Abstract
Male sex hormones—androgens—regulate male physique development. Without androgen signaling, genetic males appear female. During puberty, increasing androgens harness the hair follicle’s unique regenerative ability to replace many tiny vellus hairs with larger, darker terminal hairs (e.g., beard). Follicle response is epigenetically varied: some remain unaffected (e.g., eyelashes) or are inhibited, causing balding. How sex steroid hormones alter such developmental processes is unclear, despite high incidences of hormone-driven cancer, hirsutism, and alopecia. Unfortunately, existing development models are not androgen sensitive. Here, we use hair follicles to establish an androgen-responsive human organ culture model. We show that women’s intermediate facial follicles respond to men’s higher androgen levels by synthesizing more hair over several days, unlike donor-matched, androgen-insensitive, terminal follicles. We demonstrate that androgen receptors—androgen-activated gene transcription regulators—are required and are present in vivo within these follicles. This is the first human organ that involves multiple cell types that responds appropriately to hormones in prolonged culture, in a way which mirrors its natural behavior. Thus, intermediate hair follicles offer a hormone-switchable human model with exceptional, unique availability of genetically identical, but epigenetically hormone-insensitive, terminal follicles. This should enable advances in understanding sex steroid hormone signaling, gene regulation, and developmental and regenerative systems and facilitate better therapies for hormone-dependent disorders.—Miranda, B. H., Charlesworth, M. R., Tobin, D. J., Sharpe, D. T., Randall, V. A. Androgens trigger different growth responses in genetically identical human hair follicles in organ culture that reflect their epigenetic diversity in life.
Keywords: alopecia, balding, hirsutism, model system, intermediate hair follicles
Male sex hormones are key regulators of male body development before birth, at puberty, and throughout adulthood (1). They also promote common morphologic changes later in life that cause significant medical problems and reduce quality of life, including benign prostatic hyperplasia, prostatic carcinoma, and male pattern baldness (2). Androgens stimulate more visible body hair during puberty (e.g., beard) and continue increasing various hair follicle and hair sizes for many years (3). Paradoxically, in marked contrast, androgens may simultaneously slowly inhibit specific scalp follicles, which causes androgenetic alopecia (balding) (4,–6). Androgen-dependent hair disorders—hirsutism (male pattern hair distribution) in women and androgenetic alopecia in both sexes—cause significant psychologic distress as a result of the important social communication roles of human hair (5,–8). Unfortunately, androgen-mediated disorders are not generally well controlled. Our understanding of the mechanisms of androgen action and the creation of novel therapies are hampered by a lack of robust androgen-responsive models. Most mammals do not develop prostate disorders, nor do they show sexual differences in hair growth, and healthy androgen-dependent human tissues, such as the prostate, are generally difficult to study. Because many human hair follicles change the size and color of their hairs in response to androgens (Fig. 1A), we hypothesized that they could offer a suitable androgen-sensitive model.
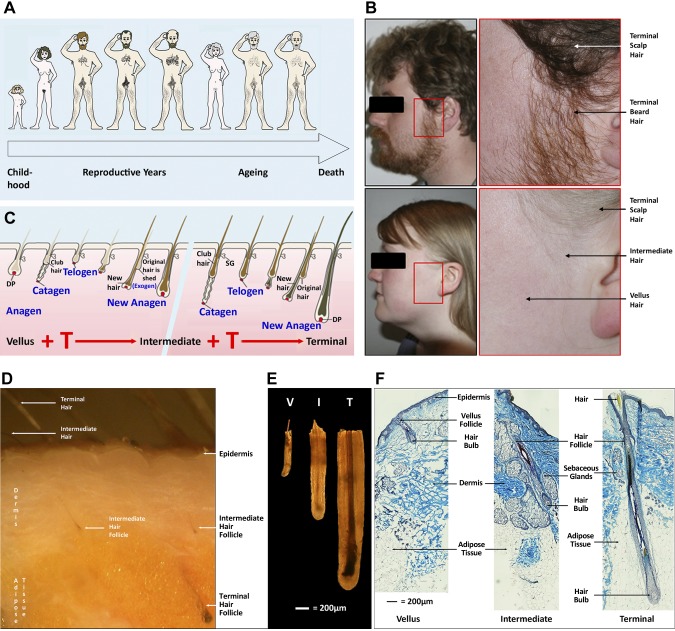
Figure 1.
Human hairs and hair follicles vary in size in different body areas during human lifespan. A) Hair changes during the human life cycle. B) Male androgen levels stimulate facial hair follicles to form large beard hairs, whereas female levels only promote intermediate hair growth in the preauricular (before the ear) region. Photographs of a 27-yr-old man and 26-yr-old woman demonstrate terminal scalp and beard hair, intermediate preauricular female hair, and vellus hair (photographer: M.R.C.). C) Androgens drive changes in hair follicle size and, thus, the new hair size over successive hair growth cycles. This diagrammatic representation of the hair cycle shows a vellus hair follicle that is changing its size and producing a larger more pigmented hair during successive hair cycles. The gap represents additional cycles that may be necessary to encompass the major changes between vellus and terminal follicles. D) Human female facial skin showing terminal and intermediate hair follicles growing in the skin. Terminal hair follicles and their pigmented hairs are more visible than the smaller, shorter intermediate follicles and their paler hairs (photographed under a dissecting microscope). E) Comparison of human vellus, intermediate, and terminal follicles. Different types of follicles that were isolated from human skin by microdissection photographed under a dissecting microscope showing differences in diameter and pigmentation. F) Histologic sections of facial skin showing the structure of tiny vellus, larger intermediate, and large terminal follicles. Frozen sections of female facial skin were stained histologically with SACPIC stain to compare the different types of anagen follicles, particularly the depth beneath the skin and diameter. Note that only terminal follicles project into the s.c. adipose tissue (9). I, intermediate; T, terminal; V, vellus.
Hair follicles are found in most areas of human skin, although the tiny, almost colorless vellus hairs that many produce are often unnoticeable, in particular in childhood where only the protective hairs of the scalp, eyebrows, and eyelashes are obvious. The gradual replacement of some vellus hairs by larger intermediate hairs announces puberty (9). By the end of male puberty, highly visible, larger, darker terminal hairs succeed them in many sites (Fig. 1) (4, 10, 11). Androgens exploit the exceptional regenerative capacity of the hair follicle’s regular growth cycles to carry out these striking phenotypic changes (Fig. 1C). These cycles allow repeated rounds of regeneration that involve the destruction of most of the lower follicle (catagen), a period of rest and recovery (telogen), and shedding of existing hairs (exogen) followed by the development of replacement lower follicles and hairs (anagen) (12). New hairs may resemble the previous versions, as on young adult scalps or mice backs, or differ from them in size and/or color. Alterations in external (e.g., day length) and/or internal stimuli, such as hormones, influence which hair type each follicle produces (4, 13, 14). Final hair size and length depends on anagen duration (15). The androgen-driven transformation over several hair growth cycles of many tiny vellus hair follicles to big, deeply penetrating terminal follicles (Fig. 1C–F) (4,–6) produces the large terminal hairs (16). Girls’ lower androgen levels only stimulate such major changes in axillary and pubic regions (Fig. 1A) (4). Limited expansion of vellus follicles to intermediate-sized follicles occurs elsewhere, particularly in women (e.g., preauricular sides of the face; Fig. 1B–F) (9, 10); however, if women’s androgen levels rise, intermediate follicles will continue to enlarge, causing hirsutism (6).
