Abstract
In many non-human species, including primates, gestational reproductive hormones play an essential role in the onset of maternal motivation and behaviors. We investigated the associations between prepartum estradiol and progesterone and maternal behavior at 1-year postpartumin 177 women. Blood was obtained at five gestational time points and an index of quality of maternal care was determined using a well-validated mother-child interaction protocol. Women who exhibited higher quality maternal care at 1-year postpartum were characterized by unique gestational profiles of estradiol, progesterone and the estrogen to progesterone ratio; specifically by slower accelerations and levels of these hormone trajectories beginning in midgestation. Further, it appeared that both fetal sex and parity moderated these findings, with first time mothers and mothers of females showing stronger associations. In sum, these data document persisting associations between prepartum hormone profiles and human maternal behavior. More broadly, these findings add to the growing literature highlighting the perinatal period as one of critical neurodevelopment in the lifespan of the human female.
Keywords: Estrogens, Progesterone, Maternal behavior, Maternal care, Maternal sensitivity, Maternal brain, Pregnancy, Prenatal
1. Introduction
Human offspring are vulnerable and require intensive and prolonged parental care. Further, humans produce relatively few offspring over the course of the lifespan. As such, it is not surprising that some evolutionary biologists have argued that the development of maternal behaviors represents one of the primary forces shaping evolution of the mammalian brain (Hrdy, 1999; MacLean, 1990). In addition, because historically, the human father was often absent, others have argued that the burden has fallen on the female nervous system to care for our vulnerable young (Kinsley and Amory-Meyer, 2011). To meet the complex and varied challenges of motherhood, the female nervous system exhibits a remarkable neuroplasticity as a result of pregnancy, parturition, lactation and offspring exposure (Hillerer et al., 2014; Kinsley, 2008). In a range of non-human mammals, compelling evidence further suggests that the perinatal endocrine milieu plays a role in this extensive neurological transformation, setting the stage for maternal motivation and responsiveness (Bridges, 1984; Terkel and Rosenblatt, 1968, 1972).
A rich and well-articulated non-primate animal literature describes the important role of sex steroid hormones in the onset and maintenance of maternal behaviors (Brunton and Russell, 2008; Numan and Insel, 2003; Saltzman and Maestripieri, 2011; Bridges, 2015). Across many species during gestation, predictable and dramatic increases in estrogens and progesterone are observed. These sex steroids are synthesized by the gonads, adrenal cortex and placenta and act on target tissues throughout the body including the brain. Among non-human primates, prepartum estrogens and the ratio of estradiol to progesterone have been linked to responsiveness to infants and rates of interaction with unrelated infants during gestation (Maestripieri and Wallen, 1995; Maestripieri and Zehr, 1998; Ramirez et al., 2004). Among nulliparous, reproductively-suppressed female marmosets, endocrine treatment that simulates the estradiol and progesterone profile of late pregnancy will result in more bar pressing to obtain visual access to a replica of an infant marmoset and to turn off an audio recording of infant distress compared to untreated females (Pryce et al., 1993). Persisting associations between gestational sex steroid levels and postpartum maternal behaviors also have been observed in non-human primate models. Late pregnancy estradiol concentrations have been linked to postpartum maternal behaviors and infant survival in tamarins, marmosets, titi monkeys, macaques and baboons (Bardi et al., 2003; Fite and French, 2000; French et al., 2004; Jarcho et al., 2012; Pryce et al., 1988).
To our knowledge, only one previous study has examined the link between gestational reproductive hormone exposures and postnatal maternal behavior in humans. Fleming et al. (1997) examined estradiol and progesterone levels four times across gestation as predictors of mothers' reports of attachment to their infants at 4-days postpartum. Mothers who exhibited higher levels of estradiol at both 5 and 7 months' gestation reported lower feelings of attachment to their infants in the early postpartum period. This same inverse association was observed for the estrogen to progesterone ratio. To date, it is not known whether or not prepartum sex hormone profiles predict maternal behavior or attachment beyond the immediate postpartum period.
The current study, which utilizes data from a large, prospective cohort of women, examines whether or not associations between gestational hormone profiles and observations of human maternal behavior can be detected as late as the end of the first postpartum year (no existing study has examined this question beyond 1-month postpartum).
2. Materials and methods
2.1. Participants
Participants included 177 women and their infants (97 boys and 80 girls) enrolled in a large, longitudinal study of early life influences on development. Mothers were recruited early in pregnancy from a large university medical center based on the following criteria: 1) singleton pregnancy 2) over the age of 18 3) English speaking 4) non-smoking 5) absence of any condition that could dysregulate neuroendocrine function. Exclusion criteria for the present study also included: 1) attended <3 prepartum visits 2) missing data at the 12 month postpartum visit. Demographic characteristics of the participants can be seen in Table 1. The women who met the inclusion criteria for this study did not differ from the larger sample in terms of race/ethnicity, maternal age, education level, income, cohabitation with the baby's father or parity. It was the case however, that women who had delivered preterm were less likely to have been included in the present sent of analyses. This research was approved by the University of California, Irvine, institutional review board, carried out in accordance with The Code of Ethics of the World Medical Association for experiments involving humans and all participants provided informed consent.
Table 1.
Participant characteristics (N = 177).
| Race/ethnicity (%) | |
|---|---|
| Latina | 27 |
| Non-Hispanic White | 51 |
| Asian | 8 |
| Other | 14 |
| Maternal age (mean years) | 30.0 |
| Education (%) | |
| High school or less | 38 |
| Associates or vocational degree | 18 |
| 4-Year college degree | 25 |
| Graduate degree | 19 |
| Annual household income (mean US Dollars) | 61,203 |
| Parity (% primiparous) | 41.8 |
| Length of gestation (mean weeks) | 39.2 |
| Infant sex (% female) | 45.2 |
| Infant age at assessment (mean months) | 12.2 |
| Maternal sensitivity composite (mean) | 9.6 |
| Depression (mean) | 4.1 |
Note: The range of possible scores for the maternal sensitivity composite is 3 to 12 and the range for depression is 0 to 27.
