Abstract
Superoxide dismutase (SOD) gene expression was investigated to elucidate its role in drought and freezing tolerance in spring and winter wheat (Triticum aestivum). cDNAs encoding chloroplastic Cu/ZnSODs and mitochondrial MnSODs were isolated from wheat. MnSOD and Cu/ZnSOD genes were mapped to the long arms of the homologous group-2 and -7 chromosomes, respectively. Northern blots indicated that MnSOD genes were drought inducible and decreased after rehydration. In contrast, Cu/ZnSOD mRNA was not drought inducible but increased after rehydration. In both spring and winter wheat seedlings exposed to 2°C, MnSOD transcripts attained maximum levels between 7 and 49 d. Transcripts of Cu/ZnSOD mRNA were detected sooner in winter than in spring wheat; however, they disappeared after 21 d of acclimation. Transcripts of both classes of SOD genes increased during natural acclimation in both spring and winter types. Exposure of fully hardened plants to three nonlethal freeze-thaw cycles resulted in Cu/Zn mRNA accumulation; however, MnSOD mRNA levels declined in spring wheat but remained unchanged in winter wheat. The results of the dehydration and freeze-thaw-cycle experiments suggest that winter wheat has evolved a more effective stress-repair mechanism than spring wheat.
Active oxygen species such as superoxide, H2O2, and hydroxyl radicals are by-products of normal cell metabolism. These active oxygen species result in the peroxidation of membrane lipids (Mead, 1976), breakage of DNA strands (Brawn and Fridovich, 1981), and inactivation of enzymes (Fucci et al., 1983). The conditions leading to damage caused by active oxygen species are referred to as oxidative stress. Both chloroplasts and mitochondria can produce active oxygen species either under normal growth conditions or during exposure to various stresses. The PSI electron-transport chain contains a number of autooxidizable enzymes that reduce O2 to superoxide (Badger, 1985; Asada and Takahashi, 1987; Asada, 1994), and evidence indicates that superoxide and H2O2 can also be produced by PSII under high-light intensities (Landgraf et al., 1995). During mitochondrial respiration, reactive oxygen species are also generated via the reactions of the electron-transport chain (Rich and Bonner, 1978; Bowler et al., 1991).
Active oxygen species are also generated during chemical and environmental stresses, including chilling and freezing (Wise and Naylor, 1987; Senaratna et al., 1988; Kendall and McKersie, 1989; Tsang et al., 1991; McKersie et al., 1993), drought (Perl-Treves and Galun, 1991; Price and Hendry, 1991), desiccation (Senaratna et al., 1985; Leprince et al., 1990), flooding (Hunter et al., 1983; Van Toai and Bolles, 1991), herbicide treatment (Malan et al., 1990; Kurepa et al., 1997), pathogen attack (Montalbini and Buonaurio, 1986; Buonaurio et al., 1987; Koch and Slusarenko, 1990), and ionizing radiation (Niwa et al., 1977).
SODs are a group of metalloenzymes that protect cells from superoxide radicals by catalyzing the dismutation of the superoxide radical to molecular O2 and H2O2. SODs are categorized into two families with unrelated DNA sequences. Cu/ZnSOD is located mainly in the cytosol and/or chloroplasts of plants, whereas the other family contains either Mn (MnSOD) in the mitochondria or Fe (FeSOD) in the chloroplast (Fridovich, 1986). For monocotyledonous plants, only cDNAs for chloroplastic Cu/ZnSOD and mitochondrial MnSOD have been isolated from rice or corn (Sakamoto et al., 1993; Zhu and Scandalios, 1993; Kaminaka et al., 1997).
Cold and drought are the most common environmental stresses, but the regulation of SOD gene expression under these conditions is not well documented (Perl-Treves and Galun, 1991; Price and Hendry, 1991; McKersie et al., 1993). Wheat (Triticum aestivum) is the largest cultivated crop in the world and has distinct spring and winter genotypes. To our knowledge, no study has been done of the SOD gene response to prolonged periods of cold and drought stress or of the differential regulation of SOD gene expression between spring and winter genotypes in wheat. In this paper we report the isolation, chromosomal localization, and differential expression of chloroplastic Cu/ZnSOD and mitochondrial MnSOD genes in a spring and a winter wheat subjected to both cold and drought stresses.
MATERIALS AND METHODS
Plant Materials
Spring and winter wheat (Triticum aestivum cvs Katepwa and Norstar, respectively, both hexaploid) were used for northern analyses. The cv Chinese Spring wheat and 35 ditelosomic lines derived from cv Chinese Spring were used for chromosomal localization of SOD genes. In the nomenclature of the ditelosomic lines, the numeral indicates the chromosome number, the A, B, or D represents the specific genome, and the L or S indicates which chromosome arm is present. Ditelosomic lines from the E.R. Sears collection were provided by Drs. B. Fowler and A. Limin (Crop Development Center, University of Saskatchewan, Saskatoon, Canada).
Drought Treatment
Seedlings of both the spring and winter wheat were grown in a sandy-loam soil at a density of five or six plants per pot (15 cm in diameter) in a greenhouse. Water was withheld from plants at the three-leaf stage for 6 d to simulate a drought stress, and then the plants were watered to saturation. During the drought period a fan was used to increase the rate of transpiration. Plants were harvested at 0, 2, 4, and 6 d during the drought treatment and 1 and 3 d after rehydration. Leaf water potentials (bar) were measured with a water-potential meter (PMS Instrument Co., Corvallis, OR).
