Abstract
Background
Increasing evidence indicates that long noncoding RNAs (LncRNAs) play a key role in multiple pathological processes. It has been shown that LncRNA steroid receptor RNA activator (SRA) is elevated in peripheral blood of patients with polycystic ovary syndrome (PCOS). The aim of this study was to assess the effect of elevated LncRNA SRA on ovarian granular cells of mice in vitro.
Material/Methods
We firstly isolated granular cells from mouse ovaries and over-expressed the LncRNA SRA by means of lentiviral transfection in this cell line. Then, we assessed the effects of LncRNA SRA on granular cells through real-time PCR, CCK-8 assay, flow cytometry, Hoechst staining, and Western blot assay.
Results
We demonstrated that elevated LncRNA SRA stimulated cell growth, changed distribution of cell cycle phases with increase of Cyclin B, Cyclin E, and Cyclin D1, and inhibited cell apoptosis with up-regulation of bcl2 and down-regulation of bax, cleaved-caspase 3, and cleaved-PARP. Moreover, the contents of estradiol (E2) and progesterone (PG) and expressions of their key enzymes (CYP19A1 and CYP11A1) were up-regulated following over-expression of LncRNA SRA.
Conclusions
Taken together, our results indicate that abnormal LncRNA SRA may be a risk factor for evoking PCOS.
MeSH Keywords: Apoptosis; Cell Proliferation; Polycystic Ovary Syndrome; RNA, Long Noncoding
Background
Polycystic ovarian syndrome (PCOS), one of most common endocrine disorders among women of childbearing age, has various clinical symptoms, including abnormal menstruation, infertility, hyperandrogenism with subsequent hirsutism, insulin resistance (IR), acne, weight gain, and cystic ovaries [1,2]. PCOS is thought to be a result of an interaction between genetic factors and environmental factors [3]. Although the etiology of PCOS is very complex and not yet fully understood, it has been reported that hormonal abnormalities and ovarian dysfunction play important role in PCOS development [4,5]. It could be valuable to explore new targets that may mediate growth and hormone secretion of ovarian cells.
Long noncoding RNAs (LncRNAs) are widely defined as RNA molecules greater than 200 nt in length, and participate in a variety of pathological processes, including cancers [6,7], autism spectrum disorders [8], inflammatory responses [9], cardiovascular disease [10], and neurodegenerative diseases [11]. LncRNA steroid receptor RNA activator (SRA) was originally identified as an LncRNA that coordinates the functions of various transcription factors and enhances steroid nuclear receptor-dependent gene expression, such as estrogen receptor alpha (ERα) and androgen receptor (AR) [12,13]. A previous study has shown that LncRNA SRA is elevated in peripheral blood leukocytes of patients with PCOS, suggesting the association of LncRNA SRA with PCOS [14]. Nevertheless, the role of LncRNA SRA in occurrence of PCOS is not clear and needs further study.
In the present study, to explore whether LncRNA SRA plays a role in the development of PCOS, we firstly over-expressed the LncRNA SRA in granular cells derived from mouse ovaries and assessed its effects on cell proliferation, apoptosis, and estradiol (E2) and progesterone (PG) secretions. Our findings demonstrate a new function of LncRNA SRA in ovarian granular cells.
Material and Methods
Animal
Female KM mice, 21 days old, were purchased from the Animal Experimental Center of Xi’an Jiaotong University and raised with a 12-hour light: 12-hour dark cycle and in a temperature-controlled environment (22±1°C), and food and water were available ad libitum. The study was approved by the Animal Care and Usage committee of Xi’an Jiaotong University and was in accordance with the National Institutes of Health guidelines regulating the care and use of animals.
Isolation and culture of ovarian granular cells
The mice were anesthetized by intraperitoneal injection with 3% pentobarbital sodium (100 mg/kg). Then, the ovaries, separated from the mouse under sterile conditions, was washed twice with PBS and cut into pieces. Fifteen ml of type I collagenase (0.1%) (Biosharp, USA) was used to digest the sample at 37°C for 5 min. Thereafter, cells were selected through a 100-mesh sieve. The residual tissue was digested and selected repeatedly until the tissue was completely digested. Afterwards, discard of supernatant, addition of RPMI-1640 medium (Gibco, Grand Island, NY, USA) with 10% fetal bovine serum (Hyclone, Logan, UT, USA). The cells were cultured in an incubator at 37°C with atmosphere of 5% CO2.