2.2. Overview of study design
Pregnant participants were recruited by a research nurse during the first trimester of pregnancy. Each woman then participated in a study visit at 14–16 (M = 15.31, SD = 0.92), 24–26 (M = 25.55, SD = 0.93), 30–32 (M = 30.96, SD = 0.77), 36+ weeks' gestation (M = 36.7, SD = 0.83) and also at 12-months postpartum (M = 13.23, SD = 0.53). At each prepartum visit, an interview was conducted and a blood sample obtained. At the postpartum visit, structured interviews were conducted, a blood sample was collected in a subset of women (N = 116) and maternal behaviors were observed and coded.
2.3. Endocrine measures
In the afternoon, blood samples (20 ml/draw) were withdrawn by antecubital venipuncture into red top vacutainers for serum collection. Blood samples sat at room temperature until clotted. Samples were then centrifuged at 2000 ×g (15 min). Serum was decanted into polypropylene tubes and stored at −80 °C until assayed.
17β-Estradiol levels were determined by a microtiter well competitive binding enzyme immunoassay. Serum samples were diluted 10-fold prior to testing and mixed well. Diluted samples (25 µl) were incubated with enzyme conjugate (200 µl) for 2 h at room temperature in each well. After decanting and rinsing wells with diluted wash solution three times (400 µl per well), substrate reagent (100 µl) was added to the blotted wells and incubated for 15 min at room temperature. Within 10 min after addition of stop reagent (50 µl), absorbance readings were taken at 450 nm. The assay has <0.2% cross-reactivity with estriol and estrone, and non-detectable cross-reactivity with 17α-estradiol, progesterone and 25 other naturally occurring steroids. Reported inter-and intra-assay coefficients of variance are <10% and 7%, respectively, with analytical sensitivity at 9.71 pg/ml.
Progesterone levels were determined quantitatively by competitive binding enzyme immunoassay. Thawed and thoroughly mixed serum samples were diluted 10-fold prior to assay. Diluted samples (25 µl) were dispensed into wells and incubated with enzyme conjugate (200 µl) for 1 h at room temperature. Decanted microtiter plates were washed 3 times with diluted wash solution (400 µl per well). Substrate reagent (200 µl) was added to each well and incubated for 15 min at room temperature. Enzymatic reaction was stopped with stop solution (50 µl) and absorbance readings were taken at 450 nm within 10 min. Cross reactivity of this assay is reported as 1.1% with 11-desoxycorticosterone; <0.4% with pregnenolone, 17α –OH progesterone, corticosterone; and <0.1% with estriol, 17β-estradiol, cortisol, and 3 other naturally occurring steroids. Inter-and intra-assay coefficients of variance are reported as <10% and 7% respectively with analytical sensitivity at 0.045 ng/ml.
In addition, because in humans and other species the balance of sex steroids appears to play a role in the onset of maternal behavior (Fleming et al., 1997; Jarcho et al., 2012; Maestripieri and Zehr, 1998; Rosenblatt et al., 1988), the ratio of estrogen to progesterone (E/P) was calculated and also used as a predictor of maternal behavior.
2.4. Assessment of maternal behavior
Mothers were videotaped interacting with their infants in a semi-structured 10-minute play episode when the infant was 12-months of age. From these videotapes maternal behavior coded using a laboratory protocol developed for the NICHD Study of Early Child Care and Youth Development (NICHD Early Child Care Research Network, 1999a). During this play interaction, mothers were given a standard set of age-appropriate toys and told to play with their infant as they would at home.
Categories of maternal behavior were scored on a 4-point global rating scale (1 = not at all characteristic to 4 = highly characteristic). Following the NICHD procedure, a composite rating of maternal sensitivity was created by summing ratings of sensitivity to nondistress, positive regard, and intrusiveness (reverse-coded). Coding was done by intensively trained and reliable teams of coders. All coders were blind to other data gathered on study participants. Twenty percent of sessions were selected at random, without coder knowledge, and coded again by a second independent coder to obtain an index of inter-rater reliability. Reliability for each of the three subscales comprising the composite were: sensitivity to nondistress (90%), intrusiveness (90%), and positive regard (93%).
This paradigm is an objective, behaviorally-based laboratory assessment tool for studying maternal sensitivity with standardized procedures for coding behavior. It was developed for a large NICHD multisite study that followed over 1200 children and their families from 1 month postpartum through early adolescence. The composite measure of maternal sensitivity is positively associated with measures of mothers' interactive behaviors and inventory scores derived from the Home Observation of Measurement of the Environment (Caldwell and Bradley, 1984; Spiker et al., 1993), indicating that this assessment relates to maternal behavior when assessed in more naturalistic settings. Additionally, it is predictive specifically of the quality of mother-child attachment and also predicts child socioemotional, language and cognitive development (Brooks-Gunn et al., 2002; NICHD Early Child Care Research Network, 1999b, 2006).
2.5. Additional measures
Maternal demographics (e.g. race/ethnicity, parity, income age and education) and breastfeeding status were obtained from structured interview. Length of gestation was determined by combining early ultrasound measures and medical chart review according to ACOG guidelines (American Congress of Obstetricians and Gynecologists, 2009). In addition, maternal depressive symptoms at 12-months postpartum were assessed with the short-version of the Center for Epidemiologic Studies Depression Scale (CESD), an instrument that demonstrates both good reliability and validity (Irwin et al., 1999; Knight et al., 1997; Santor and Coyne, 1997).