Cold Acclimation in a Controlled Environment
The wheat seeds were germinated on moist paper towels and the seedlings were transferred to a foam grid for hydroponic culture. The planting grids were used to suspend the seedlings over one-half-strength Hoagland solution, with the roots immersed in a continuously aerated solution (Tyler et al., 1981). At the three-leaf stage, the temperature of the controlled-environment chamber was decreased to a constant day/night temperature of 2°C, with a 16-h photoperiod. The plants were harvested after 0, 2, 7, 21, and 49 d of cold acclimation.
Cold Acclimation under Natural Autumn Conditions
Seeds of spring and winter wheat were sown into flax stubble at the University of Saskatchewan on September 9, 1996. Seeding depth was 3 to 5 cm, and 10 kg ha−1 fertilizer (11–15-1) was applied at the time of seeding. Field-acclimated seedlings at the three- to five-leaf stage were harvested on September 26, September 30, October 9, October 15, and October 22 and subjected to a controlled-freeze test as described previously (O'Conner et al., 1993). After freezing, the crowns were thawed at 4°C overnight and transplanted to flats containing Redi-Earth (W.R. Grace & Co., Boca Raton, FL) for regrowth analysis after 3 weeks at 23°C in a greenhouse. Freezing tolerance was determined as LT50 (Gusta et al., 1982).
Plants harvested on October 22 were subjected to a freeze-thaw cycle. Seedlings of both cultivars were placed on moist tissue paper in the bottom of 30- × 140-mm glass test tubes, nucleated with ice at −2.5°C, held isothermal for 1 h, and cooled to −3°C overnight. The next morning, the seedlings were thawed at 4°C for 8 h and recooled to −3°C overnight. After three freeze-thaw cycles, the seedlings were transplanted to flats containing Redi-Earth for regrowth analysis for 3 weeks as described above.
Screening of Wheat cDNA Library
A cDNA library constructed in λZAPII (Stratagene) from mRNA isolated from cold-acclimated cv Norstar winter wheat (Houde et al., 1992) was obtained from Dr. F. Sarhan (University of Montreal, Quebec, Canada). The library was screened with heterologous probes from wild tobacco (Nicotiana plumbaginifolia): a mitochondrial MnSOD cDNA (Bowler et al., 1988) and a chloroplastic Cu/ZnSOD cDNA (Tsang et al., 1991). Approximately 5.0 × 104 phage were plated on 150-mm NYZ agar (5 g of NaCl, 2 g of MgSO4·7 H2O, 5 g of yeast extract, and 10 g/L NZ amine) plates and grown at 37°C for 6 to 9 h. After the plates were cooled at 4°C overnight, plaques were lifted onto nylon membranes (Hybond N+, Amersham). The hybridization and washing procedures were as outlined by the manufacturer. The remainder of the isolation procedure was conducted as described in the λZAPII cDNA library protocol.
DNA Sequencing and Sequence Analysis
Six MnSOD cDNAs and five chloroplastic Cu/ZnSOD cDNAs were sequenced completely using primers and synthetic oligonucleotides from cycle DNA-sequencing reactions performed with fluorescent dye terminators on an automated DNA sequencer (model 373A, Applied Biosystems) at the Plant Biotechnology Institute (National Research Council, Saskatoon, Saskatchewan, Canada). Overlapped sequence data from both strands for each clone were assembled and analyzed using DNAStar computer software (Madison, WI).
Mapping of SOD Genes Using Southern Analysis
Genomic DNA was isolated from seedlings of the available ditelosomic wheat lines (cv Chinese Spring) using the cetyl-trimethyl-ammonium bromide method (Procunier et al., 1990). The genomic DNA (7.5 μg) was digested with DraI, electrophoresed in 1.0% agarose gels, and transferred onto nylon membranes (GeneScreen Plus, NEN Life Science) by capillary blotting. Hybridization and washing were carried out according to the manufacturer's instructions for aqueous hybridization.
Northern Analysis
Total RNA was isolated from crown tissue of wheat using a modified hot-phenol method (Robertson et al., 1994). After isolation, total RNA (30 μg per lane) was separated by electrophoresis on formaldehyde gels. The remainder of the RNA blotting and hybridization was as described previously (Robertson et al., 1994), except that 200 μg of sonicated herring DNA was used instead of yeast tRNA.
Each lane of the autoradiography films from all northern blots were passed through a scanning densitometer three times to obtain an average value for relative intensity. Zero was determined by passing the beam through a clear region of the film, which had not been in contact with the membrane. The values obtained for each treatment from the various gels were then averaged and plotted to show relative changes in mRNA accumulation. The relative intensity of mRNA transcripts in northern blots was expressed as treatment intensity divided by control intensity.