At the same time as these marked androgen-driven transformations take place during puberty, other follicles, such as eyelashes and some scalp follicles, remain unaffected, and some areas that respond by increasing hair size show differing response times (up to >30 yr for ear canal hair) (3, 11, 16). In marked contrast, androgens inhibit follicle size and anagen length in specific scalp areas of genetically susceptible individuals, which causes the reverse slow replacement of terminal scalp hairs by vellus hairs and balding (17, 18). The essential role of androgens and androgen receptors in these changes is highlighted by men with complete androgen insensitivity syndrome—lacking functional androgen receptors—who display no body hair changes at puberty, nor androgenetic alopecia (1). As an individual’s follicles are exposed to the same androgen levels, how does this stimulate hair growth in many areas, have no effect in others, and inhibit some scalp follicles (Fig. 1A)? These unique, contrasting responses to the same hormone are presumably a result of intrinsic differences within individual hair follicles, which demonstrates that each follicle is a discrete organ. This is confirmed when follicles from nonbalding occipital sites that are transplanted into balding regions during transplant surgery retain their original androgen-independent characteristics (19). It is also highlighted by differences in androgen receptor content between androgen-sensitive and androgen-independent human and red deer hair follicle cells (20,–22). These variations in androgen response are presumably a result of location-specific epigenetic differences in gene expression, likely established within developing hair follicles by site-specific variations in exposure to paracrine regulatory molecules during embryogenesis. Although mice do not show sex-specific diversity in hair growth, epigenetic differences were reported recently in the various types of coat follicles, likely influenced by the timing of their embryonic development, rather than spatial location (23, 24).
In addition to epigenetic variations in androgen responsiveness, human follicles have other attributes that suggest they would make a useful model for androgen action. Their position in skin makes them a relatively accessible human tissue that is available as a byproduct of surgical procedures. Their mechanism of androgen action also shows striking similarities with that of the prostate. The mesenchyme-derived dermal papilla in the center of the hair bulb (Fig. 1F) (4,–6, 25) initiates and regulates much follicular growth activity via paracrine signaling (26). Androgens are believed to act via androgen receptors in dermal papilla cells that form hormone-receptor complexes that act as gene switches, which activate cell-specific gene transcription and alter dermal papilla production of short-distance paracrine signals that influence other follicular cells. This closely resembles androgen action via the mesenchyme/stroma in developing and cancerous prostates (2, 27).
Cultured nonbalding scalp follicles respond to external factors, such as drugs, by altered growth (28, 29). Unfortunately, nonbalding scalp follicles are not androgen dependent; children have well-developed head hair. Most androgen-sensitive follicles have significant limitations in availability, like beard, or suitability for organ culture (e.g., tiny, mostly resting balding follicles). We recently characterized for the first time intermediate hair follicles from preauricular regions of adult women’s faces (Fig. 1B) (9). They are a distinct hair follicle type, intermediate in size and depth below the epidermis between vellus and terminal follicles, that produce slightly pigmented, short, thin hairs (Fig. 1C–F). These follicles are vellus in childhood but increase in size in response to adult female androgen levels. If exposed to male levels, these follicles would enlarge further to terminal beard-like follicles.
Therefore, we hypothesized that female preauricular intermediate hair follicles offer a unique androgen-responsive, multicellular, human organ that involves mesenchyme-, epiderm-, and neural crest–derived cells where gene expression could be switched on or off naturally by adding hormones in organ culture, with widespread, important relevance. We also hypothesized that adjacent genetically identical, but epigenetically androgen-independent, terminal follicles from the same women would not respond to testosterone by increasing growth in culture. To determine whether such intermediate hair follicles would respond to testosterone in the male physiologic range in organ culture, we isolated and cultured them with or without testosterone for 9 d, carrying out parallel experiments with matched genetically identical terminal follicles that were taken from the same women at the same time. We followed this with additional culture, molecular biologic, and immunohistologic investigations to determine whether androgen receptors were present in the follicles in vivo and whether they were necessary for any response.
MATERIALS AND METHODS
Skin samples and hair follicle isolation
Preauricular hair-bearing skin samples from 22 healthy Caucasian women were collected, with full ethical permission and informed consent, during surgery into Falcon tubes (50 ml) that contained sterile transport medium (25 ml), transported at 4°C, and microdissected within 4 h, as described previously (28). Transport medium was composed of William’s E medium that contained 10 U/ml penicillin, 100 ng/ml hydrocortisone, 5 µg/ml insulin (all Sigma-Aldrich, St. Louis, MO, USA) and 2 mM l-glutamine (Thermo Fisher Scientific, Waltham, MA, USA). Matched terminal and intermediate hair follicles were isolated by careful microdissection in sterile PBS in a plastic Petri dish (60 mm) on ice under cool fiberoptic illumination by using a Leica MZ8 dissecting microscope (Leica Microsystems, Wetzlar, Germany). Hair follicles were isolated by carefully following each follicle from the hair bulb toward the epidermis, removing adjacent epidermis, dermis, and subcutaneous fat (Fig. 1D) (9), and collected into sterile Petri dishes (30 mm) that contained culture medium for organ culture or RNAlater (Sigma-Aldrich) for molecular biologic analysis. Any remaining extraneous tissue was removed by using 27.5-gauge sterile syringe needles (Tyco Health Care, Gosport, United Kingdom).
Testosterone effects on facial hair follicle growth in organ culture
To investigate whether isolated preauricular facial hair follicles would respond to testosterone in organ culture, matched intermediate and terminal follicles from 6 women age 59 ± 3 yr (means ± sem) were cultured as previously described (28). More intermediate hair follicles were cultured from each participant (20 follicles/condition) than terminal follicles (15 follicles/condition), as the tiny size of the intermediate follicles made isolation more difficult. Damaged follicles do not grow successfully in organ culture (28, 29). Isolated anagen follicles were transferred to individual wells of a 24-well plate (Corning, Corning, New York, USA) that contained medium (1 ml) and were incubated at 37°C in 5% CO2 and 95% air in a humidified incubator for 9 d. Culture media were composed of transport medium plus 0.001% DMSO that was supplemented by the vehicle alone (0.0001% ethanol) or 10 nM testosterone (Sigma-Aldrich). Sterile filtered media (0.2 µm pore; Sarstedt, Nümbrecht, Germany) were changed every 3 d. Each follicle was measured, classified as in anagen or not, and photographed every 24 h, as described previously (28). Any follicle that failed to grow in the first 3 d was classed as nonviable and excluded. Hair follicles that began to enter a catagen-like state as indicated by separation of the dermal papilla from the hair matrix were deemed to have completed anagen. Final length was obtained from the last anagen measurement to avoid length increases during movement of the hair while entering a catagen-like state (29).
Analysis of androgen receptor requirement for testosterone responses
To determine whether the response to testosterone was via androgen receptor–mediated mechanism, hormone-stimulation experiments were repeated and expanded to include an antiandrogen using follicles from an additional 6 women age 57 ± 3 yr (means ± sem). This antiandrogen, cyproterone acetate, is a competitive inhibitor of androgen receptor binding that is used to treat hirsutism in many countries (30). Matched intermediate and terminal follicles were incubated in culture media that were supplemented with either vehicle alone (0.0001% ethanol), 10 nM testosterone, 1 µM cyproterone acetate, or 10 nM testosterone and 1 µM cyproterone acetate combined, as used previously in cultured dermal papilla cells (22).