Cell transfection
To over-express the LncRNA SRA, the full-length sequence of LncRNA SRA was subcloned into the lentiviral vector LV5 (GenePharma, Shanghai, China) and an empty vector as a control. The harvested viruses were employed to infect ovarian granular cells for over-expression of LncRNA SRA. The infection efficiency was confirmed by real-time PCR.
Immunofluorescence
The cells were cultured on a glass slide and immobilized using 4% paraformaldehyde for 15 min. The fixed cells were permeabilized with 0.1% Triton X-100 for 30 min. Then, goat serum was used to block nonspecific binding sites for 15 min. The cells were incubated with primary antibody against follicle-stimulating hormone receptor (FSHR) (22665-1-AP, Proteintech, Wuhan, China) at 4°C overnight. Afterwards, Cy3-labeled goat anti-rabbit IgG (A0516, Beyotime, Shanghai, China) was used to probe the primary antibody for 1 h at room temperature. Thereafter, cell nuclei were counterstained with DAPI. The slides were incubated with quenching agent and were imaged with a fluorescence microscope (BX-53; Olympus, Tokyo, Japan).
Real-time PCR
Total RNAs were extracted with the high purity total RNA extraction kit (BioTeke, Beijing, China) according to the manufacturer’s instructions and reverse-transcribed into cDNAs with the super M-MLV reverse transcriptase (BioTeke). Then, real-time quantitative PCR was conducted to analyze SRA, CYP11A1, CYP19A1, and β-actin expression levels. The primer sequences were synthesized as follows: SRA forward, CAAACGCACTCCCCTTACTA, reverse, TGTTCCAGAGGTCTCAGCAC; CYP11A1 forward, TGGGTGCCGTGGATAACAG, reverse, ACAGATGGTCGCAGATACTAAA; CYP19A1 forward, GGATGACGTAA TTGACGGCTA, reverse, CACCTGGAATCGTCTCAAAA; β-actin forward, CTGTG CCCATCTACGAGGGCTAT, reverse, TTTGATGTCACGCACGATTTCC. The expression of genes was calculated based on 2−ΔΔCt method and was normalized to the loading control, β-actin.
CCK-8 assay
The cells were seeded into 96-well plates at a density of 4×103 cells per well in quintuplicate and incubated for 0, 24, 48, and 72 h at 37°C. At indicated time points, the supernatant was replaced by 100 μl of complete medium, 10 μl of CCK-8 solution was added to each well, and the plates were incubated at 37°C for another 1 h. Then, the absorbance value was measured at 450 nm with a microplate reader (ELx-800, Biotek Instruments, Winooski VT, USA).
Flow cytometry detection of cell cycle
Cell cycle was analyzed with a commercial kit (Beyotime) according to the manufacturer’s instructions. Briefly, the cells were collected and fixed with pre-cooling 70% alcohol for 2 h at 4°C. After discarding the supernatant, the precipitate was washed with PBS and recentrifuged. We used 500 μl of staining buffer to resuspend cells. We added a total of 25 μl propidium iodide staining solution followed by 10 μl RNase A to the resuspended cells and mixed them well, followed by incubation for 30 min at 37°C away from light. Then, the samples were detected immediately with a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Flow cytometry detection of cell apoptosis
Cell apoptosis was evaluated using a commercial kit (Wanlei, Shenyang, Liaoning Province, China) according to the manufacturer’s instructions. The cells were centrifuged and resuspended in 500 μl binding buffer with sufficient mixing. Then, 5 μl of Annexin V-Light650 and 10 μl propidium iodide were added and mixed well, and the mixture was allowed to react for 15 min at room temperature away from light. Afterwards, the samples were detected with a flow cytometer (BD Biosciences).
Hoechst stain
For Hoechst stain, the cells cultured on the glass slide were washed with PBS and fixed with 4% paraformaldehyde for 20 min. After discarding fixative and washing cells with PBS, 0.5 ml Hoechst staining solution was applied to stain cells for 5 min. After adding anti-quenching agent and covering with coverslips, the slides were observed with a fluorescence microscope (BX-53).
