Abstract
Cyanide-resistant (“alternative”) respiration was studied in Arabidopsis during incompatible and compatible infection with Pseudomonas syringae pv tomato DC3000. Total leaf respiration increased as the leaves became necrotic, as did the cyanide-resistant component that was sensitive to salicylhydroxamic acid. Infiltration of leaves with an avirulent strain rapidly induced alternative oxidase (AOX) mRNA, whereas the increase was delayed in the compatible combination. The increase in mRNA correlated with the increase in AOX protein. Increased expression was confined to the infected leaves, in contrast to the pathogenesis-related protein-1, which was induced systemically. Virtually all of the AOX protein was in the reduced (high-activity) form. Using transgenic NahG and mutant npr1-1 and etr1-1 plants, we established that the rapid induction of the AOX was associated with necrosis and that ethylene, but not salicylic acid, was required for its induction. Increased pyruvate levels in the infected leaves suggested that increased substrate levels were respired through the alternative pathway; however, in the control leaves and the infected leaves, respiration was not inhibited by salicylhydroxamic acid alone. Increased respiration appeared to be associated primarily with symptom expression rather than resistance reactions.
Mitochondrial respiration provides the energy necessary to drive metabolic and transport processes in cells. During electron transport along the Cyt pathway a proton gradient is generated across the inner mitochondrial membrane, which allows ATP synthesis. Plant mitochondria contain an additional alternative electron-transport pathway that branches off from the Cyt pathway at the level of ubiquinone; it is not inhibited by cyanide and does not contribute to the formation of a proton gradient. This pathway is therefore not coupled to ATP synthesis (Siedow and Umbach, 1995). Electron transport along this alternative pathway involves only a single quinol oxidase, termed the AOX. The energy of electron flow through the alternative pathway is mainly lost as heat (Moore and Siedow, 1991; Siedow and Umbach, 1995). This process seems wasteful and the physiological significance of the alternative pathway in the metabolism of these plants is still unclear. One example of alternative pathway activity during an essential part of the life cycle is in the reproduction of Araceae. The spadix is heated 10°C or more to volatilize odoriferous compounds that attract pollinators (Meeuse, 1975; Raskin et al., 1987). The alternative pathway is also found in most other plant species and plant organs and thus must serve other functions as well.
Cyanide-resistant plant respiration is increased during various stress conditions, e.g. low temperature, wounding, and plant diseases (Uritani and Asahi, 1980; Hiser and McIntosh, 1990; Vanlerberghe and McIntosh, 1992; Purvis and Shewfelt, 1993). AOX protein levels are also increased after wounding, infection, and low temperature conditions (Hiser and McIntosh, 1990; Vanlerberghe and McIntosh, 1992; Lennon et al., 1997), suggesting a role for the alternative respiration in stress alleviation. However, we do not know whether or to what extent the cyanide-resistant pathway also contributes to respiration under these conditions. Enhanced operation of the alternative pathway might relieve the Cyt pathway and prevent overreduction, thus reducing the formation of harmful radicals (Purvis and Shewfelt, 1993; Wagner and Krab, 1995). Nevertheless, the significance of the alternative respiratory pathway during stress remains to be elucidated. It is interesting that increased expression of both the AOX in Arum lily spadices during flowering and of PRs during resistance responses in, for example, tobacco, requires SA as a signal (Raskin et al., 1987; Rhoads and McIntosh, 1992; Delaney et al., 1994; Lennon et al., 1997). The latter is associated with the occurrence of SAR against further infections. Addition of SA to cell suspensions or intact leaves of tobacco also induces aox gene expression (Rhoads and McIntosh, 1993; Lennon et al., 1997). Together, these results suggest that SA acts as a signal in inducing both AOX and resistance responses in infected plants and that the alternative pathway might be associated with the resistant state.
The observations described above prompted us to investigate the possible relationship between the induction of the alternative pathway and resistance expression in Arabidopsis infection and whether SA is involved as a signal in both responses. Arabidopsis infected with the leaf-spotting bacterium Pseudomonas syringae is a well-described plant-pathogen system in which several features of the resistance response, as well as the involvement of SA, have been established (Whalen et al., 1991; Uknes et al., 1993; Cameron et al., 1994; Delaney et al., 1994). Using an avirulent and a virulent strain of P. syringae we studied whether the resistance response of the plant was associated with induction of the alternative pathway. The expression of the aox gene was compared with the expression of the gene encoding PR-1, which is a good marker for SA-dependent expression of SAR in Arabidopsis (Uknes et al., 1993).
To study the significance of SA for the induction of AOX in infected plants, transgenic and/or mutant Arabidopsis plants were used. Plants expressing the bacterial nahG gene, encoding the enzyme salicylate hydroxylase, which converts SA to catechol, are unable to accumulate SA. These transgenics exhibit an increased susceptibility to pathogens and are unable to express SAR (Delaney et al., 1994). Mutant npr1-1 plants that are unable to express PRs or SAR upon infection (Cao et al., 1994) are defective in the subsequent signaling pathway. Expression of AOX was monitored in these plants in response to infection. Because ethylene stimulates cyanide-resistant respiration in plant organs (Laties, 1982), we also investigated the possible involvement of ethylene. This hormone plays a role in the responses to infections in the plant and its production is strongly increased in infected plant tissues, coinciding with necrosis (Uritani and Asahi, 1980; De Laat and Van Loon, 1982, 1983; Brederode et al., 1991). Expression of AOX was monitored upon infection of the Arabidopsis etr1-1 mutant, in which the perception of ethylene was strongly reduced (Schaller and Bleecker, 1995).
MATERIALS AND METHODS
Plant Material, Bacterial Strains, and Growth Conditions
Seeds of Arabidopsis ecotype Columbia (Col-0) wild-type, nahG transgenic (Delaney et al., 1994), npr1-1 (Cao et al., 1994), etr1-1 (Bleecker et al., 1988), and rps2-201 (Kunkel et al., 1993) mutants were sown on quartz sand. After 2 weeks the seedlings were transferred to a mixture of autoclaved potting soil and sand (12:5). Plants were cultivated in a growth chamber with 9-h d (200 μmol m−2 s−1 at 24°C) and 15-h night (20°C) cycles and 65% RH. Twice a week plants were supplied with water or modified one-half-strength Hoagland nutrient solution: 2 mm KNO3, 5 mm Ca(NO3)2, 1 mm KH2PO4, 1 mm MgSO4, and trace elements, pH 7.0 (Hoagland and Arnon, 1938), containing 10 μm Sequestreen (CIBA-Geigy, Basel, Switzerland).
