Abstract
In order to prepare the mother for the demands of pregnancy and lactation, the maternal brain is subjected to a number of adaptations. Maternal behaviors are regulated by complex neuronal interactions. Here, we show that the melanin concentrating hormone (MCH) system is an important regulator of maternal behaviors.
First, we report that melanin concentrating hormone receptor 1 knockout (MCHR1 KO) mice display a disruption of maternal behavior. Early postpartum MCHR1 KO females exhibit poor nesting, deficits in pup retrieval and maternal aggression. In addition, ablation of MCH receptors results in decreased milk production and prolactin mRNA levels. Then we show that these results are in line with those obtained in wild type mice (WT) treated with the specific MCHR1 antagonist GW803430. Furthermore, following pups retrieval, MCHR1 KO mice display a lower level of Fos expression than WT mice in the ventral tegmental area, and nucleus accumbens. With the progression of the lactation period, however, the MCHR1 KO mice improve maternal care towards their pups. This is manifested by an increase in the pups' survival rate and the decrease in pups' retrieval time beyond the second day after parturition.
In conclusion, we show that the MCH system plays a significant role in the initiation of maternal behavior. In this context, MCH may play a role in integrating information from multiple sources, and connecting brain reward, homeostatic and regulatory systems.
Keywords: Melanin concentrating hormone, Maternal behavior, Knockout, Mice, Antagonist
1. Introduction
There have been separate lines of evidence that support a role of melanin concentrating hormone (MCH) system in various aspects of maternal behavior (Adams et al., 2011; Benedetto et al., 2014).
The anatomical connections and physiological functions of the melanin concentrating hormone (MCH) system are at the basis of its role in regulating maternal behavior. MCH is a hypothalamic neuropeptide that is released by neurons in the lateral hypothalamus and incerto-hypothalamic area (Bittencourt et al., 1992; Sita et al., 2007) and acts through a G protein-coupled receptor, MCHR1 (Chambers et al., 1999; Saito et al., 1999, 2000). The MCHergic neurons project widely throughout the brain. The best-characterized function of MCH system is its regulation of energy homeostasis and food intake (Qu et al., 1996; Rossi et al., 1997). The MCH system, however, regulates other diverse physiological functions such as sleep, stress, anxiety, mood, aggression, and cognition (Blouin and Siegel, 2013; Chung et al., 2011; Fraigne and Peever, 2013; Roy et al., 2006, 2007).
There is a strong anatomical connection between MCH neurons and the maternal neuronal circuit including the medial preoptic area (mPOA) and oxytocin neurons in the paraventricular nucleus (PVN), ventral tegmental area (VTA), nucleus accumbens-shell (NAc-sh), olfactory bulb, lateral septum (LS), and amygdala (Bittencourt et al., 1992; Hervieu et al., 2000; Numan, 2012; Saito et al., 2001) Oxytocin and vasopressin have been shown to excite hypothalamic MCH neurons but not other LH GABAergic neurons (Yao et al., 2012). This suggests that MCH neurons may mediate or modulate some of the oxytocin and vasopressin actions such as maternal behavior. MCHR1 expression in the medial amygdala has been implicated for an important role in maternal aggression towards an unfamiliar male intruder (Niu et al., 2012).
That the MCH system may be involved in maternal behavior is supported by several observations. First, high mortality and cannibalism rate were observed among offspring of MCH KO mothers, an indication of poor mothering (Adams et al., 2011).
Then, the mPOA, which does not express MCH in male or non-lactating female rats, displays mRNA expression and peptide synthesis only during late lactation stage (Knollema et al., 1992; Rondini et al., 2010). The MCH synthesis in mPOA neurons gradually increases during the lactation period, reaching its maximal levels at the end of this period during the weaning stage (Knollema et al., 1992; Rondini et al., 2010).
Finally, Benedetto et al. (2014) have shown that when injected into the medial preoptic area (mPOA) of early postpartum females, MCH inhibits the active components of maternal behavior (Benedetto et al., 2014), suggesting an inhibitory role for the MCH in the mPOA on maternal behavior.
In order to further understand the functional significance of MCH, we study the effects that the genetic deletion of MCHR1 and the pharmacological blockade of MCHR1 have in regulating maternal behavior.
2. Experimental procedures
2.1. Animals and experimental design
Eight-eleven week-old female MCHR1 KO mice (n = 48) and background matched wild type B6NTac (n = 55) were used.
For the maternal behavior assays, female WT and MCHR1 KO mice were allowed to mate with genotype matched male mice for a period of 3 days. Following this mating period male mice were removed from the cage and the female mice were subsequently monitored daily for signs of pregnancy by visual examination and weight measurements. The date of birth of pups was considered postpartum day 0 (PPD0). We studied the role of the MCH system on nest building, pups' retrieval and maternal aggression in the early period of lactation because this is the time when these behaviors are expressed at their highest levels as described in previous studies (Hennessy et al., 1980; Pedersen et al., 2006; Sato et al., 2010; Thomas and Palmiter, 1997).
Maternal behavior tests (nest building, pups retrieval and maternal aggression) were carried out on the same animals in a sequence: nest building on PPD1, pups retrieval on PPD1–PPD3, and maternal aggression on PPD7. Milk production measurement was carried out on animals that were not tested in maternal behavior tests. Milk production test was carried out on PPD9–PPD11 because it relies on measuring the changes in the pups' daily body weights, which increase with the growth of the pups (Nagai, 1971; Roepke et al., 2009; Sampson and Jansen, 1984).
All experimental procedures were approved by the Institutional Animal Care and Use Committee of University of California, Irvine and were performed in compliance with national and institutional guidelines for the care and use of laboratory animals.
2.2. Drugs
The MCHR1 antagonist GW803430 (gift from Dr. Donald R. Gehlert, Eli Lilly) was dissolved in 2% Tween 80 solution. The effects of GW803430 were studied on two assays: pups' retrieval and milk production. Doses and time of administration of GW803430 were selected based on previous study (Cippitelli et al., 2010). Where GW803430 was used, the control group received the vehicle (2% Tween 80 solution) at a volume of 10 µ/gr of mouse weight.
2.3. Maternal behaviors in post-partum mice
Pup mortality between PPD0–PPD3 was presented as the survival percentage of initial litter size, 149–162 pups of 19–22 dams. Both wild type and knockout mothers were observed for instances of cannibalism.
The following maternal behaviors were assessed by observers who are blinded to the condition of the experimental subjects.
2.3.1. Nest building
The quality of the nest building was scored on PPD1 using a 5-point nest-rating scale (Deacon, 2006). Cages were changed once per week and contained 3 fresh nestlets to ensure proper nest building.