2.6. Data analysis plans
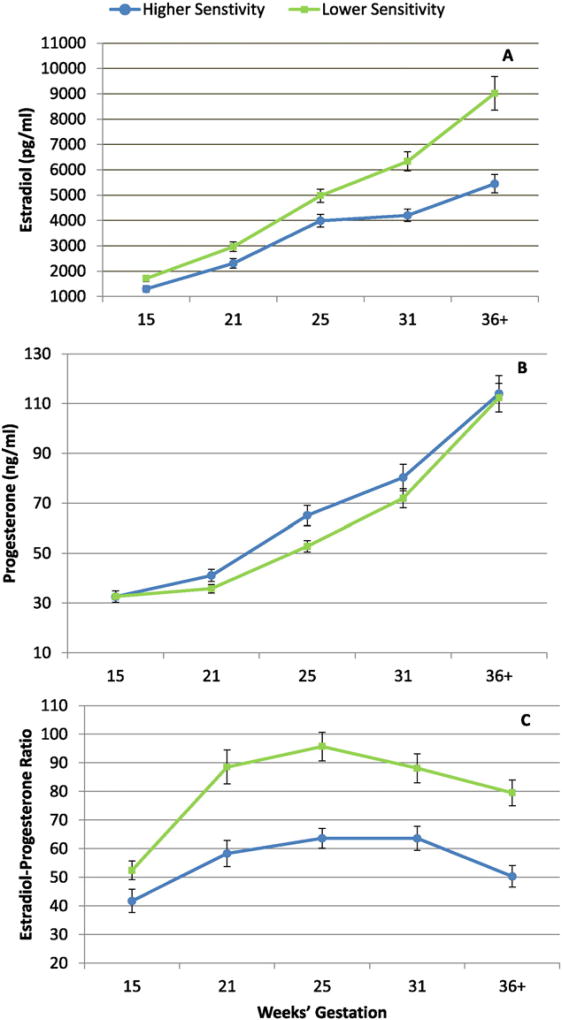
To assess the association between maternal sensitivity and hormone trajectories across gestation, multilevel modeling using hierarchical linear modeling (HLM) growth curve analysis (Raudenbush and Bryk, 2002) was conducted. Initial testing indicated that quadratic models provided the best fit for the gestational estradiol, progesterone and E/P trajectories, consistent with the fact that none of the three hormones show steady linear increases across gestation (Fig. 1). In each full two-level model, the effects of maternal sensitivity on hormone levels at the initial assessment (14 weeks) and change across time were evaluated. Specifically, the level-1 variables (or the time-variant variables) included hormone levels at each study visit and timing of these study visits in weeks. The level-2 variables (or time-invariant variables) included maternal sensitivity (entered as a continuous variable) and relevant covariates (see below). The full models were then repeated at one-week intervals for each week until the last study visit occurred (38 weeks' gestation).
Fig. 1.
Gestational trajectories of estradiol (A), progesterone (B) and the estrogen to progesterone ratio (C). Means and standard errors of top and bottom quartiles of maternal sensitivity at 12-months post-partum are plotted only for illustrative purposes. In all statistical analyses, sensitivity was treated as a continuous variable.
Variables evaluated as potential covariates included: maternal age, parity, race/ethnicity, education, income, cohabitation with baby's father, duration of breastfeeding, depressive symptoms at the 12-month assessment, infant sex, infant gestational age at birth and also infant age at assessment. Third variables were included in the models if they exhibited a statistically significant association with both the predictor (hormone) and outcome (maternal sensitivity). Variables that met this criterion for estradiol and E/P included: maternal age, ethnicity, education, income and duration of breastfeeding. For progesterone the variables that met criteria were: maternal age, parity, education and ethnicity. All of the bivariate associations between hormones, maternal sensitivity and the potential covariates can be seen in Table S1. In addition, because there are theoretical and empirical reasons to expect that parity (Gatewood et al., 2005; Glynn, 2012; Love et al., 2005) and fetal sex (Clifton, 2010; Glynn and Sandman, 2012) could moderate associations between prenatal hormone exposures and maternal behavior, we tested potential parity and sex interactions in the models.
3. Results
3.1. Sample characteristics
Basic demographic characteristics of the sample as well as maternal sensitivity scores can be seen in Table 1. Levels of maternal sensitivity were consistent with those from a nationally representative sample (NICHD Early Child Care Research Network, 1999b, 2005) and mean postpartum depression values also were consistent with published studies in other cohorts of women studied in the perinatal period (c.f. Paulson et al., 2006; Stapleton et al., 2012).
3.2. Gestational endocrine profiles
As expected, both estradiol and progesterone showed reliable increases from one gestational assessment to the next (see Table 2). Further, both estradiol and progesterone levels were consistent with gestational data from other similar studies (c.f. Fleming et al., 1997; O'Hara et al., 1991). The estradiol to progesterone ratio exhibited a u-shaped function across gestation with the peak at 25 weeks' gestation. The estradiol to progesterone ratio did not differ when comparing 19 to 31 weeks. However, with that exception, all other gestational time points did differ from one another for each hormone and the ratio (all p's < 0.05). Estradiol and progesterone were modestly correlated at each gestational time point (r's ranged from 0.24 to 0.75; See Table S2). Gestational levels of estradiol, progesterone and the E/P were not predictive of hormone levels at 12-months postpartum (Table S2).
Table 2.
Mean levels of estradiol, progesterone and the estradiol to progesterone ratio across gestation (mean and standard deviation).
| Week of gestation | |||||
|---|---|---|---|---|---|
|
|
|||||
| 15 | 19 | 25 | 31 | 36+ | |
| Estradiol (pg/ml) | 1500.6 (762.7) | 2562.0 (1113.0) | 4209.8 (1562.8) | 5111.1 (2081.5) | 7009.2 (3251.5) |
| Progesterone (ng/ml) | 31.5 (10.6) | 36.3 (10.9) | 56.0 (17.8) | 76.7 (26.1) | 120.7 (41.5) |
| Estradiol/progesterone | 50.4 (25.6) | 74.7 (41.5) | 80.0 (32.5) | 72.3 (33.8) | 61.7 (27.4) |
3.3. Analysis of hormone trajectories and maternal behavior
As shown in Fig. 1A and B, multilevel mixed modeling revealed that change in estradiol and progesterone profiles across gestation were associated with maternal behavior 12 months later. Women who were rated as more sensitive exhibited trajectories of estradiol that accelerated at a slower rate beginning at 23 weeks' gestation (Bs ranged from −12.4 to −90.1; p's < 0.05) and also had lower levels of estradiol beginning at 28 weeks' compared to women who exhibited less sensitivity (Bs ranged from −165.5 to −729.9; p's < 0.05). These differences persisted until the end of gestation. Women who were characterized as more sensitive also showed slower rates of progesterone increase beginning at 36weeks until the end of gestation (Bs ranged from −0.51 to −0.59; p's < 0.05). In addition, differences in the E/P trajectory during gestation were associated with maternal sensitivity at 12 months. More sensitive mothers were characterized by a lower E to P ratio from 20 to 34 weeks' gestation (Bs ranged from −2.3 to −2.5; p's < 0.05; see Fig. 1C).
3.4. Evaluation of potential moderating effects of fetal sex and parity
Women who were giving birth for the first time exhibited higher levels of progesterone beginning at 32 weeks' gestation (Bs ranged from −0.41.1 to −103.0; p's < 0.05). In addition, the multilevel models revealed statistically significant interactions between parity and maternal behavior such that the effects were driven by the first time mothers. Beginning at 32 weeks' until the end of gestation, higher levels of progesterone were associated with less sensitivity only among the primiparous women (Bs ranged from −4.2 to −12.7; p's < 0.05).