Pst (Pseudomonas syringae pv tomato) (Dong et al., 1991; Whalen et al., 1991) strains DC3000 (pLH12Ω; virulent) and DC3000 (pLH12; avirulent) were provided by Dr. A.F. Bent (University of Illinois, Urbana). These strains were grown at 28°C on King's medium B (King et al., 1954), containing 40 mg L−1 tetracycline. Nonpathogenic Pseudomonas fluorescens strains WCS417 and WCS374, originally isolated from the rhizosphere of wheat (Lamers et al., 1988) and potato (Geels and Schippers, 1983), respectively, were grown similarly in the absence of the antibiotic. Escherichia coli strain DH5α, harboring pGEM-3Z constructs, was grown in Luria-Bertani medium supplemented with 100 mg L−1 ampicillin.
Plants were inoculated for 5 weeks after sowing. For treatment of the plants, bacterial cultures were washed and resuspended in 10 mm MgCl2. Sterile 10 mm MgCl2 was used as a control. The bacterial suspension or the control solution was then pressure infiltrated into the abaxial side of the leaves using a syringe without a needle (Swanson et al., 1988). For treatment of leaves with SA, leaves were infiltrated with neutralized SA solutions. Treatment of leaves with ACC was carried out by dipping the leaves in a solution of 1 mm ACC and 0.01% Silwet L77 (v/v) in water (Van Meeuwen Chemicals, Weest, The Netherlands). Controls were performed using 0.01% Silwet (v/v) in water.
Respiration of Infected Leaves
Leaves were detached, cut into four pieces with a razor blade, and kept in the dark for 15 min before measurement. Pieces were transferred into an air-tight cuvette containing 20 mm Hepes (pH 7.2) and 0.2 mm CaCl2 (Atkin et al., 1993), and O2 uptake was measured as a decrease of O2 concentration using a Clark-type electrode (YSI, Yellow Springs, OH). Cyanide-resistant O2 uptake was measured in the presence of 0.5 mm KCN. To assess whether the cyanide-resistant component was due to the presence of the alternative pathway, we used an appropriate concentration (2 mm) of the inhibitor SHAM. The effect of SHAM was also assessed in the absence of KCN.
Quantification of Transcript Levels of AOX and PR-1
The competitive RT-PCR (Siebert and Larrick, 1992) was used to semiquantitatively determine AOX and PR-1 transcript levels in leaves. Poly(A+)RNA was isolated from several leaves of three plants using the QuickPrep Micro mRNA Purification kit (Pharmacia Biotech, Uppsala, Sweden); 200 ng was used for reverse transcription with the Ready-To-Go T-Primed First-Strand kit (Pharmacia Biotech). Competitive RT-PCR was then carried out using two gene-specific oligonucleotides as primers in the amplification reaction, 0.8 μL of the first-strand mixture containing the cDNA and 0.8 μL containing 1, 5, 10, 50, 100, and 500 pg of competitor DNA. The gene-specific oligonucleotides were based on the published sequence of Arabidopsis AOX (Kumar and Söll, 1992) and yield a fragment of approximately 350 bp. A 400-bp heterologous competitor DNA fragment, competing for the same set of primers, was obtained as described by Siebert and Larrick (1992). After agarose-gel electrophoresis in the presence of ethidium bromide, the resulting PCR products were quantified upon UV illumination. The dilution of the competitor DNA, yielding an approximate equimolar amount of product as the target cDNA, was taken as a measure of target mRNA level. Transcript levels were expressed as relative values, taking the level in noninfected control treatments as 1. A similar procedure was carried out to determine PR-1 mRNA levels using the primer set and competitor DNA as described by Pieterse et al. (1996). Northern blots, probed with the Arabidopsis AOX-specific PCR product described above, failed to show specific bands, probably because of low AOX transcript levels. Northern analysis using total RNA extracts, however, agreed with competitive RT-PCR results of PR-1 mRNA levels (Pieterse et al., 1996), which confirmed the validity of the RT-PCR results. All experiments were carried out at least twice.
Western Blotting
Total leaf extracts were prepared from 100 mg (fresh weight) of frozen leaf material obtained from several plants. The material was ground in liquid N2 using a mortar and pestle and suspended in a total volume of 400 μL of a protein sample mixture (62.5 mm Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, and 0.001% bromphenol blue). After the sample was centrifuged for 10 min at 14,000 rpm in an Eppendorf centrifuge to precipitate cell debris, the proteins were separated by SDS-PAGE according to the method of Laemmli (1970) and subsequently electrotransferred to nitrocellulose filters using a blot-transfer buffer (25 mm Tris, 192 mm Gly, and 20% [v/v] methanol). Immunodetection of the AOX protein was carried out according to the product protocol of the AOX monoclonal antibody, aminooxyacetic acid (GT monoclonal antibodies kindly provided by Dr. T.E. Elthon, University of Nebraska, Lincoln), which was used as a primary antibody (1:50). A conjugate of Fab fragments of anti-mouse IgG from sheep and peroxidase (Boehringer Mannheim) was used as the secondary antibody (1:25,000). AOX protein was then detected by chemiluminescence using a substrate (SuperSignal ULTRA, Pierce), according to the protocol provided by the manufacturer.
Determination of Pyruvate Concentration in Leaves
For the determination of pyruvate concentrations in leaves, about 1 g of fresh leaves was ground as described above. Preparation of the samples and measurement of the pyruvate concentration was carried out essentially as described by Wagner and Wagner (1995), with the addition of an extra purification step in which the final sample mixture was mixed with active C and subsequently filtered. Conversion of NADH to NAD+ in the presence of lactate dehydrogenase (Boehringer Mannheim) was recorded at a wavelength of 340 nm.
RESULTS
Total and Cyanide-Resistant, SHAM-Sensitive Respiration in Leaves Infiltrated with Avirulent or Virulent Bacterial Strains
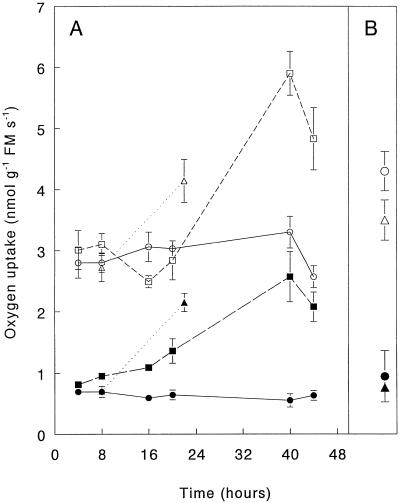
To determine whether infiltration of Arabidopsis leaves with either a virulent or an avirulent strain of Pst affected respiratory activity, we measured rates of O2 uptake of infected leaves and mock-infiltrated leaves. Leaves infiltrated with the avirulent strain collapsed within 1 d because of the HR, whereas those infiltrated with the virulent strain started to necrose 2 d after infiltration. Infiltration of the leaves with 107 CFU mL−1 avirulent pathogen resulted in a rapid increase in total and in cyanide-resistant, SHAM-sensitive O2 uptake (Fig. 1A). SHAM did not affect leaf respiration in the absence of KCN (data not shown). The compatible plant-pathogen combination showed a similar, but delayed, change in the total and cyanide-resistant, SHAM-sensitive O2 uptake (Fig. 1A). Noninfected leaves of plants that were infiltrated with the avirulent strain did not show a systemic increase in cyanide-resistant, SHAM-sensitive respiration (Fig. 1B). The increase of total O2 uptake in both the compatible and the incompatible combination coincided with an increase in the KCN-resistant component that was sensitive to SHAM (i.e. the alternative pathway). However, the lack of an effect of SHAM in the absence of KCN provides no information concerning the contribution of the alternative pathway to respiration in the absence of inhibitors.
