Abstract
Background
The aim of this study was to undertake a histological evaluation of the presence of eosinophils in esophageal muscle in patients with achalasia before treatment with peroral endoscopic myotomy (POEM), with clinical follow-up at one year.
Material/Methods
Before treatment, esophageal biopsies including mucosa and esophageal muscle were obtained from 28 patients with achalasia. Nine patients who had undergone esophagectomy for esophageal carcinoma were included in the control group. The Eckardt Score was used to evaluate the clinical symptoms of achalasia. Histology of routinely processed tissue sections was used to perform eosinophil cell counts (0 to +++), and immunohistochemistry was used to detect expression of eosinophil major basic protein (MBP), eosinophil-derived neurotoxin (EDN), and S100 protein in cases of achalasia (n=28) and controls (n=9). The findings in patients with achalasia were compared before and one year following POEM.
Results
Esophageal tissue from patients with achalasia showed eosinophils infiltrating into the muscularis externa in 85.7% (24/28), into the muscularis propria in 28.6% (8/28), and in 89% (25/28) there were few remaining myenteric ganglion cells, before POEM. The extent of inflammation was similar in all regions of the esophagus and between subtypes of achalasia. At one year following POEM, the Eckardt Scores between the former eosinophil (0) group and the eosinophil (+++) group were significantly different (Z=3.50, P=0.030).
Conclusions
Achalasia of the esophagus was associated with infiltration of the esophageal muscle by activated eosinophils and a decrease in the density of ganglion cells in the myenteric esophageal plexus.
MeSH Keywords: Eosinophils, Esophageal Achalasia, Myenteric Plexus
Background
Achalasia of the esophagus is a motility disorder characterized by reduced or absent lower esophageal sphincter relaxation in response to swallowing, and lack of peristalsis of the body of the esophagus as detected by manometry, which is used to divide achalasia into types I, II, and III [1,2]. Achalasia of the esophagus affects both sexes equally and affects all age groups [1,2]. This condition is associated with loss of ganglion cells of the esophageal myenteric plexus, mainly in the distal esophagus and the lower esophageal sphincter [3,4]. Although several theories have been proposed to explain the etiology, the reasons for the development of the abnormal neural response in achalasia of the esophagus remain unknown.
A previously published study by Landres et al. showed that infiltration of the esophageal muscle was present in a patient with severe achalasia [5]. These authors proposed that eosinophilic infiltration of the esophagus might have resulted in esophageal motility dysfunction [5]. Other studies have shown that in achalasia of the esophagus, inflammation involving the esophageal myenteric plexus involves eosinophils and lymphocytes with injury to and loss of ganglion cells [3,6]. However, data from these previous studies were derived from surgical biopsies in severe cases where patients were unresponsive to surgical management [3,6].
In 2010, the technique of peroral endoscopic myotomy (POEM) was developed for the treatment of achalasia of the esophagus, which is a less invasive surgical approach using a trans-oral approach into the esophageal muscle layer [7]. Also, this approach has allowed less invasive biopsies of muscularis propria of the esophagus to be obtained [8,9]. The information obtained using this technique is expected to provide new insights into the underlying pathology of achalasia, but histological examination of esophageal specimens from patients with achalasia undergoing POEM should still be undertaken.
The aim of the present study was to undertake a histological evaluation of the presence of eosinophils in esophageal muscle in patients with achalasia before treatment with POEM, with clinical follow-up at one year.
Material and Methods
Study population
This study included 28 patients who were newly diagnosed with achalasia of the esophagus and who were referred to our gastroenterology department for peroral endoscopic myotomy (POEM) between September 2014 and March 2015. All patients underwent esophageal biopsy. A comprehensive preoperative clinical evaluation was completed for all 28 patients, which included a clinical assessment according to the Eckardt Score for symptoms of achalasia. All patients were asked about the frequency of dysphagia, regurgitation, and chest pain, as well as the degree of weight loss. Upper gastrointestinal endoscopy, high-resolution manometry, and timed barium swallow were performed in all cases. Manometry was used to divide the patients into achalasia type I (with minimal esophageal pressurization), type II (with esophageal compression), and type III (with spasm). During POEM, esophageal muscle biopsies and mucosal tissue samples were obtained from the 28 patients included in the study.