Fetal sex also appeared to play a moderating role in determining associations between prepartum hormone profiles and maternal behavior. Women pregnant with female fetuses exhibited higher levels of both progesterone and estradiol beginning at 28 weeks' (Bs ranged from 30.6 to 99.7; p's < 0.05) and 29 weeks (Bs ranged from 3279.1 to 7766.0; p's < 0.05) gestation respectively, and these differences persisted until the end of the study period. Further, in pregnancies resulting in daughters, the associations between both hormones and maternal behavior were apparent, whereas they were not in pregnancies resulting in sons. Among those who gave birth to daughters, higher levels of estradiol and progesterone were associated with decreased sensitivity. These differences in levels reached statistical significance beginning at 25 weeks for estradiol (Bs ranged from −299.5 to −1054.4; p's < 0.05) and 30 weeks for progesterone (Bs ranged from −3.07 to −10.6; p's < 0.05) and were apparent until the end of gestation.
4. Discussion
Our data demonstrate that gestational hormone exposures are associated with lasting effects on human maternal behavior. Women who were rated as less sensitive mothers at 1-year postpartum were characterized by unique prepartum profiles of estradiol, progesterone and the estrogen to progesterone ratio; specifically by accelerating trajectories and higher levels of these hormones beginning in midgestation. These findings further add to the existing literature because they also document associations between sex steroid hormones and objective observations of human maternal behavior (as opposed to self-reports as shown previously). The onset and development of human maternal behaviors clearly are multiply determined and these critical influences include psychosocial, environmental, biological and genetic mechanisms (Chittleborough et al., 2012; Mileva-Seitz et al., 2013; Raby et al., 2015; Rilling and Young, 2014; Zelkowitz et al., 2014). Here we provide additional support for the importance of the biological pathway in this process.
The findings are largely consistent with the single other study in humans that also reported negative associations between gestational estradiol and the estradiol to progesterone ratio and feelings of attachment to the infant during the first postpartum week (Fleming et al., 1997). Fleming et al. did not detect relations between progesterone and attachment reports, unlike the present study, however. This could be due to the time at which the indicators of maternal behaviors were measured (one week versus one year), to the fact that the measures of maternal behavior differed (behavioral observation versus reports of attachment), to the fact that neither fetal sex or parity were examined as potential moderators, or to the differences in the statistical analyses approach (the present study approach allowed assessment of hormone trajectories across pregnancy as opposed to analysis of single time points). Our findings also are consistent with non-human primate work documenting associations between gestational sex steroid exposures and maternal motivation and behavior. It has been shown, for example, that higher levels of prepartum estradiol predict less optimal postpartum maternal behaviors and decreased infant survivorship in marmosets (Fite and French, 2000) and that baboons and macaques that exhibit a higher estrogen to progesterone ratio during pregnancy are at increased risk for exhibiting neglectful or abusive behaviors towards their infants (French et al., 2004;Maestripieri and Megna, 2000).
Although an extensive discussion of the precise neural mechanisms through which gestational sex steroid exposures might influence maternal sensitivity or responsiveness is beyond the scope of this paper (Bridges, 2015; Pfaff et al., 2011), the pathways are likely both direct and indirect. Estrogen exerts effects on maternal behavior and motivation through actions in the medial preoptic area and nucleus accumbens (Numan et al., 1977; Numan and Stolzenberg, 2009; Stolzenberg et al., 2007). In addition, estrogen influences the synthesis of oxytocin and regulation of oxytocin receptors (Bale et al., 1995; Fahrbach et al., 1985;McCarthy et al., 1996; Thomas et al., 1999). Progesterone similarly may exert direct influences via the MPOA and it has been documented that progesterone withdrawal following sustained and chronic elevations increases oxytocin mRNA in the paraventricular nucleus (Amico et al., 1997; Blyth et al., 1997). It also is plausible that these hormones exert influences not only by altering activity, but through alterations in neuronal structure in the adult female brain. There is evidence from pharmacological models that both estrogen and progesterone induce structural modifications, especially in dendritic spines (McEwen and Woolley, 1994; Gould et al., 1990), and also that the nature of these effects of estrogen and progesterone are dependent on whether or not they occur in the context of the other (which offers some insight into the links between E/P and maternal behaviors influence of the estrogen to progesterone ratio; Gould et al., 1990;Woolley and McEwen, 1993). Consistent with these pharmacological models cited above, there are analogous changes in neuronal structure in the hippocampus, medial prefrontal cortex, medial preoptic area, paraventricular nucleus and caudate nucleus in rodents during pregnancy and the postpartum (Kinsley et al., 2006; Leuner and Gould, 2010; Pawluski and Galea, 2006; Pawluski et al., 2012). Further, in the few studies in which these structural changes are examined as predictors of behavioral changes associated with reproduction, they do appear to play a role (Leuner and Gould, 2010; Shams et al., 2012).
It is notable and intriguing that we identified sex differences in the relation between prenatal hormone exposures and maternal behavior. It was the case that elevations in progesterone and estradiol late in pregnancy were predictive of lower maternal sensitivity only among women pregnant with female fetuses and it also was the case that pregnancies with female fetuses exhibited higher levels of these hormones during that gestational period. Thus, these differences may reflect the restricted range of exposures among male fetuses, a finding that has been previously demonstrated for estradiol (Gol et al., 2004; Toriola et al., 2011), with the progesterone findings being somewhat less consistent (Gol et al., 2004; Toriola et al., 2011;Wuu et al., 2002). However, it also is interesting to consider these findings in the context that sex differences in trajectories of development and interactions between the maternal and fetal unit are detectable beginning very early in gestation. For example, in pregnancies with female fetuses, hCG levels (HCG is a glycoprotein hormone produced by the developing embryo and later by the syncytiotrophoblasts) in the maternal circulation are elevated compared to those with male fetuses and this difference is apparent as early as three weeks after conception and persists throughout gestation (Obiekwe and Chard, 1982; Santolaya-Forgas et al., 1997; Yaron et al., 2002). Further, pervasive sex differences exist in responsivity to maternal signals in shaping fetal neurological development (Glynn and Sandman, 2012; Sandman et al., 2013) and these new findings suggest, as we have posited previously, that fetal sex may affect pregnancy-related maternal neural plasticity as well (Glynn, 2010).