2.3.2. Pup retrieval
The retrieval behavior test measured the mother's latency to retrieve the first pup and the total time required to retrieve 3 pups daily for 3 consecutive days. Pup retrieval test was performed at PPD1, PPD2, and PPD3. All tests were videotaped for a total time of 5 min and analyzed. The mother was removed for one minute from her cage and her pups were removed from the nest. Three pups were placed in each corner of the cage. The female was returned back to her nest, and the latency to retrieve the first pup (in seconds) and the duration of retrieval for the three pups were recorded in 5 min (Wang and Storm, 2011).
The effect of GW803430 (3, 10, 30 mg/kg, i.p.) on retrieval was evaluated on PPD2, by injecting the WT mothers with one of the above doses 30 min prior to the retrieval testing. The control group received the vehicle (2% Tween 80 solution) that was used to dissolve GW803430 at a volume of 10 µl/gr of mouse weight.
2.3.3. Maternal aggression
Maternal aggression tests were performed at PPD7 as described previously (Wang et al., 2011). During the maternal aggression test, a male intruder was introduced to the home cage of the female for 10 min. The total numbers of attacks including aggressive moves, flank/back, head/neck, or combination were measured.
2.4. Milk production
Pup weight and pup weight gain were used to estimate milk yield according to Sampson and Jansen using the following equation (Sampson et al., 1984): Yield (g/day/pup) = 0.0322 + (0.0667 × weight) + (0.877 × gain) Weight: pup weight (g), gain (g/day). Milk production starting from PPD9–PPD11 was assessed in WT females injected on P10 with vehicle (control), or with GW803430 (30 mg/kg); KO females were injected on PPD10 with vehicle (6–8 pups).
2.5. Immunohistochemistry
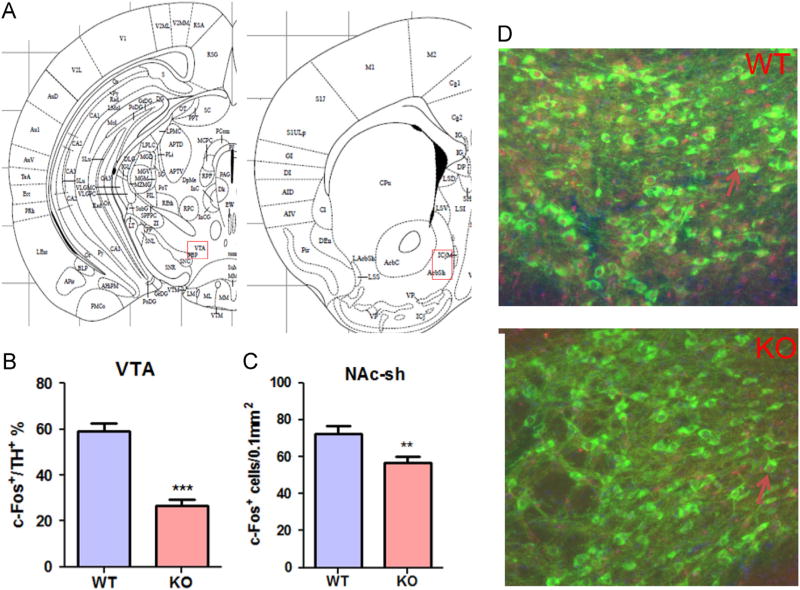
Immunostaining was performed to localize the Fos- immunoreactive neurons (Fos-ir-neurons) in brain sections at the levels of VTA and NAc-sh following pups retrieval test. In the VTA, to determine the Fos immunereactivity in dopamine neurons, double immunostaining was performed using Fos antibody and, tyrosine hydroxylase (TH) antibody. On PPD2, 90 min after the pup retrieval test, three WT and three MCHR1 KO mice that have not been subjected to any treatment were anesthetized with halothane and perfused intracardially with 40 ml saline followed by 50 ml of 4% formaldehyde in phosphate buffer saline (PBS). Brains were removed and 20 m coronal sections were cut using cryostat. Three sections were selected from each of the VTA and NAc-sh according to the mouse brain atlas (Paxinos, 2001). Sections were blocked with 4% normal donkey serum in PBS with 0.3% Triton X-100 for 60 min. For double immunostaining, brain sections at the level of the VTA were simultaneously incubated for 24 h at room temperature with mouse anti-Fos (1:500, Abcam) and chicken anti-TH (1:500, Aves). The sections were then washed with PBS, and incubated for one hour with Alexa488 labeled anti-chicken and Alexa560 labeled anti-mouse secondary antibodies (1:500, Invitrogen). Sections were washed with PBS, incubated for five minutes with 4′,6-diamidino-2-phenylindole (DAPI) solution (1:10,000) to stain the nuclei, and mounted with Anti-fade solution. Zeiss fluorescent microscope and Zeiss LSM image browser software (Carl Zeiss, USA) were used for image acquisition and analysis (Alachkar et al., 2013). Fos-ir-neurons were counted in the bilateral areas of each section, and the mean values of three non-consecutive sections per brain of three brains were calculated. A surface of 0.2 to 0.4 mm2 was acquired from each section. In the VTA, the number of Fos-ir-neurons was presented as a percentage of the total TH positive neurons.
2.6. qt-PCR
To identify the changes of the Pro-MCH mRNA during pregnancy and postpartum period, quantitative real time-PCR was used to measure hypothalamic Pro-MCH mRNA levels of naive WT mice (animals that have never been subjected to any behavioral experiments or pharmacological treatment): non-pregnant, pregnant, PPD2, and PPD16. Prolactin mRNA levels were measured in naïve WT and MCHR1 KO mice on PPD2 and PPD16. qt-PCR was carried out as previously described (Nagasaki et al., 2006), using gene-specific primers to mouse Pro-MCH, prolactin, and mouse GAPDH (Pro-MCH: Forward: GGGGATGAAGAAAACTCAGCTAAA, Reverse: TGCGG ACCAGCAGGTATCA, prolactin: Forward: TCCGAGAGCTGTTTGACCGTGTGG, Reverse: CAGGGAAGAAGTGGGGCAGTC AT, GAPDH1: Forward: TGGCACAGTCAAGGCTGAGA, Reverse: CGCTCCTGGAAGATGGT GAT. All the primer sets were designed to span exon–intron boundaries. Total RNA was extracted from the hypothalamus (for pro-MCH) and pituitary (for prolactin) using phenol/guanidine isothiocyanate (TRIZOL; Invitrogen, Carlsbad, CA), and 2 mg total RNA was reverse transcribed using Superscript III ribonuclease H reverse transcriptase kit (Invitrogen) in the presence of 100 ng random hexamers. Reactions for the quantification of mRNAs by PCRs were carried out using iTag Universal SYBR Green Supermix (Bio Rad), and analyzed by Bio Rad CFX Connect Real-Time System (Bio Rad, California, USA). The expression of genes was calculated relative to that in the non-pregnant female mice for pro-MCH and to that in the matching WT female for prolactin, and normalized for the GAPDH mRNA, using the 2ΔΔCT method (GAPDH mRNA levels were not different between WT and KO non-pregnant mice) (Nagasaki et al., 2006).