Patients were excluded from the study who had achalasia with previous esophageal treatment, patients with Barrett’s esophageal lesions, esophageal stricture, liver cirrhosis and/or esophageal varices, active esophagitis, connective tissue diseases, allergic diseases, hematological disease or coagulopathy, women who were pregnant, and patients with hiatus hernia or any abdominal discomfort. Other exclusion criteria included the use of non-steroidal anti-inflammatory drugs, corticosteroids, or other immunosuppressive drugs in the six months before examination. Control samples of normal esophageal tissue were obtained from nine patients who had undergone esophagectomy for esophageal carcinoma.
The study was reviewed and approved by the Medical Ethics Committee of Tianjin Medical University General Hospital (IRB 2014-YX-064). All patients provided written informed consent to participate in this study.
Histopathology
Tissue samples of the lower esophageal sphincter, distal esophagus, approximately 5 cm above the esophagogastric junction, and mid-esophagus, approximately 10–20 cm above the esophagogastric junction, were fixed in formalin and embedded in paraffin wax before sectioning onto glass slides.
In the nine patients who had normal esophageal tissues sampled from esophagectomy specimens resected for esophageal carcinoma, histology was performed to confirm that the esophageal tissue sampled was histologically normal, with tissue sampling from regions at least 5 cm beyond the area of the tumor.
Tissue sections from the 28 patients with achalasia of the esophagus and nine patients with normal esophageal tissue from resection specimens were routinely stained with hematoxylin and eosin (H&E) and examined by light microscopy. The number of eosinophils in the mucosal and muscle layers was counted per high-power field (HPF) at x 400 objective magnification. The mean number of eosinophils per three HPFs was recorded. The eosinophils in the esophageal mucosa were quantified histologically as none (0), few (+), focal (++), or diffuse (+++) [6,10].
Immunohistochemistry
Immunohistochemical staining was performed using primary antibodies to eosinophil major basic protein (MBP) (Abcam, Cambridge, MA, USA) (Cat. number: ab187523) (dilution, 1: 100) and eosinophil-derived neurotoxin (EDN) (Novus, Canada) (Cat. number: NBP1-91872) (dilution, 1: 100) to identify activated eosinophils. A primary antibody to S100 protein (Dako, Denmark) (Cat. number: Z0311) (dilution, 1: 500) was used as a neural marker.
Histological examination of the histology and immunohistochemistry of the esophageal tissues was performed by two pathologists and a physician who were blinded to the clinical data. Any disagreement in their opinions were settled by consensus.
The POEM procedure and definitions of clinical outcome
Two experienced endoscopists performed the POEM procedure, as described by Inoue et al. [7]. Patients were followed-up at one, three, six, and 12 months by clinical interview and using the Eckardt Score. Post-treatment Eckardt Scores >3 were defined as primary failure of the POEM procedure. Recurrence of achalasia of the esophagus was defined as an initial Eckardt Score of ≤3, followed by an increase to >3 during the follow-up period. Both primary failure and recurrence were considered as a failure of the POEM procedure [11,12].
Statistical analysis
Patient demographics and histological findings were expressed as continuous variables and as the mean ± standard deviation (SD), and range. Differences between groups were analyzed using an unpaired Student’s t-test or the Mann–Whitney U-test. Differences in the distribution of eosinophils between groups were evaluated with the chi-squared (χ2) test. Statistical associations were assessed using Spearman’s correlation analysis. A two-tailed P-value <0.05 was considered to indicate statistical significance. Statistical analysis was performed using SPSS software version 21 (IBM, NY, USA).
Results
Study population and esophageal tissue sampling
The demographic and clinical characteristics of the 28 patients with achalasia of the esophagus and nine controls are summarized in Table 1. No significant differences in the distribution of age or sex were found between the groups (age: t=−1.836, P=0.075; sex distribution: χ2=0.007; P=0.615). None of the participants had food allergies or food intolerance.
Table 1.
Clinical characteristics.
| Achalasia patients (n=28) | |
|---|---|
| Gender (F: M) | 12: 16 |
| Age, years (range) | 51.1±14.5 (24–78) |
| Symptom duration, months (range) | 28.0±18.4 (5–80) |
| Type of achalasia (I/II/III) | 12/14/2 |
| Eckardt score before POEM (range) | 8.1±1.6 (5–12) |
| Eckardt score 1 year after POEM (range) | 2.3±1.3 (0–4) |
| Controls (n=9) | |
| Gender (F: M) | 4: 5 |
| Age, years (range) | 60.8±9.6 (45–75) |
Esophageal biopsies that included mucosal and muscle tissues were taken during the peroral endoscopic myotomy (POEM) procedure from 28 patients with achalasia. From the nine patients who had esophagectomy resection specimens, full-thickness sections were taken from the normal areas of the esophagus, as normal controls. Endoscopic examination and histology showed no epithelial eosinophils in patients with achalasia or the controls.