Similar to offspring sex, parity was associated with differential associations between prepartum hormone profiles and maternal behavior. Specifically, the correlations between progesterone and maternal behaviors were present only among the primiparous females. Again, like the effects of sex, this could be due to differences in potential for exposures (primiparous women exhibited higher levels of progesterone consistent with previous reports; Toriola et al., 2011; Wuu et al., 2002). However, it also is the case that there is some evidence that parity moderates the changes in maternal brain and behaviors associated with reproductive history. For example, multiparous rodent and human mothers who have given birth more times exhibit more profound changes in cognitive function (Gatewood et al., 2005; Glynn, 2012). These findings also are consistent with the assertion that pregnancy exerts a lasting imprint on the female that will affect subsequent hormone exposures (Bridges, 2016; Fox et al., 2015; Glynn, 2012; Musey et al., 1987), an assertion for which some additional evidence exists. For instance, nulliparous rats are more sensitive to estrogen exposures at a molecular level than are multiparous rats (Bridges and Byrnes, 2006). Further, nulliparous rodents are more dependent on hormone exposures of pregnancy for the development of responsiveness to pups, whereas multiparous females are not similarly reliant on these exposures (Moltz and Wiener, 1966), suggesting that maternal behaviors may be more emancipated from hormonal control among multiparous females because of previous maternal experience (Maestripieri and Zehr, 1998).
The hormone exposures of pregnancy are but one component of the endocrine-neurological process involved in the onset and regulation of maternal behavior (Bridges, 2015). Simply put, programming of the maternal brain does not end with parturition. As discussed above, gestational hormone exposures augment or prime the system, but then female is further influenced by the experiences of lactation and offspring exposures (Hillerer et al., 2014; Saltzman and Maestripieri, 2011). Examination of independent effects of pregnancy, lactation and interaction with progeny suggest that these experiences interact synergistically resulting in the most profound changes to the mother's brain and behavioral repertoire (Lambert et al., 2005; Pawluski et al., 2006; Wartella et al., 2003). Further, these synergistic and cumulative effects appear to permanently alter the structure and function of the female brain with implications for responsiveness to juveniles, stress responding, aggression and cognitive function and aging that persist throughout the lifespan (de Almeida et al., 2014; Gatewood et al., 2005; Tu et al., 2005).
The quality of the early mother-offspring relationship is a key contributor to offspring health and development. In animal models, variation in maternal care have been linked to permanent alterations in progeny of fear behavior and HPA-axis regulation, pain sensitivity, vulnerability to addiction, learning and memory, and brain structure and function (Kaffman and Meaney, 2007; Sanchez, 2006). Similarly, in humans, the quality of maternal behavior is associated with social competence, emotion regulation, cognitive and language development, school readiness, physical health and psychopathology (Belsky and Fearon, 2002; Carlson, 1998; NICHD Early Child Care Resarch Network, 1999b, 2003; Rhee et al., 2006; Stams et al., 2002;Warren and Simmens, 2005). Further, compelling evidence suggests that the quality of maternal care itself also is transmitted across generations and that one of the primary biological mechanisms for this transmission involves estrogen pathways (Champagne et al., 2001). For example, female rodents that experienced more sensitive rearing show an enhanced sensitivity to reproductive hormone exposures in the onset of their own maternal behaviors (Novakov and Fleming, 2005) and animals that are reared by more sensitive mothers possess higher levels of estrogen-inducible oxytocin receptors and also estrogen receptors in the mPOA (Champagne et al., 2003). It is likely, given the findings here and the conservation of physiological regulation of maternal behavior across species, that sex steroids play an important role in control of these intergenerational processes in humans as well.
Supplementary Material
Acknowledgments
The authors thank the families who participated in these projects. We also thank the dedicated staff at the Women and Children's Health and Well-Being project, and Kendra Leak in particular, for her efforts on the mother-child coding. This research was supported by grants from the National Institutes of Health (HD-40967, NS-41298, MH-86062 and MH-96889).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.yhbeh.2016.07.002.
References
- American Congress of Obstetricians and Gynecologists. ACOG practice bulletin no 101: ultrasonography in pregnancy. Obstet. Gynecol. 2009;113:451–461. doi: 10.1097/AOG.0b013e31819930b0. [DOI] [PubMed] [Google Scholar]
- Amico JA, Thomas A, Hollingshead DJ. The duration of estradiol and progesterone exposure prior to progesterone withdrawal regulates oxytocin mRNA levels in the paraventricular nucleus of the rat. Endocr. Res. 1997;23:141–156. doi: 10.3109/07435809709031849. [DOI] [PubMed] [Google Scholar]
- Bale TL, Pedersen CA, Dorsa DM. CNS oxytocin receptor mRNA expression and regulation by gonadal steroids. Adv. Exp. Med. Biol. 1995;395:269–280. [PubMed] [Google Scholar]
- Bardi M, Shimizu K, Barrett GM, Borgognini-Tarli SM, Huffman MA. Peripartum sex steroid changes and maternal style in rhesus and Japanese macaques. Gen. Comp. Endocrinol. 2003;133:323–331. doi: 10.1016/s0016-6480(03)00193-x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Fearon RM. Early attachment security, subsequent maternal sensitivity, and later child development: does continuity in development depend upon continuity of caregiving? Attach Hum. Dev. 2002;4:361–387. doi: 10.1080/14616730210167267. [DOI] [PubMed] [Google Scholar]
- Blyth BJ, Hollingshead DJ, Amico JA. Time course of induction of oxytocin messenger ribonucleic acid levels in the hypothalamic paraventricular nucleus of ovariectomized rats following gonadal steroid administration. Life Sci. 1997;60:2427–2433. doi: 10.1016/s0024-3205(97)00327-5. [DOI] [PubMed] [Google Scholar]
