Abstract
A small heat-shock protein (sHSP) that shows molecular chaperone activity in vitro was recently purified from mature chestnut (Castanea sativa) cotyledons. This protein, renamed here as CsHSP17.5, belongs to cytosolic class I, as revealed by cDNA sequencing and immunoelectron microscopy. Recombinant CsHSP17.5 was overexpressed in Escherichia coli to study its possible function under stress conditions. Upon transfer from 37°C to 50°C, a temperature known to cause cell autolysis, those cells that accumulated CsHSP17.5 showed improved viability compared with control cultures. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of cell lysates suggested that such a protective effect in vivo is due to the ability of recombinant sHSP to maintain soluble cytosolic proteins in their native conformation, with little substrate specificity. To test the recent hypothesis that sHSPs may be involved in protection against cold stress, we also studied the viability of recombinant cells at 4°C. Unlike the major heat-induced chaperone, GroEL/ES, the chestnut sHSP significantly enhanced cell survivability at this temperature. CsHSP17.5 thus represents an example of a HSP capable of protecting cells against both thermal extremes. Consistent with these findings, high-level induction of homologous transcripts was observed in vegetative tissues of chestnut plantlets exposed to either type of thermal stress but not salt stress.
Both prokaryotes and eukaryotes synthesize a set of proteins that can interact with nonnative polypeptide chains to prevent irreversible aggregation reactions and/or nonproductive folding pathways. Most of these so-called molecular chaperones are induced as part of the ubiquitous heat-shock response and accordingly have been classified into HSP families (Vierling, 1991; Parsell and Lindquist, 1993; Boston et al., 1996). Although HSP induction is correlated with the acquisition of thermotolerance in a variety of organisms, the roles played by individual components in such processes are often not well understood (Boston et al., 1996; Hartl, 1996). Different organisms accumulate different HSPs in response to similar levels of stress. For example, HSP104 has been shown to play a major protective role upon heat shock in yeast but not in Drosophila melanogaster, which does not even synthesize an HSP100 protein in response to thermal stress (Sanchez and Lindquist, 1990). Stress conditions other than elevated temperatures can also lead to HSP induction (Vierling, 1991; Parsell and Lindquist, 1993).
The heat-shock response in plants has been extensively investigated for more than a decade (Vierling, 1991; Boston et al., 1996; Waters et al., 1996). In contrast to other eukaryotes, the most prominent heat-induced proteins of plants are the sHSPs, a structurally diverse family of polypeptides with sizes ranging from approximately 15 to 30 kD. sHSPs are encoded in higher plants by at least six multigene families and have been localized to the cytoplasm, ER, mitochondria, and chloroplast (Boston et al., 1996; Waters et al., 1996). Considerably fewer sHSPs have been identified in animals, yeast, and prokaryotes, where the most important HSPs belong to the families HSP60, HSP70, HSP90, and HSP110 (Hartl, 1996).
The in vivo function of plant sHSPs is largely unknown at present. A few studies have demonstrated that at least some members can function as molecular chaperones in vitro (Jinn et al., 1989, 1995; Lee et al., 1995, 1997; Collada et al., 1997), as is the case for mammalian sHSPs and the related α-crystallin eye-lens proteins (Horwitz, 1992; Buchner, 1996). As a result of analyses of the progeny of heat-tolerant and nontolerant variants of Agrostis palustris, a thermoprotective role has been proposed for specific HSP25 proteins (Park et al., 1996). More recently, enhanced thermotolerance has been reported in recombinant Escherichia coli cells expressing a glutathione S-transferase/rice HSP16.9 fusion protein (Yeh et al., 1997). Other studies with heat-stressed tomato fruits have shown a correlation between the accumulation of sHSPs (as well as other heat-induced proteins) and the acquisition of chilling tolerance (Sabehat et al., 1996, 1998; Kadyrzhanova et al., 1998). Aside from heat stress, other environmental or developmental signals regulate the expression of plant sHSPs. The best-characterized example of developmental regulation is the induction of specific members during seed maturation at normal growth temperatures (Hernandez and Vierling, 1993; Coca et al., 1994; DeRocher and Vierling, 1994; zur Nieden et al., 1995). Recently, a sunflower sHSP promoter that is activated during zygotic embryogenesis but not by heat stress was characterized (Carrasco et al., 1997).
In contrast to other seeds, which typically accumulate low to moderate levels of sHSPs, recalcitrant chestnut (Castanea sativa) seeds contain a highly abundant sHSP (Collada et al., 1997). Recalcitrant seeds have unusually high water contents and are in principle more sensitive to certain types of stress. Like other sHSPs, the chestnut protein forms high-molecular-mass complexes under nondissociating conditions and can function as a molecular chaperone in vitro (Collada et al., 1997). We report here the isolation of a full-length cDNA for this protein, CsHSP17.5, as well as its immunocytochemical localization in chestnut cotyledonary cells. The main finding is that CsHSP17.5 expressed in E. coli enhances cell viability not only under heat stress but also at chilling temperatures. In contrast, overproduction of the major heat-induced GroEL/ES chaperone has been shown to reduce E. coli viability at 4°C (Kandror and Goldberg, 1997).