Histological evaluation of eosinophils in achalasia of the esophagus
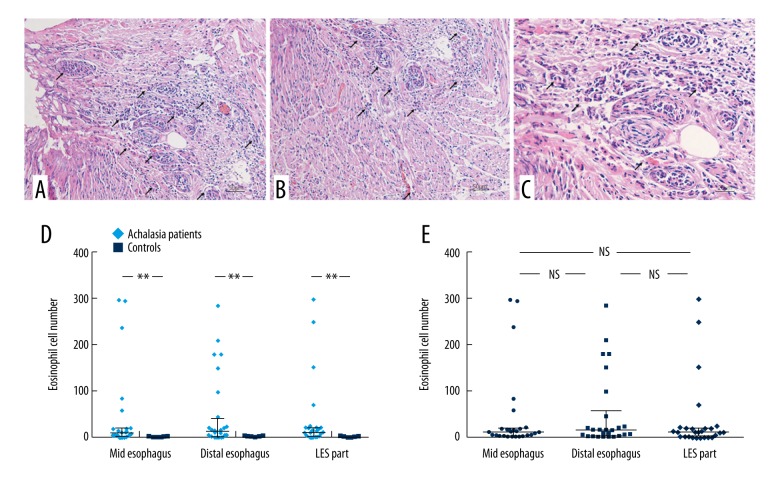
Routine histology using hematoxylin and eosin (H&E) staining of esophageal tissue from patients with achalasia showed eosinophils infiltrating into the muscularis externa in 85.7% (24/28), and into the muscularis propria in 28.6% (8/28) (Figure 1A–1C). In the muscle of all three regions of the esophagus, significantly more eosinophils were present in patients with achalasia compared with the normal control esophageal tissue: middle, 10 (15.75) vs. 1 (1), Z=−3.87, P=0.000; distal, 15 (55.75) vs. 1 (1), Z=−3.32, P=0.000; lower esophageal sphincter, 12 (18) vs. 1 (1.5), Z=−3.50, P=0.000 (Figure 1D) (Table 2). The inflammatory reaction was similar in the middle esophageal, distal esophageal, and lower esophageal sphincter samples from patients with achalasia (P>0.05) (Figure 1E).
Figure 1.
The muscularis externa of the esophagus in patients with achalasia showing eosinophilia. (A) Photomicrograph of the histology of the muscularis externa of the esophagus in a patient with achalasia shows severe inflammation with eosinophilia. Hematoxylin and eosin (H&E). Magnification ×200. (B) Photomicrograph of the histology of the muscularis externa of the esophagus in a patient with achalasia shows severe inflammation with eosinophilia. H&E. Magnification ×200. (C) Photomicrograph of the histology of the muscularis externa of the esophagus in a patient with achalasia shows severe inflammation with eosinophilia. H&E. Magnification ×400. (D) The number of eosinophils in regions of the esophagus from patients with achalasia, compared with controls. (E) The number of eosinophils in the muscularis layer in regions of the esophagus in patients with achalasia. ** P<0.05; NS – not significant.
Table 2.
Distribution of eosinophils in the esophageal muscle.