2.7. Statistical analysis
Graphpad Prism (GraphPad Software, Inc.) was used for statistical analysis. Data were presented as means ± S.E.M. Results were analyzed by student t-test or ANOVA followed by the appropriate post-hoc comparisons, and P < 0.05 was considered statistically significant.
3. Results
3.1. Hypothalamic MCH mRNA during pregnancy and lactation
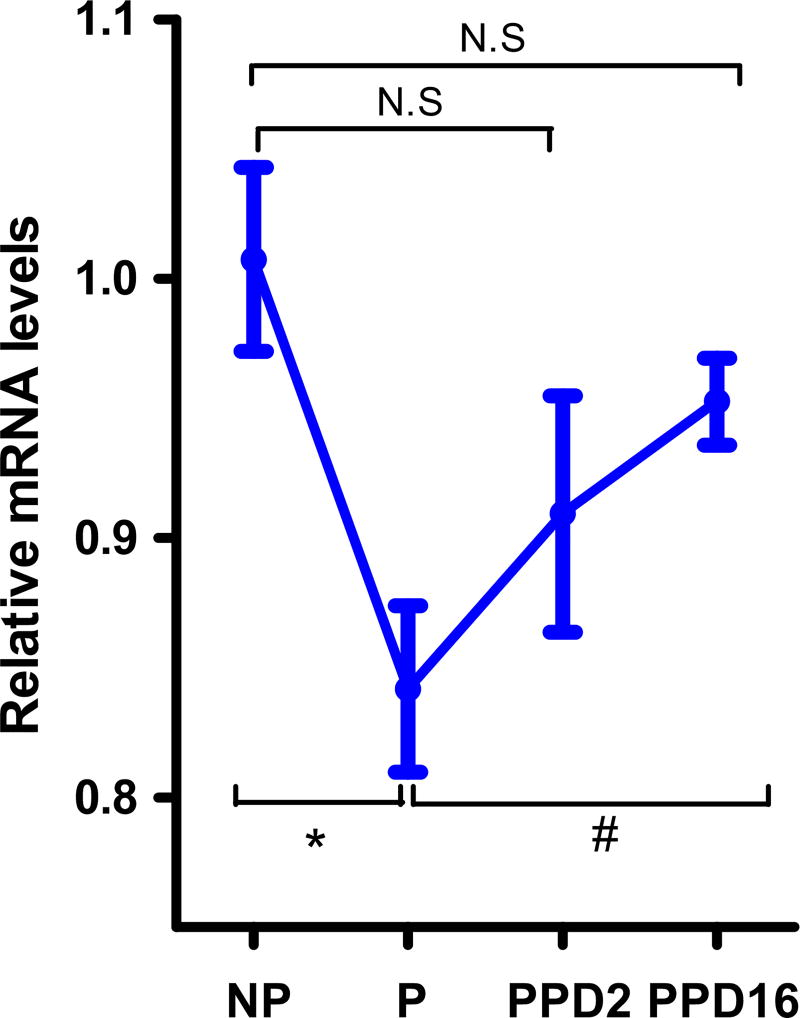
An indication that the MCH system may have a role in regulating maternal behaviors stems from our analysis of Pro-MCH mRNA in pregnancy and lactation. In WT mice, hypothalamic Pro-MCH mRNA levels were significantly lower in pregnant than in non-pregnant mice, P < 0.05 (Figure 1). In the early lactation period (post-partum day 2:PPD2), MCH mRNA levels began to increase but were not significantly different from that found in the pregnancy (P > 0.05). However, in the late postpartum period (PPD16), these levels were significantly increased when compared to the pregnancy levels (P < 0.05) and were restored to non-pregnant values, P > 0.05.
Figure 1.
Hypothalamic mRNA levels of MCH by qt-PCR in non-pregnant (NP), pregnant (P), and postpartum (PPD2 and PPD16) female WT mice (n = 3–4). One way ANOVA followed by Tukey posthoc test, F3,14 = 15.69, P vs. NP, *P < 0.05; P vs. PPD16, # P < 0.05.
3.2. Genetic ablation of the MCHR1 leads to increased mortality and cannibalism, poor nest building, and impaired maternal aggression
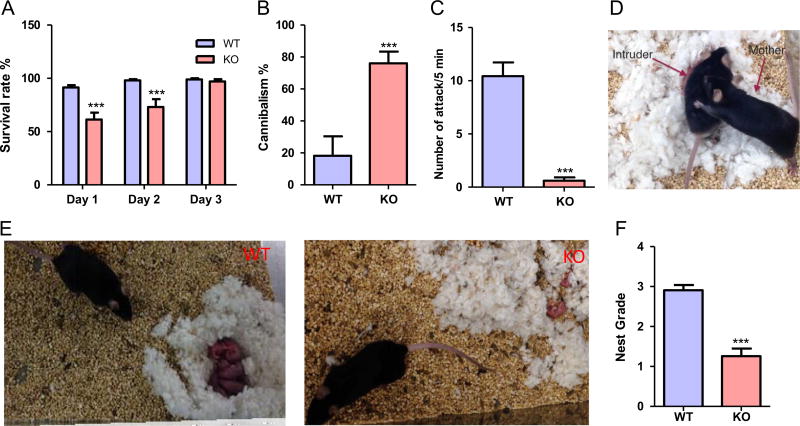
Then, WT and MCHR1 KO mice were observed over a 12 month breeding period. The breeding success rate was almost 100% of the bred mice in both the WT and MCHR1 KO dams, with average litter size of 9 ± 2 pups for WT and 6 ± 2 pups for MCHR1 KO mice. The survival rate among MCHR1 KO pups was evaluated on the first three postpartum days (PPD), and was found to be lower on PPD1 (61.3 ± 6.5) and PPD2 (73.1 ± 7.3%) than for the wild type neonates (91.4 ± 2.2 and 98.1 ± 1% on PPD1 and PPD2 respectively), original number of pups: 149 (MCHR1 KO) and 162 (WT), (P < 0.001, and < 0.01 PPD1 and PPD2 respectively, Figure 2A). This rate was, however, restored to normal levels on PPD3, P > 0.05. Cannibalism by MCHR1 KO mothers was one cause for the increased mortality rate of the pups (Figure 2B), and accounted for 76 ± 7% of the mortality cases compared with 18 ± 12% in WT, P < 0.001. MCHR1 KO postpartum females also displayed a significantly lower propensity to attack male intruders, P < 0.0001 unpaired t-test, (Figure 2C and D). MCHR1 KO mothers displayed poor nest building, an indicator of poor mothering. The scores for the nests were 2.9 ± 0.1 and 1.3 ± 0.2 in WT and KO mice in a 5-point nest-rating scale, P < 0.001, n = 22–24, (Figure 2E and F). Together these data indicate that the lack of an active MCH system leads to a defect in maternal behavior.