Identification of androgen receptor gene expression
To analyze androgen receptor gene expression, total RNA was extracted aseptically from the isolated lower follicles of matched anagen terminal (60/person) and intermediate hair follicles (80/person) from 5 women age 60 ± 3 yr (mean ± sem) and prostate glands of healthy adult male rats (Wistar albino Rattus norvegicus) immediately after performing microdissection using a GenElute Mammalian Total RNA kit (Sigma-Aldrich) as described previously (28). cDNAs were synthesized from poly(A)+ RNAs, isolated by using a GenElute mRNA Miniprep kit (Sigma-Aldrich), and quality checked by gel electrophoresis and β-actin gene expression for RT-PCR as described previously (28) or for analysis with NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific) for quantitative real-time RT-PCR.
RT-PCR analysis using androgen receptor primers (forward: AAGAGGAACAGCAGCCTTCACA. reverse: ATGGGGCAGCTGAGTCATCCT) (31) that were confirmed suitable for human and rat samples using National Center for Biotechnology Information Primer Blast and Ensembl Genomic Browser was carried out by using a PCR Sprint thermal cycler (Thermo Hybaid, Ashford, United Kingdom) to identify androgen receptor gene expression and analyzed and sequenced as described previously (28). DNA denaturation for 5 min at 95°C was followed by 35 cycles of the following: 95°C for 1 min, 65°C for 1 min, 72°C for 1 min; elongation for 11 min at 72°C was followed by cooling at 4°C. Androgen receptor identity was confirmed by using the National Center for Biotechnology Information BLAST program (http://www.ncbi.nlm.nih.gov/blast/bl2seq/wblast2.cgi).
Comparison of the amounts of androgen receptor gene expression in terminal and intermediate hair follicles
To compare the relative amounts of androgen receptor in intermediate and terminal hair follicles, we performed quantitative real-time RT-PCR in triplicate for each sample in a Bio-Rad iCycler (Bio-Rad, Hercules, CA, USA) using established primers (32) that were synthesized by Sigma Genosys Biotechnologies (Pampisford, United Kingdom). Reaction mixtures contained 12.5 μl 1× iQ SYBR Green Supermix (Bio-Rad), 0.25 μl forward and 0.25 μl reverse primers, 11 μl water, and 1 μl cDNA. Optimized thermal cycler program settings followed an initial denaturation step (95°C, 5 min), by 50 cycles of denaturation (95°C, 15 s), annealing (65°C, 1 min), and elongation (72°C, 15 s). Melt curves analysis over 60–90°C confirmed specific binding. Relative differences in expression—measured as ΔΔCt relative to β-actin—were calculated by using the Gene Expression Macro program (Bio-Rad).
Localization of androgen receptor protein
Androgen receptor protein was localized by immunohistochemistry in frozen vertical sections of partially dissected preauricular follicles that were embedded in optimum cutting temperature compound (OCT) (Raymond A. Lamb; Thermo Fisher Scientific) from 5 Caucasian women age 52 ± 3 yr (mean ± sem) by using rat prostate (detailed above) as positive control. Sections (5 μm) on poly-l-lysine–coated slides (28) were fixed in Zamboni’s fixative (10 min), incubated in 0.3% (v/v) hydrogen peroxide in methanol (30 min) to block endogenous peroxidase activity, and permeabilized by using 0.1% Triton X-100 at 4°C (10 min) as described previously (21). Nonspecific protein binding was blocked with 5% (v/v) horse serum in PBS (20 min) before incubation overnight at 4°C with human androgen receptor mouse mAb [NCL-AR-318, Novocastra; Leica Biosystems, Newcastle, United Kingdom; diluted 1:50 with 1.5% (v/v) horse serum in PBS]. Secondary Ab/visualization system was horse biotinylated anti-mouse IgG (5 μg/ml, BA-2001; Vector Laboratories, Peterborough, United Kingdom; 30 min), ExtraAvidin peroxidase (Sigma-Aldrich; 30 min), and chromogen 3-amino-9-ethylcarbazole substrate (Vector Laboratories). Sections were mounted with aqueous mountant (Aquamount; VWR International, Leicester, United Kingdom), dried overnight, and sealed with clear nail varnish (Laval, Warrington, United Kingdom) to prevent evaporation. Staining was examined and photographed by using a Nikon Eclipse 80i light microscope with a Nikon ACT-2U photographic system (Nikon, Tokyo, Japan).
Statistical analysis
Results were plotted and comparisons were analyzed as means ± sem of each person’s average values, not of all the individual follicles measured; therefore, when analyzing the effect of testosterone on intermediate follicles (Fig. 2), sample number was 6 from the 6 donors, not 120 from the total number of follicles studied per condition. After confirming normal distribution by using Kolmogorov Smirnov’s test, growth data were analyzed by using Student’s paired t test, and the percentage of hair follicle samples remaining in anagen over 9 d were analyzed by using ANOVA.
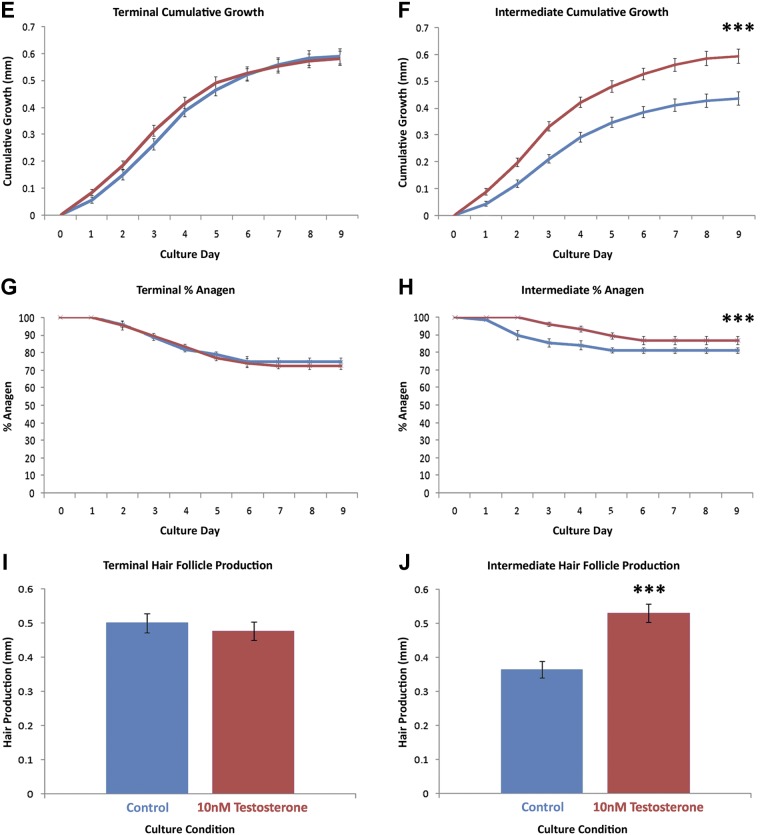
Figure 2.