Western blot
Total protein was extracted from samples using RIPA lysis buffer (Beyotime). Protein concentration was determined with a BCA Protein Assay Kit (Beyotime). Protein was separated through SDS-PAGE, transferred into PVDF membranes, and blocked with 5% skim milk for 1 h. Then, the membranes were incubated with primary antibodies against Cyclin B (bs-23016R, Bioss, China), Cyclin E (bs-0573R, Bioss, Chin), Cyclin D1 (BA0770, Boster, China), bcl-2 (BA0412, Boster, China), bax (D120073, Shenggong, China), cleaved-caspase-3 (#9661, CST, USA), cleaved-PARP (#9548, CST, USA), and β-actin (bsm-33139M, Bioss, China) at 4°C overnight. Afterwards, horseradish peroxidase-conjugated secondary antibodies were used to probe the primary antibodies. The blots were visualized using enhanced chemiluminescence (Beyotime) with a gel imaging system (WD-9413B, Beijing Liuyi, China). The proteins were quantified and normalized to the corresponding internal control (β-actin).
Detection of E2 and PG
The levels of E2 and PG were detected using 2 commercially available ELISAs (Uscn Life Science Inc., Wuhan, Hubei Province, China), according to the manufacturer’s directions.
Statistical analysis
We used the t test and two-factor variance analysis for difference analysis with GraphPad Prism 5.0 software (GraphPad, San Diego, CA, USA). The data are presented as mean ± standard deviation (SD). Each experiment was repeated 3 times. A P value less than 0.05 was considered statistically significant and are shown as follows: “*” indicates less than 0.05, “**” indicates less than 0.01, and “***” indicates less than 0.001.
Results
Over-expression of LncRNA SRA in ovarian granular cells of mice
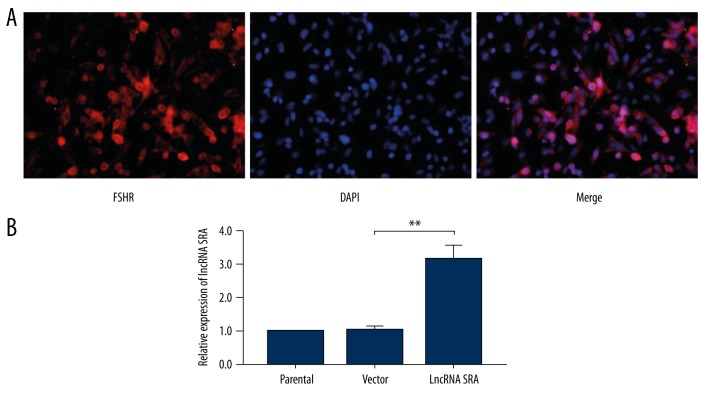
To investigate whether LncRNA SRA plays a role in development of PCOS, we separated ovarian granular cells from the mice, and detected the expression of FSHR through immunofluorescence (Figure 1A), which is the specific maker of granular cells in the ovary. Then, we up-regulated LncRNA SRA in ovarian granular cells using lentiviral transfection. As shown in Figure 1B, the level of LncRNA SRA was significantly increased after transfection compared with in the control group.
Figure 1.
Up-regulation of LncRNA SRA in ovarian granular cells of mice. (A) Identification of ovarian granular cells. (B) Real-time PCR analysis of LncRNA SRA expression. Data shown as mean ±SD. ** P<0.01.