Figure 2.
MCHR1 ablation affects several aspects of maternal behaviors. (A) Survival rates in the progeny of WT and MCHR1 KO mice (n = 19–22). Two way ANOVA followed by Bonferroni post-hoc test (F1,111 = 27.83, P < 0.001): WT vs. KO, PPD1: ***P < 0.001, PPD2: ***P < 0.001, PPD3: P > 0.05. (B) Cannibalism rates in the progeny of WT and MCHR1 KO mice as a percentage of the mortalities (n = 19–22). Unpaired t-test (t = 4.3): WT vs. KO, ***P < 0.001. (C) Number of attacks by WT and MCHR1 KO females of male intruder mice (n = 23). Unpaired t-test (t = 7.4): WT vs. KO, ***P < 0.001. (D) Representative graph of maternal aggression in the WT female mice on PPD7. (E) Representative graph of the nest build by WT (left panel) and MCHR1 KO (right panel) mice. (F) The score of the nesting quality of WT and MCHR1 KO mice (n = 22–24). Unpaired t-test (t = 6.8): WT vs. KO, ***P < 0.001.
3.3. Pup retrieval is impaired in postpartum MCHR1 KO female mice
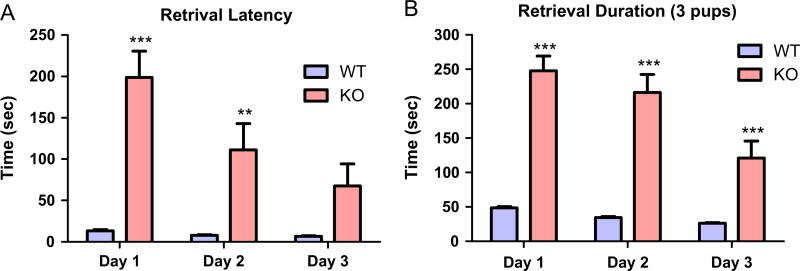
Pup retrieval is an important indicator of maternal care. At PPD1, 100% of the WT mothers retrieved at least one pup within the test time (5 minutes) while only 35% of MCHR1 KO did it. At PPD2 and PPD3, 45% and 80% of the MCHR1 KO mice respectively retrieved at least one pup. The latencies to retrieve the first pups by MCHR1 KO decreased on PPD2 and PPD3 compared with PPD1, P < 0.001, but were still higher in all days than the WT postpartum female mice, P < 0.001, P < 0.01 on PPD1 and PPD2 (Figure 3A). The retrieval duration (total time to retrieve 3 pups) in MCHR1 KO mice decreased on PPD2 and PPD3 compared to PPD1, P < 0.001. However, it was significantly larger in all three days when compared to the corresponding time in the WT mice, P < 0.001, n = 19–20, (Figure 3B).
Figure 3.
Effect of MCHR1 genetic ablation on pups’ retrieval in postpartum female mice. (A) The retrieval latency of WT and MCHR1 KO female mice (n = 19–20). Two way ANOVA followed by followed by Bonferroni post-hoc test (F1,111 = 42.6, P < 0.001): WT vs. KO, PPD1: ***P < 0.001, PPD2: **P < 0.01, PPD3: P > 0.05. (B) The retrieval duration of WT and MCHR1 KO female mice (n = 19–20). Two way ANOVA followed by Bonferroni post-hoc test (F1,111 = 129, P < 0.001): WT vs. KO, PPD1: ***P < 0.001, PPD2: ***P < 0.001, PPD3: ***P < 0.001.
3.4. Acute pharmacological inhibition of the MCH system leads to poor pup retrieval in the postpartum period
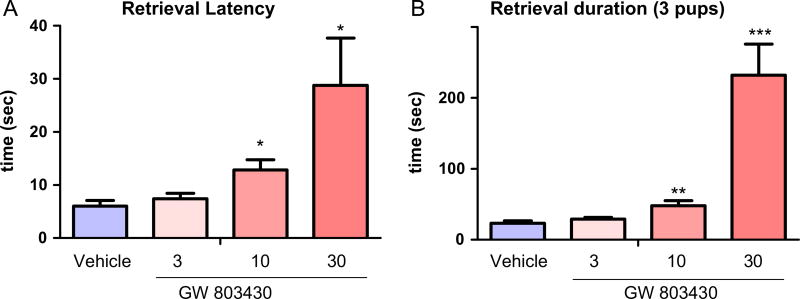
To test whether acute inhibition of the MCH system affects maternal behavior, the selective MCHR1 antagonist GW803430 was administered intraperitoneally on PPD2 at increasing doses (3, 10, 30 mg/kg). Pup retrieval was recorded and found to be inhibited in a dose dependent manner. Retrieval latencies were prolonged significantly at doses 10 mg/kg and 30 mg/g but not 3 mg/kg, P < 0.001 (Figure 4A). The retrieval duration was also increased by GW803430 10 and 30 mg/kg but not 3 mg/kg, P < 0.001 (Figure 4B).
Figure 4.
Effect of MCHR1 antagonist GW803430 on pups’ retrieval in postpartum female mice. (A) The dose response of MCHR1 antagonist GW803430 in the retrieval latency (n = 5–6). One way ANOVA followed by Dunnett's test, (F3,19 = 5.14): vehicle vs. drug, **P < 0.01, ***P < 0.001. (B) The dose response of MCHR1 antagonist GW803430 in the retrieval duration (n = 5–6). One way ANOVA followed by Dunnett's test (F3,19 = 16.1): vehicle vs. drug, *P < 0.05.
3.5. Genetic ablation of the MCHR1 leads to lowered Fos immunoreactivity in the dopamine reward system
To study whether the poor pups’ retrieval observed in MCHR1 KO mice correlates with a decrease in the dopamine system activity, we examined the induction of Fos following pups retrieval test in the NAc-sh and VTA. The density of Fos-ir-neurons (Fos-ir-neurons) in the MCHR1 KO mice was significantly lower in the NAc-sh, P < 0.01 (Figure 5) when compared to the WT mice. In the VTA, while the number of dopaminergic neurons was not different between the WT and MCHR1 KO, the number of dopaminergic, Fos-ir-neurons was significantly lower in the MCHR1 KO mice when compared to the WT mice, P < 0.001 (Figure 5).