Testosterone stimulates the growth of intermediate hair follicles in organ culture, but terminal follicles from the same women remain unaffected. Matched terminal (n = 15 follicles/condition per subject) and intermediate (n = 20 follicles/condition per subject) follicles from 6 women age 59 ± 3 yr were incubated in organ culture for 9 d with control medium (blue) or medium that contained 10 nM testosterone (red). A–D) Sequential photomicrographs, taken every 24 h for 9 d, of typical individual terminal and intermediate follicles show growth of hair fiber (Fiber), but not the connective tissue sheath (CTS) in both types of media. Testosterone significantly stimulated the growth rate (F), length of time the follicles remained in anagen (growing) (H), and the actual length of hair produced (D, J) in intermediate follicles (means ± sem; n = 6). It had no effect on terminal follicles (C, E, G, I). Testosterone-stimulated intermediate follicles (D, J) produced the same amount of hair as terminal follicles in either medium (A, C, I). IRS, inner root sheath; ORS, outer root sheath.
RESULTS
Androgens affected the growth of female preauricular intermediate hair follicles, but not matched terminal follicles
Daily observations and growth measurements of each hair follicle enabled precise analysis of the rate of growth, the length of time that follicles were able to remain in anagen (i.e., continue to grow), and the overall amount of hair produced in organ culture. Single measurements after a set number of days would not indicate whether anagen—the key factor for increasing hair length in vivo (15)—had been affected and would include false increases of length because of hairs being pushed up by the follicle during entry into the catagen-like stage. Only follicles that were in anagen were included in any day’s results, and the final length that was recorded for any follicle was that achieved on the last day in anagen.
Both terminal hair follicles and their matched intermediate follicles—microdissected from preauricular skin samples from the same 6 women—were able to grow in organ culture in control media, synthesizing new hair. Some follicles of each type maintained anagen throughout the 9-d experimental period (Fig. 2); however, their growth patterns differed. Intermediate follicles grew less quickly as assessed by daily length measurements (0.048 ± 0.010 mm/d; means ± sem) than did terminal follicles (0.066 ± 0.013 mm/d) under control conditions, particularly during the early stages of culture (P = 0.019). This resulted in the synthesis of significantly less hair (0.432 ± 0.022 compared with 0.596 ± 0.023 mm; P < 0.001) during the experiment (Fig. 2).
Addition of 10 nM testosterone to the organ cultures had no effect on any of the measured growth parameters of the terminal hair follicles (Fig. 2). In marked contrast, testosterone increased the percentage of intermediate follicles that remained in anagen until d 9 from 81 ± 1.8 to 87 ± 2.3% (P < 0.001) and boosted their rate of growth from 0.048 ± 0.010 to 0.065 ± 0.014 mm/d (P < 0.001). Overall, testosterone stimulated hair production by intermediate hair follicles to increase 45% from 0.366 ± 0.024 to 0.532 ± 0.027 mm (P < 0.001; Fig. 2). Of interest, intermediate follicles that were treated with testosterone grew as well as terminal follicles (P = 0.691; Fig. 2).
Preauricular intermediate hair follicle response to testosterone requires androgen receptors
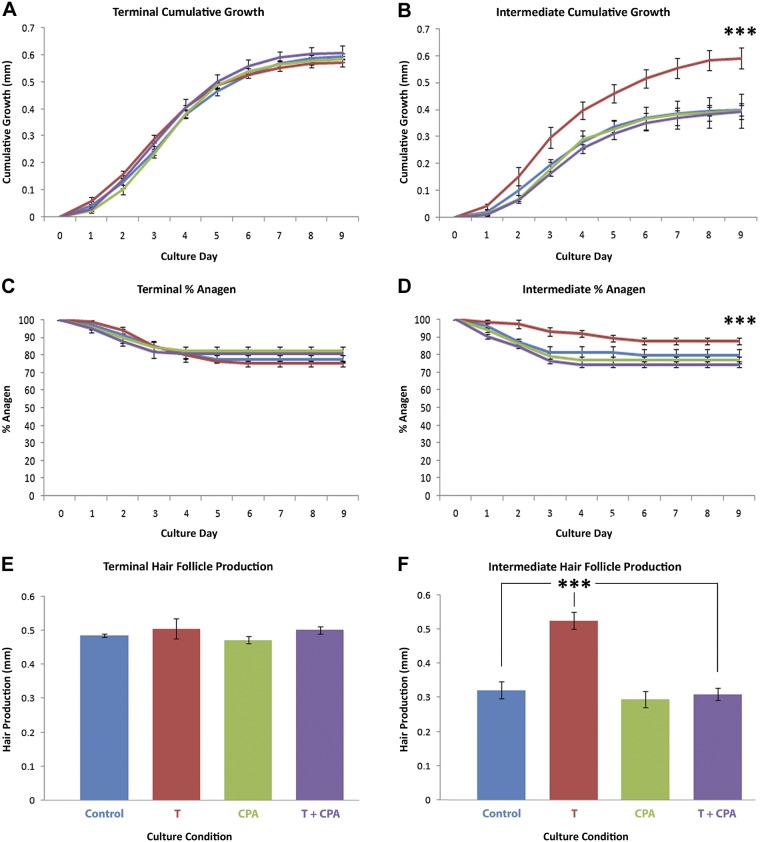
To determine whether increased growth of intermediate hair follicles in organ culture in response to testosterone is receptor mediated, we investigated whether the antiandrogen, cyproterone acetate, blocked testosterone’s effects by using samples from another 6 women (33). Testosterone increased growth from 0.044 ± 0.012 to 0.066 ± 0.015 mm/d and prolonged anagen from 78 ± 2.0 to 88 ± 1.9% (P = 0.04) in intermediate hair follicles from this second group of women, which resulted in increased cumulative growth over 9 d (0.590 ± 0.04 compared with 0.400 ± 0.02 mm; P < 0.01), but had no effect on their matched terminal ones (Fig. 3). Cyproterone acetate (1 μM) alone or in combination with testosterone (10 nM) had no effect on any growth parameters in terminal hair follicles in organ culture (Fig. 3). Intermediate hair follicle growth was also not affected by cyproterone acetate alone; however, when cyproterone acetate was supplied to intermediate follicles in combination with testosterone, it prevented all stimulatory effects that were observed with testosterone alone (Fig. 3).
Figure 3.
Androgens stimulate the growth of intermediate facial hair follicles in organ culture by an androgen receptor–mediated mechanism. Matched terminal (n = 15 follicles/condition per subject) and intermediate (n = 20 follicles/condition per subject) follicles from 6 additional women age 57 ± 3 yr were incubated for 9 d with control medium (blue), 10 nM testosterone (red), 1 μM cyproterone acetate (green), or testosterone and cyproterone acetate combined (purple). Testosterone-stimulated growth rate (B), length of time the follicles remained in anagen (growing; D), and the actual length of hair produced (F) by intermediate follicles very significantly (means ± sem; n = 6). It had no effect on any parameter in terminal follicles (A, C, E). Cyproterone acetate alone caused no change, but when administered in combination with testosterone blocked all the effects of testosterone on intermediate follicles (B, D, F). ***P < 0.0001.