Effects of elevated LncRNA SRA on cell proliferation
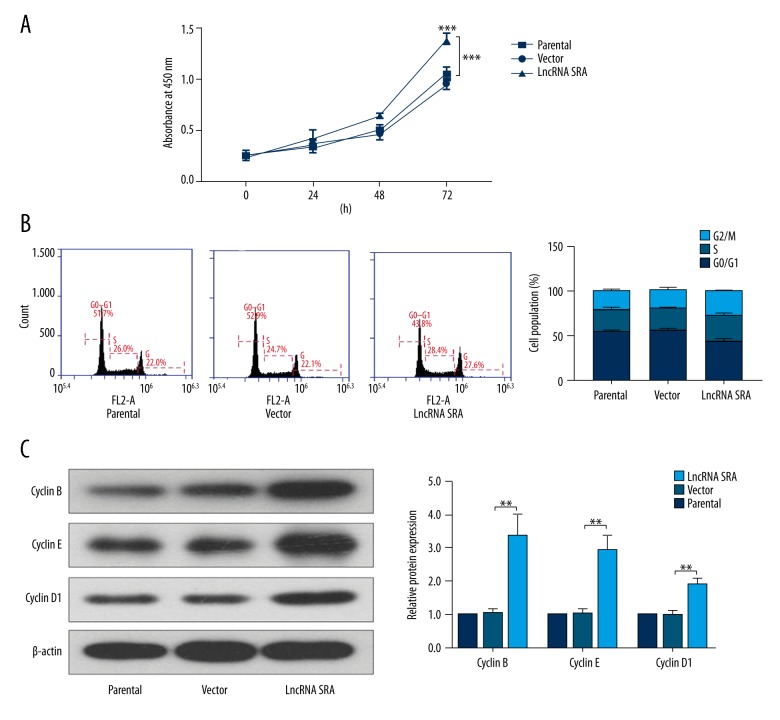
Next, we evaluated the functional role of LncRNA SRA in ovarian granular cells. As shown in Figure 2A, the result of CCK-8 assay clearly indicated an enhancement in proliferative capacity of ovarian granular cells after over-expression of LncRNA SRA. Moreover, the distribution of cell cycle phases in LncRNA SRA-elevated granular cells was altered with flow cytometry, as evidence by the decrease of G0/G1 phase compared with those of control cells (Figure 2B). Western blot assay showed that the expression levels of Cyclin B, Cyclin E, and Cyclin D1 were up-regulated following transfection of LncRNA SRA.
Figure 2.
Up-regulation of LncRNA SRA promoted cell proliferation. (A) Cell proliferation ability was determined by CCK-8 assay. (B) Cell cycle was assessed by flow cytometry. (C) Western blot analysis of Cyclin B, Cyclin E, and Cyclin D1 expression. Data shown as mean ±SD. ** P<0.01; *** P<0.001.
Effects of elevated LncRNA SRA on cell apoptosis
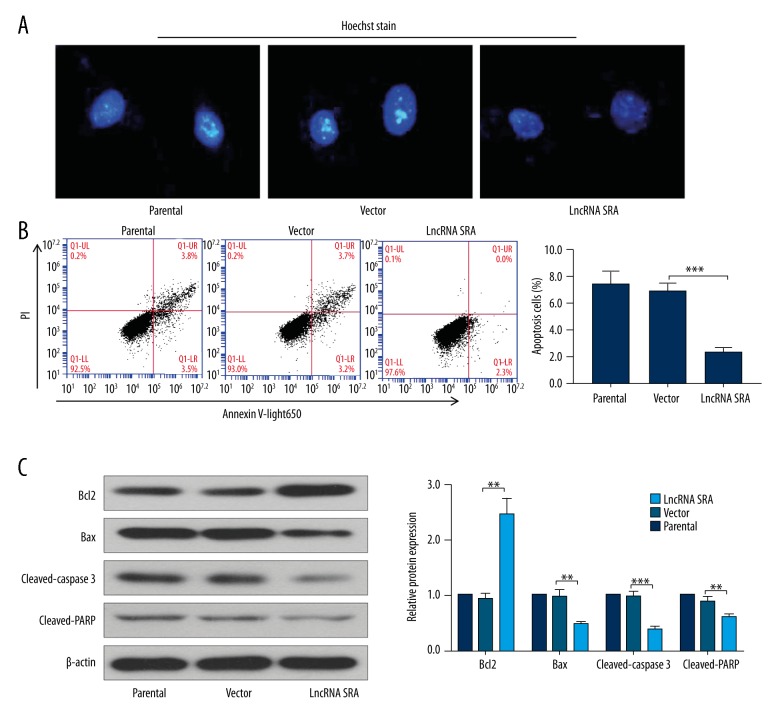
We also explored the impact of LncRNA SRA on cell apoptosis. Cell morphology was evaluated by Hoechst staining. As shown in Figure 3A, apoptosis was distinctly inhibited in LncRNA SRA-elevated cells compared with control cells. The result of flow cytometry was similar to that of Hoechst staining, showing that the number of apoptotic cells was significantly reduced after over-expression of LncRNA SRA (Figure 3B). At the molecular level, elevated LncRNA SRA promoted anti-apoptosis protein of bcl2 expression, and restrained apoptosis proteins of bax, cleaved-caspase 3, and cleaved-PARP expressions (Figure 3C).