MATERIALS AND METHODS
Plant Material and Stress Treatments
European chestnut (Castanea sativa Mill.) seeds were harvested at either the mature or the late-mature stage (approximately 4 weeks before shedding) in Zarzalejo, a village northwest of Madrid, Spain. After germination plantlets were kept in a growth chamber (16-h day/8-h night, 22°C/18°C, 70% RH). Heat-stress experiments were performed with 14- to 20-week-old plantlets at either 32°C or 40°C and 80% RH for 8 h. Cold treatments were carried out at 4°C for up to 4 weeks. For salt-stress experiments, plantlets were watered profusely with 200 mm NaCl for up to 48 h.
cDNA Cloning
A cDNA library in λ-Uni-ZAP XR was made from immature chestnut cotyledon poly(A+) RNA using the ZAP-cDNA Gigapack III Gold Cloning kit (Stratagene). Before ligation, the cDNA was enriched in small fragments (between approximately 2000 and 400 bp) by gel filtration on Sepharose CL-2B. The library was screened at reduced stringency with the full-length cDNA for sunflower HSP17.6 (Almoguera and Jordano, 1992). Positive cDNA clones were isolated and subjected to in vivo excision into Escherichia coli SOLR cells to render recombinant pBluescript SK(−) phagemids. For each insert both strands were completely sequenced using an automated DNA sequencer (model 373, Perkin-Elmer).
RNA Isolation and Northern Hybridization
Total RNA was obtained from chestnut plantlets as described previously (Chang et al., 1993). Poly(A+) RNA was prepared using oligo(dT)-cellulose spun columns (Pharmacia Biotech). Northern analyses were carried out following standard procedures (Maniatis et al., 1982). After hybridization, membranes (Magna, MSI, Westborough, MA) were washed twice in 2× SSC (1× SSC is 0.15 m NaCl and 15 mm sodium citrate, pH 7.0) and 0.1% (w/v) SDS at room temperature for 15 min, twice in 1× SSC and 0.1% SDS for 15 min, and twice in 0.2× SSC and 0.1% SDS for 15 min. Autoradiographs were taken on Kodak X-Omat-S film exposed overnight.
Silent Mutagenesis and Bacterial Expression of CsHSP17.5
The coding sequence for CsHSP17.5 was subcloned into the expression vector pRSET (Invitrogen, Carlsbad, CA). Previously, an internal NdeI site (nucleotides 114–119 in Fig. 1) was eliminated by silent mutagenesis involving two sequential PCR steps. For the first reaction, we used the forward primer 5′-CTCACTGGATATATGGGACCC-3′ (nucleotides 105–125, mutation underlined) and the reverse primer 5′-CATGCCATACGGATCCACACTCC-3′ (nucleotides 611–589, BamHI site underlined). The second reaction yielded the desired mutation by using the product of the first PCR as a primer and a second primer flanking the 5′ end of the gene, 5′-GCAGATCATATGGCGCTCAGT-3′ (NdeI site underlined; start codon in italics). A polymerase with 3′→5′ proofreading activity (Pfu, Stratagene) and experimental conditions described previously (Garcia-Casado et al., 1998) was used in all amplifications. The final product was cut with NdeI and BamHI and ligated to pRSET open with the same enzymes to yield pRSET-HSP. The engineered mutation was confirmed by sequencing both strands. For bacterial expression, E. coli BL21(DE3) cells (Novagen, Madison, WI) transformed with pRSET-HSP were grown at 37°C and 250 rpm to an A600 of 1.0, then 1 mm IPTG was added, and growth was continued for up to 4 h.
Figure 1.
Nucleotide and predicted amino acid sequence of Cs hsp17.5 cDNA. The cDNA is 733 bp long and encodes a polypeptide of 154 residues (predicted Mr = 17,482). The 3′-untranslated region consists of 217 nucleotides and contains a putative polyadenylation signal (indicated in boldface) 158 nucleotides upstream of the poly(A+) tail. The deduced amino acid sequence includes, with agreement at every residue, two internal peptides (underlined) obtained by endoproteinase Asp-N cleavage of the purified seed protein (Collada et al., 1997). CsHSP17.5 also includes two motifs (residues 61–88 and 111–140) conserved in all plant sHSPs (Vierling, 1991), as well as an N-terminal domain (residues 10–24) characteristic of class I sHSPs (Waters et al., 1996).
Protein Purification and Immunodetection
CsHSP17.5 was purified from chestnut seeds as described previously (Collada et al., 1997). SDS-PAGE fractionation and protein immunoblotting were carried out as described by Garcia-Casado et al. (1998) using a 1:500 dilution of monospecific polyclonal antibodies to CsHSP17.5 (Collada et al., 1997).
Immunoelectron Microscopy
For immunoelectron microscopy experiments, immature chestnut cotyledons were fixed with 4% (v/v) paraformaldehyde in PBS immediately after collection. Low-temperature embedding and mounting of ultrathin sections were carried out as described previously (Rodriguez-Cerezo et al., 1997). Sections were blocked for 60 min in 30 mm Tris-HCl, pH 7.5, 0.1% (w/v) BSA, and 1% (w/v) gelatin and then incubated for 2 h with a 1:50 dilution of monospecific antibodies against CsHSP17.5. Sections were washed, colloidal gold labeled (10-nm particles), and stained with uranyl acetate and lead citrate (Rodriguez-Cerezo et al., 1997).