Figure 1.
Total and cyanide-resistant, SHAM-sensitive O2-uptake rates of wild-type Arabidopsis leaves infiltrated with avirulent or virulent strains of Pst, expressed in units of fresh mass (FM). A, Leaves infiltrated with suspensions containing 107 CFU of the pathogens per mL. Circles, Control; triangles, avirulent pathogen; and squares, virulent pathogen. Open symbols indicate total O2-uptake of the leaves; closed symbols indicate cyanide-resistant, SHAM-sensitive O2 uptake. B, Systemic, noninfiltrated leaves of mock plants infiltrated on lower leaves 3 d earlier. Results are mean values ± se; n = 4.
Induction of AOX in Response to Infection
AOX transcript levels were determined at intervals in leaves after infiltration with Pst, with AOX-specific primers yielding a 350-bp DNA fragment. To test whether this fragment was specific for AOX, the fragment was ligated into a pGEM-3Z vector and transferred into E. coli strain DH5α. From the resulting clones, 20 were subjected to a second amplification round, using degenerate aox-specific nested primers based on highly conserved sequences of plant AOX, as reported by Whelan et al. (1996). All clones gave a PCR product of the expected size (about 180 bp). Two of the clones containing the 350-bp fragment were also subjected to DNA-sequence analysis. The DNA sequence of the fragment was identical to the matching part of the published aox sequence from Arabidopsis, confirming that the amplification product of 350 bp corresponded to AOX.
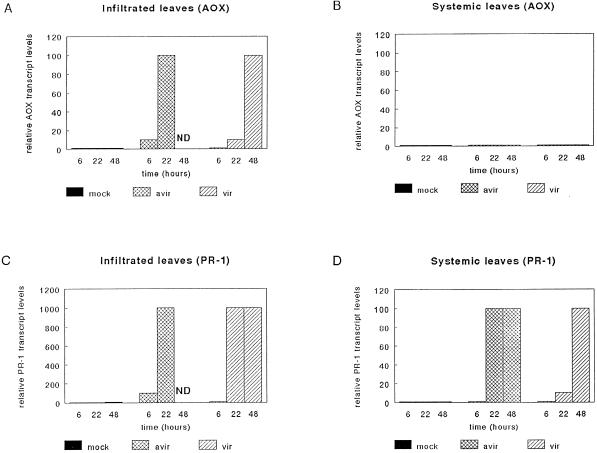
In the incompatible combination, AOX transcript levels were enhanced 10-fold within 6 h after infiltration (Fig. 2A). After 22 h, just before total collapse of the leaves (after which mRNA could no longer be isolated), AOX transcript levels were increased 100-fold. Leaves infiltrated with the virulent strain showed a similar increase of AOX transcripts; however, it was delayed by about 1 d (Fig. 2A). These differential time-dependent induction patterns were associated with the rate of necrosis development in the infiltrated leaves. Noninfected leaves of infected plants did not show any induction after infiltration with either the avirulent or the virulent Pst strain, indicating that there was no measurable systemic increase in AOX transcripts (Fig. 2B).
Figure 2.
AOX and PR-1 transcript levels in Arabidopsis treated with avirulent (avir) or virulent (vir) Pst. Leaves were infiltrated with bacterial suspensions containing 107 CFU, and transcript levels were determined in the infiltrated leaves and in the unaffected, systemic leaves of the same plants. Values are given relative to the control level (set at 1) in mock-infiltrated leaves. ND, Not detectable.
To compare the induction of AOX transcripts with that of SAR-associated PR-1 mRNA, we measured PR-1 transcript levels in the same samples. The results (Fig. 2, C and D) show that, like AOX transcripts, the PR-1 transcript levels increased upon infiltration of the Pst strains into the leaves. Induction of PR-1 occurred earlier after infiltration with the avirulent strain than with the virulent strain, but it reached similar levels by 22 h. In contrast to the AOX transcript levels, however, PR-1 transcript levels were increased systemically. This increase developed more rapidly in the incompatible than in the compatible plant-pathogen combination (Fig. 3, C and D). Identical results were obtained in independent replicates.
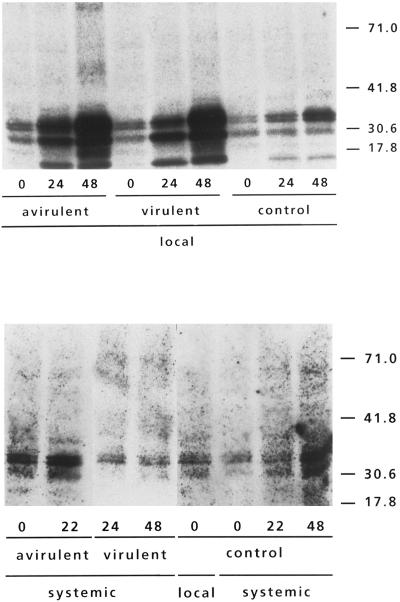
Figure 3.
Detection of AOX protein in Arabidopsis leaves treated with avirulent or virulent Pst. AOX was detected on western blots, using immunological staining of the protein. Top, Treated leaves: lanes 1 to 3, at 0, 24, and 48 h, respectively, after infiltration with the avirulent Pst strain; lanes 4 to 6, at 0, 24, and 48 h, respectively, after infiltration with the virulent Pst strain; and lanes 7 to 9, at 0, 24, and 48 h, respectively, after mock infiltration. Bottom, Nontreated leaves (except lane 5): lanes 1 and 2, at 0 and 22 h, respectively, after infiltration with the avirulent Pst strain; lanes 3 and 4, at 24 and 48 h, respectively, after infiltration with a virulent Pst strain; lane 5, control level in treated leaf immediately after mock infiltration (0 h); and lanes 6 to 8, at 0, 22, and 48 h. respectively, after mock infiltration.
Two Pseudomonas fluorescens strains (WCS374 and WCS417) were used as nonpathogenic controls to determine whether the induction of AOX was specific for an interaction with a pathogen. Neither of these two nonpathogenic strains induced AOX within 2 d after infiltration of the leaves with 107 CFU mL−1 (not shown), nor did they cause any visible symptoms in the leaves for the duration of the experiment. These results indicate that AOX induction is associated with infection of either a susceptible host by a virulent strain or a resistant host by an avirulent strain of a pathogen rather than with bacterial infiltration.