RESULTS
Isolation and Characterization of Chloroplastic Cu/ZnSOD Genes
A Cu/ZnSOD cDNA from wild tobacco (Tsang et al., 1991) was used to probe a cold-acclimated winter wheat cv Norstar λZAPII (Stratagene) cDNA library (Houde et al., 1992). Five positive clones were isolated, and both strands of each clone were sequenced completely. Three of the cDNAs had complete open reading frames. The first clone (SOD1.1) was 883 bp (accession no. U69536), whereas the other two were identical and were designated SOD1.2 (781 bp) (accession no. U69632). Both genes were missing poly(A+) tails but contained polyadenylation signals (AAATAAA). The two genes had 98% identity and encoded two nearly identical (98% identity) Cu/ZnSOD proteins of 201 amino acids with a calculated molecular mass of approximately 20.3 kD and a calculated pI of 5.46. The wheat chloroplast Cu/ZnSOD proteins were 80% to 86% identical to the chloroplast Cu/ZnSODs from other plant species (e.g. pea, tomato, and spinach) but had only 60% to 68% sequence identity to cytosolic Cu/ZnSODs (e.g. maize and rice). An identical 47-amino acid peptide was present at the N terminus of both proteins encoded by the SOD1.1 and SOD1.2 cDNAs and is a putative chloroplast transit peptide.
Isolation and Characterization of Mitochondrial Mn Genes
The wheat cDNA library was also screened with a wild tobacco MnSOD cDNA (Bowler et al., 1988). Six positive clones were isolated and sequenced. Four of the cDNAs had complete open reading frames; three were identical (934 bp) and designated SOD3.1 (accession no. U72212), and the fourth (SOD3.2) was 891 bp (accession no. U73172). The poly(A+) tails of both genes were missing, but both had two polyadenylation signals (AATAAA). The two genes were 98% identical in the open reading frames, were 88% identical in the noncoding region, and encoded two nearly identical (98%) MnSOD proteins of 231 amino acids, with a calculated molecular mass of approximately 25.3 kD and a calculated pI of 8.25. The wheat MnSOD proteins were 84% to 88% identical to MnSOD proteins from other monocotyledonous species (e.g. rice and maize) but only 74% to 79% identical to MnSOD proteins from dicotyledonous species (e.g. pea, tobacco, and rubber tree). A putative 27-amino acid mitochondrial transit peptide was present at the N terminus of both proteins encoded by the wheat SOD3.1 and SOD3.2 cDNAs.
Mapping of Cu/ZnSOD (SOD 1) and MnSOD (SOD 3) Genes
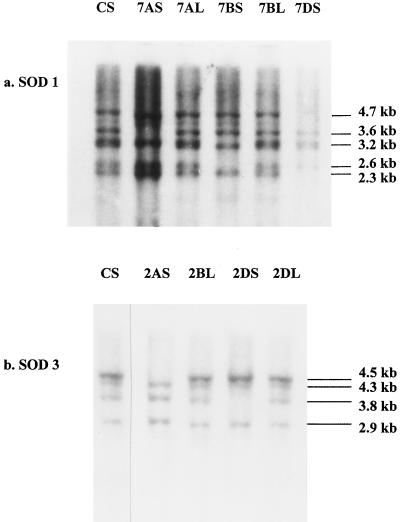
Ditelosomics are euploid lines in which one arm in a given chromosome is missing; they can be used to map a gene to a specific chromosome arm in wheat (Neuman and Hart, 1986). Using the 35 ditelosomic lines that were available for this study, we were able to determine that the chloroplast Cu/ZnSOD genes were located on the long arms of the group-7 chromosomes (Fig. 1a). Restriction fragments were absent in DraI-digested DNA from the ditelosomic lines 7AS (3.6 kb), 7BS (2.6 kb), and 7DS (2.3 and 4.7 kb) compared with the fragment pattern obtained using the 7L ditelosomic lines and cv Chinese Spring DNA. Because the S ditelosomic lines are missing the long arms of the corresponding chromosomes, the missing restriction fragments indicated the presence of these DNA fragments on the long arms.
Figure 1.
Southern analyses of genomic DNA isolated from cv Chinese Spring (CS) wheat ditelosomic lines. Wheat genomic DNA was digested with DraI, separated by agarose-gel electrophoresis, transferred to nylon membranes, probed with chloroplastic Cu/ZnSOD (a) and mitochondrial MnSOD (b) genes, and washed under high-stringency conditions.
When a Southern blot of DraI-digested DNA from group-2 ditelosomic lines was probed with the MnSOD cDNA, fragments were absent in the 2AS (4.5 kb) and 2DS (3.8 kb) lines that were present in the 2L lines and in the cv Chinese spring DNA (Fig. 1b). Although the 2BS line was not available, it is reasonable to conclude that the MnSOD genes are located on the long arms of the homologous group-2 chromosomes.
Differential Expression of Cu/ZnSOD and MnSOD Genes during Drought Stress
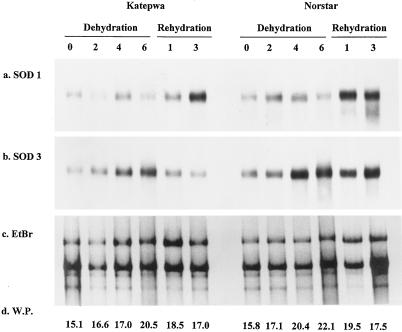
Drought stress was induced by withholding water from greenhouse-grown winter and spring wheat seedlings. A fan was placed near the seedlings during the drought treatment to increase the transpiration rate. MnSOD mRNA increased gradually during the drought period, with maximum transcript accumulation observed in the d-6 samples in both the spring and winter wheat seedlings (Fig. 2; Table I). A decline in the MnSOD mRNA levels occurred when the stress was relieved by rewatering the seedlings in the spring genotype, whereas transcript levels did not decline in the winter seedlings. Although MnSOD genes were drought inducible in both the spring and winter wheat, it is of interest that MnSOD transcript accumulation was higher in the winter wheat cultivar (3-fold increase) than in the spring wheat cultivar (2-fold increase). Cu/ZnSOD did not appear to be drought inducible; however, a 3- to 4-fold increase in Cu/ZnSOD mRNA was noted after rehydration of the drought-stressed plants of both types (Fig. 2). Water potentials of wheat leaves increased during the drought in both spring and winter wheat. However, no correlation was found between the SOD gene expression and the water potentials (Fig. 2).