Figure 3.
Up-regulation of LncRNA SRA inhibited cell apoptosis. Hoechst staining (A) and flow cytometry (B) were performed to evaluate cell apoptosis. (C) The expression levels of bcl2, bax, cleaved-caspase 3, and cleaved-PARP were determined by Western blotting. Data shown as mean ±SD. ** P<0.01; *** P<0.001.
Effects of elevated LncRNA SRA on the secretion of E2 and PG
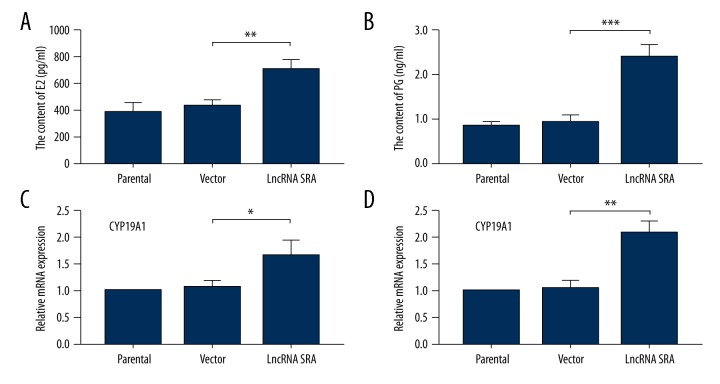
We further determined whether LncRNA SRA can regulate hormone secretion of ovarian granular cells. As shown in Figure 4A and 4B, the contents of E2 and PG were clearly increased following up-regulation of LncRNA SRA versus that of the control group. Real-time PCR assay showed that CYP19A1 and CYP11A1, 2 key enzymes for production of E2 and PG, respectively, were also significantly increased in LncRNA SRA-up-regulated cells compared to those in the control group (Figure 4C, 4D).
Figure 4.
Up-regulation of LncRNA SRA stimulated secretion of E2 and PG. The contents of E2 (A) and PG (B) were detected by commercially available ELISAs. The mRNA expression levels of CYP19A1 (C) and CYP11A1 (D) were assessed using real-time PCR. Data shown as mean ±SD. * P<0.05; ** P<0.01; *** P<0.001.
Discussion
The role of LncRNA SRA has been extensively investigated in a variety of physiological and pathological processes in previous studies, including breast cancer [15], post-pubertal mammary gland [16], myogenic differentiation [17], and hepatic steatosis [13]. The expression level of LncRNA SRA in PCOS patients is significantly up-regulated, but its function and the molecular mechanism involved in PCOS are still unclear [14]. In the present study, we report a new finding that up-regulation of LncRNA SRA promotes cell proliferation, disturbs cell cycle distribution, inhibits cell apoptosis, and stimulates the secretion of E2 and PG.
Granulosa cells, the main functional cells in the ovaries, are involved in steroid secretion and the development of follicles [18]. Ovary enlargement, hormone imbalance, and abnormal follicles are features of PCOS [19–21]. In the present study, to explore whether LncRNA SRA plays a role in the progression of PCOS, we isolated ovarian granular cells from the mice, based on previous research [22]. The results of functional experiments suggested that increased LncRNA SRA promotes cell growth and inhibits cell apoptosis. Previous studies reported that the PCOS model exhibited elevated serum E2 and PG [23,24], and excess estrogen in turn can lead to PCOS [21]. Our study demonstrated that E2 and PG levels, and CYP19A1 and CYP11A1 expression (2 key proteins for their synthesis) [25,26], were up-regulated after over-expression of LncRNA SRA. In the majority of PCOS models, the contents of E2 and PG were up-regulated; however, their levels were not changed, even reduced in letrozole and DHT-induced PCOS models, because letrozole and DHT possess the ability to inhibit their corresponding synthetase [27–30]. Moreover, it also shows that PCOS is a very complicated disease controlled by a variety of factors, so it is likely that E2 and PG are not the only factors leading to PCOS [31,32]. Taken together, our results indicate that LncRNA SRA may be a causative factor in PCOS.