- Bridges RS. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology. 1984;114:930–940. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- Bridges RS. Neuroendocrine regulation of maternal behavior. Front. Neuroendocrinol. 2015;36:178–196. doi: 10.1016/j.yfrne.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS. Long-term alterations in neural and endocrine processes induced by motherhood in mammals. Horm. Behav. 2016;77:193–203. doi: 10.1016/j.yhbeh.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS, Byrnes EM. Reproductive experience reduces circulating 17beta-estradiol and prolactin levels during proestrus and alters estrogen sensitivity in female rats. Endocrinology. 2006;147:2575–2582. doi: 10.1210/en.2005-0917. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Han WJ, Waldfogel J. Maternal employment and child cognitive outcomes in the first three years of life: the NICHD study of early child care. National Institute of Child Health and Human Development. Child Dev. 2002;73:1052–1072. doi: 10.1111/1467-8624.00457. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nat. Rev. Neurosci. 2008;9:11–25. doi: 10.1038/nrn2280. [DOI] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH. Home Observation for Measurement of the Environment. University of Arkansas Press; Little Rock: 1984. [Google Scholar]
- Carlson EA. A prospective longitudinal study of attachment disorganization/disorientation. Child Dev. 1998;69:1107–1128. [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003;144:4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- Chittleborough CR, Lawlor DA, Lynch JW. Prenatal prediction of poor maternal and offspring outcomes: implications for selection into intensive parent support programs. Matern. Child Health J. 2012;16:909–920. doi: 10.1007/s10995-011-0818-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton VL. Review: sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(Suppl):S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- de Almeida RM, Ferreira A, Agrati D. Sensory, hormonal, and neural basis of maternal aggression in rodents. Curr. Top. Behav. Neurosci. 2014;17:111–130. doi: 10.1007/7854_2014_312. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Morrell JI, Pfaff DW. Possible role for endogenous oxytocin in estrogen-facilitated maternal behavior in rats. Neuroendocrinology. 1985;40:526–532. doi: 10.1159/000124125. [DOI] [PubMed] [Google Scholar]
- Fite JE, French JA. Pre- and postpartum sex steroids in female marmosets (Callithrix kuhlii): is there a link with infant survivorship and maternal behavior? Horm. Behav. 2000;38:1–12. doi: 10.1006/hbeh.2000.1607. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Ruble D, Krieger H, Wong PY. Hormonal and experiential correlates of maternal responsiveness during pregnancy and the puerperium in human mothers. Horm. Behav. 1997;31:145–158. doi: 10.1006/hbeh.1997.1376. [DOI] [PubMed] [Google Scholar]
- Fox M, Sandman CA, Davis EP, Glynn LM. Intra-individual consistency in endocrine profiles across successive pregnancies. J. Clin. Endocrinol. Metab. 2015;100:4637–4647. doi: 10.1210/jc.2015-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Koban T, Rukstalis M, Ramirez SM, Bardi M, Brent L. Excretion of urinary steroids in pre- and postpartum female baboons. Gen. Comp. Endocrinol. 2004;137:69–77. doi: 10.1016/j.ygcen.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Gatewood JD, Morgan MD, Eaton M, McNamara IM, Stevens LF, Macbeth AH, Meyer EA, Lomas LM, Kozub FJ, Lambert KG, Kinsley CH. Motherhood mitigates aging-related decrements in learning and memory and positively affects brain aging in the rat. Brain Res. Bull. 2005;66:91–98. doi: 10.1016/j.brainresbull.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Glynn LM. Implications of maternal programming for fetal neurodevelopment. In: Zimmermann AW, Connors SL, editors. Maternal Influences on Fetal Neurodevelopment: Clinical and Research Aspects. Springer; 2010. [Google Scholar]
- Glynn LM. Increasing parity is associated with cumulative effects on memory. J. Women's Health (Larchmt) 2012;21:1038–1045. doi: 10.1089/jwh.2011.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Sandman CA. Sex moderates associations between prenatal glucocorticoid exposure and human fetal neurological development. Dev. Sci. 2012;15:601–610. doi: 10.1111/j.1467-7687.2012.01159.x. [DOI] [PubMed] [Google Scholar]
- Gol M, Altunyurt S, Cimrin D, Guclu S, Bagci M, Demir N. Different maternal serum hCG levels in pregnant women with female and male fetuses: does fetal hypophyseal—adrenal—gonadal axis play a role? J. Perinat. Med. 2004;32:342–345. doi: 10.1515/JPM.2004.064. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J. Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillerer KM, Jacobs VR, Fischer T, Aigner L. The maternal brain: an organ with peripartal plasticity. Neural Plas. 2014;2014:574159. doi: 10.1155/2014/574159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdy SB. Mother Nature: Maternal Instincts and How They Shape the Human Species. Ballantine Books; New York: 1999. [Google Scholar]
- Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D) Arch. Intern. Med. 1999;159:1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- Jarcho MR, Mendoza SP, Bales KL. Hormonal and experiential predictors of infant survivorship and maternal behavior in a monogamous primate (Callicebus cupreus) Am. J. Primatol. 2012;74:462–470. doi: 10.1002/ajp.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J. Child Psychol. Psychiatry. 2007;48:224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- Kinsley CH. The neuroplastic maternal brain. Horm. Behav. 2008;54:1–4. doi: 10.1016/j.yhbeh.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Amory-Meyer E. Why the maternal brain? J. Neuroendocrinol. 2011;23:974–983. doi: 10.1111/j.1365-2826.2011.02194.x. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Trainer R, Stafisso-Sandoz G, Quadros P, Marcus LK, Hearon C, Meyer EA, Hester N, Morgan M, Kozub FJ, Lambert KG. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm. Behav. 2006;49:131–142. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Knight RG, Williams S, McGee R, Olaman S. Psychometric properties of the Centre for Epidemiologic Studies Depression Scale (CES-D) in a sample of women in middle life. Behav. Res. Ther. 1997;35:373–380. doi: 10.1016/s0005-7967(96)00107-6. [DOI] [PubMed] [Google Scholar]