Figure 2.
Differential expression of wheat chloroplastic Cu/ZnSOD (a) and mitochondrial MnSOD (b) genes during drought stress. Total RNA from spring wheat cv Katepwa and winter wheat cv Norstar after 0, 2, 4, or 6 d of dehydration and 1 or 3 d of rehydration was electrophoresed and transferred to a nylon membrane. The same blot was sequentially hybridized with Cu/ZnSOD 1.1 and MnSOD 3.1 and was washed under high-stringency conditions. EtBr (c) and W.P. (d) represent ethidium bromide-stained gels and water potential (bar) of the fully expanded leaf, respectively.
Table I.
Relative intensity of mRNA transcripts in northern blots during drought stress
| cv Katepwa
|
cv Norstar
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dehydration
|
Rehydration
|
Dehydration
|
Rehydration
|
|||||||||
| d 0 | d 2 | d 4 | d 6 | d 1 | d 3 | d 0 | d 2 | d 4 | d 6 | d 1 | d 3 | |
| SOD 1 | 1.00 | 0.90 | 1.36 | 1.02 | 1.41 | 3.39 | 1.20 | 1.59 | 1.43 | 1.06 | 4.00 | 3.55 |
| SOD 3 | 1.00 | 1.31 | 2.39 | 2.65 | 1.49 | 0.99 | 1.38 | 1.90 | 3.47 | 3.27 | 2.61 | 3.31 |
The relative intensity was expressed as treatment intensity divided by control intensity. Details of the treatments are given in the figure legends.
Differential Expression of Cu/ZnSOD and MnSOD Genes during Cold Stress
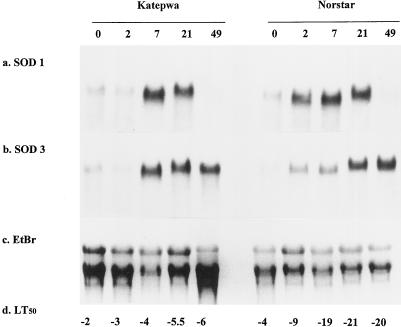
For plants cold acclimated in a controlled environment, transcripts for both MnSOD and Cu/ZnSOD genes increased during the acclimation period (Fig. 3; Table II). MnSOD transcripts gradually increased in the winter wheat seedlings and were maximal after 49 d of low-temperature stress. Maximum levels of MnSOD mRNA were detected within 7 d of cold acclimation in the spring wheat and remained constant during the entire acclimation period.
Figure 3.
Differential accumulation of wheat chloroplastic Cu/ZnSOD (a) and mitochondrial MnSOD (b) genes during cold stress in a controlled environment. Total RNA extracted from spring wheat cv Katepwa and winter wheat cv Norstar seedlings after 0, 2, 7, 21, or 49 d of cold acclimation at a constant day/night temperature of 2°C with a 16-h photoperiod was electrophoresed in 1.2% agarose gel and transferred to a nylon membrane. The same blot was sequentially hybridized with Cu/ZnSOD 1.1 and MnSOD 3.1 and washed under high-stringency conditions. EtBr (c) and LT50 (d) represent ethidium bromide-stained gels and temperatures (°C) that killed 50% of plants, respectively.
Table II.
Relative intensity of mRNA transcripts in northern blots during drought stress and during cold stress in a controlled environment
| cv Katepwa
|
cv Norstar
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| d 0 | d 2 | d 7 | d 21 | d 49 | d 0 | d 2 | d 7 | d 21 | d 49 | |
| SOD 1 | 1.00 | 0.92 | 4.84 | 4.47 | 0.37 | 1.20 | 4.17 | 4.85 | 4.56 | 0.73 |
| SOD 3 | 1.00 | 0.78 | 3.47 | 3.22 | 1.49 | 1.38 | 1.90 | 3.47 | 3.27 | 2.61 |
The relative intensity was expressed as treatment intensity divided by control intensity. Details of the treatments are given in the figure legends.
A major difference was noted in the expression of Cu/ZnSOD genes compared with MnSOD genes in response to low temperature. Cu/ZnSOD mRNA increased and attained maximal levels after 21 d of exposure to low temperature; however, the transcripts were not detected after 49 d of cold treatment in either the winter or the spring wheat cultivar (Fig. 3). In contrast, the MnSOD transcripts remained high for both cultivars for 49 d (Fig. 3; Table II).