It is well established that there is a linear relationship between the concentration of avirulent bacteria infiltrated into a leaf and the extent of necrosis (Turner and Novacky, 1974). It has been estimated that leaves collapse completely when about 25% of the plant cells are involved in the HR, which occurs upon infiltration with a concentration of about 107 CFU mL−1. To determine the extent of the correlation between the concentration of the bacterial suspension and AOX expression, leaves were infiltrated with a range of bacterial concentrations. When 5 × 105 CFU of the avirulent strain per mL was used, the leaves did not show visible symptoms, whereas a concentration of 2 to 4 × 106 CFU mL−1 resulted in a delayed necrosis becoming visible 4 d after infiltration. Nevertheless, infiltration of no more than 2 × 106 CFU of the avirulent pathogen per mL resulted in a rapid, substantial increase in AOX transcripts 6 h after infiltration. No increase in AOX transcripts was detectable in leaves infiltrated with 5 × 105 CFU of the virulent pathogens per mL (Table I). Leaves infiltrated with less than 107 CFU of the compatible pathogen per mL also showed delayed disease symptoms, which was associated with a delayed AOX induction (Table I).
Table I.
Relative AOX transcript levels in leaves of Arabidopsis ecotype Col-0 infiltrated with different concentrations of avirulent or virulent Pst
| Treatment | Concentration | Time
|
||
|---|---|---|---|---|
| 6 h | 22 h | 48 h | ||
| CFU mL−1 | ||||
| Mock | 1 | 1 | 1 | |
| Avirulent Pst | 107 | 10 | 100 | NDa |
| 4 × 106 | 5 | 10 | 5 | |
| 2 × 106 | 5 | 5 | 1 | |
| 5 × 105 | 1 | 1 | 1 | |
| Virulent Pst | 107 | 1 | 10 | 100 |
| 2 × 106 | 1 | 1 | 5 | |
The AOX transcript level in the control treatment (mock) is set at 1.
ND, Not detectable. mRNA could no longer be isolated because of complete necrosis of the leaves.
Immunodetection of AOX Protein Reveals That It Mainly Occurs in a Reduced (High-Activity) Form in Infected Plant Leaves
To study whether the induction of the AOX mRNA after bacterial infiltration is accompanied by a higher concentration of the AOX protein, AOX was analyzed by western blotting. Leaves infiltrated with avirulent or virulent Pst showed an increase in AOX protein (Fig. 3A) in accordance with the increase in AOX transcript levels. Two major bands were visible on western blots, one larger than the 30.6-kD marker and one with an apparent molecular mass that was smaller than the 30.6-kD marker. As was found previously (Elthon et al., 1989), a band that is smaller than the 17.8-kD marker appeared as well; we will not further discuss this smallest band. As suggested by Kumar and Söll (1992), the smaller protein might represent a partial degradation product of the larger protein. However, since AOX in Arabidopsis is encoded by a multigene family (Saisho et al., 1997), as it is in soybean (Whelan et al., 1996), the different bands may reflect the expression of different genes. The intensity of both larger bands was enhanced in the pathogen-infiltrated leaves, and two smaller bands became visible. No readily detectable increase of AOX protein was found in noninfiltrated leaves of treated plants, in agreement with the finding that AOX transcript levels were not increased systemically.
In vivo the AOX enzyme occurs as a dimer, the subunits of which are linked by disulfide bridges. The oxidized form, in which the two monomers are covalently linked, is much less active than the reduced form of the AOX dimer (Umbach and Siedow, 1993). A large pool of reduced AOX was detectable in the samples from leaves infiltrated with either avirulent or virulent Pst (Fig. 3), indicating that a large portion of the enzyme was in its activated form. As in the intact roots of Poa annua (Millenaar et al., 1998), the oxidized form of the protein could be visualized by this method only after a prolonged exposure of the film.
Induction of AOX in Compatible and Incompatible Plant-Pathogen Combinations Is Not Dependent on SA but Is Associated with Necrosis
The observation that PR-1 is strongly induced systemically, whereas AOX is not, indicates that the induction pathways are different or that the threshold level for the induction of AOX is not reached in systemic noninfected leaves. To investigate a possible relationship between systemic effects of SA and the induction of AOX mRNA, leaves of an Arabidopsis npr1-1 mutant that is unable to express PRs or SAR upon infection with avirulent Pst were infiltrated with the pathogen. AOX was normally induced in the infiltrated leaves (Table II), whereas, as expected, no PR-1 mRNA was detected (not shown). This result clearly demonstrates that the induction pathways of PR-1 (and SAR) and AOX after infection are different.
Table II.
Relative AOX transcript levels in leaves of different transgenic and/or mutant Arabidopsis plants infiltrated with avirulent (Avir) or Virulent (Vir) Pst
| Arabidopsis | Type of Infiltration | Time
|
||
|---|---|---|---|---|
| 6 h | 22 h | 48 h | ||
| Wild type | Mock | 1 (1) | 1 (1) | 1 (1) |
| Avir Pst | 10 (102) | 100 (103) | NDa | |
| Vir Pst | 1 (1) | 10 (103) | 100 (103) | |
| NahG | Mock | 1 (10−2) | 1 (10−2) | 1 (10−2) |
| Avir Pst | 1 (10−2) | 10 (10−1) | ND | |
| Vir Pst | 1 (10−2) | 10 (10−2) | ND | |
| npr1-1 | Mock | 1 | 1 | –b |
| Avir Pst | 10 | ND | – | |
| Vir Pst | 1 | 10 | – | |
| rps2-201 | Mock | 1 | 1 | – |
| Avir Pst | 1 | 10 | – | |
| Vir Pst | 1 | 10 | – | |
Leaves were infiltrated with 107 CFU per mL or, as a control, mock infiltrated with 10 mm MgCl2. For a description of the different transgenics and/or mutants, see the text. The AOX transcript level of the control (mock-treated) leaves of the wild type is set at 1. For wild-type and NahG plants, PR-1 transcript levels are presented in parentheses, with control (mock-treated) leaves of the wild type set at 1.
ND, Not detectable. mRNA from this tissue could no longer be isolated because of complete necrosis.
–, Not determined.
To investigate whether exogenously applied SA induces AOX in Arabidopsis, leaves were infiltrated with solutions containing 0.01, 0.1, 0.5, or 1 mm SA. Higher concentrations resulted in toxic effects. At the applied SA concentrations, there was no increase in AOX transcript levels after 6, 24, or 48 h (not shown). In contrast, 1 d after infiltration with 1 mm SA, PR-1 transcript levels had increased approximately 10-fold; 0.1 mm was the lowest SA concentration that gave a slight increase (5-fold) in PR-1 transcripts, which was detected 1 d after infiltration (not shown).