Androgen receptors are present in women’s preauricular intermediate hair follicles in vivo
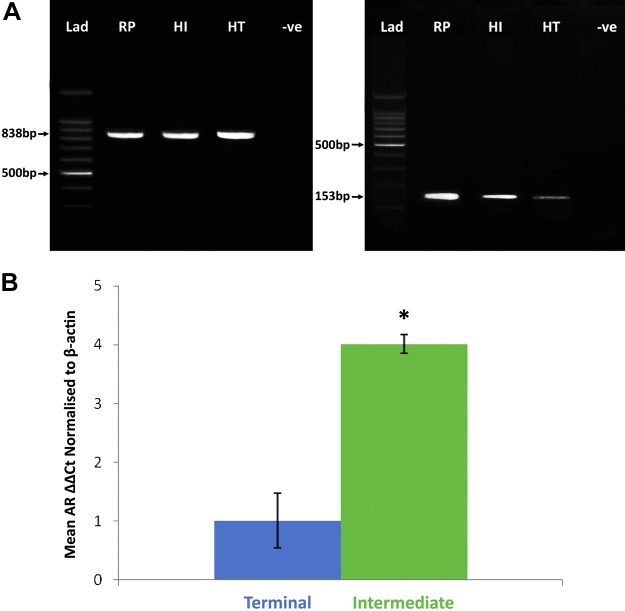
To detect whether androgen receptors were actually present within hair follicles in vivo, we examined cDNA that was extracted from matched preauricular terminal and intermediate hair follicles that were isolated from 5 women and positive control tissue (rat prostate). RT-PCR with both control β-actin and androgen receptor primers produced gene products from the rat prostate and both terminal and intermediate hair follicles with appropriately sized bands when separated by agarose gel electrophoresis (Fig. 4). Gene product sequencing confirmed the identity of androgen receptor gene expression, and the expression of control β-actin established equality of cDNA loading of terminal and intermediate hair follicle samples. Visibly larger amounts of androgen receptor gene product were observed in intermediate follicles samples on agarose gels after RT-PCR compared with terminal follicles (Fig. 4A). When a more quantitative comparison was carried out by using quantitative RT-PCR, expression of the androgen receptor gene relative to that of β-actin by preauricular intermediate hair follicles was confirmed as approximately 4 times greater than that of donor-matched terminal follicles (P = 0.04; Fig. 4B).
Figure 4.
Female facial intermediate hair follicles express more androgen receptor genes than do matched terminal follicles. Androgen receptor and control β-actin gene expression were identified in cDNA that was extracted from intermediate (90 follicles/subject) and terminal hair follicles (60/subject) from 5 women age 60 ± 3 yr by RT-PCR. A) Typical gels of RT-PCR results from 1 individual show control β-actin expression (left) and androgen receptor (right) expression. B) Quantitative RT-PCR analyses confirmed that intermediate hair follicles expressed approximately 4 times more androgen receptor (means ± sem) than did terminal follicles when related to β-actin expression. HI, intermediate hair follicles; HT, terminal hair follicles; Intermediate, intermediate hair follicles; Lad, standards ladder; RP, rat prostate; Terminal, terminal hair follicles; -ve, negative control. *P < 0.05.
Immunohistochemistry of control frozen rat prostate sections demonstrated distinct positive staining for androgen receptor protein in nuclei at the base of columnar epithelial cells (Fig. 5) by using a mAb to the androgen receptor. Sectioning the tiny intermediate hair follicles proved technically challenging. Nevertheless, positive staining was also demonstrated in hair bulbs of both terminal and intermediate hair follicles in frozen vertical sections cut through the center of female preauricular hair follicles (Fig. 5). Androgen receptor protein was clearly located in the nuclei of dermal papilla cells of both intermediate and terminal hair follicles as well as in the cells of nearby dermal papilla stalk and the dermal sheath; however, it was not observed in the adjacent keratinocytes of the hair bulb that divide and differentiate to produce hair, nor the melanocytes that produce hair pigment.
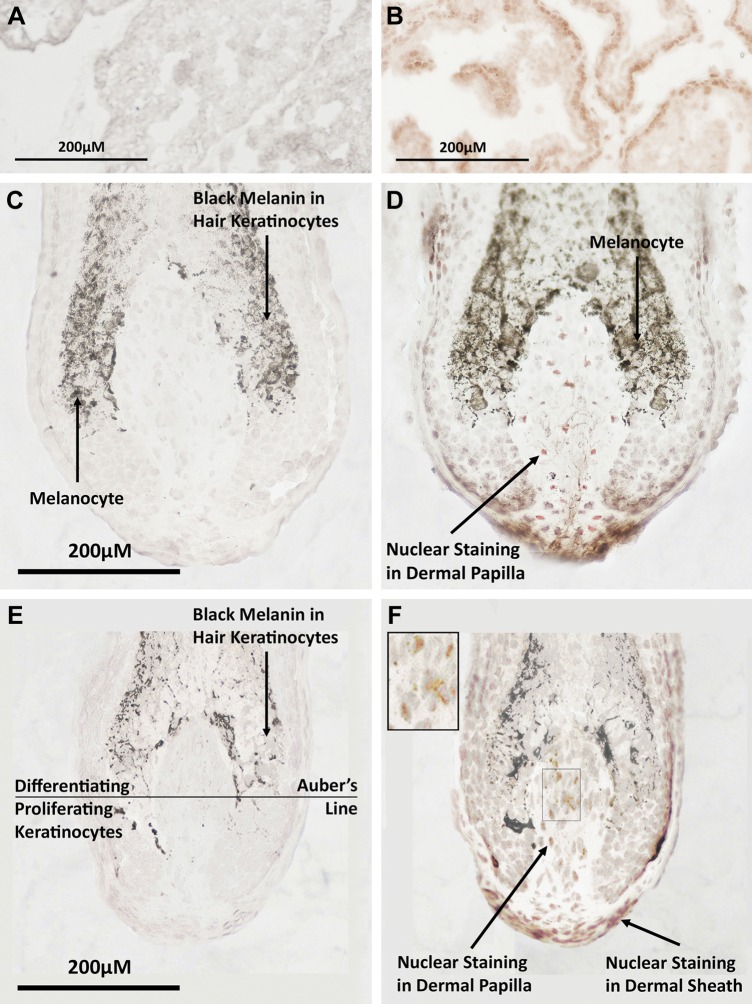
Figure 5.
Androgen receptors are present in the hair bulbs of female preauricular hair follicles. A, B) Photomicrographs of frozen sections of positive control adult rat prostate incubated with an mAb to the androgen receptor showed red positive staining in the nuclei of columnar epithelial cells (A), but no staining in the negative control (B). D, F) Immunohistochemistry of human preauricular hair follicle cryosections cut longitudinally through the approximate follicle midpoint located androgen receptors in the nuclei of dermal papilla cells and dermal sheath cells in both terminal (D) and intermediate (F) hair follicles. High-power insert in panel F is at ×2 higher magnification. No staining was observed in keratinocytes in the hair bulbs of either type of follicle. Keratinocytes in the hair bulb below the representative Auber’s line are predominantly dividing, whereas those above are differentiating (52). C, E) Dark brown areas in follicle sections are naturally occurring melanin within hair keratinocytes or melanocytes in the bulb. Negative control sections showed no background staining in either terminal or intermediate follicles.