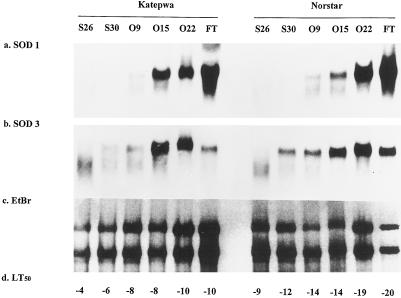
Under natural conditions, transcripts of the Cu/ZnSOD genes gradually increased and reached a maximum level on October 22 (Fig. 4). The seedlings at this date were not exposed to a natural frost; however, the night temperature already approached 0°C. MnSOD genes in winter wheat responded faster to the natural acclimating conditions than genes in spring wheat. The levels of the transcripts were maximum on October 22 in both the spring and winter wheat seedlings. The MnSOD and Cu/ZnSOD genes responded differently to the artificially induced freeze-thaw cycles. A large 1400% to 1500% increase in Cu/Zn mRNA was detected in both the spring and winter wheat seedlings after three repeated freeze-thaw cycles (Fig. 4; Table III). In contrast, the expression pattern of the MnSOD genes differed between spring and winter wheat. MnSOD mRNA levels declined after three repeated freeze-thaw cycles in the spring wheat, whereas the level of MnSOD transcripts remained relatively unchanged in the winter wheat cultivar (Fig. 4, lanes O22 and FT).
Figure 4.
Northern analyses of wheat chloroplastic Cu/ZnSOD (a) and mitochondrial MnSOD (b) genes during cold stress under natural conditions. Total RNA extracted from spring wheat cv Katepwa and winter wheat cv Norstar seedlings harvested on September 26 (S26), September 30 (S30), October 9 (O9), October 15 (O15), and October 22 (O22) or freeze-thaw plants harvested on October 22 (FT) was separated by 1.2% agarose-gel electrophoresis and transferred to a nylon membrane. The same blot was sequentially hybridized with Cu/ZnSOD 1.1 and MnSOD 3.1 and washed under high-stringency conditions. EtBr (c) and LT50 (d) represent ethidium bromide-stained gels and temperatures (°C) that killed 50% of plants, respectively.
Table III.
Relative intensity of mRNA transcripts in northern blots during drought stress and cold stress under natural conditions
| cv Katepwa
|
cv Norstar
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S26a | S30 | O9 | O15 | O22 | FT | S26 | S30 | O9 | O15 | O22 | FT | |
| SOD 1 | 1.00 | 0.93 | 1.02 | 6.33 | 6.69 | 13.96 | 1.17 | 0.99 | 1.59 | 2.43 | 10.28 | 15.28 |
| SOD 3 | 1.00 | 1.19 | 1.58 | 5.99 | 5.37 | 1.97 | 0.91 | 2.89 | 2.84 | 5.48 | 7.05 | 4.39 |
The relative intensity was expressed as treatment intensity divided by control intensity. Details of the treatments are given in the figure legends.
S26, September 26; S30, September 30; O9, October 9; O15, October 15; O22, October 22; and FT, freeze-thawed plants harvested on October 22.
Freezing tolerance was determined as LT50. Although there was a 10°C to 14°C difference in freezing tolerance between the spring and winter wheat cultivars, there was no correlation between LT50 and SOD gene expression for seedlings hardened either by artificial or natural conditions (Figs. 3 and 4).
DISCUSSION
Analysis of the wheat MnSOD and Cu/ZnSOD cDNAs showed a high degree of sequence identity with equivalent SOD proteins from other plant species. The analysis also revealed a highly conserved putative transit-peptide sequence between the different MnSOD genes in wheat. A highly conserved putative transit peptide was also present in the Cu/ZnSOD genes.
Mapping a gene to a specific chromosome is important for plant genetics and breeding. Genes of interest that do not result in a clear phenotypic or biochemical trait in breeding lines can be followed and manipulated if the chromosomal segments on which they are located are known. The ditelosomic wheat lines are an excellent tool for mapping the location of a gene to a specific chromosome arm either through DNA-blot, isozyme, or protein-blot analyses. The fact that wheat is hexaploid permits the removal of an entire chromosome arm on both homologous chromosomes. For example, the removal of the long arm on both group-6 chromosomes of the A genome does not significantly affect the viability of the ditelosomic line that is formed. Using these lines we were able to map the MnSOD gene to the long arms of the group-2 chromosomes and the Cu/ZnSOD gene to the long arms of the group-7 chromosomes. Given the high-stringency conditions used in the mapping study and the absence of missing fragments in the other ditelosomic groups (data not shown) probed with the Cu/ZnSOD cDNA, it is not likely that the missing fragment on the group-7 chromosomes (Fig. 1) corresponded to cytosol Cu/ZnSOD genes.
Our mapping studies also support the results obtained by other researchers. Netsvetaev and Krestinkov (1993) mapped a Cu/ZnSOD gene to barley chromosome 1 using isozyme analysis. Barley chromosome 1 is homologous to chromosome 7A, 7B, and 7D in hexaploid wheat (Laurie et al., 1992). Neuman and Hart (1986) used SOD isozyme analysis to map the MnSOD gene in wheat and concluded that the MnSOD genes were located on the group-2 chromosomes.