Cell-Viability Experiments
For heat-shock experiments cell cultures were grown at 37°C to an A600 of 1.0 and then diluted once with fresh Luria-Bertani medium supplemented with ampicillin at 100 μg/mL and IPTG to a final concentration of 1 mm. Two hours after induction, cultures were diluted to 6 × 106 cells mL−1, and 1-mL samples were shifted to 50°C. Aliquots (100 μL) were taken at 0, 30, and 60 min, and serial dilutions were plated in triplicate onto Luria-Bertani plus ampicillin plates. Cell viability was estimated by counting the number of colony-forming units after incubation of the plates overnight at 37°C. For cold treatments, appropriate dilutions from induced cultures were plated onto Luria-Bertani agar supplemented with ampicillin and 1 mm IPTG. Plates were then incubated at 4°C for different periods and cell viability was estimated as described above. For both treatments (heat and cold), the means of three experiments were determined from at least two independent transformants (with sd being less than 5% in all cases).
Thermostability of Soluble Proteins in E. coli
The effect of heat shock on protein stability was analyzed in recombinant IPTG-induced E. coli cells according to the method of Muchowski and Clark (1998). At various times during the 50°C treatments, aliquots were centrifuged to collect cells. The pellets were washed once with 20 mm Tris-HCl, pH 7.5, and 1 mm EDTA and extracted with the same buffer. Crude cell lysates were then centrifuged at 16,000g for 30 min, and the supernatants were analyzed by SDS-PAGE.
RESULTS
Characterization of a cDNA Encoding Chestnut Seed sHSP
An abundant protein was recently purified from mature chestnut cotyledons that shows homology to class I sHSPs from plant sources (Collada et al., 1997). To isolate its cDNA, a library from late-mature chestnut cotyledons (1.1 × 106 plaque-forming units) was screened at moderate stringency with a class I sunflower HSP cDNA. Five positive clones were randomly selected and their inserts sequenced. All inserts correspond to a single nucleotide sequence (Fig. 1), which includes a 462-bp reading frame flanked by 5′- and 3′-noncoding sequences of 54 and 217 nucleotides, respectively. A putative polyadenylation signal, AATAAA, is located 158 nucleotides upstream of the poly(A+) tail. The encoded polypeptide, which is 154 residues long, has been designated CsHSP17.5. Its predicted Mr (17,482) and pI (5.95) are similar to those experimentally determined for chestnut seed sHSP (Collada et al., 1997). Moreover, CsHSP17.5 includes the two known internal peptides of the seed protein with agreement at every residue (Fig. 1). Heterologous expression in E. coli and western and northern analyses further supported the correspondence between these proteins (see below).
Database searches revealed that CsHSP17.5 had the highest amino acid sequence similarity to class I cytosolic sHSPs. Aside from the two motifs conserved in all plant sHSPs (Vierling, 1991; Waters et al., 1996), CsHSP17.5 contains an N-terminal region (residues 10–24) characteristic of class I cytosolic proteins. Like other sHSPs, CsHSP17.5 also shows a weak but significant similarity to mammalian eye-lens α-crystallins. Molecular chaperone activity has been demonstrated for members of both protein groups (Jakob et al., 1993; Lee et al., 1995).
Subcellular Location of CsHSP17.5
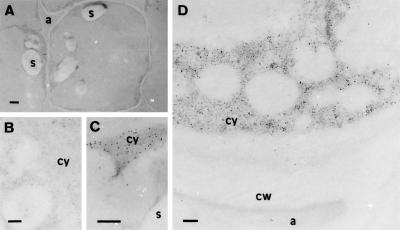
In mature chestnut seeds the greatest amount of CsHSP17.5 is localized in the cotyledons (Collada et al., 1997). Because good sections are difficult to obtain from mature seed tissue, cotyledonary cells of the late-mature stage were subjected to immunoelectron microscopic analysis. For these experiments monospecific antibodies against purified CsHSP17.5 were prepared as described previously (Collada et al., 1997). These antibodies recognize a single polypeptide when crude protein extracts from chestnut seeds are subjected to immunoblot analysis. Electron micrographs (Fig. 2) show that in cotyledonary cells the CsHSP17.5-specific label was localized exclusively in the cytoplasm. The apoplastic space, cell wall, storage vacuoles, and starch granules did not contain significant levels of specific label. Likewise, sections treated with preimmune serum did not show substantial labeling (Fig. 2B).
Figure 2.
Immunocytochemical localization of CsHSP17.5 in late-mature (approximately 4 weeks before shedding) cotyledonary cells of chestnut. A low-magnification view (A) shows the anatomy of a typical, untreated cell at this stage of seed development. Before colloidal gold labeling (10-nm particles), similar sections were incubated with preimmune serum (B) or with rabbit monospecific antibodies to CsHSP17.5 (C and D). In the latter case, gold label was found exclusively in the cytosol, with no significant labeling of starch granules (C), apoplastic space, vacuoles, or cell walls (D). No significant labeling was observed in sections treated with preimmune serum (B). a, Apoplastic space; cw, cell wall; cy, cytoplasm; s, starch granules. Bars in A = 2 μm; bars in B to D = 200 nm.