| Group | Distribution of eosinophils* | χ2 | P | ||||
|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | ||||
| Mid esophageal | Achalasia patients | 3 | 10 | 6 | 9 | 8.479 | 0.037 |
| Control group | 3 | 6 | 0 | 0 | |||
| Distal esophageal | Achalasia patients | 4 | 7 | 9 | 8 | 11.099 | 0.011 |
| Control group | 5 | 4 | 0 | 0 | |||
| Low esophageal sphincter | Achalasia patients | 3 | 8 | 9 | 8 | 10.972 | 0.012 |
| Control group | 4 | 5 | 0 | 0 | |||
Immunohistochemical markers of eosinophil activation and degranulation and neuronal markers in achalasia of the esophagus
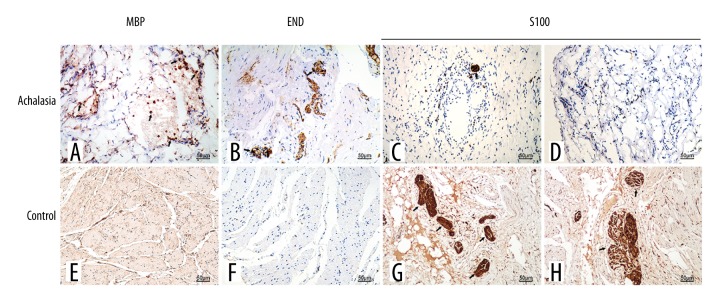
Immunohistochemistry showed that eosinophil major basic protein (MBP) and eosinophil-derived neurotoxin (EDN) were expressed in esophageal muscle from 85.7% (24/28) patients with achalasia (Figure 2A, 2B). In the normal control esophageal tissue, eosinophil MBP and EDN were not expressed or were rarely expressed (Figure 2E, 2F). For the patients with esophageal achalasia, immunohistochemical staining for the localization of S100 showed loss of esophageal myenteric plexus ganglion cells within the body of the esophagus in 25 patients with achalasia (Figure 2C). Scattered nerve cells remained in the region of the intramural cell plexus, where few eosinophils were present (Figure 2D). Immunostaining for S100 in the esophageal control tissue showed no decrease in the number of nerve and ganglion cells, and there was a regular distribution of ganglion cells associated with myenteric nerves (Figure 2G, 2H).
Figure 2.
Photomicrographs showing the immunohistochemical staining of the esophageal tissue for degranulating eosinophils and nerve cells. (A) Esophageal tissue from a patient with achalasia. Immunohistochemical staining shows localization of the primary antibody to eosinophil major basic protein (MBP), confirming that the cells are eosinophils. (B) Esophageal tissue from a patient with achalasia. Immunohistochemical staining shows localization of the primary antibody to eosinophil-derived neurotoxin (EDN), confirming that the cells are eosinophils. (C) Esophageal tissue from a patient with achalasia. Immunohistochemical staining for the localization of the primary antibody to S100, which shows loss of ganglion cells. (D) Esophageal tissue from a patient with achalasia. Immunohistochemical staining for the localization of the primary antibody to S100, which shows loss of ganglion cells. (E) Normal (control) esophageal tissue. Immunohistochemical staining with the primary antibody to eosinophil MBP is negative. (F) Normal (control) esophageal tissue. Immunohistochemical staining with the primary antibody to EDN is negative. (G) Normal (control) esophageal tissue. Immunohistochemical staining of the localization of the primary antibody to S100, which shows a regular distribution of ganglion cells associated with myenteric nerves. (H) Normal (control) esophageal tissue. Immunohistochemical staining of the localization of the primary antibody to S100, which shows a regular distribution of ganglion cells associated with myenteric nerves.
Eosinophil count and short-term (one year) clinical outcome following POEM
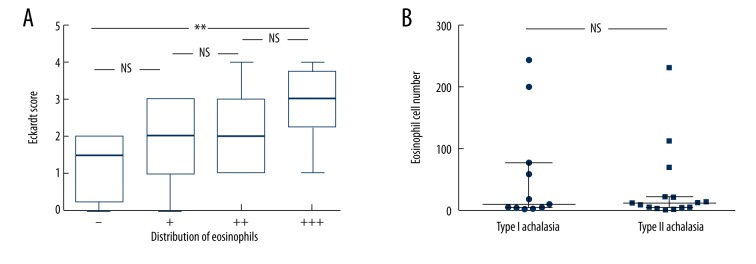
In the 28 patients with achalasia of the esophagus, before POEM, comparison of the number of eosinophils present with the Eckardt Score showed no significant differences (r=−0.164, P=0.404). There were no significant differences between the Eckardt Score before POEM and the distribution of eosinophils in the esophageal muscle of patients with achalasia (χ2=2.361, P=0.501). There were no significant differences found in the Eckardt Score between the groups (P>0.05), but the differences between the eosinophil (0) group and the eosinophil (+++) group were statistically significant at one year following POEM (Z=3.50, P=0.030) (Figure 3A). At one year following POEM, the number of eosinophils in the esophageal muscle of patients with the different types of achalasia were similar (P>0.05) (Figure 3B). The degree of esophageal inflammation, including the number of eosinophils, was similar between type I and type II achalasia. However, there were only two patients with type III achalasia in this study.