Figure 5.
Fos-ir- neurons following pups retrieval test on PPD2. (A) Histological locations of the mice brain analyzed for Fos-like immunoreactivity. VTA: Ventral Tegmental Area, NAc-sh: Nucleus accumbens shell. (B) The ratio of the Fos positive cell numbers to the TH-positive cell numbers in the VTA in PPD2 WT and MCHR1 KO mice (n = 3). Unpaired t-test (t = 5.1): WT vs. KO, ***P < 0.001. (C) Numbers of Fos positive cells in the NAc-sh (C) following pups’ retrieval test on PPD2 in WT and MCHR1 KO mice (n = 3). Unpaired t-test (NAc-sh: t = 2.8): WT vs. KO, **P < 0.01. (D) Representative micrograph of the immunoreactivity for both Fos (red) and TH (green) in the VTA in PPD2 WT and MCHR1 KO mice. Arrows indicate representative cells immunoreactive for both Fos and TH. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.6. Inactivation of the MCH system reduces milk production and prolactin mRNA levels
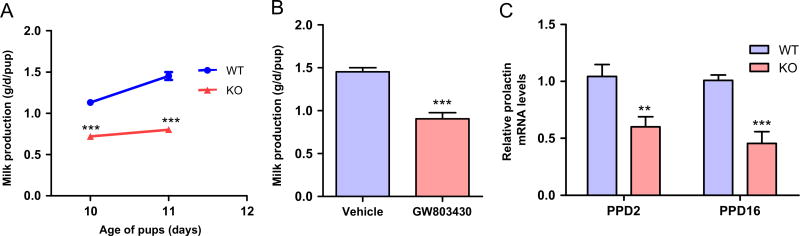
Lactation and maternal behavior are closely linked. Therefore, milk production was assessed on PPD10 and PPD11 (see material and methods). The MCHR1 KO mice produced a lower amount of milk compared with WT mice, P < 0.001, n = 5–6, (Figure 6A). GW803430 (30 mg/kg) administered 24 h before the PPD11 test significantly reduced milk production, P < 0.001 on day PPD11, n = 5, (Figure 6B). Pituitary prolactin mRNA levels, measured by qt-PCR, were decreased by 40% and 50% in MCHR1 KO mice compared with WT mice on PPD2 and PPD16 respectively, P < 0.01 and < 0.001 for PPD2 and PPD16 respectively (Figure 6C).
Figure 6.
Effect of inactivation of MCHR1 on Milk production and pituitary prolactin mRNA in postpartum female WT and MCHR1 KO mice. (A) The milk production of WT and MCHR1 KO mice (n = 5–6) on PPD10 and PPD12. Two way ANOVA followed by Bonferroni post-hoc test (F1,18 = 285.4, P < 0.001): WT vs. KO, PPD10: ***P < 0.001, PPD11: ***P < 0.001. (B) The effect of GW803430 (30 mg/kg) on the milk production of WT mice on PPD11 (n = 5). Unpaired t-test (t = 6.3): vehicle vs. GW803430, ***P < 0.001. (C) The relative prolactin mRNA levels in the pituitary of WT and MCHR1 KO mice on PPD2 and PPD16 measured by qtPCR (n = 3). Unpaired t-test (PPD2: t = 3.2; PPD16: t = 5.1): WT vs. KO, **P < 0.01, ***P < 0.001.
4. Discussion
In order to prepare the mother for the demands of pregnancy and lactation, the maternal brain is subjected to a number of adaptations. The MCH system has been viewed as important player in integrating information from multiple sources, and connecting brain reward, homeostatic and regulatory systems. This suggests that the MCH system might be important regulator of maternal behaviors.
We first measured the levels of MCH mRNA in the hypothalamus of non-pregnant, pregnant, early postpartum and late postpartum mice. In pregnant mice, MCH mRNA levels were lower compared than in the non-pregnant mice. In postpartum mice, the MCH mRNA expression was progressively increased compared to the pregnant mice, and returned to levels that are comparable to those in non-lactating mice.
Our study does not reveal which set of MCH neurons displayed the changes observed, because the qt-PCR analysis was conducted on the whole hypothalamus. However, a previous report has shown similar changes in the levels of pro-MCH mRNA in the LH in pregnant and lactating rats (Garcia et al., 2003).
It has been shown that MCH and MCHR1 protein expression levels are decreased by estradiol in rats, and that these levels are negative correlated with the serum estradiol in cycling rats (Santollo and Eckel, 2013). This suggests that the decreased and increased ppMCH mRNA levels during pregnancy and lactation respectively, might reflect the negative correlation with estradiol levels during these periods.
4.1. Activation of the MCH system is required for the initiation, but not the maintenance, of maternal behavior
The first phenotypic observations that indicated a role for the MCH system in maternal behavior were the higher mortality and cannibalism rates among the progeny of MCHR1 KO mice and the mothers poor nest building observed during the first two days after parturition. Beyond PPD2, however, the survival rate of the pups of MCHR1 KO was comparable to that of the WT mice. These findings are consistent with previous data that showed increased mortality rate among the progeny of MCH KO mice and that the survival rate was not different from that in WT beyond the PPD3 (Adams et al., 2011).
Acute blockade of the MCH system by MCHR1 selective antagonist also inhibited pup retrieval postpartum mice. Together, these data indicate that MCH plays a role in the initiation of maternal behavior. However, once initiated, the blockade of MCH system does not interfere with the maternal behavior. That was evidenced by the increase in the number of MCHR1 KO mothers that retrieved at least one pup from 35% on day PPD1 to 80% on PPD3, as well as the decrease in the duration and latencies of pups’ retrieval in these mothers from PPD1 to PPD3.
The rapid onset of maternal behavior, seen in postpartum females, is induced by both the hormonal priming during pregnancy and the stimulus from contact with pups at parturition (Numan and Stolzenberg, 2009).
Chemosensory signals (pups’ odor) stimulate female maternal behaviors (Fleming et al., 1992). MCH is known to regulate aspects of many olfactory mediated behaviors; MCH KO mice exhibit defects in olfactory mediated behaviors such as mating, estrous cycle synchronization, and enhanced aggression (Adams et al., 2011; Niu et al., 2012). Therefore, the significantly increased mortality in the offspring of MCHR1 KO as well as MCH KO mothers in the first two days after parturition might be due to the partial impairment in the detection of chemosensory signals that evoke maternal behaviors. We speculate that the continuous exposure to pups improves the detection of pups’ chemosensory signals in postpartum MCHR1 KO mice.
