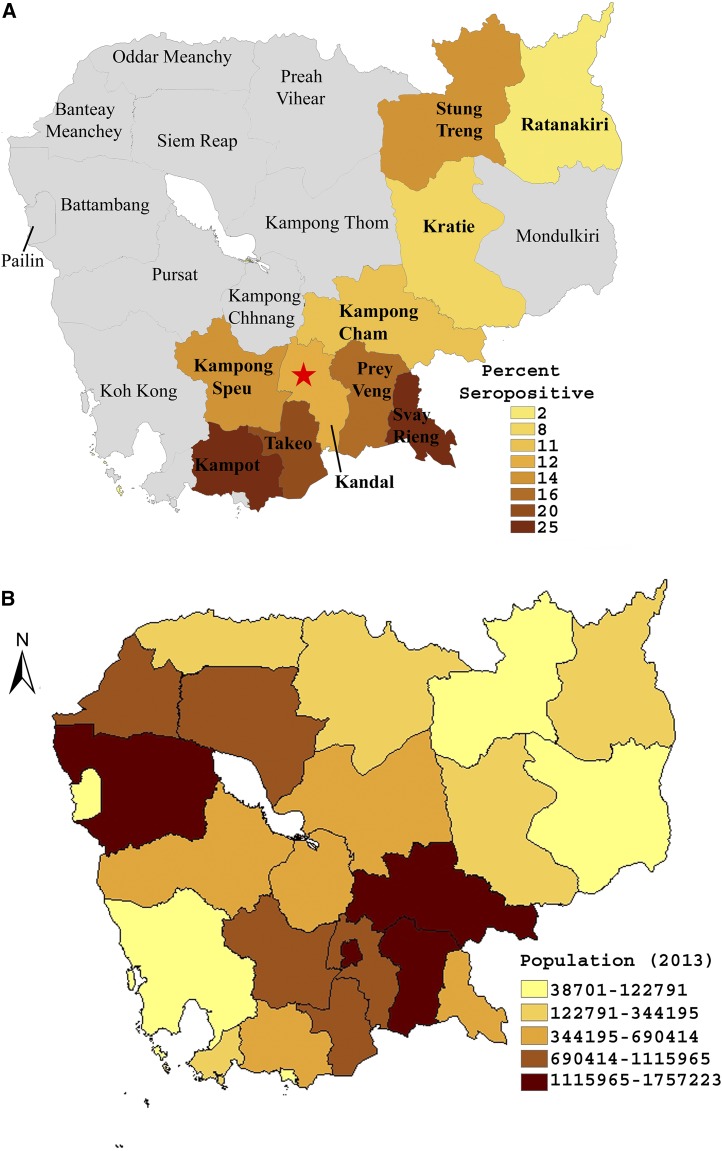
Burkholderia pseudomallei seropositivity varied significantly by province (Figures 4 and 5A), even when accounting for age and sex (Table 2). For example, the odds of being seropositive were nearly 14 times higher for subjects in Svay Rieng province compared with subjects from Ratanakiri (95% CI: 3.0–63.6, P = 0.001) with the lowest prevalence in Ratanakiri (P < 0.001). Seropositivity followed predictable elevation-dependent trends displaying an inverse relationship with elevation (spearman’s rho = −0.67, P = 0.032). For example, mountainous regions such as Ratanakiri (Figure 1) had the lowest frequency of positive samples (Figure 5A) whereas low-lying regions such as Svay Rieng and Takeo (Figure 1) had the highest rates of seropositivity (Figure 5A). Conversely, the frequency of positive samples had no correlation with population (spearman’s rho = −0.03, P = 0.934, compare Figure 5A and B) as areas such as the lightly populated Svay Rieng province presented the highest rates of seropositivity for example.
Figure 5.
Maps. The provincial rates of seropositivity (A) and population (B) were mapped using ArcGIS software version 10.3.1 (www.arcgis.com) Note: Recently, Kampong Cham province was divided into two distinct provinces—Kampong Cham and Tboung Khmum—roughly along the Mekong River. However, at the time these samples were collected, Kampong Cham existed as a single, discrete province. Maps reflect provincial boarders at the time of sample collection.
It is important to note that our samples are derived from a highly endemic region for melioidosis where prior exposure to B. pseudomallei in the population is likely quite high. Although culture remains the gold standard for diagnosis of melioidosis, rapid serological assays represent an attractive complementary approach. Investigators have been working toward development of alternative diagnostic methods, and evidence suggests that when properly validated, ELISAs can be a useful tool for detecting exposure to B. pseudomallei and predicting active infections.37 However, it is important that these assays be validated in a well-defined collection of samples before drawing conclusions on the seroprevalence of B. pseudomallei in an endemic population.38 The antigens and methods used here have been systematically validated on clinical samples in direct comparison to the gold standard serological test IHA.33,39,40 We have previously shown that B. pseudomallei Type A OPS is a promising target antigen for serodiagnosis in different groups of melioidosis patients.39 Our ELISAs have routinely demonstrated increased sensitivity and specificity and are proving to be simple serological screening tools for detection of B. pseudomallei infections.