Although our findings are meaningful, there are limitations in this study. Firstly, in vivo research on over-expression of LncRNA SRA is lacking. Secondly, the effect of down-regulation of LncRNA SRA in the PCOS model should be verified by in vitro and in vivo experiments. Moreover, as PCOS is a complex pathological process caused by many factors, such as endocrine dyscrasia, dysbolism, and genetics, more studies are needed to clarify the role of LncRNA SRA in PCOS.
Conclusions
We demonstrated that elevated LncRNA SRA can facilitate cell growth, change distribution of cell cycle phases, restrain cell apoptosis, and stimulate E2 and PG secretions, which indicates that it may be a potential risk factor for PCOS.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by grants from the National Natural Science Foundation of China (No. 81673224, 81273018, and 30700654) and the Natural Science Foundation of Shaanxi Province (No. 2015JM8436)
References
- 1.Mortada R, Williams T. Metabolic syndrome: Polycystic ovary syndrome. FP Essent. 2015;435:30–42. [PubMed] [Google Scholar]
- 2.Hardiman P, Pillay OC, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet. 2003;361:1810–12. doi: 10.1016/s0140-6736(03)13409-5. [DOI] [PubMed] [Google Scholar]
- 3.Siegel S, Futterweit W, Davies TF, et al. A C/T single nucleotide polymorphism at the tyrosine kinase domain of the insulin receptor gene is associated with polycystic ovary syndrome. Fertil Steril. 2002;78:1240–43. doi: 10.1016/s0015-0282(02)04241-3. [DOI] [PubMed] [Google Scholar]
- 4.De Leo V, Musacchio MC, Cappelli V, et al. Genetic, hormonal and metabolic aspects of PCOS: An update. Reprod Biol Endocrinol. 2016;14:38. doi: 10.1186/s12958-016-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakhshalizadeh S, Amidi F, Alleyassin A, et al. Modulation of steroidogenesis by vitamin D3 in granulosa cells of the mouse model of polycystic ovarian syndrome. Syst Biol Reprod Med. 2017;63:150–61. doi: 10.1080/19396368.2017.1296046. [DOI] [PubMed] [Google Scholar]
- 6.Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: A new paradigm. Cancer Res. 2017;77:3965–81. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36(41):5661–67. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang J, Yu Y, Yang W. Long noncoding RNA and its contribution to autism spectrum disorders. CNS Neurosci Ther. 2017;23:645–56. doi: 10.1111/cns.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathy NW, Chen XM. Long non-coding RNAs (lncRNAs) and their transcriptional control of inflammatory responses. J Biol Chem. 2017;292:12375–82. doi: 10.1074/jbc.R116.760884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazidi M, Penson P, Gluba-Brzozka A, et al. Relationship between long noncoding RNAs and physiological risk factors of cardiovascular disease. J Clin Lipidol. 2017;11:617–23. doi: 10.1016/j.jacl.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Riva P, Ratti A, Venturin M. The long non-coding RNAs in neurodegenerative diseases: Novel mechanisms of pathogenesis. Curr Alzheimer Res. 2016;13:1219–31. doi: 10.2174/1567205013666160622112234. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh SK, Patton JR, Spanjaard RA. A small RNA derived from RNA coactivator SRA blocks steroid receptor signaling via inhibition of Pus1p-mediated pseudouridylation of SRA: Evidence of a novel RNA binding domain in the N-terminus of steroid receptors. Biochemistry. 2012;51:8163–72. doi: 10.1021/bi300602r. [DOI] [PubMed] [Google Scholar]
- 13.Chen G, Yu D, Nian X, et al. LncRNA SRA promotes hepatic steatosis through repressing the expression of adipose triglyceride lipase (ATGL) Sci Rep. 2016;6:35531. doi: 10.1038/srep35531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z, Hao C, Huang X, et al. Peripheral blood leukocyte expression level of lncRNA steroid receptor RNA activator (SRA) and its association with polycystic ovary syndrome: A case control study. Gynecol Endocrinol. 2015;31:363–68. doi: 10.3109/09513590.2014.999763. [DOI] [PubMed] [Google Scholar]