DISCUSSION
Our primary aim for this research was to determine whether preauricular intermediate hair follicles from healthy women’s faces could respond to adult male levels of androgens in organ culture by increasing growth as they can in vivo. Under control conditions, these intermediate follicles were able to grow in organ culture for several days, producing additional hair fiber. Their growth patterns over the 9-d culture period resembled those of the same women’s matched terminal follicles that were isolated from the same skin samples studied in parallel as well as those reported previously in nonbalding scalp hair follicles (29); however, the intermediate follicles had slower rates of growth than did terminal follicles and produced less hair. Both types of follicles were incubated with testosterone to investigate their ability to respond in organ culture to levels of androgen in the male physiologic range. Large numbers of intermediate follicles were studied for each hormonal condition (120 follicles), with 20 follicles taken from each of 6 donors to avoid any variations as a result of differences in individual follicles. The level of testosterone used had previously been employed to measure androgen receptors in primary lines of cultured human dermal papilla cells by dose-response saturation analysis (22) and was shown to cause significant proliferation of hair follicle keratinocyte outer root sheath cells when cocultured with dermal papilla cells (34). Intermediate follicles significantly increased their growth in organ culture in response to testosterone in a similar manner to their natural responses in the body. In marked contrast, terminal follicles that were obtained from the same women, which were androgen independent in vivo, remained unaffected by the addition of testosterone when studied in parallel. A notable observation was that intermediate follicles treated with testosterone grew as fast as their corresponding terminal follicles. Whether this reflects a biologic maximum rate as terminal follicles all over the body grow at similar rates in vivo (15) or a limit as a result of culture conditions is unclear.
This androgen response by female facial intermediate follicles in organ culture was blocked by competition with an antiandrogen—cyproterone acetate—that is used clinically for the treatment of hirsutism (30) and here at levels used in earlier hair follicle cell culture experiments (22, 34). This inhibition, combined with the absence of any effect of the antiandrogen alone, demonstrates that increased growth is an androgen-stimulated effect. It also indicates that the increased growth involves an androgen receptor–mediated mechanism rather than one of the more recently established rapid response alternative mechanisms because it was blocked by competition with an antiandrogen (35,–37). Whether this is the classic pathway or another mechanism that involves the androgen receptor cannot be clarified fully from these data; however, the effects of androgens on hair growth here in organ culture as well as in vivo are slow, rather than rapid, and involve alterations in paracrine signaling molecules (4). This suggests the involvement of the slower classic pathway (25). Detection of androgen receptor protein by immunohistochemistry only in the nuclei of dermal papilla cells and dermal sheath fibroblasts in hair bulbs of intact preauricular hair follicles strongly supports this classic mechanism. It is also in agreement with previous reports that located androgen receptors in the dermal papilla cell nuclei of beard and other types of hair follicles, but not in keratinocytes or melanocytes (4, 34). Their presence in dermal sheath cells also fits with our previous experiments (unpublished results) and with the ability of dermal sheath cells to replace dermal papilla cells in initiating new hair follicle development (38,–40). This immunolocalization does not completely exclude possible additional actions via nonclassic androgen receptor–mediated pathways as more diffuse receptors in the cytoplasm would be difficult to detect (36, 41). Nevertheless, a classic androgen receptor–mediated mechanism would also concur with the slow clinical effects of antiandrogens, such as cyproterone acetate and spirolactone, in treating hirsutism in women (30).
Androgen receptor gene expression was also examined by quantitative real-time RT-PCR as the low density of dermal papilla cells in their surrounding extracellular matrix, the pear-shaped 3-dimensional structure of the dermal papilla, and the tiny size of the follicles make any form of quantitative comparison from sections impractical. Androgen-sensitive intermediate follicles expressed approximately 4 times more androgen receptor gene than did their parallel androgen-insensitive terminal follicles. This is in agreement with earlier reports that detected more androgen receptor in androgen-sensitive follicles compared with matched androgen-insensitive terminal follicles. Dermal papilla cells that were cultured from both androgen-stimulated beard follicles and those from androgen-inhibited balding scalp contained higher levels of androgen receptor protein and showed greater expression of androgen receptor gene than did those from androgen-insensitive, nonbalding scalp follicles (20, 22, 42). The location of androgen receptors in the dermal papilla nuclei of the hair bulbs of intact preauricular intermediate follicles also concurs with the accepted view that androgens regulate human hair follicle size by acting via androgen receptors in the cells of the dermal papilla (4). In this mechanism, which is derived primarily from experiments that used isolated primary cells from human hair follicles alone or in coculture systems, androgens act via androgen receptors in the mesenchyme-derived dermal papilla cells, stimulating alterations in gene expression. These alterations then cause alterations in the activities of epithelial keratinocyte cells and melanocytes, which means that the effect of androgens on these cells is via an indirect mechanism (4, 34). This is similar to actions in the androgen-dependent prostate via the mesenchyme-derived stroma during morphogenesis and the recent understanding of androgen actions in cancerous changes (2, 27).
These exciting results confirm that preauricular intermediate facial follicles from normal women are androgen-sensitive, complex organs that can retain their androgen-responsive characteristics outside the body. They can respond to testosterone by using androgen receptor–mediated pathways to increase the formation of their normal product, pigmented, keratinized hair, for several days in organ culture. This response in organ culture mirrors the natural response within the human body. The amount of pigmented hair fiber that is produced by hair follicles in response to androgen in organ culture was selected as the target to measure here, rather than any single gene, because this is the actual response in vivo observed when such intermediate follicles produce larger visible hairs in hirsutism that is caused by androgen-secreting tumors (6). Production of a measurably greater amount of pigmented hair fiber requires increased highly controlled, complex hair fiber synthesis, which necessitates altered gene expression in several cell types. This includes altering dermal papilla cell gene expression to modify the regulatory signals they provide for other cell types in the bulb to coordinate increasing cell division and differentiation of keratinocytes in the hair bulb to make more hair fiber and raising melanin synthesis and/or pigment transfer rates in melanocytes.
Although microdissection and culture of preauricular intermediate hair follicles is technically challenging, the facial skin involved is more readily available as cosmetic surgery byproducts than most human material. Female preauricular intermediate hair follicles have significant advantages over the previous androgen-sensitive model—cultured dermal papilla cells that are derived from appropriate hair follicles, such as large beard follicles (4). Unlike beard follicles, these female intermediate follicles are readily capable of additional androgen response in the body, enlarging if androgens rise abnormally. In addition, isolated dermal papilla cells undergo rapid changes of gene expression once isolated and cultured in standard 2-dimensional cell culture conditions, which can be somewhat ameliorated by using a 3-dimensional culture method (24, 43). In marked contrast, dermal papilla cells in the intact hair bulbs of these cultured follicles remain in a more natural structure surrounded by, and interacting with, their own normal cells.