Mitochondrial MnSOD activity parallels the activity of the respiratory electron-transport chain of mitochondria in wild tobacco (Bowler et al., 1989). Respiration increases dramatically during stress and the production of superoxide radicals is rigorously coupled with the rate of mitochondrial respiration (Zhu and Scandalios, 1993). Therefore, it was not surprising to note that MnSOD expression increased with increasing drought stress and decreased after rehydration in wheat seedlings. It is unclear why a difference in the kinetics of MnSOD gene expression was noted between the spring and winter wheat cultivars. However, it may be related to the increased potential of winter wheat to partially cold acclimate during dehydration (Siminovitch and Cloutier, 1982) or it may be related to the higher stress tolerance of winter cereals.
In contrast to the results with the MnSOD genes, the Cu/ZnSOD genes did not respond to drought, a finding that is consistent with the report by Perl-Treves and Galun (1991). It was also interesting that the MnSOD and Cu/ZnSOD genes responded differently to rehydration (Fig. 2). It is not clear why drought did not enhance the expression of chloroplast Cu/ZnSOD genes. It is possible that the inhibition of photosynthesis, which occurs during drought, may result in reduced production of superoxide radicals in the chloroplast. After the drought stress is relieved, photosynthesis increases, and superoxide radicals are again generated because of leakage of electrons from PSI and Fd (Asada and Takahashi, 1987).
Both MnSOD and Cu/ZnSOD genes were induced in both winter and spring plants subjected to cold-acclimating conditions either in controlled environments or in the field (Figs. 3 and 4). As was observed in response to drought, the induction of MnSOD genes was more rapid in the seedlings of the winter wheat than the spring wheat cultivar under both growth conditions. This similarity in response to the different stresses may be related to the fact that cold acclimation results in cellular dehydration (Malone, 1993). The increased expression of MnSOD genes in seedlings exposed to various stresses may in part explain the difference in the cold-acclimation capabilities of spring and winter cereals. Overexpression of SOD genes in transgenic plants has been reported to increase chilling or freezing tolerance in several species (Gupta et at al., 1993; McKersie et al., 1993; Van Camp et al., 1996).
The Cu/ZnSOD genes were induced by exposure to cold temperature in seedlings of both spring and winter wheat in contrast to the absence of gene expression in response to drought. A possible explanation may be that cold-acclimating conditions did not result in the cessation of photosynthesis and, thus, superoxide radicals were still being generated. After 21 d of acclimation cellular dehydration levels may result in the shutdown of photosynthesis in the controlled environments and explain the decrease in Cu/ZnSOD gene expression after this time. In field-acclimated seedlings the onset of Cu/ZnSOD transcript accumulation was slower compared with that in seedlings acclimated in a controlled environment (Fig. 4, lane O22).
In this study a major difference between MnSOD and Cu/ZnSOD gene expression was the response of these genes to a repeated freeze-thaw cycle. A freeze-thaw cycle near the end of the acclimation period is known to enhance the freezing tolerance of winter cereals (Gusta et al., 1982). The winter wheat seedlings in later October and early November had an LT50 of −20°C when exposed to three repeated freeze-thaw cycles. Therefore, it is highly unlikely that a frost of −3°C would be injurious to the thylakoid membrane. Although the spring wheat cultivar can tolerate −8°C when cooled at 2°C h−1, seedlings are injured after repeated exposure to frosts of −4°C (L.V. Gusta, unpublished data). This would result in an increased production of superoxide radicals and may explain the increase in Cu/ZnSOD gene expression. Tsang et al. (1991) observed that chloroplastic FeSOD gene expression increased during chilling treatment of wild tobacco plants, and FeSOD mRNA levels further increased after the plants were returned to their normal growth temperature. However, in contrast to our results, Tsang et al. (1991) found that the expression of MnSOD and cytosol Cu/ZnSOD genes were unaffected by chilling but that SOD gene expression increased 5- to 10-fold after the plants were returned to their normal growth temperature.
These differences in response may reflect the difference in the cold hardiness of the two species. Wild tobacco is chilling sensitive and has little or no freezing tolerance, whereas spring wheat is moderately freezing tolerant and winter wheat is very cold hardy. The presence of MnSOD transcripts in the winter wheat plants after the freeze-thaw treatments represents the only significant difference in the expression pattern of either SOD gene between spring and winter wheat. Whether the persistence of MnSOD mRNA in winter varieties is involved in the increased freezing tolerance inherent in winter cultivars is unknown. However, the increase in stress tolerance in several transgenic plants resulting from the overexpression of other SOD genes in a number of species suggests that it could be important (Gupta et at al., 1993; McKersie et al., 1993; Van Camp et al., 1996).
The accession numbers for the sequences reported in this article are U69536 (Cu/ZnSOD 1.1), U69632 (Cu/ZnSOD 1.2), U72212 (MnSOD 3.1), and U73172 (MnSOD 3.2).
Abbreviations:
- LT50
median lethal temperature (causing 50% death)
- SOD
superoxide dismutase
Footnotes
This research was partially supported by operating and strategic grants from the Natural Sciences and Engineering Research Council of Canada.