Also, our results suggest that the lack of an active MCH system leads to maternal responses that are deficient, but not totally impaired. Therefore, once initiated, the maternal responses become comparable to those in the WT mothers. Indeed, another neuropeptide system, oxytocin, has been demonstrated to play a role in the initiation but not the maintenance of maternal behavior (Rich et al., 2014). Our current data may not reveal whether MCH system mediate the evoking effect of oxytocin on maternal behavior. However, strong evidence from previous reports suggests that MCH neurons might partially mediate certain functions of oxytocin (Yao et al., 2012).
4.2. MCH system inactivation impairs maternal behavior in the early lactation period, partially, by disrupting the dopamine reward system
Pup retrieval is a motivated maternal behavior that is regulated by the reward system (Robinson et al., 2011). Dopamine projections from the VTA to the NAc have been established as key modulators of the circuitry mediating active and goal-directed maternal behaviors, such as pup retrieval, licking, and grooming (Henschen et al., 2013; Miller and Lonstein, 2005; Numan, 2007; Robinson et al., 2011; Stolzenberg et al., 2007). Increased dopamine release in the NAc is associated with stronger maternal responses (Shahrokh et al., 2010). The mPOA, which is important for the motivational aspects of maternal behavior, has been shown to stimulate the VTA and increase NAc activity during maternal behavior (Numan et al., 2009; Shahrokh et al., 2010). Compared to cocaine, pup stimuli have been shown to be more reinforcing and more powerful in activating the reward system in females during the early lactating period (Ferris et al., 2005). MCHR1 are expressed densely throughout the reward circuit particularly the VTA and the NAc-sh (Saito et al., 2001). MCH effects on the reward system have been well established; MCH directs reward-motivated behaviors such as food rewards, cocaine- and amphetamine-induced rewards, and reward learning (Chung et al., 2009; Geuzaine et al., 2014; Sherwood et al., 2012). In the current study, we show that genetic ablation and pharmacologic inactivation of MCHR1 in the early postpartum period inhibit pups retrieval.
The reduced pups’ retrieval behavior in the MCHR1 KO mother mice was associated with lower density of Fos-ir-neurons in the dopaminergic neurons (TH positive) of the VTA when compared with the wild type mice. The density of Fos-ir-neurons also was lower in the NAc-sh, another region of the reward system compared with the WT mice. These results suggest that the dopamine reward system is downregulated in the early postpartum period in MCHR1 KO, and that MCH may exert part of its action in regulating maternal behavior through the reward system.
MCH expression at the mRNA and peptide levels has been shown to be induced in neurons of the medial preoptic area (MPO) uniquely in postpartum females (Knollema et al., 1992; Rondini et al., 2010). This transiently induced MCH expression in the postpartum mPOA is progressively up-regulated, reaching its peak expression at the end of lactation period, when maternal responsiveness has diminished (Rondini et al., 2010). Interestingly, when injected into the mPOA in early postpartum female rats, MCH selectively decreased the expression of active (rewarded) maternal behaviors in these rats to levels that are typically characteristic of the late postpartum period (Benedetto et al., 2014), when endogenous levels of MCH in the mPOA are naturally high (Rondini et al., 2010). These results suggest that the uniquely induced MCH in the mPOA in the late, but not early, postpartum period, may play a role in suppressing motivated aspects of maternal behavior through inhibitory projections to dopamine reward circuit. These results also suggest a facilitatory role of MCH neurons in the lateral hypothalamus and/or incerto-hypothalamic area in early postpartum period and an inhibitory role for MCH in the mPOA in the expression of maternal behavior during late postpartum period. On the other hand, spatial activities and tasks are involved in maternal care (Cost et al., 2014), and MCH has been recently implicated in spatially oriented food seeking behavior (Sita et al., 2016).
4.3. MCH system inactivation impairs milk production by reducing prolactin levels
Nursing is important aspect of maternal behavior, which is largely dependent on milk production. Lactation is an energy-demanding condition that is associated with massive food and water intake as an adaptation to meet the requirement of milk production (Smith and Grove, 2002). We found a significant decrease in milk production in MCHR1 KO lactating female mice compared to the WT mice. We relied in our milk production calculations on the weight gain over 24 h. This method is not out of limitation because the difference in body weight might reflect changes in the metabolic rate rather than milk production. However, we measured the milk production on days when pups were still not moving, and thus the oxygen consumption should not be significantly different from the wild type pups. Also, the effect of the MCHR1 antagonist in reducing milk production supports a direct role on the mothers rather than the pups. Our results on the decrease of milk production, upon genetic and pharmacological inactivation of MCHR1, are strongly supported by the finding that prolactin mRNA levels were significantly lower in the MCHR1 KO female mice compared with WT mice.
The decrease in milk production might be due to the prolactin deficiency. MCH neurons may exert direct and/or indirect action upon prolactin secretion; rat pituitary expresses MCHR1 in prolactin cells (unpublished data), supporting a direct role of MCH system in milk production, while MCH injections have been shown to increase serum prolactin in rats possibly through tuberoinfundibular dopaminergic (TIDA) neurons (Yang and Shieh, 2004).
Furthermore, prolactin, acting at its receptors in the mPOA, has been implicated in the induction of maternal behaviors in rodents (Consiglio and Bridges, 2009; Melo et al., 2009). Thus, another mechanism for MCH in regulating maternal behavior could be through stimulating the secretion of prolactin. The higher rates of mortality and cannibalism among the progeny of MCHR1 KO mice might then be simply attributed to the decrease in prolactin release due to the lack of an active MCH system.
5. Conclusion
In the current study, we have shown that the MCH system plays a significant role in the initiation of maternal behavior, and that it regulates maternal responses through modulating a number of brain circuits involved in maternal behavior. We have to note, however, that behaviors tested in this study do not reflect consummatory behaviors such as grooming, hovering, and nursing. In this context, MCH may play a role in integrating information from multiple sources, and connecting brain reward, homeostatic and regulatory systems.
Acknowledgments
Funding
This work was supported by National Institute of Health (DA024746), CART and Eric L. and Lila D. Nelson Chair in Neuropharmacology. Amal Alachkar is supported by the Institute of International Education IIE-SRF fellowship.
We thank our colleagues Drs. Geoff Abbott and Naoto Hoshi for helpful discussions.
Footnotes
Contributors
Amal Alachkar and Olivier Civelli designed the study, wrote the protocol, carried out the statistical analysis, and wrote the manuscript. Lamees Alhassen and Kara Onouye undertook the behavioral experiments. Zhiwei Wang undertook the qtPCR analyses. Lien Wang and Nayna Sanathara managed the literature searches and analyses, and contributed to the manuscript preparation. All authors contributed to and have approved the final manuscript.
Conflict of interest
None.