In this way, female facial intermediate hair follicles offer a novel, exciting, hormone-responsive, human organ model that can be cultured and manipulated in a simple medium in accessible dishes in a standard culture laboratory. In addition to this powerful model system, hair follicles studied here offer the exceptional opportunity of comparison with matched androgen-insensitive terminal hair follicles obtainable from the same women at the same time. These are genetically identical terminal hair follicles with different epigenetic mechanisms that retain their normal, nonandrogen-responsive character in organ culture. This offers the excellent and unique opportunity for experiments that investigate androgen effects on androgen-sensitive, human intermediate hair follicles with parallel control experiments in follicles that carry out the same processes of hair synthesis with the identical individual’s genes, but with epigenetic differences that prevent androgen responses. These models will facilitate significant experiments that can harness powerful genetic analyses or live-cell imaging tools to advance our understanding of androgen action (44). Although these intermediate follicles should be valuable for basic scientific research, they are unlikely to be useful for drug testing because of the challenges posed by their specialist sources and tiny size; however, studies on androgen action using intermediate hair follicles should also be relevant to other sex steroid-dependent tissues (e.g., the human prostate, which has several similarities with human hair follicles). Both androgen-sensitive tissues show medically significant changes with increased age (e.g., androgenetic alopecia and benign prostatic hypertrophy), and both use indirect androgen signaling via mesenchyme-derived cells of the dermal papilla or prostate stroma, which is important during prostatic carcinoma development (45), and employ similar signaling pathways, such as WNT/B-catenin (46, 47). Greater understanding of androgen mechanisms of action in a multicellular living human organ in culture that involves mesenchyme and epithelial cell interactions should enable improved treatments for widespread hormone-dependent disorders, such as androgenetic alopecia, hirsutism, benign prostatic hypertrophy, and prostate cancer, likely by targeting post–androgen receptor aspects of the mechanism. Androgens also play significant roles in human development, both before birth and during puberty. Rodent hair follicles and bird feather follicles are already frequently studied as models for developmental processes and stem cell niches (13, 48,–51), because the development of a new lower hair follicle in the anagen stage of the hair follicle growth cycle begins with lower follicle regeneration from epithelial and melanocyte stem cells under the influence of the dermal papilla, thereby recapitulating hair follicle morphogenesis. Therefore, studies of these human androgen-sensitive intermediate follicles may also prove relevant to other important biologic systems, such as cell signaling, development, stem cell manipulation, and tissue engineering.
ACKNOWLEDGMENTS
The authors thank the Plastic Surgery and Burns Research Unit and the Faculty of Life Sciences at the University of Bradford (Bradford, United Kingdom) for financial support for B.H.M. and M.R.C., respectively. The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
V. A. Randall conceived, designed, and supervised the project and cowrote the paper; B. H. Miranda was the primary investigator, selected appropriate skin donors, analyzed the data, and cowrote the paper; M. R. Charlesworth contributed the remaining experimental work; D. T. Sharpe and D. J. Tobin selected appropriate skin donors; and all authors contributed to manuscript preparation.
REFERENCES
- 1.Hughes I. A., Werner R., Bunch T., Hiort O. (2012) Androgen insensitivity syndrome. Semin. Reprod. Med. 30, 432–442 [DOI] [PubMed] [Google Scholar]
- 2.Ricke E. A., Williams K., Lee Y. F., Couto S., Wang Y., Hayward S. W., Cunha G. R., Ricke W. A. (2012) Androgen hormone action in prostatic carcinogenesis: stromal androgen receptors mediate prostate cancer progression, malignant transformation and metastasis. Carcinogenesis 33, 1391–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamilton J. B. (1958) Age, sex, and genetic factors in the regulation of hair growth in man: a comparison of Caucasian and Japanese populations. The Biology of Hair Growth (Montagna W., Ellis R. A., ), 399–433, Academic Press, New York [Google Scholar]
- 4.Randall V. A. (2007) Hormonal regulation of hair follicles exhibits a biological paradox. Semin. Cell Dev. Biol. 18, 274–285 [DOI] [PubMed] [Google Scholar]
- 5.Randall V. A. (2012) Androgens and hair: a biological paradox with clinical consequences. Testosterone: Action, Deficiency, Substitution (Nieschlag E., Behre H. M., ), 154–176, Cambridge University Press, Cambridge, UK [Google Scholar]
- 6.Randall V. A. (2008) Androgens and hair growth. Dermatol. Ther. (Heidelb.) 21, 314–328 [DOI] [PubMed] [Google Scholar]
- 7.Cash T. F. (2001) The psychology of hair loss and its implications for patient care. Clin. Dermatol. 19, 161–166 [DOI] [PubMed] [Google Scholar]
- 8.Girman C. J., Rhodes T., Lilly F. R., Guo S. S., Siervogel R. M., Patrick D. L., Chumlea W. C. (1998) Effects of self-perceived hair loss in a community sample of men. Dermatology (Basel) 197, 223–229 [DOI] [PubMed] [Google Scholar]
- 9.Miranda B. H., Tobin D. J., Sharpe D. T., Randall V. A. (2010) Intermediate hair follicles: a new more clinically relevant model for hair growth investigations. Br. J. Dermatol. 163, 287–295 [DOI] [PubMed] [Google Scholar]
- 10.Marshall W. A., Tanner J. M. (1969) Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 44, 291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall W. A., Tanner J. M. (1970) Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 45, 13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins C. A., Westgate G. E., Jahoda C. A. (2009) From telogen to exogen: mechanisms underlying formation and subsequent loss of the hair club fiber. J. Invest. Dermatol. 129, 2100–2108 [DOI] [PubMed] [Google Scholar]
- 13.Chuong C. M., Randall V. A., Widelitz R. B., Wu P., Jiang T. X. (2012) Physiological regeneration of skin appendages and implications for regenerative medicine. Physiology (Bethesda) 27, 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lei M., Chuong C. M. (2016) Stem cells. Aging, alopecia, and stem cells. Science 351, 559–560 [DOI] [PubMed] [Google Scholar]
- 15.Saitoh M., Uzuka M., Sakamoto M. (1970) Human hair cycle. J. Invest. Dermatol. 54, 65–81 [DOI] [PubMed] [Google Scholar]
- 16.Jansen V. A., van Baalen M. (2006) Altruism through beard chromodynamics. Nature 440, 663–666 [DOI] [PubMed] [Google Scholar]
- 17.Hamilton J. B. (1951) Patterned loss of hair in man; types and incidence. Ann. N. Y. Acad. Sci. 53, 708–728 [DOI] [PubMed] [Google Scholar]
- 18.Randall V. A. (2005) Physiology and pathophysiology of androgenetic alopecia. Endocrinology (Degroot L. J., Jameson J. L., ), 3295–3309, W. B. Saunders Co., Philadelphia [Google Scholar]
- 19.Bunagan M. J., Banka N., Shapiro J. (2013) Hair transplantation update: procedural techniques, innovations, and applications. Dermatol. Clin. 31, 141–153 [DOI] [PubMed] [Google Scholar]
- 20.Hibberts N. A., Howell A. E., Randall V. A. (1998) Balding hair follicle dermal papilla cells contain higher levels of androgen receptors than those from non-balding scalp. J. Endocrinol. 156, 59–65 [DOI] [PubMed] [Google Scholar]