Heterologous Expression in E. coli Cells
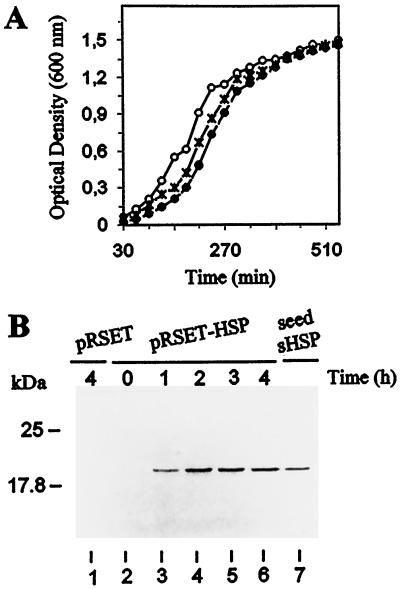
To analyze its possible in vivo function under stress conditions, the complete coding sequence for seed CsHSP17.5 was introduced into E. coli using the pRSET expression vector. The vector alone was also introduced into E. coli as a control. Under normal culture conditions, similar growth rates were observed for both types of recombinant cells (pRSET-HSP and pRSET) and for untransformed wild-type cells (Fig. 3A). After IPTG addition, SDS-PAGE analysis showed the overproduction of recombinant CsHSP17.5 (apparent Mr approximately 20,000) in extracts from pRSET-HSP cells but not in extracts from cells carrying the control plasmid. The recombinant protein reached maximal expression levels 2 to 4 h after induction and had the same apparent size as seed sHSP, as shown by SDS-PAGE (Fig. 3B).
Figure 3.
Heterologous expression of CsHSP17.5 in E. coli BL21(DE3). A, Growth of wild-type (○) and transformed pRSET (×) and pRSET-HSP (•) cells at 37°C. At the times indicated, 1-mL culture aliquots were taken and the A600 was determined. Before induction (time 0) and at different times after IPTG addition (1, 2, 3, and 4 h), samples were taken from pRSET (control) and pRSET-HSP cultures, and crude cell lysates were prepared. Proteins (25 μg) were then fractionated by SDS-PAGE and immunoblotted with monospecific antibodies to purified seed CsHSP17.5 (B). The lysate from pRSET cells (lane 1) was prepared 4 h after the addition of IPTG. An authentic sample of chestnut seed sHSP (0.5 μg) was included for comparison (lane 7).
Heat- and Cold-Stress Experiments
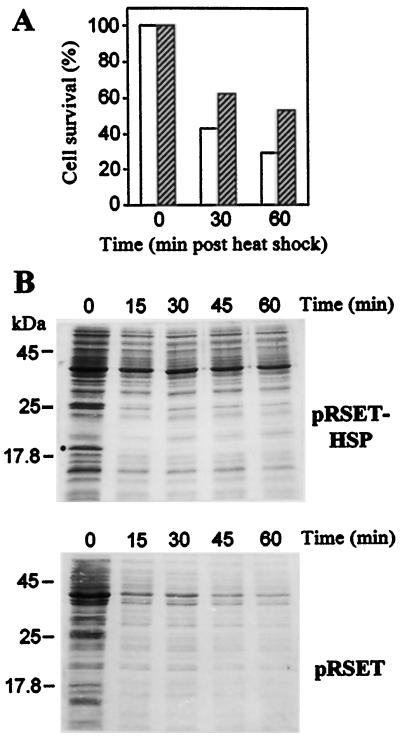
The effect of recombinant CsHSP17.5 on cell survival was evaluated in cultures subjected to heat stress. Two hours after IPTG addition, cultures were diluted to 6 × 106 cells mL−1 and then transferred to 50°C, a temperature that is known to cause cell autolysis. At various times after the temperature shift, cell viability was measured by counting colony-forming units in serially diluted culture aliquots. Whereas cell viability decreased rapidly in both pRSET and pRSET-HSP cultures upon heat shock (Fig. 4A), the measured survival rates were significantly higher in cells overexpressing CsHSP17.5 (approximately 2-fold after 60 min at 50°C). Similar differences were observed when untransformed wild-type cells were used for comparison (not shown). Because in vitro molecular chaperone activity has been demonstrated previously for seed sHSP (Collada et al., 1997), we investigated whether the expression of recombinant CsHSP17.5 had any effect on the thermostability of bacterial soluble proteins. At various times after the shift to 50°C, culture aliquots were taken and the cells pelleted, washed, and extracted as described in Methods. SDS-PAGE analysis of these extracts showed that, although many soluble proteins precipitated or were rapidly degraded in control cells during the heat shock, this effect was delayed and quantitatively less pronounced in pRSET-HSP cells (Fig. 4B). Such an increase in protein thermostability is associated with a rapid insolubilization of recombinant CsHSP17.5. The inclusion of 6 m urea and 2% (w/v) SDS in the extraction buffer (not shown) ruled out degradation of CsHSP17.5 during the 50°C treatment.