References
- Adams AC, Domouzoglou EM, Chee MJ, Segal-Lieberman G, Pissios P, Maratos-Flier E. Ablation of the hypothalamic neuropeptide melanin concentrating hormone is associated with behavioral abnormalities that reflect impaired olfactory integration. Behav. Brain Res. 2011;224(1):195–200. doi: 10.1016/j.bbr.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alachkar A, Jiang D, Harrison M, Zhou Y, Chen G, Mao Y. An EJC factor RBM8a regulates anxiety behaviors. Curr. Mol. Med. 2013;13(6):887–899. doi: 10.2174/15665240113139990019. [DOI] [PubMed] [Google Scholar]
- Benedetto L, Pereira M, Ferreira A, Torterolo P. Melanin-concentrating hormone in the medial preoptic area reduces active components of maternal behavior in rats. Peptides. 2014;58:20–25. doi: 10.1016/j.peptides.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, et al. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J. Comp. Neurol. 1992;319(2):218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Blouin AM, Siegel JM. Relation of melanin concentrating hormone levels to sleep, emotion and hypocretin levels. Sleep. 2013;36(12):1777. doi: 10.5665/sleep.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J, Ames RS, Bergsma D, Muir A, Fitzgerald LR, Hervieu G, et al. Melanin-concentrating hormone is the cognate ligand for the orphan G-protein-coupled receptor SLC-1. Nature. 1999;400(6741):261–265. doi: 10.1038/22313. [DOI] [PubMed] [Google Scholar]
- Chung S, Hopf FW, Nagasaki H, Li CY, Belluzzi JD, Bonci A, et al. The melanin-concentrating hormone system modulates cocaine reward. Proc. Natl. Acad. Sci. USA. 2009;106(16):6772–6777. doi: 10.1073/pnas.0811331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Verheij MM, Hesseling P, van Vugt RW, Buell M, Belluzzi JD, et al. The melanin-concentrating hormone (MCH) system modulates behaviors associated with psychiatric disorders. PLoS One. 2011;6(7):e19286. doi: 10.1371/journal.pone.0019286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Karlsson C, Shaw JL, Thorsell A, Gehlert DR, Heilig M. Suppression of alcohol self-administration and reinstatement of alcohol seeking by melanin-concentrating hormone receptor 1 (MCH1-R) antagonism in Wistar rats. Psychopharmacology. 2010;211(4):367–375. doi: 10.1007/s00213-010-1891-y. [DOI] [PubMed] [Google Scholar]
- Consiglio AR, Bridges RS. Circulating prolactin, MPOA prolactin receptor expression and maternal aggression in lactating rats. Behav. Brain Res. 2009;197(1):97–102. doi: 10.1016/j.bbr.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cost KT, Lobell TD, Williams-Yee ZN, Henderson S, Dohanich G. The effects of pregnancy, lactation, and primiparity on object-in-place memory of female rats. Horm. Behav. 2014;65(1):32–39. doi: 10.1016/j.yhbeh.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Deacon RM. Assessing nest building in mice. Nat. Protoc. 2006;1(3):1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM, Jr, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J. Neurosci.: Off. J. Soc. Neurosci. 2005;25(1):149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, Gavarth K, Sarker J. Effects of transections to the vomeronasal nerves or to the main olfactory bulbs on the initiation and long-term retention of maternal behavior in primiparous rats. Behav. neural Biol. 1992;57(3):177–188. doi: 10.1016/0163-1047(92)90122-k. [DOI] [PubMed] [Google Scholar]
- Fraigne JJ, Peever JH. Melanin-concentrating hormone neurons promote and stabilize sleep. Sleep. 2013;36(12):1767–1768. doi: 10.5665/sleep.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos KFG. The Mouse Brain in Stereotaxic Coordinates. 2. Academic Press; 2001. [Google Scholar]
- Garcia MC, Lopez M, Gualillo O, Seoane LM, Dieguez C, Senaris RM. Hypothalamic levels of NPY, MCH, and prepro-orexin mRNA during pregnancy and lactation in the rat: role of prolactin. FASEB J.: Off. Publ. Fed. Am. Soc. Exp. Biol. 2003;17(11):1392–1400. doi: 10.1096/fj.02-0933com. [DOI] [PubMed] [Google Scholar]
- Geuzaine A, Tyhon A, Grisar T, Brabant C, Lakaye B, Tirelli E. Amphetamine reward in food restricted mice lacking the melanin-concentrating hormone receptor-1. Behav. Brain Res. 2014;262:14–20. doi: 10.1016/j.bbr.2013.12.052. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Li J, Lowe EL, Levine S. Maternal behavior, pup vocalizations, and pup temperature changes following handling in mice of 2 inbred strains. Dev. Psychobiol. 1980;13(6):573–584. doi: 10.1002/dev.420130603. [DOI] [PubMed] [Google Scholar]
- Henschen CW, Palmiter RD, Darvas M. Restoration of dopamine signaling to the dorsal striatum is sufficient for aspects of active maternal behavior in female mice. Endocrinology. 2013;154(11):4316–4327. doi: 10.1210/en.2013-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Harrison D, Meakin J, Maycox P, Nasir S, et al. The distribution of the mRNA and protein products of the melanin-concentrating hormone (MCH) receptor gene, slc-1, in the central nervous system of the rat. Eur. J. Neurosci. 2000;12(4):1194–1216. doi: 10.1046/j.1460-9568.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- Knollema S, Brown ER, Vale W, Sawchenko PE. Novel hypothalamic and preoptic sites of prepro-melanin-concentrating hormone messenger ribonucleic Acid and Peptide expression in lactating rats. J. Neuroendocr. 1992;4(6):709–717. doi: 10.1111/j.1365-2826.1992.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Melo AI, Perez-Ledezma M, Clapp C, Arnold E, Rivera JC, Fleming AS. Effects of prolactin deficiency during the early postnatal period on the development of maternal behavior in female rats: mother's milk makes the difference. Horm. Behav. 2009;56(3):281–291. doi: 10.1016/j.yhbeh.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Miller SM, Lonstein JS. Dopamine d1 and d2 receptor antagonism in the preoptic area produces different effects on maternal behavior in lactating rats. Behav. Neurosci. 2005;119(4):1072–1083. doi: 10.1037/0735-7044.119.4.1072. [DOI] [PubMed] [Google Scholar]
- Nagai J. Preweaning weight as a measure of milk production in mice. Can. J. Genet. Cytol. J. Can. Genet. Cytol. 1971;13(2):354–361. doi: 10.1139/g71-054. [DOI] [PubMed] [Google Scholar]
- Nagasaki H, Wang Z, Jackson VR, Lin S, Nothacker HP, Civelli O. Differential expression of the thyrostimulin subunits, glycoprotein alpha2 and beta5 in the rat pituitary. J. Mol. Endocrinol. 2006;37(1):39–50. doi: 10.1677/jme.1.01932. [DOI] [PubMed] [Google Scholar]