A possible involvement of SA in AOX induction during pathogenesis was further investigated in leaves infiltrated with the virulent pathogen using SA-degrading transgenic nahG plants. No major differences in disease development were observed compared with those in wild-type Arabidopsis. Determination of the AOX transcript levels showed that the AOX induction during the first 22 h was similar in wild-type and nahG plants (Table II). In contrast, there were clear differences in symptom development between wild-type and nahG plants that were infiltrated with the avirulent pathogen. In the transgenic nahG plants, symptoms appeared later and necrosis developed as in the compatible combination. Also, AOX induction in these leaves was delayed and similar to the compatible combination (Table II). PR-1 transcript levels remained very low in NahG plants, in contrast to those in the wild type (Table II), confirming that SA levels in the transgenic plants do not increase after infection. Thus, the finding that AOX transcript levels were significantly increased during both the compatible and incompatible interaction indicates that SA is not essential for AOX induction during these infections.
On the other hand, the finding that in the NahG plants the “fast” AOX induction 6 h after infiltration with the avirulent pathogen was abolished (Table II) suggested that SA-dependent processes must be involved in AOX induction during the incompatible interaction. Because the HR was also delayed in the transgenic NahG (Delaney et al., 1994), HR might be associated with the fast AOX induction. To test this hypothesis, leaves of the Arabidopsis mutant rps2-201 were infiltrated with the normally avirulent pathogen Pst DC3000 (avrRpt2). This mutant plant contains a defect in the rps2 resistance gene (Kunkel et al., 1993; Yu et al., 1993) and is therefore compatible with Pst DC3000 (avrRpt2). Upon infiltration of the leaves with this pathogen, the fast AOX induction observed in the leaves of wild-type Arabidopsis was absent in the rps2-201 plants. Instead, the induction of AOX in the mutant plant was similarly timed as that upon infiltration with the virulent pathogen (Table II). This result implies that the fast AOX induction, as with the incompatible interaction, is indeed associated with the rapid cell death that occurs during an HR. The comparatively slow induction of AOX in the compatible combinations is most likely associated with delayed necrosis of the plant tissue.
Total Respiration and Cyanide-Resistant, SHAM-Sensitive O2-Uptake Rates Are Not Affected in Leaves of etr1-1 Plants Infiltrated with an Avirulent Pst Strain
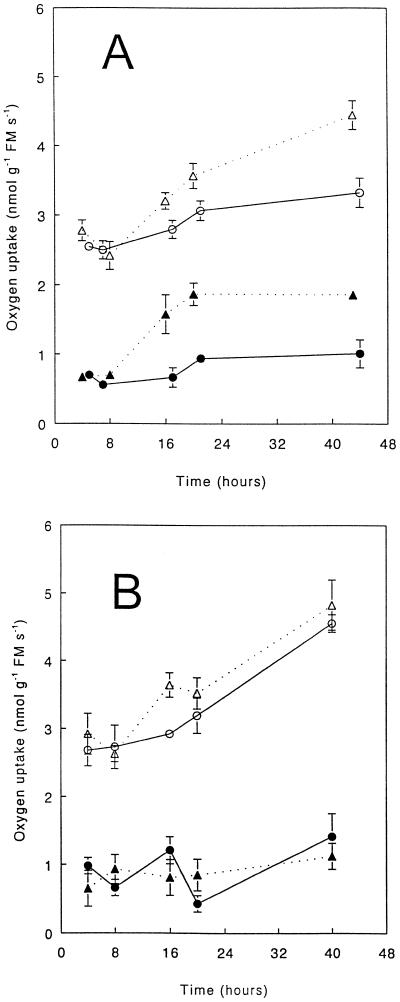
To study the possible involvement of ethylene-dependent processes in AOX induction upon infection, O2 uptake was measured using wild-type and etr1-1 mutant plants. Leaves were infiltrated with the avirulent Pst strain, because the respiratory increase of infected leaves of the wild type was most predictable with this pathogen. To measure O2 uptake during a prolonged period (2 d), leaves of wild-type plants were infiltrated with bacterial suspensions, containing from 5 × 105 to 107 CFU. With 5 × 105 CFU, no disease symptoms were visible until 6 d after infiltration; however, there was a considerable and rapid increase in cyanide-resistant, SHAM-sensitive O2 uptake, which remained constant between 20 and 48 h (Fig. 4A). In contrast to wild-type Arabidopsis, leaves of etr1-1 mutant plants, infiltrated with the same bacterial suspensions, did not show an increase in the total or cyanide-resistant, SHAM-sensitive rate of O2 uptake with time (Fig. 4B). These results indicate that ethylene-dependent processes are required for the induction of AOX during infection of Arabidopsis with avirulent Pst.
Figure 4.
Total and cyanide-resistant, SHAM-sensitive O2-uptake rates of wild-type (A) and etr1-1 (B) Arabidopsis leaves infiltrated with an avirulent strain of Pst. Leaves were infiltrated with suspensions containing 5 × 105 CFU of the pathogens per mL. Circles, Control; triangles, avirulent pathogen. Open symbols, Total O2 uptake of the leaves; closed symbols, cyanide-resistant, SHAM-sensitive O2 uptake. Results are mean values ± se; n = 4. FM, Fresh mass.
Expression of AOX Is Dependent on Ethylene
For a molecular analysis of the possible involvement of ethylene in the induction of AOX, AOX transcript levels were monitored in leaves of wild-type and etr1-1 plants infiltrated with the avirulent or virulent Pst strain. In the etr1-1 plants the AOX transcript levels were only slightly increased upon infection with the avirulent Pst strain, compared with those in the wild type. The compatible combination did not result in a detectable increase in AOX transcript levels in etr1-1(data not shown).
Pyruvate Accumulates in Tissue Infiltrated with the Pathogenic Bacteria
Infected plant tissues accumulate hexoses (Farrar, 1992), which can be used in several defense-related biosynthetic pathways and/or converted into large amounts of respiratory substrate. When the Cyt pathway is unable to cope with the latter, one might imagine that the alternative pathway is required. Flooding of the Cyt pathway with a respiratory substrate is likely to result in the accumulation of pyruvate (Vanlerberghe and McIntosh, 1996). Pyruvate is also a strong activator of AOX in vitro (Millar et al., 1993) and thus might be a feed-forward regulator of the alternative pathway in vivo. Therefore, levels of pyruvate in the leaf tissues infiltrated with the avirulent pathogen were determined. Necrotizing leaves showed a 4-fold increase of the pyruvate concentration 22 h after infiltration (Table III). It is unlikely that the bacteria contributed significantly to these increased pyruvate levels in the infected leaves, because their fresh weight was less than 0.01% of the total fresh weight of the infected leaves. These results suggested that the mitochondria was flooded with respiratory substrate and that the alternative electron-transport pathway was fully activated.
Table III.