Figure 3.
The Eckardt Score and the distribution of eosinophils in the esophageal muscle of patients with achalasia one year following peroral endoscopic myotomy (POEM). (A) No significant differences are found in the Eckardt Score between the groups (P>0.05), but the differences between eosinophil (0) group and the eosinophil (+++) group are statistically significant at one year following peroral endoscopic myotomy (POEM) (Z=3.50, P=0.030). (B) One year following POEM, the number of eosinophils in the esophageal muscle of patients in the different types of achalasia is similar. ** P<0.05. NS – not significant.
Discussion
Achalasia is a common primary esophageal motility disorder. Although this condition was first described centuries ago, the etiology and pathogenesis remain unknown. In achalasia of the esophagus, the neurons and ganglion cells of the esophageal myenteric plexus are reduced in number or are absent, resulting in impaired or absent esophageal peristalsis and impaired relaxation of the lower esophageal sphincter. The myenteric neurons and ganglia are thought to be lost due to the effects of inflammation [3,6,13,14]. Goldblum et al. evaluated the morphological features of 42 esophageal resection specimens taken from patients with achalasia and found inflammatory cells, consisting of lymphocytes and eosinophils, within the myenteric plexus in all cases [6]. In another study, the degree of inflammation, including eosinophils, was correlated with an increased duration of symptoms among patients with achalasia [13].
The first two cases of eosinophilic esophagitis were reported in patients with primary motility disorders (achalasia and esophageal spasm) more than 30 years ago [5,15]. More recently, esophageal motility disorders, which may present as achalasia or hypercontractile (nutcracker) esophagus, have been reported in a subset of patients with eosinophilic esophagitis [16–21]. A retrospective study conducted by Mandaliya et al. suggested that a concurrence of achalasia and eosinophilic esophagitis, though rare, is associated with poor long-term post-treatment outcome [22]. One case report of a patient with achalasia and eosinophilic esophagitis showed improved esophageal motility and disappearance of dysphagia after treatment with 50 mg prednisolone [14,21]. These previous studies indicate a possible association esophageal eosinophils and achalasia.
The results of the present study showed that eosinophils infiltrated the muscularis externa of the esophagus in 82.1% (23/28) of patients with achalasia, and there was involvement of the muscularis propria in 28.6% (8/28) of patients. These findings are higher than those reported previously in achalasia by Goldblum et al. who reported eosinophils in 52.4% (22/42) of patients, and by Gockel et al. who reported eosinophils in 17.6% (3/17) of patients, respectively [3,13]. The reason for the relatively increased prevalence of eosinophils in the present study might be due to the more rigorous tissue sampling used.
Activated eosinophils can induce tissue damage by releasing cytotoxic eosinophil granule proteins, including eosinophil major basic protein (MBP), eosinophilic cationic protein (ECP), and eosinophil-derived neurotoxin (EDN) [23–25]. Studies on eosinophil-induced neurotoxicity have also described neurological dysfunction in both the central and peripheral nervous systems [25]. In the present study, increased number of eosinophils and degranulated eosinophils, as indicated by increased levels of immunostaining for eosinophil MBP and EDN, were observed in the esophageal muscle of patients with achalasia along with a marked decrease or absence of ganglion cells. It is possible to speculate that the myenteric ganglion cell changes in achalasia might be linked to eosinophilic infiltration and degranulation. However, the underlying mechanism that causes eosinophils to infiltrate the esophageal muscle layer in achalasia is unknown, and it is still not clear whether the recruited and activated eosinophils are primary causative factors or a secondary cellular response to achalasia.
In the present study, there were no differences in the histological findings between different parts of the esophagus in achalasia. Also, negative correlations were found between the number of eosinophils and the duration of disease, which appears to contradict previously reported findings of more severe inflammation in patients with a longstanding history of achalasia. However, there are several reasons why there are discordant findings between studies, including differences in sample size, the heterogeneity of the patient population being studied, and methodological differences between studies. In some cases in the present study, the severity of eosinophil infiltration was focal and in some cases eosinophils were found in perimysial and perivascular areas. Particularly in biopsy-based studies, such focal distribution of eosinophils may have resulted in false-negative study findings.