Although this work represents the most comprehensive epidemiological survey of melioidosis in Cambodia to date, there is always room for improvement. Our study populations were derived from studies with vastly different inclusion criteria, each Province was not evenly represented and large portions of Cambodia remain unrepresented. To continue to improve our understanding of melioidosis in Cambodia, future epidemiological surveys should emphasize harmonized patient inclusion criteria and data collection in an all-inclusive nation-wide approach.
CONCLUSIONS
We analyzed 1,316 samples from 10 provinces and found serological evidence of B. pseudomallei infection in approximately 163 individuals. This work, coupled with recent prospective and retrospective studies,19–26 demonstrates that melioidosis represents a considerably higher burden in Cambodia than the present scientific literature would suggest. More studies are needed to raise awareness of melioidosis and continue to define the actual morbidity and mortality of this severe infectious disease.
Disclaimer: The views expressed in this manuscript are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
REFERENCES
- 1.Cheng AC, Currie BJ, 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev 18: 383–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inglis TJ, Garrow SC, Henderson M, Clair A, Sampson J, O’Reilly L, Cameron B, 2000. Burkholderia pseudomallei traced to water treatment plant in Australia. Emerg Infect Dis 6: 56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Currie BJ, Mayo M, Anstey NM, Donohoe P, Haase A, Kemp DJ, 2001. A cluster of melioidosis cases from an endemic region is clonal and is linked to the water supply using molecular typing of Burkholderia pseudomallei isolates. Am J Trop Med Hyg 65: 177–179. [DOI] [PubMed] [Google Scholar]
- 4.Lim C, Peacock SJ, Limmathurotsakul D, 2016. Association between activities related to routes of infection and clinical manifestations of melioidosis. Clin Microbiol Infect 22: 79.e1–79.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howe C, Sampath A, Spotnitz M, 1971. The pseudomallei group: a review. J Infect Dis 124: 598–606. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AC, Currie BJ, Dance DA, Funnell SG, Limmathurotsakul D, Simpson AJ, Peacock SJ, 2013. Clinical definitions of melioidosis. Am J Trop Med Hyg 88: 411–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Currie BJ, 2015. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin Respir Crit Care Med 36: 111–125. [DOI] [PubMed] [Google Scholar]
- 8.Wiersinga WJ, Currie BJ, Peacock SJ, 2012. Melioidosis. N Engl J Med 367: 1035–1044. [DOI] [PubMed] [Google Scholar]
- 9.Limmathurotsakul D, et al. 2016. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol 1: 15008. [DOI] [PubMed] [Google Scholar]
- 10.Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, Wongsuvan G, Chaisuksant S, Chetchotisakd P, Chaowagul W, Day NP, Peacock SJ, 2010. Increasing incidence of human melioidosis in northeast Thailand. Am J Trop Med Hyg 82: 1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitmore A, 1913. An account of a glanders-like disease occurring in Rangoon. J Hyg (Lond) 13: 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanton AT, Fletcher W, 1925. Melioidosis and its relation to glanders. J Hyg (Lond) 23: 347–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanton AT, Fletcher W, Kanagarayer K, 1924. Two cases of melioidosis. J Hyg (Lond) 23: 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamber PA, 1930. Un cas de melioidose observe a Phnom Penh. Bull Soc Pathol Exot 23: 436–441. [Google Scholar]
- 15.Chan CK, Hyland RH, Leers WD, Hutcheon MA, Chang D, 1984. Pleuropulmonary melioidosis in a Cambodian refugee. Can Med Assoc J 131: 1365–1367. [PMC free article] [PubMed] [Google Scholar]
- 16.Weller PF, Dickersin GR, Mueller PR, Mark EJ, Ryan ET, Naimi T, 1992. A 43-year-old Cambodian man with several years of recurrent bouts of fever and abdominal-pain—melioidosis of spleen, with multiple granulomas. N Engl J Med 327: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 17.Thonn S, Lebon E, Triau RS, 1960. An epizootic of melioidosis in pigs in Cambodge. Rev Élev Méd Vét Pays Trop 13: 175–179 (French). [Google Scholar]
- 18.Overtoom R, Khieu V, Hem S, Cavailler P, Te V, Chan S, Lau P, Guillard B, Vong S, 2008. A first report of pulmonary melioidosis in Cambodia. Trans R Soc Trop Med Hyg 102 (Suppl 1): S21–S25. [DOI] [PubMed] [Google Scholar]