- Lambert KG, Berry AE, Griffins G, Amory-Meyers E, Madonia-Lomas L, Love G, Kinsley CH. Pup exposure differentially enhances foraging ability in primiparous and nulliparous rats. Physiol. Behav. 2005;84:799–806. doi: 10.1016/j.physbeh.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E. Dendritic growth in medial prefrontal cortex and cognitive flexibility are enhanced during the postpartum period. J. Neurosci. 2010;30:13499–13503. doi: 10.1523/JNEUROSCI.3388-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love G, Torrey N, McNamara I, Morgan M, Banks M, Hester NW, Glasper ER, Devries AC, Kinsley CH, Lambert KG. Maternal experience produces long-lasting behavioral modifications in the rat. Behav. Neurosci. 2005;119:1084–1096. doi: 10.1037/0735-7044.119.4.1084. [DOI] [PubMed] [Google Scholar]
- MacLean PD. The Triune Brain in Evolution: Role in Paleocerebral Functions. Plenum Press; New York: 1990. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Megna NL. Hormones and behavior in rhesus macaque abusive and nonabusive mothers 2. Mother-infant interactions. Physiol. Behav. 2000;71:43–49. doi: 10.1016/s0031-9384(00)00338-3. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Wallen K. Interest in infants varies with reproductive condition in group-living female pigtail macaques (Macaca nemestrina) Physiol. Behav. 1995;57:353–358. doi: 10.1016/0031-9384(94)00222-q. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Zehr JL. Maternal responsiveness increases during pregnancy and after estrogen treatment in macaques. Horm. Behav. 1998;34:223–230. doi: 10.1006/hbeh.1998.1470. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, McDonald CH, Brooks PJ, Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol. Behav. 1996;60:1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Woolley CS. Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Exp. Gerontol. 1994;29:431–436. doi: 10.1016/0531-5565(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Mileva-Seitz V, Steiner M, Atkinson L, Meaney MJ, Levitan R, Kennedy JL, Sokolowski MB, Fleming AS. Interaction between oxytocin genotypes and early experience predicts quality of mothering and postpartum mood. PLoS One. 2013;8:e61443. doi: 10.1371/journal.pone.0061443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moltz H, Wiener E. Effects of ovariectomy on maternal behavior of primiparous and multiparous rats. J. Comp. Physiol. Psychol. 1966;62:382–387. doi: 10.1037/h0022565. [DOI] [PubMed] [Google Scholar]
- Musey VC, Collins DC, Brogan DR, Santos VR, Musey PI, Martino-Saltzman D, Preedy JR. Long term effects of a first pregnancy on the hormonal environment: estrogens and androgens. J. Clin. Endocrinol. Metab. 1987;64:111–118. doi: 10.1210/jcem-64-1-111. [DOI] [PubMed] [Google Scholar]
- NICHD Early Childcare Research Network. Child care and mother-child interaction in the first 3 years of life. Dev. Psychol. 1999a;35:1399–1413. [PubMed] [Google Scholar]
- NICHD Early Childcare Research Network. Chronicity of maternal depressive symptoms, maternal sensitivity, and child functioning at 36 months. Dev. Psychol. 1999b;35:1297–1310. doi: 10.1037//0012-1649.35.5.1297. [DOI] [PubMed] [Google Scholar]
- NICHD Early Childcare Research Network. Does amount of time spent in child care predict socioemotional adjustment during the transition to kindergarten? Child Dev. 2003;74:976–1005. doi: 10.1111/1467-8624.00582. [DOI] [PubMed] [Google Scholar]
- NICHD Early Childcare Research Network. Predicting individual differences in attention, memory, and planning in first graders from experiences at home, child care, and school. Dev. Psychol. 2005;41:99–114. doi: 10.1037/0012-1649.41.1.99. [DOI] [PubMed] [Google Scholar]
- NICHD Early Childcare Research Network. Infant-mother attachment classification: risk and protection in relation to changing maternal caregiving quality. Dev. Psychol. 2006;42:38–58. doi: 10.1037/0012-1649.42.1.38. [DOI] [PubMed] [Google Scholar]
- Novakov M, Fleming AS. The effects of early rearing environment on the hormonal induction of maternal behavior in virgin rats. Horm. Behav. 2005;48:528–536. doi: 10.1016/j.yhbeh.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The Neurobiology of Parental Behavior. Springer; 2003. [New York]. [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front. Neuroendocrinol. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Numan M, Rosenblatt JS, Komisaruk BR. Medial preoptic area and onset of maternal behavior in the rat. J. Comp. Physiol. Psychol. 1977;91:146–164. doi: 10.1037/h0077304. [DOI] [PubMed] [Google Scholar]
- Obiekwe BC, Chard T. Human chorionic gonadotropin levels in maternal blood in late pregnancy: relationship to birthweight, sex and condition of the infant at birth. BJOG. 1982;89:543–546. doi: 10.1111/j.1471-0528.1982.tb03656.x. [DOI] [PubMed] [Google Scholar]
- O'Hara MW, Schlechte JA, Lewis DA, Varner MW. Controlled prospective study of postpartum mood disorders: psychological, environmental, and hormonal variables. J. Abnorm. Psychol. 1991;100:63–73. doi: 10.1037//0021-843x.100.1.63. [DOI] [PubMed] [Google Scholar]
- Paulson JF, Dauber S, Leiferman JA. Individual and combined effects of postpartum depression in mothers and fathers on parenting behavior. Pediatrics. 2006;118:659–668. doi: 10.1542/peds.2005-2948. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Galea LA. Hippocampal morphology is differentially affected by reproductive experience in the mother. J. Neurobiol. 2006;66:71–81. doi: 10.1002/neu.20194. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Vanderbyl BL, Ragan K, Galea LA. First reproductive experience persistently affects spatial reference and working memory in the mother and these effects are not due to pregnancy or ‘mothering’ alone. Behav. Brain Res. 2006;175:157–165. doi: 10.1016/j.bbr.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Valenca A, Santos AI, Costa-Nunes JP, Steinbusch HW, Strekalova T. Pregnancy or stress decrease complexity of CA3 pyramidal neurons in the hippocampus of adult female rats. Neuroscience. 2012;227:201–210. doi: 10.1016/j.neuroscience.2012.09.059. [DOI] [PubMed] [Google Scholar]