In the present study, the clinical relevance of the study findings was evaluated using the Eckardt Score together with an evaluation of the difference in distribution of eosinophils in the esophageal muscle of patients with achalasia before and one year following peroral endoscopic myotomy (POEM). Although the number of eosinophils and the grades of achalasia were not significantly correlated with the Eckardt Score before POEM, a significant difference was found between the eosinophil (0) group and the eosinophil (+++) group, and the degree of inflammation was similar between type I and type II achalasia. However, in this study, the number of patients with type III achalasia was limited to two, and no associations could be determined. From the findings of this study, it might be possible to propose that activated eosinophils infiltrate into the muscularis myenteric plexus of the esophagus, and influence the outcome following POEM. However, the present study was preliminary, with insufficient patient numbers to eliminate confounding factors and to make a full assessment of the efficacy of POEM in patients with achalasia of the esophagus with varying degrees of eosinophil infiltrates and of eosinophil activation.
This study also had several other limitations, in addition to the limited number of patients studied. As mentioned in a previous study [9], the degree of ganglion cell loss may have been misrepresented in esophageal tissue specimens obtained by POEM. Although residual ganglion cells were observed in three cases out of the 28 cases of esophageal achalasia studied, larger study samples are required. Also, the alterations in the muscle seen on biopsy specimens may not have accurately represented the full scope of the esophageal pathology for each patient. Because achalasia is a rare disease, the number of patients who underwent POEM was limited, and because of the small size of the present study, it is difficult to analyze the possible relationship between subtypes of achalasia and the degree and distribution of esophageal eosinophil infiltration. Therefore, further studies are recommended with a larger sample size to validate the findings of the present study with regard to the histology of the esophageal muscle layer in achalasia.
Conclusions
The findings of this study showed that achalasia of the esophagus was associated with infiltration of the esophageal muscle by activated eosinophils and a decrease in the density of ganglion cells in the myenteric esophageal plexus. However, the study findings also support the view that achalasia is a heterogeneous disease, and that achalasia with eosinophil infiltration in esophageal muscle may represent a subtype of eosinophilic esophagitis. Larger, multi-center, controlled studies are still needed to characterize the etiology and pathogenesis of achalasia and to further investigate how the underlying mechanisms that lead to achalasia might affect patient outcome following treatments such as peroral endoscopic myotomy (POEM).
Footnotes
Conflict of interest
None.
Source of support: This study was supported by National Natural Science Foundation of China (81600425)
References
- 1.Francis DL, Katzka DA. Achalasia: Update on the disease and its treatment. Gastroenterology. 2010;139:369–74. doi: 10.1053/j.gastro.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Ghoshal UC, Daschakraborty SB, Singh R. Pathogenesis of achalasia cardia. World J Gastroenterol. 2012;18:3050–57. doi: 10.3748/wjg.v18.i24.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldblum JR, Whyte RI, Orringer MB, Appelman HD. Achalasia. A morphologic study of 42 resected specimens. Am J Surg Pathol. 1994;18:327–37. [PubMed] [Google Scholar]
- 4.Pandolfino JE, Gawron AJ. Achalasia: A systematic review. JAMA. 2015;313:1841–52. doi: 10.1001/jama.2015.2996. [DOI] [PubMed] [Google Scholar]
- 5.Landres RT, Kuster GG, Strum WB. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology. 1978;74:1298–301. [PubMed] [Google Scholar]
- 6.Goldblum JR, Rice TW, Richter JE. Histopathologic features in esophagomyotomy specimens from patients with achalasia. Gastroenterology. 1996;111:648–54. doi: 10.1053/gast.1996.v111.pm8780569. [DOI] [PubMed] [Google Scholar]
- 7.Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265–71. doi: 10.1055/s-0029-1244080. [DOI] [PubMed] [Google Scholar]