Pyruvate concentration in leaves of Arabidopsis 22 h after infiltration of the leaves with avirulent Pst
| Experiment | Controla | Pst(pLH12) | Percentage |
|---|---|---|---|
| nmol g−1 fresh wt | |||
| 1 | 120 | 492 | 410 |
| 2 | 98 | 383 | 391 |
The data are from two independent experiments. The bacterial suspension contained 107 CFU mL−1.
Mock-infiltrated leaves (10 mm MgCl2).
DISCUSSION
An increase in respiration is a widespread phenomenon in plant-pathogen interactions (Farrar, 1992; Lennon et al., 1997). The present study suggests that the alternative pathway might be a major contributor to the increase in respiration in Arabidopsis upon infection with avirulent and virulent P. syringae strains. This was evidenced by strongly increased AOX transcript levels, increased amounts of reduced (high-activity) AOX protein, and increased cyanide-resistant, SHAM-sensitive O2-uptake rates in the infected leaves. Because SHAM did not affect leaf respiration in the absence of KCN, we have no evidence that the alternative pathway contributed to the enhanced leaf respiration. Infection with the tobacco mosaic virus in tobacco also enhances AOX protein levels, but it has no effect on the contribution of the alternative pathway to leaf respiration (Lennon et al., 1997).
Because SA acts as a signal in the induction of AOX in Arum lily flower stalks and in tobacco leaves and as a signal of PRs and SAR during resistance responses, the following questions have been addressed: Is AOX induced during plant diseases? Is such induction associated with the expression of PRs? Do SA and/or ethylene act as a signal(s) in the induction of AOX during infection in Arabidopsis leaves? In Arabidopsis AOX was strongly induced in the infected tissue with both the incompatible and the compatible combination. In treated leaves the expression of AOX and PR-1 mRNA upon infection was correlated in time, but in systemic leaves AOX transcript levels were not increased, in contrast to the transcript levels in PR-1. This is at variance with the results in tobacco, in which AOX protein levels increased both in tobacco mosaic virus-infected and in noninfected systemic leaves (Lennon et al., 1997). Application of SA did not induce AOX in Arabidopsis, and AOX induction was not abolished in NahG plants, indicating that SA accumulation is not essential in the induction of AOX due to bacterial infection in Arabidopsis. In contrast to AOX transcript levels, PR-1 transcript levels remained low in the NahG plants, even in the infiltrated leaves. Because PR-1 expression is associated with SA accumulation, AOX and PR-1 expression in Arabidopsis must be regulated by different signals. Direct evidence showing the involvement of different signaling pathways for induction of PR-1 and AOX came from experiments with the npr1-1 mutant, which is unable to express PRs and SAR. Immediately upon infiltration of the leaves with either the avirulent or the virulent Pst strain, this mutant showed AOX-induction patterns (in time and place) similar to those in the wild-type plant.
Although SA was clearly not essential for induction of AOX, fast induction of AOX during the avirulent plant-pathogen combination was abolished in both nahG and npr1-1 plants, indicating that SA-dependent processes do play a role. Fast induction of AOX in the avirulent plant-pathogen combination was associated with HR. Lower amounts of the avirulent pathogen resulted in lower AOX transcript levels in the leaves (Table II), indicating a direct relationship between the level of induction and the amount of tissue affected. The delayed induction of AOX in the compatible combination suggests that HR accelerates but is not essential for the induction of the AOX. Apparently the AOX induction is associated with the development of necrosis. It is well known that respiratory changes occurring as a result of infection in plants are not limited to the infected cells but also take place in the surrounding tissue (Farrar, 1992). Therefore, the increases in AOX transcript levels, AOX protein, and total and cyanide-resistant, SHAM-sensitive respiration in Arabidopsis leaves infected with Pst most likely take place in the vicinity of necrotizing plant cells. Previous reports have shown that the increases in total and cyanide-resistant plant respiration can be stronger in a susceptible plant compared with those in a resistant one (Farrar and Rayns, 1987). Such increases are caused by the localization of the pathogen in the avirulent plant-pathogen combination; consequently, less tissue is affected. The major difference between the reaction of a resistant and a susceptible plant appears to be the speed at which respiration increases and AOX induction occurs.
Ethylene plays a major role in the induction of AOX upon infection. Based on the transcript levels, AOX induction in the etr1-1 mutant plants was almost completely abolished in leaves infiltrated with either the virulent or avirulent pathogen. Moreover, O2-uptake rates in the presence of cyanide were not increased in the leaves of the etr1-1 plant infiltrated with the avirulent pathogen. Taken together, the results suggest that strong, local AOX induction during infections is associated with ethylene production at the site of infection. Ethylene is strongly increased at an early stage in an HR (De Laat and Van Loon, 1983; Boller, 1991), indicating a functional role for the rapid action of AOX in the incompatible combination.
Although local increases of AOX transcripts corresponded well with increased AOX protein levels and increased cyanide-resistant, SHAM-sensitive respiration, the actual contribution of the alternative pathway to the respiration in infected tissue was not determined. This would require the use of the O2 discrimination technique (Day et al., 1996). The AOX pool in the plant tissue ultimately determines the maximum contribution of the alternative pathway to total respiration. The actual contribution can be strongly modulated, however, by the state of the disulfide bridge between two AOX subunits, because the reduced dimer is much more active than the oxidized, covalently bound form. Our western blotting results show that, at least during the later stage of infection, the amount of the reduced (high-activity) AOX dimer was increased. This suggests that AOX could significantly contribute to respiration in these tissues. Furthermore, pyruvate levels in the Arabidopsis leaves infiltrated with the avirulent pathogen were strongly increased. This suggests that the mitochondria are flooded with respiratory substrate and activator, a condition that is likely to favor full operation of the alternative pathway in the affected cells.
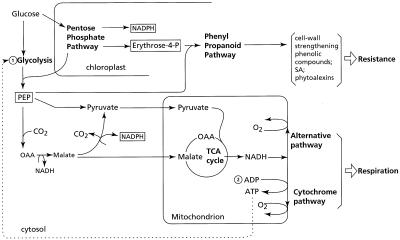
How can the resistance response and the energy-wasting alternative pathway be linked at the physiological level? A schematic representation of the main components and pathways involved in the physiological response of plant cells to infection and other stresses, such as wounding and chilling injury, are depicted in Figure 5. Infections and other stresses are associated with enhanced biosynthesis of aromatic compounds. The oxidative pentose-phosphate pathway provides erythrose-4-phosphate, which condenses with PEP into the precursor of numerous phenylpropanoids that are implicated in resistance reactions. The NADPH that is produced might act as a reductant in numerous stress-related reactions. Another source for NADPH is the cytosolic NADP-malic enzyme, which catalyzes the oxidation of malate to pyruvate and CO2. This enzyme is induced upon the addition of elicitors, suggesting that it is involved in primary metabolism changes after infection (Schaaf et al., 1995). The pentose-phosphate pathway, which may account for 90% of the breakdown of Glc during infections (Shaw and Samborski, 1957), bypasses the allosteric adenylate control of glycolysis. In combination with the enhanced activity of the cytosolic NADP-malic enzyme, this can lead to an accumulation of pyruvate, particularly if the Cyt pathway in the affected tissue is somehow restricted. That the operation of the alternative pathway does not contribute to the formation of ATP might not be disadvantageous, because many defense-related reactions require NADPH rather than ATP, as shown in Figure 5.