LITERATURE CITED
- Asada K. Production and action of active oxygen species in photosynthetic tissues. In: Foyer CH, Mullineaux PM, editors. Causes of Photooxidative Stress and Amelioration of Defense System in Plants. Boca Raton, FL: CRC Press; 1994. pp. 77–104. [Google Scholar]
- Asada K, Takahashi M. Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osmond CB, Arntzen CJ, editors. Photoinhibition. Amsterdam: Elsevier Science Publishers; 1987. pp. 227–287. [Google Scholar]
- Badger MR. Photosynthetic oxygen exchange. Annu Rev Plant Physiol. 1985;36:27–53. [Google Scholar]
- Bowler C, Alliotte T, Bulcke M, Bauw G, Vandekerckhove J, Van Montagu M, Inze D. A plant manganese superoxide dismutase is efficiently imported and correctly processed by yeast mitochondria. Proc Natl Acad Sci USA. 1989;86:3237–3241. doi: 10.1073/pnas.86.9.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C, Alliotte T, De Loose M, Van Montagu M, Inze D. The induction of manganese superoxide dismutase in response to stress in Nicotiana plumbaginifolia. EMBO J. 1988;8:31–38. doi: 10.1002/j.1460-2075.1989.tb03345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C, Slooten L, Vandenbranden S, Rycke RD, Botterman J, Sybesma C, Montagu M, Inze D. Manganese superoxide dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants. EMBO J. 1991;10:1723–1732. doi: 10.1002/j.1460-2075.1991.tb07696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawn K, Fridovich I. DNA strand scission by enzymically generated oxygen radicals. Arch Biochem Biophys. 1981;206:414–419. doi: 10.1016/0003-9861(81)90108-9. [DOI] [PubMed] [Google Scholar]
- Buonaurio R, Della Torre G, Montalbini P. Soluble superoxide dismutase (SOD) in susceptible and resistant host-parasite complexes of Phaseolus vulgaris and Uromyces phaseoli. Physiol Mol Plant Pathol. 1987;31:173–184. [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol. 1986;41:35. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- Fucci L, Oliver CN, Coon MJ, Stadtman ER. Inactivation of metabolic enzymes by mixed-function oxidation reaction: possible implication in protein turnover and ageing. Proc Natl Acad Sci USA. 1983;80:1521–1525. doi: 10.1073/pnas.80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AS, Webb RP, Holaday AS, Allen RD. Overexpression of superoxide dismutase protects plants from oxidative stress. Induction of ascorbate peroxidase in superoxide dismutase-overexpressing plants. Plant Physiol. 1993;103:1067–1073. doi: 10.1104/pp.103.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusta LV, Fowler DB, Tyler NJ (1982) Factors influencing hardening and survival in winter wheat. In PH Li, A Sakai, eds, Plant Cold Hardiness and Freezing Stress: Mechanism and Crop Implications, Vol 2. Academic Press, New York, pp 23–40
- Houde M, Danyluk J, Laliberte JF, Rassart E, Dhindsa RS, Sarhan F. Cloning, characterization and expression of a cDNA encoding a 50-kiladalton protein specifically induced by cold acclimation in wheat. Plant Physiol. 1992;99:1381–1387. doi: 10.1104/pp.99.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MIS, Hetherington AM, Crawford RMM. Lipid peroxidation: a factor in anoxia intolerance in Iris species. Phytochemistry. 1983;22:1145–1147. [Google Scholar]
- Kaminaka H, Morita S, Yohoi H, Masumura T, Tanaka H. Molecular cloning and characterization of a cDNA for plastidic copper/zinc-superoxide dismutase in rice (Oryza sativa L.) Plant Cell Physiol. 1997;38:65–69. doi: 10.1093/oxfordjournals.pcp.a029086. [DOI] [PubMed] [Google Scholar]
- Kendall EJ, McKersie BD. Free radical and freezing injury to cell membranes of winter wheat. Physiol Plant. 1989;76:86–94. [Google Scholar]
- Koch E, Slusarenko AJ. Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell. 1990;2:437–445. doi: 10.1105/tpc.2.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurepa J, Herouart D, Van Montagu MV, Inze D. Differential expression of Cu/Zn- and Fe-superoxide dismutase genes of tobacco during development, oxidative stress, and hormonal treatments. Plant Mol Biol. 1997;38:463–470. doi: 10.1093/oxfordjournals.pcp.a029190. [DOI] [PubMed] [Google Scholar]
- Landgraf P, Doeger M, Ohmann E, Tschiersch H. Light stress and reactive oxygen species: consequences for photosynthesis in Euglena gracilis. In: Mathis P, editor. Photosynthesis: From Light to Biosphere, Vol IV. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 465–468. [Google Scholar]
- Laurie DA, Snape JW, Gale MD (1992) DNA marker techniques for genetic analysis in barley. In PR Shewry, ed, Barley: Genetics, Biochemistry, Molecular Biology and Biotechnology. CAB International, Wallingford, UK, pp 115–132
- Leprince O, Deltour R, Thorpe PC, Atherton NM, Hendry GAF. The role of free radical and radical processing systems in loss of desiccation tolerance in germinating maize (Zea mays L.) New Phytol. 1990;116:573–580. [Google Scholar]
- Malan C, Greyling MM, Gressel J. Correlation between Cu/Zn superoxide dismutase and glutathione reductase and environmental and xenobiotic stress tolerance in maize inbreds. Plant Sci. 1990;69:157–166. [Google Scholar]