- 8.Sato H, Inoue H, Ikeda H, et al. In vivo histopathological assessment of the muscularis propria in achalasia by using endocytoscopy (with video) Endosc Int Open. 2014;2:E178–82. doi: 10.1055/s-0034-1377319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakajima N, Sato H, Takahashi K, et al. Muscle layer histopathology and manometry pattern of primary esophageal motility disorders including achalasia. Neurogastroenterol Motil. 2017;29(3) doi: 10.1111/nmo.12968. [DOI] [PubMed] [Google Scholar]
- 10.Mueller S, Aigner T, Neureiter D, Stolte M. Eosinophil infiltration and degranulation in oesophageal mucosa from adult patients with eosinophilic oesophagitis: A retrospective and comparative study on pathological biopsy. J Clin Pathol. 2006;59:1175–80. doi: 10.1136/jcp.2005.031922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werner YB, Costamagna G, Swanstrom LL, et al. Clinical response to peroral endoscopic myotomy in patients with idiopathic achalasia at a minimum follow-up of 2 years. Gut. 2016;65:899–906. doi: 10.1136/gutjnl-2014-308649. [DOI] [PubMed] [Google Scholar]
- 12.Ren Y, Tang X, Chen Y, et al. Pre-treatment Eckardt Score is a simple factor for predicting one-year peroral endoscopic myotomy failure in patients with achalasia. Surg Endosc. 2017;31(8):3234–41. doi: 10.1007/s00464-016-5352-5. [DOI] [PubMed] [Google Scholar]
- 13.Gockel I, Bohl JR, Doostkam S, et al. Spectrum of histopathologic findings in patients with achalasia reflects different etiologies. J Gastroenterol Hepatol. 2006;21:727–33. doi: 10.1111/j.1440-1746.2006.04250.x. [DOI] [PubMed] [Google Scholar]
- 14.Boeckxstaens GE, Zaninotto G, Richter JE. Achalasia. Lancet. 2014;383:83–93. doi: 10.1016/S0140-6736(13)60651-0. [DOI] [PubMed] [Google Scholar]
- 15.Dobbins JW, Sheahan DG, Behar J. Eosinophilic gastroenteritis with esophageal involvement. Gastroenterology. 1977;72:1312–16. [PubMed] [Google Scholar]
- 16.Moawad FJ, Maydonovitch CL, Veerappan GR, et al. Esophageal motor disorders in adults with eosinophilic esophagitis. Dig Dis Sci. 2011;56:1427–31. doi: 10.1007/s10620-011-1655-5. [DOI] [PubMed] [Google Scholar]
- 17.Murray IA, Joyce S, Palmer J, et al. Incidence and features of eosinophilic esophagitis in dysphagia: A prospective observational study. Scand J Gastroenterol. 2016;51:257–62. doi: 10.3109/00365521.2015.1093166. [DOI] [PubMed] [Google Scholar]
- 18.Roman S, Hirano I, Kwiatek MA, et al. Manometric features of eosinophilic esophagitis in esophageal pressure topography. Neurogastroenterol Motil. 2011;23:208–14. e111. doi: 10.1111/j.1365-2982.2010.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korsapati H, Babaei A, Bhargava V, et al. Dysfunction of the longitudinal muscles of the oesophagus in eosinophilic oesophagitis. Gut. 2009;58:1056–62. doi: 10.1136/gut.2008.168146. [DOI] [PubMed] [Google Scholar]
- 20.Cools-Lartigue J, Chang SY, McKendy K, et al. Pattern of esophageal eosinophilic infiltration in patients with achalasia and response to Heller myotomy and Dor fundoplication. Dis Esophagus. 2013;26:766–75. doi: 10.1111/j.1442-2050.2012.01385.x. [DOI] [PubMed] [Google Scholar]
- 21.Savarino E, Gemignani L, Zentilin P, et al. Achalasia with dense eosinophilic infiltrate responds to steroid therapy. Clin Gastroenterol Hepatol. 2011;9:1104–6. doi: 10.1016/j.cgh.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Mandaliya R, DiMarino AJ, Cohen S. Association of achalasia and eosinophilic esophagitis. Indian J Gastroenterol. 2013;32:54–57. doi: 10.1007/s12664-012-0255-4. [DOI] [PubMed] [Google Scholar]
- 23.Erjefalt JS, Greiff L, Andersson M, et al. Allergen-induced eosinophil cytolysis is a primary mechanism for granule protein release in human upper airways. Am J Respir Crit Care Med. 1999;160:304–12. doi: 10.1164/ajrccm.160.1.9809048. [DOI] [PubMed] [Google Scholar]
- 24.Schoepfer AM, Straumann A, Panczak R, et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1255–66.e21. doi: 10.1053/j.gastro.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarro S, Boix E, Cuchillo CM, Nogués MV. Eosinophil-induced neurotoxicity: The role of eosinophil cationic protein/RNase 3. J Neuroimmunol. 2010;227:60–70. doi: 10.1016/j.jneuroim.2010.06.012. [DOI] [PubMed] [Google Scholar]