- 15.Yan R, Wang K, Peng R, et al. Genetic variants in lncRNA SRA and risk of breast cancer. Oncotarget. 2016;7:22486–96. doi: 10.18632/oncotarget.7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shore AN, Herschkowitz JI, Rosen JM. Noncoding RNAs involved in mammary gland development and tumorigenesis: there’s a long way to go. J Mammary Gland Biol Neoplasia. 2012;17:43–58. doi: 10.1007/s10911-012-9247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tye CE, Gordon JA, Martin-Buley LA, et al. Could lncRNAs be the missing links in control of mesenchymal stem cell differentiation? J Cell Physiol. 2015;230:526–34. doi: 10.1002/jcp.24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Havelock JC, Rainey WE, Carr BR. Ovarian granulosa cell lines. Mol Cell Endocrinol. 2004;228:67–78. doi: 10.1016/j.mce.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Hopkinson ZE, Sattar N, Fleming R, Greer IA. Polycystic ovarian syndrome: The metabolic syndrome comes to gynaecology. BMJ. 1998;317:329–32. doi: 10.1136/bmj.317.7154.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bednarska S, Siejka A. The pathogenesis and treatment of polycystic ovary syndrome: What’s new? Adv Clin Exp Med. 2017;26:359–67. doi: 10.17219/acem/59380. [DOI] [PubMed] [Google Scholar]
- 21.Ghafurniyan H, Azarnia M, Nabiuni M, Karimzadeh L. The effect of green tea extract on reproductive improvement in estradiol valerate-induced polycystic ovarian syndrome in rat. Iran J Pharm Res. 2015;14:1215–33. [PMC free article] [PubMed] [Google Scholar]
- 22.M’Baye M, Hua G, Khan HA, Yang L. RNAi-mediated knockdown of INHBB increases apoptosis and inhibits steroidogenesis in mouse granulosa cells. J Reprod Dev. 2015;61:391–97. doi: 10.1262/jrd.2014-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yaba A, Demir N. The mechanism of mTOR (mammalian target of rapamycin) in a mouse model of polycystic ovary syndrome (PCOS) J Ovarian Res. 2012;5:38. doi: 10.1186/1757-2215-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabel AM, Al-Shehri AH, Al-Talhi RA, Abd Elmaaboud MA. The promising effect of linagliptin and/or indole-3-carbinol on experimentally-induced polycystic ovarian syndrome. Chem Biol Interact. 2017;273:190–99. doi: 10.1016/j.cbi.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Sorensen AE, Wissing ML, Salo S, et al. MicroRNAs related to polycystic ovary syndrome (PCOS) Genes (Basel) 2014;5:684–708. doi: 10.3390/genes5030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comim FV, Hardy K, Franks S. Adiponectin and its receptors in the ovary: Further evidence for a link between obesity and hyperandrogenism in polycystic ovary syndrome. PLoS One. 2013;8:e80416. doi: 10.1371/journal.pone.0080416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manneras L, Cajander S, Holmang A, et al. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148:3781–91. doi: 10.1210/en.2007-0168. [DOI] [PubMed] [Google Scholar]
- 28.Maliqueo M, Benrick A, Stener-Victorin E. Rodent models of polycystic ovary syndrome: Phenotypic presentation, pathophysiology, and the effects of different interventions. Semin Reprod Med. 2014;32:183–93. doi: 10.1055/s-0034-1371090. [DOI] [PubMed] [Google Scholar]
- 29.Maliqueo M, Sun M, Johansson J, et al. Continuous administration of a P450 aromatase inhibitor induces polycystic ovary syndrome with a metabolic and endocrine phenotype in female rats at adult age. Endocrinology. 2013;154:434–45. doi: 10.1210/en.2012-1693. [DOI] [PubMed] [Google Scholar]
- 30.Burt Solorzano CM, Beller JP, Abshire MY, et al. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids. 2012;77:332–37. doi: 10.1016/j.steroids.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. doi: 10.1038/nrdp.2016.57. [DOI] [PubMed] [Google Scholar]
- 32.Feichtinger M, Stopp T, Gobl C. [Metabolic and reproductive consequences of the polycystic ovary syndrome (PCOS)]. Wien Med Wochenschr. 2016;166:139–42. doi: 10.1007/s10354-016-0439-0. [in German] [DOI] [PubMed] [Google Scholar]