- Malone M. Rapid inhibition of leaf growth by root cooling in wheat: kinetics and mechanism. J Exp Bot. 1993;44:1663–1669. [Google Scholar]
- McKersie BD, Chen Y, de Beus M, Bowley SR, Bowler C, Inze D, D'Halluin, Botterman J. Superoxide dismutase enhances tolerance of freezing stress in transgenic alfalfa (Medicago sativa L.) Plant Physiol. 1993;103:1155–1163. doi: 10.1104/pp.103.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead JF (1976) Free radical mechanisms of lipid damage and consequences for cellular membranes. In WA Pryor, ed, Free Radicals in Biology, Vol 1. Academic Press, New York, pp 51–68
- Montalbini P, Buonaurio R. Effect of tobacco mosaic virus infection on levels of soluble superoxide dismutase (SOD) in Nicotiana tabacum and Nicotiana glutinosa leaves. Plant Sci Lett. 1986;47:135–143. [Google Scholar]
- Netsvetaev VP, Krestinkov IS. Chromosomal position of the superoxide dismutase locus, Sod1 (=Sod B), in barley. Barley Genet Newsl. 1993;22:44–45. [Google Scholar]
- Neuman PR, Hart GE. Genetic control of the mitochondrial form of superoxide dismutase in hexaploid wheat. Biochem Genet. 1986;24:435–446. doi: 10.1007/BF00499098. [DOI] [PubMed] [Google Scholar]
- Niwa T, Yamaguchi H, Yano K (1977) Radioprotection by superoxide dismutase: reduction of oxygen effect. In O Higaishi, K Asada, eds, Biochemical and Medical Effects of Active Oxygen. University of Tokyo Press, Tokyo, pp 209–225
- O'Conner BJ, Reaney MJT, Gusta LV. A practical method of assessing the freezing tolerance of large populations of field-grown winter cereals. Can J Plant Sci. 1993;75:149–153. [Google Scholar]
- Perl-Treves R, Galun E. The tomato Cu,Zn superoxide dismutase genes are developmentally regulated and respond to light and stress. Plant Mol Biol. 1991;17:745–760. doi: 10.1007/BF00037058. [DOI] [PubMed] [Google Scholar]
- Price AH, Hendry GAF. Iron-catalyzed oxygen radical formation and its possible contribution to drought damage in nine native grasses and three cereals. Plant Cell Environ. 1991;14:477–484. [Google Scholar]
- Procunier JD, Xu J, Kasha KS. A rapid reliable DNA extraction method for higher plants. Barley Genet Newsl. 1990;20:74–75. [Google Scholar]
- Rich PR, Bonner WD., Jr The sites of superoxide anion generation in higher plant mitochondria. Arch Biochem Biophys. 1978;188:205–213. doi: 10.1016/0003-9861(78)90373-9. [DOI] [PubMed] [Google Scholar]
- Robertson AJ, Reaney MJT, Wilen RW, Lamb N, Abrams SR, Gusta LV. Effects of abscisic acid metabolites and analogs on freezing tolerance and gene expression in bromegrass (Bromus inermis Leyss.) cell cultures. Plant Physiol. 1994;105:823–830. doi: 10.1104/pp.105.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto A, Nosaka Y, Tanaka K. Cloning and sequence analysis of a complementary DNA for manganese-superoxide dismutase from rice (Oryza sativa L.) Plant Physiol. 1993;103:1477–1478. doi: 10.1104/pp.103.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senaratna T, Mackay CE, McKersie BD, Fletcher RA. Unicanazole-induced chilling tolerance in tomato and its relationship to antioxidant content. J Plant Physiol. 1988;133:56–61. [Google Scholar]
- Senaratna T, McKersie BD, Stinson RH. Simulation of dehydration injury to membranes from soybean axes by free radicals. Plant Physiol. 1985;77:472–477. doi: 10.1104/pp.77.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch D, Cloutier K. Twenty-four hour induction of freezing and drought tolerance in plumules of winter rye seedlings by desiccation stress at room temperature in dark. Plant Physiol. 1982;69:250–259. doi: 10.1104/pp.69.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang EWT, Bowler C, Herouart D, Van Camp W, Villarroel R, Genetello C, Van Montagu M, Inze D. Differential regulation of superoxide dismutases in plants exposed to environmental stress. Plant Cell. 1991;3:783–792. doi: 10.1105/tpc.3.8.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler NJ, Fowler DB, Gusta LV. The effects of salt stress on the cold hardiness of winter wheat. Can J Plant Sci. 1981;61:543–548. [Google Scholar]
- Van Camp W, Capiau K, Van Montagu M, Inze D, Slooten L. Enhancement of oxidative stress tolerance in transgenic tobacco plants overproducing Fe-superoxide dismutase in chloroplasts. Plant Physiol. 1996;112:1703–1714. doi: 10.1104/pp.112.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Toai T, Bolles CS. Postanoxia injury in soybean (Glycine max) seedling. Plant Physiol. 1991;97:588–592. doi: 10.1104/pp.97.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RR, Naylor AW. Chilling-enhanced photooxidation. Evidence for the role of single oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol. 1987;83:278–282. doi: 10.1104/pp.83.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Scandalios JG. Maize mitochondrial manganese superoxide dismutases are encoded by a differentially expressed multigene family. Proc Natl Acad Sci USA. 1993;90:9310–9314. doi: 10.1073/pnas.90.20.9310. [DOI] [PMC free article] [PubMed] [Google Scholar]