Figure 4.
Protective effect of recombinant CsHSP17.5 on cell viability and protein stability upon heat stress in vivo. A, Viability of E. coli transformants for pRSET-HSP (hatched bars) and pRSET (white bars) constructs subjected to 50°C treatments. At the times indicated after the temperature shift, culture samples were taken, serially diluted, and plated onto Luria-Bertani plus ampicillin plates. Cell viability is plotted as the percentage of colony-forming units relative to the starting number of colonies at time 0. Means of three independent experiments are shown (sd was less than 5%). B, SDS-PAGE analysis of bacterial soluble proteins during heat shock at 50°C. Based on the results described above, culture samples corresponding to similar amounts of viable cells were taken at 0, 15, 30, 45, and 60 min after heat shock. Cells (pRSET-HSP and pRSET) were pelleted and soluble proteins extracted as described in Methods. The position of recombinant CsHSP17.5 is marked (•).
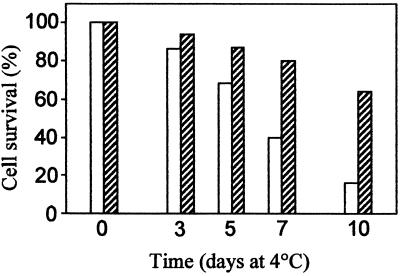
We also tested whether recombinant CsHSP17.5 might be relevant for cell viability at chilling temperatures, as hypothesized for heat-induced tomato sHSPs (Sabehat et al., 1996, 1998; Kadyrzhanova et al., 1998). For these experiments aliquots from IPTG-induced cultures were plated and kept at 4°C. At different times cell viability was measured by counting colony-forming units in triplicate plates. As shown in Figure 5, both control pRSET cells and cells overexpressing CsHSP17.5 lost viability upon storage in the cold, although at significantly different rates. The control cells died with a half-life of 5 to 6 d, and after 10 d at 4°C only about 10% remained alive; conversely, approximately 60% of the pRSET-HSP cells survived after the same period.
Figure 5.
Effect of recombinant CsHSP17.5 on cell viability at 4°C. Cultures grown normally and induced with IPTG for 2 h at 37°C were diluted and plated onto Luria-Bertani agar supplemented with 100 μg/mL ampicillin and 1 mm IPTG. Plates were then kept at 4°C. At the times indicated after temperature downshift, plates were transferred to 37°C and cell viability was estimated as in Figure 4A. Means of three independent experiments are shown (sd was less than 5%). White bars, pRSET cells; hatched bars, pRSET-HSP cells.
Expression of Cs hsp17.5 Homologs in Chestnut Plantlets
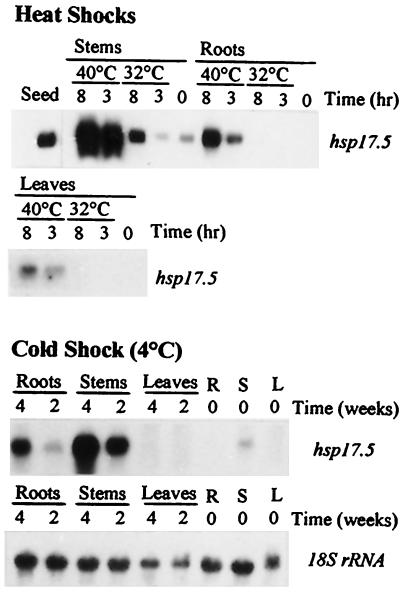
A single hybridizing band was detected when total RNA from late-mature chestnut cotyledons was probed at moderate stringency with the cDNA for CsHSP17.5 (Fig. 6). Under the same conditions a weak band of similar size could be observed in RNA from stems, but not from roots or leaves, of nonstressed chestnut plantlets. However, when plantlets of the same age were subjected to thermal stress, increased transcript abundance was observed for Cs hsp17.5 homologs in all organs analyzed. Expression was higher at 40°C than at 32°C in all cases and appeared to be especially pronounced in stems.
Figure 6.
Induction of Cs hsp17.5 homologs in 18-week-old chestnut plantlets subjected to heat or cold stress. RNA was extracted and analyzed by northern hybridization using as a probe the first 662 bp of Cs hsp17.5 cDNA (EcoRI-SalI fragment). For heat-stress experiments, plantlets were treated at either 32°C or 40°C for 8 h. Cold-stress experiments were carried out at 4°C for up to 4 weeks. The amount of RNA loaded per lane was approximately 3 μg (seeds) or 10 μg. As a control, the same filters were hybridized with a barley 18S ribosomal probe (shown here for the cold treatment). All hybridizing bands were approximately 730 nucleotides in size. R, Roots; S, stems; L, leaves.
On the other hand, induction of Cs hsp17.5 homologs could be observed in chestnut plantlets kept at 4°C for up to 4 weeks (Fig. 6). Although the highest transcript levels were also found in stems, the time course of induction was much slower than under heat-stress conditions. It is noteworthy that no hybridizing bands could be detected under the same experimental conditions in leaves of cold-stressed plants. Likewise, no transcripts were detected when total RNA from leaves, stems, or roots of salt-stressed chestnut plantlets was hybridized with the same probe (not shown). The patterns observed in control samples (leaves, stems, or roots) at the beginning of the cold shock (shown in Fig. 6) were consistent throughout the experiment and several months later. Hybridization of the same filters with an 18S ribosomal probe was performed to verify that similar amounts of RNA were loaded in each lane.