Figure 5.
Major metabolic pathways involved in the resistance response to pathogens and its association with respiration. Important intermediates involved in the biosynthesis of several defense compounds are depicted in boxes. Numbers refer to major control points for glycolysis due to allosteric inhibition of phosphofructokinase by ATP (1) and inhibition of oxidative phosphorylation by limiting amounts of ADP (2). TCA cycle, Tricarboxylic acid cycle; OAA, oxaloacetic acid.
It has been proposed that operation of the alternative pathway during environmental stresses might (partly) relieve the mitochondrial electron-transport pathway (Purvis and Shewfelt, 1993; Wagner and Krab, 1995). This could prevent overreduction that might result in the formation of harmful radicals. Formation of O2 radicals is instrumental in necrotization, but limited lesion formation during avirulent reactions requires that surrounding tissues be protected. This is clearly illustrated by the occurrence of disease lesion mimics, in which spontaneous necrosis may spread because of mutations (Dietrich et al., 1994). During infections the formation of radicals might worsen disease symptoms. For a successful resistance response the production of radicals might be an important feature, because the performance of a plant during disease is determined not only by its ability to localize the pathogen but also by the its capacity to minimize tissue damage. Because several other stress conditions induce AOX, we speculate that cyanide-resistant respiration is important to the plant for acclimation to adverse conditions. How much this enables the plant to cope with such stresses awaits experiments with plants that cannot express AOX.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Patrick M. Finnigan (Australian National University, Canberra) for critically reading the manuscript, Dr. Jim Whelan (University of Western Australia, Nedlands) for providing advice and the AOX-specific degenerant primers, Dr. Thomas E. Elthon (University of Nebraska, Lincoln) for providing the AOX-specific monoclonal antibodies, and Dr. Andrew Bent (University of Illinois, Urbana) for providing the Pst strains. We would also like to thank Erik De Vlieger for his help with obtaining the data concerning the effects of SHAM on leaf respiration.
Abbreviations:
- AOX
alternative oxidase
- CFU
colony-forming unit(s)
- HR
hypersensitive reaction
- KCN
potassium cyanide
- PR
pathogenesis-related protein
- RT-PCR
reverse transcriptase-PCR
- SA
salicylic acid
- SAR
systemic acquired resistance
- SHAM
salicylhydroxamic acid
Footnotes
This work was supported by grants from the Netherlands Organization for Scientific Research.
LITERATURE CITED
- Atkin OK, Cummings WR, Collier DE. Light induction of alternative pathway capacity in leaf slices of Belgium endive. Plant Cell Environ. 1993;16:231–235. [Google Scholar]
- Bleecker AB, Estelle M, Somerville C, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Boller T. Ethylene in pathogenesis and disease resistance. In: Mattroo AK, Suttle JD, editors. The Plant Hormone Ethylene. Boca Raton, FL: CRC Press; 1991. pp. 293–314. [Google Scholar]
- Brederode FT, Linthorst HJM, Bol JF. Differential induction of acquired resistance and PR gene expression on tobacco by virus infection, ethephon treatment, UV light and wounding. Plant Mol Biol. 1991;17:1117–1125. doi: 10.1007/BF00028729. [DOI] [PubMed] [Google Scholar]
- Cameron RK, Dixon RA, Lamb CJ. Biological induced systemic acquired resistance in Arabidopsis thaliana. Plant J. 1994;5:715–725. [Google Scholar]
- Cao H, Bowling SA, Gordon AS, Dong X. Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell. 1994;6:1583–1592. doi: 10.1105/tpc.6.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Krab K, Lambers H, Moore AL, Siedow JN, Wagner AM, Wiskich JT. The cyanide-resistant oxidase. To inhibit or not to inhibit, that is the question. Plant Physiol. 1996;110:1–2. doi: 10.1104/pp.110.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Laat AMM, Van Loon LC. Regulation of ethylene biosynthesis in virus-infected tobacco leaves. II. Time course of levels of intermediates and in vivo conversion rates. Plant Physiol. 1982;69:240–245. doi: 10.1104/pp.69.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Laat AMM, Van Loon LC. The relationship between stimulated ethylene production and symptom expression in virus-infected tobacco leaves. Physiol Plant Pathol. 1983;22:261–273. [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E and others. A central role of salicylic acid in plant disease resistance. Science. 1994;266:1247–1250. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- Dietrich R, Delaney T, Uknes S, Ward E, Ryals J, Dangl J. Arabidopsis mutants simulating disease response. Cell. 1994;77:565–577. doi: 10.1016/0092-8674(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Dong X, Mindrinos M, Davis KR, Ausubel FM. Induction of Arabidopsis defense genes by virulent and avirulent Pseudomonas syringae strains and by a cloned avirulence gene. Plant Cell. 1991;3:61–72. doi: 10.1105/tpc.3.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon TE, Nickels RL, McIntosh L. Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiol. 1989;89:1311–1317. doi: 10.1104/pp.89.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar JF (1992) Beyond photosynthesis: the translocation and respiration of diseased leaves. In PG Ayres, ed, Pests and Pathogens. BIOS Scientific Publishers, Oxford, UK, pp 107–127
- Farrar JF, Rayns FW. Respiration of barley infected with powdery mildew: increased engagement of the alternative oxidase. New Phytol. 1987;107:119–125. [Google Scholar]
- Geels FP, Schippers B. Selection of antagonistic fluorescent Pseudomonas spp. and their root colonization and persistence following treatment of seed potatoes. Phytopathol Z. 1983;108:193–206. [Google Scholar]
- Hiser C, McIntosh L. Alternative oxidase of potato is an integral membrane protein synthesized de novo during aging of tuber slices. Plant Physiol. 1990;93:312–318. doi: 10.1104/pp.93.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. The water culture method for growing plants without soil. Calif Agric Exp Stn Bull. 1938;347:36–39. [Google Scholar]