- 19.Nhem S, Letchford J, Meas C, Thann S, McLaughlin JC, Baron EJ, West TE, 2014. Detection of Burkholderia pseudomallei in sputum using selective enrichment broth and Ashdown’s medium at Kampong Cham provincial hospital, Cambodia. F1000 Res 3: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vlieghe E, Kruy L, De Smet B, Kham C, Veng CH, Phe T, Koole O, Thai S, Lynen L, Jacobs J, 2011. Melioidosis, phnom penh, Cambodia. Emerg Infect Dis 17: 1289–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rammaert B, et al. 2011. Pulmonary melioidosis in Cambodia: a prospective study. BMC Infect Dis 11: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chheng K, et al. 2013. A prospective study of the causes of febrile illness requiring hospitalization in children in Cambodia. PLoS One 8: e60634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pagnarith Y, Kumar V, Thaipadungpanit J, Wuthiekanun V, Amornchai P, Sin L, Day NP, Peacock SJ, 2010. Emergence of pediatric melioidosis in Siem Reap, Cambodia. Am J Trop Med Hyg 82: 1106–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoesser N, et al. 2012. Pediatric suppurative parotitis in Cambodia between 2007 and 2011. Pediatr Infect Dis J 31: 865–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner P, et al. 2016. A retrospective analysis of melioidosis in Cambodian children, 2009–2013. BMC Infect Dis 16: 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schully KL, et al. 2017. Melioidosis in lower provincial Cambodia: a case series from a prospective study of sepsis in Takeo province. PLoS Negl Trop Dis 11: e0005923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wuthiekanun V, Pheaktra N, Putchhat H, Sin L, Sen B, Kumar V, Langla S, Peacock SJ, Day NP, 2008. Burkholderia pseudomallei antibodies in children, Cambodia. Emerg Infect Dis 14: 301–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexander AD, Huxsoll DL, Warner AR, Jr, Shepler V, Dorsey A, 1970. Serological diagnosis of human melioidosis with indirect hemagglutination and complement fixation tests. Appl Microbiol 20: 825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashdown LR, Johnson RW, Koehler JM, Cooney CA, 1989. Enzyme-linked immunosorbent assay for the diagnosis of clinical and subclinical melioidosis. J Infect Dis 160: 253–260. [DOI] [PubMed] [Google Scholar]
- 30.Cheng AC, O’Brien M, Freeman K, Lum G, Currie BJ, 2006. Indirect hemagglutination assay in patients with melioidosis in northern Australia. Am J Trop Med Hyg 74: 330–334. [PubMed] [Google Scholar]
- 31.O’Brien M, Freeman K, Lum G, Cheng AC, Jacups SP, Currie BJ, 2004. Further evaluation of a rapid diagnostic test for melioidosis in an area of endemicity. J Clin Microbiol 42: 2239–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Appassakij H, Silpapojakul KR, Wansit R, Pornpatkul M, 1990. Diagnostic value of the indirect hemagglutination test for melioidosis in an endemic area. Am J Trop Med Hyg 42: 248–253. [DOI] [PubMed] [Google Scholar]
- 33.Suttisunhakul V, Chantratita N, Wikraiphat C, Wuthiekanun V, Douglas Z, Day NP, Limmathurotsakul D, Brett PJ, Burtnick MN, 2015. Evaluation of polysaccharide-based latex agglutination assays for the rapid detection of antibodies to Burkholderia pseudomallei. Am J Trop Med Hyg 93: 542–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rolim DB, Vilar DC, de Goes Cavalcanti LP, Freitas LB, Inglis TJ, Nobre Rodrigues JL, Nagao-Dias AT, 2011. Burkholderia pseudomallei antibodies in individuals living in endemic regions in northeastern Brazil. Am J Trop Med Hyg 84: 302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kronmann KC, Truett AA, Hale BR, Crum-Cianflone NF, 2009. Melioidosis after brief exposure: a serologic survey in US Marines. Am J Trop Med Hyg 80: 182–184. [PMC free article] [PubMed] [Google Scholar]
- 36.Chantratita N, Wuthiekanun V, Thanwisai A, Limmathurotsakul D, Cheng AC, Chierakul W, Day NP, Peacock SJ, 2007. Accuracy of enzyme-linked immunosorbent assay using crude and purified antigens for serodiagnosis of melioidosis. Clin Vaccine Immunol 14: 110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Limmathurotsakul D, Chantratita N, Teerawattanasook N, Piriyagitpaiboon K, Thanwisai A, Wuthiekanun V, Day NP, Cooper B, Peacock SJ, 2011. Enzyme-linked immunosorbent assay for the diagnosis of melioidosis: better than we thought. Clin Infect Dis 52: 1024–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peacock SJ, Cheng AC, Currie BJ, Dance DA, 2011. The use of positive serological tests as evidence of exposure to Burkholderia pseudomallei. Am J Trop Med Hyg 84: 1021–1022, author reply 1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suttisunhakul V, Wuthiekanun V, Brett PJ, Khusmith S, Day NP, Burtnick MN, Limmathurotsakul D, Chantratita N, 2016. Development of rapid enzyme-linked immunosorbent assays for detection of antibodies to Burkholderia pseudomallei. J Clin Microbiol 54: 1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pumpuang A, Dunachie SJ, Phokrai P, Jenjaroen K, Sintiprungrat K, Boonsilp S, Brett PJ, Burtnick MN, Chantratita N, 2017. Comparison of O-polysaccharide and hemolysin co-regulated protein as target antigens for serodiagnosis of melioidosis. PLoS Negl Trop Dis 11: e0005499. [DOI] [PMC free article] [PubMed] [Google Scholar]