- 21.Thornton M. J., Hibberts N. A., Street T., Brinklow B. R., Loudon A. S., Randall V. A. (2001) Androgen receptors are only present in mesenchyme-derived dermal papilla cells of red deer (Cervus elaphus) neck follicles when raised androgens induce a mane in the breeding season. J. Endocrinol. 168, 401–408 [DOI] [PubMed] [Google Scholar]
- 22.Randall V. A., Thornton M. J., Messenger A. G. (1992) Cultured dermal papilla cells from androgen-dependent human hair follicles (e.g., beard) contain more androgen receptors than those from non-balding areas of scalp. J. Endocrinol. 133, 141–147 [DOI] [PubMed] [Google Scholar]
- 23.Chi W., Wu E., Morgan B. A. (2015) Earlier-born secondary hair follicles exhibit phenotypic plasticity. Exp. Dermatol. 24, 265–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Driskell R. R., Juneja V. R., Connelly J. T., Kretzschmar K., Tan D. W., Watt F. M. (2012) Clonal growth of dermal papilla cells in hydrogels reveals intrinsic differences between Sox2-positive and -negative cells in vitro and in vivo. J. Invest. Dermatol. 132, 1084–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Handelsman D. J. (2001) Androgen action and pharmacologic uses. Endocrinology (DeGroot L. J., Jameson L. J., ), Vol. 3, 2232–2242, W. B. Saunders Co., Philadelphia [Google Scholar]
- 26.Morgan B. A. (2014) The dermal papilla: an instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb. Perspect. Med. 4, a015180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cunha G. R., Ricke W. A. (2011) A historical perspective on the role of stroma in the pathogenesis of benign prostatic hyperplasia. Differentiation 82, 168–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shorter K., Farjo N. P., Picksley S. M., Randall V. A. (2008) Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. FASEB J. 22, 1725–1736 [DOI] [PubMed] [Google Scholar]
- 29.Khidhir K. G., Woodward D. F., Farjo N. P., Farjo B. K., Tang E. S., Wang J. W., Picksley S. M., Randall V. A. (2013) The prostamide-related glaucoma therapy, bimatoprost, offers a novel approach for treating scalp alopecias. FASEB J. 27, 557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin K. A., Chang R. J., Ehrmann D. A., Ibanez L., Lobo R. A., Rosenfield R. L., Shapiro J., Montori V. M., Swiglo B. A. (2008) Evaluation and treatment of hirsutism in premenopausal women: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 93, 1105–1120 [DOI] [PubMed] [Google Scholar]
- 31.Croft N. J., Randall V. A. (2003) The antler of the red deer (Cervus elaphus): an androgen target organ. Hair Science and Technology (van Neste D., ), 69–74, Skinterface, Tournai, Belgium [Google Scholar]
- 32.Bièche I., Parfait B., Tozlu S., Lidereau R., Vidaud M. (2001) Quantitation of androgen receptor gene expression in sporadic breast tumors by real-time RT-PCR: evidence that MYC is an AR-regulated gene. Carcinogenesis 22, 1521–1526 [DOI] [PubMed] [Google Scholar]
- 33.Linke T., Scholten M., Baniahmad A. (2011) Detection of ligand-selective interactions of the human androgen receptor by SELDI-MS-TOF. Methods Mol. Biol. 776, 225–251 [DOI] [PubMed] [Google Scholar]
- 34.Itami S., Kurata S., Sonoda T., Takayasu S. (1995) Interaction between dermal papilla cells and follicular epithelial cells in vitro: effect of androgen. Br. J. Dermatol. 132, 527–532 [PubMed] [Google Scholar]
- 35.Castoria G., Giovannelli P., Di Donato M., Ciociola A., Hayashi R., Bernal F., Appella E., Auricchio F., Migliaccio A. (2014) Role of non-genomic androgen signalling in suppressing proliferation of fibroblasts and fibrosarcoma cells. Cell Death Dis. 5, e1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foradori C. D., Weiser M. J., Handa R. J. (2008) Non-genomic actions of androgens. Front. Neuroendocrinol. 29, 169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C., Liu Y., Cao J. M. (2014) G protein-coupled receptors: extranuclear mediators for the non-genomic actions of steroids. Int. J. Mol. Sci. 15, 15412–15425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynolds A. J., Lawrence C., Cserhalmi-Friedman P. B., Christiano A. M., Jahoda C. A. (1999) Trans-gender induction of hair follicles. Nature 402, 33–34 [DOI] [PubMed] [Google Scholar]
- 39.McElwee K. J., Kissling S., Wenzel E., Huth A., Hoffmann R. (2003) Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J. Invest. Dermatol. 121, 1267–1275 [DOI] [PubMed] [Google Scholar]
- 40.Wang X., Marr A. K., Breitkopf T., Leung G., Hao J., Wang E., Kwong N., Akhoundsadegh N., Chen L., Mui A., Carr N., Warnock G. L., Shapiro J., McElwee K. J. (2014) Hair follicle mesenchyme-associated PD-L1 regulates T-cell activation induced apoptosis: a potential mechanism of immune privilege. J. Invest. Dermatol. 134, 736–745 [DOI] [PubMed] [Google Scholar]
- 41.Walker W. H. (2009) Molecular mechanisms of testosterone action in spermatogenesis. Steroids 74, 602–607 [DOI] [PubMed] [Google Scholar]
- 42.Ando Y., Yamaguchi Y., Hamada K., Yoshikawa K., Itami S. (1999) Expression of mRNA for androgen receptor, 5alpha-reductase and 17beta-hydroxysteroid dehydrogenase in human dermal papilla cells. Br. J. Dermatol. 141, 840–845 [DOI] [PubMed] [Google Scholar]
- 43.Higgins C. A., Chen J. C., Cerise J. E., Jahoda C. A., Christiano A. M. (2013) Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc. Natl. Acad. Sci. USA 110, 19679–19688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanders T. A., Llagostera E., Barna M. (2013) Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature 497, 628–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leach D. A., Need E. F., Toivanen R., Trotta A. P., Palethorpe H. M., Tamblyn D. J., Kopsaftis T., England G. M., Smith E., Drew P. A., Pinnock C. B., Lee P., Holst J., Risbridger G. P., Chopra S., DeFranco D. B., Taylor R. A., Buchanan G. (2015) Stromal androgen receptor regulates the composition of the microenvironment to influence prostate cancer outcome. Oncotarget 6, 16135–16150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee S. H., Johnson D. T., Luong R., Yu E. J., Cunha G. R., Nusse R., Sun Z. (2015) Wnt/β-catenin-responsive cells in prostatic development and regeneration. Stem Cells 33, 3356–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lien W. H., Polak L., Lin M., Lay K., Zheng D., Fuchs E. (2014) In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nat. Cell Biol. 16, 179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin S. J., Foley J., Jiang T. X., Yeh C. Y., Wu P., Foley A., Yen C. M., Huang Y. C., Cheng H. C., Chen C. F., Reeder B., Jee S. H., Widelitz R. B., Chuong C. M. (2013) Topology of feather melanocyte progenitor niche allows complex pigment patterns to emerge. Science 340, 1442–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen C. C., Plikus M. V., Tang P. C., Widelitz R. B., Chuong C. M. (2016) The modulatable stem cell niche: tissue interactions during hair and feather follicle regeneration. J. Mol. Biol. 428, 1423–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adam R. C., Yang H., Rockowitz S., Larsen S. B., Nikolova M., Oristian D. S., Polak L., Kadaja M., Asare A., Zheng D., Fuchs E. (2015) Pioneer factors govern super-enhancer dynamics in stem cell plasticity and lineage choice. Nature 521, 366–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lay K., Kume T., Fuchs E. (2016) FOXC1 maintains the hair follicle stem cell niche and governs stem cell quiescence to preserve long-term tissue-regenerating potential. Proc. Natl. Acad. Sci. USA 113, E1506–E1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Auber L. (1952) The anatomy of follicles producing wool fibres with special reference to keratinization. Earth Environ. Sci. Trans. R. Soc. Edinb. 62, 191–254 [Google Scholar]