- King EO, Ward MK, Raney DE. Two simple media for demonstration of phycocyanin and fluorescin. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- Kumar AM, Söll D. Arabidopsis alternative oxidase sustains Escherichia coli respiration. Proc Natl Acad Sci USA. 1992;89:10842–10846. doi: 10.1073/pnas.89.22.10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel BN, Bent AF, Dahlbeck D, Innes RW, Staskawicz BJ. RPS2, an Arabidopsis disease resistance locus specifying recognition of Pseudomonas syringae strains expressing the avirulence gene avrRpt2. Plant Cell. 1993;5:865–875. doi: 10.1105/tpc.5.8.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamers JG, Schippers B, Geels FP (1988) Soil-borne disease of wheat in The Netherlands and results of seed bacterization with pseudomonads against Gaeumannomyces graminis var. tricici. In ML Jorna, LA Slootmaker, eds, Cereal Breeding Related to Integrated Cereal Production. Pudoc, Wageningen, The Netherlands, pp 134–139
- Laties GG. The cyanide-resistant, alternative path in higher plant respiration. Annu Rev Plant Physiol. 1982;33:519–555. [Google Scholar]
- Lennon AM, Neuenschwander UH, Ribas-Carbo M, Giles L, Ryals JA, Siedow JN. The effect of salicylic acid and tobacco mosaic virus infection on the alternative oxidase of tobacco. Plant Physiol. 1997;115:783–791. doi: 10.1104/pp.115.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeuse BJD. Thermogenic respiration in aroids. Annu Rev Plant Physiol. 1975;26:117–126. [Google Scholar]
- Millar AH, Wiskich JT, Whelan J, Day DA. Organic acid activation of the alternative oxidase of plant mitochondria. FEBS Lett. 1993;329:259–262. doi: 10.1016/0014-5793(93)80233-k. [DOI] [PubMed] [Google Scholar]
- Millenaar FF, Benschop J, Wagner AM, Lambers H. The role of the alternative oxidase in stabilizing the in vivo reduction state of the ubiquinone pool and the activation state of the alternative oxidase. Plant Physiol. 1998;118:599–607. doi: 10.1104/pp.118.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AL, Siedow JN. The regulation and nature of the cyanide-resistant alternative oxidase of plant mitochondria. Biochim Biophys Acta. 1991;1059:121–140. doi: 10.1016/s0005-2728(05)80197-5. [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Van Wees SCM, Hoffland E, Pelt JA, Van Loon LC. Systemic resistance in Arabidopsis induced by biocontrol bacteria is independent of salicylic acid accumulation and pathogenesis-related gene expression. Plant Cell. 1996;8:1225–1237. doi: 10.1105/tpc.8.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis AC, Shewfelt RL. Does the alternative pathway ameliorate chilling injury in sensitive plant tissues? Physiol Plant. 1993;88:712–718. doi: 10.1111/j.1399-3054.1993.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Raskin I, Ehmann A, Melander WR, Meeuse BJD. Salicylic acid: a natural inducer of heat production in Arum lilies. Science. 1987;237:1601–1602. doi: 10.1126/science.237.4822.1601. [DOI] [PubMed] [Google Scholar]
- Rhoads DM, McIntosh L. Salicylic acid regulation of respiration in higher plants: alternative oxidase expression. Plant Cell. 1992;4:1131–1139. doi: 10.1105/tpc.4.9.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads DM, McIntosh L. Cytochrome and alternative pathway respiration in tobacco. Effects of salicylic acid. Plant Physiol. 1993;103:877–883. doi: 10.1104/pp.103.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saisho D, Nambara E, Naito S, Tsutsumi N, Hirai A, Nakazone M. Characterization of the gene family for alternative oxidase from Arabidopsis thaliana. Plant Mol Biol. 1997;35:585–596. doi: 10.1023/a:1005818507743. [DOI] [PubMed] [Google Scholar]
- Schaaf J, Walter MH, Hess D. Primary metabolism in plant defense. Regulation of a bean malic enzyme gene promoter in transgenic tobacco by developmental and environmental cues. Plant Physiol. 1995;108:949–960. doi: 10.1104/pp.108.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller GE, Bleecker AB. Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1–1 gene. Science. 1995;270:1809–1811. doi: 10.1126/science.270.5243.1809. [DOI] [PubMed] [Google Scholar]
- Shaw M, Samborski DJ. The physiology of host-parasite relations: the pattern of respiration in rusted and mildewed cereal leaves. Can J Bot. 1957;35:389–407. [Google Scholar]
- Siebert PD, Larrick JW. Competitive PCR. Nature. 1992;359:557–558. doi: 10.1038/359557a0. [DOI] [PubMed] [Google Scholar]
- Siedow JN, Umbach AL. Plant mitochondrial electron transfer and molecular biology. Plant Cell. 1995;7:821–831. doi: 10.1105/tpc.7.7.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J, Kearney B, Dahlbeck D, Staskawicz BJ. Cloned avirulence gene of Xanthomonas campestris pv vesicatoria complements spontaneous race change mutant. Mol Plant-Microbe Interact. 1988;1:5–9. [Google Scholar]
- Turner JG, Novacky A. The quantitative relation between plant and bacterial cells involved in the hypersensitive reaction. Phytopathology. 1974;64:885–890. [Google Scholar]
- Uknes S, Winter AM, Delaney T, Vernooij B, Morse A, Friedrich L, Nye G, Potter S, Ward E, Ryals J. Biological induction of systemic acquired resistance in Arabidopsis. Mol Plant-Microbe Interact. 1993;6:692–698. [Google Scholar]
- Umbach AL, Siedow JN. Covalent and noncovalent dimers of the cyanide-resistant alternative oxidase protein in higher plant mitochondria and their relationship to enzyme activity. Plant Physiol. 1993;103:845–854. doi: 10.1104/pp.103.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uritani I, Asahi T. Respiration and related metabolic activity in wounded and infected tissues. In: Stumpf PK, Conn EE, editors. The Biochemistry of Plants, Vol 2. London: Academic Press; 1980. pp. 463–485. [Google Scholar]
- Vanlerberghe GC, McIntosh L. Lower temperature increases alternative pathway capacity and alternative oxidase protein in tobacco. Plant Physiol. 1992;100:115–119. doi: 10.1104/pp.100.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Signals regulating the expression of the nuclear gene encoding alternative oxidase of plant mitochondria. Plant Physiol. 1996;111:589–595. doi: 10.1104/pp.111.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AM, Krab K. The alternative respiration pathway in plants: role and regulation. Physiol Plant. 1995;95:318–325. [Google Scholar]
- Wagner AM, Wagner MJ. Measurements of in vivo ubiquinone reduction levels in plant cells. Plant Physiol. 1995;108:277–283. doi: 10.1104/pp.108.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen MC, Innes RW, Bent AF, Staskawicz BJ. Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidopsis and soybean. Plant Cell. 1991;3:49–59. doi: 10.1105/tpc.3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan J, Millar AH, Day DA. The alternative oxidase is encoded in a multigene family in soybean. Planta. 1996;198:197–201. doi: 10.1007/BF00206244. [DOI] [PubMed] [Google Scholar]
- Yu G-L, Katagiri F, Ausubel FM. Arabidopsis mutations at the RPS2 locus result in loss of resistance to Pseudomonas syringae strains expressing the avirulence gene avrRpt2. Mol Plant-Microbe Interact. 1993;6:434–443. doi: 10.1094/mpmi-6-434. [DOI] [PubMed] [Google Scholar]