DISCUSSION
It has been shown that mature chestnut cotyledons accumulate a highly abundant sHSP under normal growing conditions. This protein (CsHSP17.5) forms oligomeric complexes under nondenaturing conditions and possesses molecular chaperone activity (Collada et al., 1997). As a first step toward analyzing its possible function in vivo, we have isolated and characterized a full-length cDNA clone that encodes CsHSP17.5 (Fig. 1). The predicted polypeptide (154 amino acids) includes the two internal peptidic sequences previously determined for the chestnut seed sHSP. Moreover, monospecific antibodies to the purified seed protein recognized recombinant CsHSP17.5 when expressed in E. coli (Fig. 3B). The highest sequence identity in databank searches was found with class I cytosolic sHSPs, including a characteristic N-terminal motif (residues 10–24) that was absent from class II sHSPs. Aside from cytosolic members (class I and II), the plant sHSP family includes proteins localized to the chloroplasts, ER, and mitochondria (Boston et al., 1996; Waters et al., 1996). Immunoelectron microscopic studies of chestnut cotyledonary cells revealed an overall cytoplasmic localization for CsHSP17.5 (Fig. 2), as predicted by sequence analysis.
In spite of their abundance and unique multiplicity, little is known at present about the specific role of plant sHSPs (Boston et al., 1996). Recent overexpression experiments involving α-crystallins and sHSPs of animal origin (for review, see Buchner, 1996), as well as a rice sHSP-glutathione S-transferase fusion (Yeh et al., 1997), have illustrated the ability of these proteins to confer thermotolerance. To investigate the possible function of CsHSP17.5 in vivo, we introduced its coding sequence into E. coli using the pRSET expression vector. It is known that this organism does not synthesize class I sHSPs in response to heat stress (Buchner, 1996; Yeh et al., 1997). In our study the coding sequence of Cs hsp17.5 cDNA was engineered so that no vector-encoded amino acids were present in the recombinant protein.
As shown in Figure 4A, we found that overexpression of CsHSP17.5 in E. coli was correlated with maintenance of viability under heat-stress conditions. Furthermore, SDS-PAGE analysis of cell lysates suggested that the protective effect of CsHSP17.5 is associated with an increase in the thermostability of soluble proteins (Fig. 4B). Whether such stabilization is due directly to the chaperone function of the sHSP or to interactions with other E. coli proteins (e.g. heat-induced chaperones) remains to be determined. Like other sHSPs, CsHSP17.5 can bind nonnative proteins in vitro and promote their renaturation in an ATP-independent manner (Collada et al., 1997; M.-A. Guevara, C. Aragoncillo, and L. Gomez, unpublished results). On the other hand, in vitro refolding experiments, as well as studies with transgenic Arabidopsis cell cultures, support the notion that sHSPs act in concert with other HSPs during the refolding process (Ehrnsperger et al., 1997; Forreiter et al., 1997; Lee et al., 1997). The increased thermotolerance of pRSET-HSP cells could also be related to a hypothetical effect of CsHSP17.5 on membrane stability under heat-shock conditions. Supportive evidence of membrane stabilization has been obtained for GroEL/ES chaperones (Török et al., 1997) and a chloroplastic sHSP from Synechocystis PCC 6803 (Horváth et al., 1998). Likewise, heat-induced membrane association was recently described for a prokaryotic sHSP homologous to CsHSP17.5 (Jobin et al., 1997).
It is well established that exposure of plants to moderately high temperatures induces thermotolerance (Vierling, 1991). Less expected was the finding that prestorage heat treatments increase the chilling tolerance of a number of marketable fruits and vegetables (e.g. Lurie and Klein, 1991; McCollum et al., 1995; Sabehat et al., 1996). In agreement with these observations, experimental evidence is presented here that recombinant CsHSP17.5 is important in maintaining cell viability at low temperatures. As shown in Figure 5, pRSET-HSP cells overexpressing the chestnut protein died more slowly upon storage at 4°C than control cells. Such an effect on bacterial viability has been demonstrated so far only for TF (trigger factor), an abundant E. coli protein up-regulated by low temperatures (Kandror and Goldberg, 1997). Molecular chaperone activity has been reported for both TF and CsHSP17.5.
Although the precise reasons why bacterial cells die at 4°C have not yet been discovered, the protective effect of CsHSP17.5 might be due to the maintenance of proteins in a functional conformation, as in the case of heat stress. That role would be especially relevant at low temperatures, at which ribosomal function is inhibited and the solubility and folding properties of many proteins are substantially altered. Chaperones of the HSP70 family that are induced by low but not high temperatures were recently described in yeast and E. coli (Craig et al., 1993; Thieringer et al., 1998). Aside from representing an example of improved chilling tolerance mediated by a nonbacterial protein, our results with pRSET-HSP cells subjected to cold stress demonstrate a novel in vivo role for sHSPs. This role had been hypothesized based on the correlation between the acquisition of chilling tolerance in heat-treated tomato fruits and the expression of sHSPs and other heat-induced proteins (Sabehat et al., 1996, 1998; Kadyrzhanova et al., 1998).