- Niu JG, Yokota S, Tsumori T, Oka T, Yasui Y. Projections from the anterior basomedial and anterior cortical amygdaloid nuclei to melanin-concentrating hormone-containing neurons in the lateral hypothalamus of the rat. Brain Res. 2012;1479:31–43. doi: 10.1016/j.brainres.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev. Psychobiol. 2007;49(1):12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- Numan M. Maternal behavior: neural circuits, stimulus valence, and motivational processes. Parent.: Sci. Pract. 2012;(12):105–114. [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front. Neuroendocr. 2009;30(1):46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Vadlamudi SV, Boccia ML, Amico JA. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes Brain Behav. 2006;5(3):274–281. doi: 10.1111/j.1601-183X.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380(6571):243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Rich ME, deCardenas EJ, Lee HJ, Caldwell HK. Impairments in the initiation of maternal behavior in oxytocin receptor knockout mice. PloS One. 2014;9(6):e98839. doi: 10.1371/journal.pone.0098839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Zitzman DL, Williams SK. Mesolimbic dopamine transients in motivated behaviors: focus on maternal behavior. Front. Psychiatry. 2011;2:23. doi: 10.3389/fpsyt.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TK, King EC, Reyna-Neyra A, Paroder M, Purtell K, Koba W, et al. Kcne2 deletion uncovers its crucial role in thyroid hormone biosynthesis. Nat. Med. 2009;15(10):1186–1194. doi: 10.1038/nm.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondini TA, Donato J, Jr, Rodrigues Bde C, Bittencourt JC, Elias CF. Chemical identity and connections of medial preoptic area neurons expressing melanin-concentrating hormone during lactation. J. Chem. Neuroanat. 2010;39(1):51–62. doi: 10.1016/j.jchemneu.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Rossi M, Choi SJ, O'Shea D, Miyoshi T, Ghatei MA, Bloom SR. Melanin-concentrating hormone acutely stimulates feeding, but chronic administration has no effect on body weight. Endocrinology. 1997;138(1):351–355. doi: 10.1210/endo.138.1.4887. [DOI] [PubMed] [Google Scholar]
- Roy M, David N, Cueva M, Giorgetti M. A study of the involvement of melanin-concentrating hormone receptor 1 (MCHR1) in murine models of depression. Biol. Psychiatry. 2007;61(2):174–180. doi: 10.1016/j.biopsych.2006.03.076. [DOI] [PubMed] [Google Scholar]
- Roy M, David NK, Danao JV, Baribault H, Tian H, Giorgetti M. Genetic inactivation of melanin-concentrating hormone receptor subtype 1 (MCHR1) in mice exerts anxiolytic-like behavioral effects. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 2006;31(1):112–120. doi: 10.1038/sj.npp.1300805. [DOI] [PubMed] [Google Scholar]
- Saito Y, Cheng M, Leslie FM, Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J. Comp. Neurol. 2001;435(1):26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- Saito Y, Nothacker HP, Civelli O. Melanin-concentrating hormone receptor: an orphan receptor fits the key. Trends Endocrinol. Metab. 2000;11(8):299–303. doi: 10.1016/s1043-2760(00)00290-3. [DOI] [PubMed] [Google Scholar]
- Saito Y, Nothacker HP, Wang Z, Lin SH, Leslie F, Civelli O. Molecular characterization of the melanin-concentrating-hormone receptor. Nature. 1999;400(6741):265–269. doi: 10.1038/22321. [DOI] [PubMed] [Google Scholar]
- Sampson DA, Jansen GR. Measurement of milk yield in the lactating rat from pup weight and weight gain. J. Pediatr. Gastroenterol. Nutr. 1984;3(4):613–617. doi: 10.1097/00005176-198409000-00023. [DOI] [PubMed] [Google Scholar]
- Santollo J, Eckel LA. Oestradiol decreases melanin-concentrating hormone (MCH) and MCH receptor expression in the hypothalamus of female rats. J. Neuroendocr. 2013;25(6):570–579. doi: 10.1111/jne.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Nakagawasai O, Tan-No K, Onogi H, Niijima F, Tadano T. Influence of olfactory bulbectomy on maternal behavior and dopaminergic function in nucleus accumbens in mice. Behav. Brain Res. 2010;215(1):141–145. doi: 10.1016/j.bbr.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151(5):2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A, Wosiski-Kuhn M, Nguyen T, Holland PC, Lakaye B, Adamantidis A, et al. The role of melanin-concentrating hormone in conditioned reward learning. Eur. J. Neurosci. 2012;36(8):3126–3133. doi: 10.1111/j.1460-9568.2012.08207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sita LV, Diniz GB, Canteras NS, Xavier GF, Bittencourt JC. Effect of intrahippocampal administration of anti-melanin-concentrating hormone on spatial food-seeking behavior in rats. Peptides. 2016;76:130–138. doi: 10.1016/j.peptides.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Sita LV, Elias CF, Bittencourt JC. Connectivity pattern suggests that incerto-hypothalamic area belongs to the medial hypothalamic system. Neuroscience. 2007;148(4):949–969. doi: 10.1016/j.neuroscience.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Smith MS, Grove KL. Integration of the regulation of reproductive function and energy balance: lactation as a model. Front. Neuroendocrinol. 2002;23(3):225–256. doi: 10.1016/s0091-3022(02)00002-x. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, McKenna JB, Keough S, Hancock R, Numan MJ, Numan M. Dopamine D1 receptor stimulation of the nucleus accumbens or the medial preoptic area promotes the onset of maternal behavior in pregnancy-terminated rats. Behav. Neurosci. 2007;121(5):907–919. doi: 10.1037/0735-7044.121.5.907. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD. Impaired maternal behavior in mice lacking norepinephrine and epinephrine. Cell. 1997;91(5):583–592. doi: 10.1016/s0092-8674(00)80446-8. [DOI] [PubMed] [Google Scholar]
- Wang Z, Storm DR. Maternal behavior is impaired in female mice lacking type 3 adenylyl cyclase. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 2011;36(4):772–781. doi: 10.1038/npp.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SC, Shieh KR. Effects of the cocaine- and amphetamine-regulated transcript peptide on the turnover of dopamine in tuberoinfundibular neurons and serum prolactin levels: studies using estrogen, melanin concentrating hormone, and melanocortin. Neuropharmacology. 2004;47(7):1070–1080. doi: 10.1016/j.neuropharm.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Yao Y, Fu LY, Zhang X, van den Pol AN. Vasopressin and oxytocin excite MCH neurons, but not other lateral hypothalamic GABA neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302(7):R815–R824. doi: 10.1152/ajpregu.00452.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]