The recalcitrant seeds of chestnut, in which CsHSP17.5 accumulates abundantly, have one of the highest moisture contents known at shedding (typically >50%). Compared with orthodox seeds, high-moisture seeds are more sensitive to certain types of environmental stresses. Found naturally in a wide area of southern Europe, the seeds of chestnut must endure extreme temperatures both during maturation (August to mid-October) and during the winter, shortly after shedding. Temperatures frequently range from above 35°C in summer to below 0°C in winter. The results reported here support a role for CsHSP17.5 in protecting seed tissues against the damaging effects of both thermal extremes. This notion is reinforced by the finding that transcripts hybridizing with the Cs hsp17.5 cDNA are induced in vegetative organs of chestnut plantlets subjected to either heat or cold stress, but not salt stress (Fig. 6). In these experiments the strongest inductions were observed in stems, in which constitutive expression also occurs. Although the thermostabilizing function of sHSPs was recently extended to lipid membranes (Jobin et al., 1997; Horváth et al., 1998), the exact biochemical mechanisms by which sHSPs, and probably other HSPs, attenuate both heat- and cold-induced cell damage remain to be determined. The differential induction kinetics observed in chestnut plantlets at high and low temperatures (Fig. 6) may reflect fundamental differences in cellular requirements at each thermal extreme.
The accession number of the sequence reported in this article is AJ009880.
ACKNOWLEDGMENTS
We thank Drs. G. Salcedo and J.M. Malpica for helpful comments, Dr. J. Jordano for the Ha hsp17.6 cDNA clone, and J. Garcia for technical assistance. The barley 18S ribosomal probe was the kind gift of Dr. A. Molina.
Abbreviations:
- HSP
heat-shock protein
- IPTG
isopropyl-1-thio-β-galactoside
- sHSP
small (low-molecular-mass) HSP
Footnotes
This research was supported by grant no. BIO96-0441 from the Ministerio de Educación y Cultura, Spain, and by grant no. 07B-012-97 from Comunidad Autónoma de Madrid, Spain. A.S. was the recipient of a predoctoral fellowship from the Ministerio de Educación y Cultura, Spain.
LITERATURE CITED
- Almoguera C, Jordano J. Developmental and environmental concurrent expression of sunflower dry-seed-stored low-molecular heat-shock protein and Lea mRNAs. Plant Mol Biol. 1992;19:781–792. doi: 10.1007/BF00027074. [DOI] [PubMed] [Google Scholar]
- Boston RS, Viitanen PV, Vierling E. Molecular chaperones and protein folding in plants. Plant Mol Biol. 1996;32:191–222. doi: 10.1007/BF00039383. [DOI] [PubMed] [Google Scholar]
- Buchner J. Supervising the fold: functional principles of molecular chaperones. FASEB J. 1996;10:10–19. [PubMed] [Google Scholar]
- Carrasco R, Almoguera C, Jordano J. A plant small heat shock protein gene expressed during zygotic embryogenesis but noninducible by heat stress. J Biol Chem. 1997;272:27470–27475. doi: 10.1074/jbc.272.43.27470. [DOI] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep. 1993;11:113–116. [Google Scholar]
- Coca MA, Almoguera C, Jordano J. Expression of sunflower low-molecular-weight heat-shock proteins during embryogenesis and persistence after germination: localization and possible functional implications. Plant Mol Biol. 1994;25:479–492. doi: 10.1007/BF00043876. [DOI] [PubMed] [Google Scholar]
- Collada C, Gomez L, Casado R, Aragoncillo C. Purification and in vitro chaperone activity of a class I small heat-shock protein abundant in recalcitrant chestnut seeds. Plant Physiol. 1997;115:71–77. doi: 10.1104/pp.115.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EA, Gambill BD, Nelson RJ. Heat shock proteins: molecular chaperones of protein biogenesis. Microbiol Rev. 1993;57:402–414. doi: 10.1128/mr.57.2.402-414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRocher AE, Vierling E. Developmental control of small heat shock protein expression during pea seed maturation. Plant J. 1994;5:93–102. [Google Scholar]
- Ehrnsperger M, Gräber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forreiter C, Kirschner M, Nover L. Stable transformation of an Arabidopsis cell suspension culture with firefly luciferase providing a cellular system for analysis of chaperone activity in vivo. Plant Cell. 1997;9:2171–2181. doi: 10.1105/tpc.9.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Casado G, Collada C, Allona I, Casado R, Pacios LF, Aragoncillo C, Gomez L. Site-directed mutagenesis of active site residues in a class I endochitinase from chestnut seeds. Glycobiology. 1998;8:1021–1028. doi: 10.1093/glycob/8.10.1021. [DOI] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Hernández LD, Vierling E. Expression of low molecular weight heat shock proteins under field conditions. Plant Physiol. 1993;101:1209–1216. doi: 10.1104/pp.101.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth I, Glatz A, Varvasovski V, Török Z, Páli T, Balogh G, Kovács E, Nádasdi L, Benkö S, Joó F and others. Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: identification of hsp17 as a “fluidity gene.”. Proc Natl Acad Sci USA. 1998;95:3513–3518. doi: 10.1073/pnas.95.7.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. α-Crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Jinn TL, Chen YM, Lin CY. Characterization and physiological function of class I low-molecular-mass, heat-shock protein complex in soybean. Plant Physiol. 1995;108:693–701. doi: 10.1104/pp.108.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinn TL, Yeh YC, Chen YM, Lin CY. Stabilization of soluble proteins in vitro by heat shock proteins-enriched ammonium sulfate fraction from soybean seedlings. Plant Cell Physiol. 1989;30:463–469. [Google Scholar]
- Jobin MP, Delmas F, Garmyn D, Diviès D, Guzzo J. Molecular characterization of the gene encoding an 18-kDa small heat shock protein associated with the membrane of Leuconostoc oenos. Appl Environ Microbiol. 1997;63:609–614. doi: 10.1128/aem.63.2.609-614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadyrzhanova DK, Vlachonasios KE, Ververidis P, Dilley DR. Molecular cloning of a novel heat induced/chilling tolerance related cDNA in tomato fruit by use of mRNA differential display. Plant Mol Biol. 1998;36:885–895. doi: 10.1023/a:1005954909011. [DOI] [PubMed] [Google Scholar]
- Kandror O, Goldberg AL. Trigger factor is induced upon cold shock and enhances viability of Escherichia coli at low temperatures. Proc Natl Acad Sci USA. 1997;94:4978–4981. doi: 10.1073/pnas.94.10.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GJ, Pokala N, Vierling E. Structure and in vitro molecular chaperone activity of cytosolic small heat shock proteins from pea. J Biol Chem. 1995;270:10432–10438. doi: 10.1074/jbc.270.18.10432. [DOI] [PubMed] [Google Scholar]
- Lee GJ, Roseman AM, Saibil HR, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie S, Klein JD. Acquisition of low temperature tolerance in tomatoes by exposure to high temperature stress. J Am Soc Hortic Sci. 1991;116:1007–1012. [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- McCollum TG, Doostdar H, Mayer RT, McDonald RE. Immersion of cucumber fruit in heated water alters chilling-induced physiological changes. Postharv Biol Technol. 1995;6:55–64. [Google Scholar]
- Muchowski PJ, Clark JI. ATP-enhanced molecular chaperone functions of the small heat-shock protein human αB crystallin. Proc Natl Acad Sci USA. 1998;95:1004–1009. doi: 10.1073/pnas.95.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Shijavi R, Krans JV, Luthe DS. Heat-shock response in heat-tolerant and non-tolerant variants of Agrostis palustris Huds. Plant Physiol. 1996;111:515–524. doi: 10.1104/pp.111.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cerezo E, Findlay K, Shaw JG, Lomonossoff GP, Qiu SG, Linstead P, Shanks M, Risco C. The coat anti cylindrical inclusion proteins of a potyvirus are associated with connections between plant cells. Virology. 1997;236:296–306. doi: 10.1006/viro.1997.8736. [DOI] [PubMed] [Google Scholar]
- Sabehat A, Lurie S, Weiss D. Expression of small heat-shock proteins at low temperatures. A possible role in protecting against chilling injuries. Plant Physiol. 1998;117:651–658. doi: 10.1104/pp.117.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabehat A, Weiss D, Lurie S. The correlation between heat-shock protein accumulation and persistence and chilling tolerance in tomato fruit. Plant Physiol. 1996;110:531–537. doi: 10.1104/pp.110.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y, Lindquist S. HSP104 required for induced thermotolerance. Science. 1990;248:1112–1115. doi: 10.1126/science.2188365. [DOI] [PubMed] [Google Scholar]
- Thieringer HA, Jones PG, Inouye M. Cold shock and adaptation. Bioessays. 1998;20:49–57. doi: 10.1002/(SICI)1521-1878(199801)20:1<49::AID-BIES8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Török Z, Horváth I, Goloubinoff P, Kovács E, Glatz A, Balogh G, Vígh L. Evidence for a lipochaperonin: association of active protein-folding GroESL oligomers with lipids can stabilize membranes under heat shock conditions. Proc Natl Acad Sci USA. 1997;94:2192–2197. doi: 10.1073/pnas.94.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling E. The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:579–620. [Google Scholar]
- Waters ER, Lee GJ, Vierling E. Evolution, structure and function of the small heat shock proteins in plants. J Exp Bot. 1996;47:325–338. [Google Scholar]
- Yeh CH, Chang PL, Yeh KW, Lin WC, Chen YM, Lin CY. Expression of a gene encoding a 16.9-kDa heat-shock protein, Oshsp16.9, in Escherichia coli enhances thermotolerance. Proc Natl Acad Sci USA. 1997;94:10967–10972. doi: 10.1073/pnas.94.20.10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Nieden U, Neumann D, Bucka A, Nover L. Tissue-specific localization of heat-stress proteins during embryo development. Planta. 1995;196:530–538. [Google Scholar]