- Pfaff D, Waters E, Khan Q, Zhang X, Numan M. Minireview: estrogen receptor-initiated mechanisms causal to mammalian reproductive behaviors. Endocrinology. 2011;152:1209–1217. doi: 10.1210/en.2010-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Abbott DH, Hodges JK, Martin RD. Maternal behavior is related to prepartum urinary estradiol levels in red-bellied tamarin monkeys. Physiol. Behav. 1988;44:717–726. doi: 10.1016/0031-9384(88)90052-2. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Dobeli M, Martin RD. Effects of sex steroids on maternal motivation in the common marmoset (Callithrix jacchus): development and application of an operant system with maternal reinforcement. J. Comp. Psychol. 1993;107:99–115. doi: 10.1037/0735-7036.107.1.99. [DOI] [PubMed] [Google Scholar]
- Raby KL, Lawler JM, Shlafer RJ, Hesemeyer PS, Collins WA, Sroufe LA. The interpersonal antecedents of supportive parenting: a prospective, longitudinal study from infancy to adulthood. Dev. Psychol. 2015;51:115–123. doi: 10.1037/a0038336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez SM, Bardi M, French JA, Brent L. Hormonal correlates of changes in interest in unrelated infants across the peripartum period in female baboons (Papio hamadryas Anubis sp.) Horm. Behav. 2004;46:520–528. doi: 10.1016/j.yhbeh.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. second. Sage Publications; Thousand Oaks: 2002. [Google Scholar]
- Rhee KE, Lumeng JC, Appugliese DP, Kaciroti N, Bradley RH. Parenting styles and overweight status in first grade. Pediatrics. 2006;117:2047–2054. doi: 10.1542/peds.2005-2259. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345:771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt JS, Mayer AD, Giordano AL. Hormonal basis during pregnancy for the onset of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:29–46. doi: 10.1016/0306-4530(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Maestripieri D. The neuroendocrinology of primate maternal behavior. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35:1192–1204. doi: 10.1016/j.pnpbp.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Horm. Behav. 2006;50:623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Glynn LM, Davis EP. Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. J. Psychosom. Res. 2013;75:327–335. doi: 10.1016/j.jpsychores.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santolaya-Forgas J, Meyer MJ, Burton BK, Scommegna A. Altered newborn gender distribution in patients with low mid-trimester maternal serum human chorionic gonadotropin (MShCG) J. Matern. Fetal Med. 1997;6:111–114. doi: 10.1002/(SICI)1520-6661(199703/04)6:2<111::AID-MFM10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Santor DA, Coyne JA. Shortening the CES-D to improve its ability to detect cases of depression. Psychol. Assess. 1997;9:233–243. [Google Scholar]
- Shams S, Pawluski JL, Chatterjee-Chakraborty M, Oatley H, Mastroianni A, Fleming AS. Dendritic morphology in the striatum and hypothalamus differentially exhibits experience-dependent changes in response to maternal care and early social isolation. Behav. Brain Res. 2012;233:79–89. doi: 10.1016/j.bbr.2012.04.048. [DOI] [PubMed] [Google Scholar]
- Spiker D, Ferguson J, Brooks-Gunn J. Enhancing maternal interactive behavior and child social competence in low birth weight, premature infants. Child Dev. 1993;64:754–768. doi: 10.1111/j.1467-8624.1993.tb02941.x. [DOI] [PubMed] [Google Scholar]
- Stams GJ, Juffer F, van IMH. Maternal sensitivity, infant attachment, and temperament in early childhood predict adjustment in middle childhood: the case of adopted children and their biologically unrelated parents. Dev. Psychol. 2002;38:806–821. doi: 10.1037//0012-1649.38.5.806. [DOI] [PubMed] [Google Scholar]
- Stapleton LR, Schetter CD, Westling E, Rini C, Glynn LM, Hobel CJ, Sandman CA. Perceived partner support in pregnancy predicts lower maternal and infant distress. J. Fam. Psychol. 2012;26:453–463. doi: 10.1037/a0028332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg DS, McKenna JB, Keough S, Hancock R, Numan MJ, Numan M. Dopamine D1 receptor stimulation of the nucleus accumbens or the medial preoptic area promotes the onset of maternal behavior in pregnancy-terminated rats. Behav. Neurosci. 2007;121:907–919. doi: 10.1037/0735-7044.121.5.907. [DOI] [PubMed] [Google Scholar]
- Terkel J, Rosenblatt JS. Maternal behavior induced by maternal blood plasma injected into virgin rats. J. Comp. Physiol. Psychol. 1968;65:479–482. doi: 10.1037/h0025817. [DOI] [PubMed] [Google Scholar]
- Terkel J, Rosenblatt JS. Humoral factors underlying maternal behavior at parturition: cross transfusion between freely moving rats. J. Comp. Physiol. Psychol. 1972;80:365–371. doi: 10.1037/h0032965. [DOI] [PubMed] [Google Scholar]
- Thomas A, Shughrue PJ, Merchenthaler I, Amico JA. The effects of progesterone on oxytocin mRNA levels in the paraventricular nucleus of the female rat can be altered by the administration of diazepam or RU486. J. Neuroendocrinol. 1999;11:137–144. doi: 10.1046/j.1365-2826.1999.00294.x. [DOI] [PubMed] [Google Scholar]
- Toriola AT, Vaarasmaki M, Lehtinen M, Zeleniuch-Jacquotte A, Lundin E, Rodgers KG, Lakso HA, Chen T, Schock H, Hallmans G, Pukkala E, Toniolo P, Grankvist K, Surcel HM, Lukanova A. Determinants of maternal sex steroids during the first half of pregnancy. Obstet. Gynecol. 2011;118:1029–1036. doi: 10.1097/AOG.0b013e3182342b7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu MT, Lupien SJ, Walker CD. Measuring stress responses in postpartum mothers: perspectives from studies in human and animal populations. Stress. 2005;8:19–34. doi: 10.1080/10253890500103806. [DOI] [PubMed] [Google Scholar]
- Warren SL, Simmens SJ. Predicting toddler anxiety/depressive symptoms: effects of caregiver sensitivity on temperamentally vulnerable children. Infant Ment Health J. 2005;26:40–55. doi: 10.1002/imhj.20034. [DOI] [PubMed] [Google Scholar]
- Wartella J, Amory E, Lomas LM, Macbeth A, McNamara I, Stevens L, Lambert KG, Kinsley CH. Single or multiple reproductive experiences attenuate neurobehavioral stress and fear responses in the female rat. Physiol. Behav. 2003;79:373–381. doi: 10.1016/s0031-9384(03)00150-1. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Wuu J, Hellerstein S, Lipworth L, Wide L, Xu B, Yu GP, Kuper H, Lagiou P, Hankinson SE, Ekbom A, Carlstrom K, Trichopoulos D, Adami HO, Hsieh CC. Correlates of pregnancy oestrogen, progesterone and sex hormone-binding globulin in the USA and China. Eur. J. Cancer Prev. 2002;11:283–293. doi: 10.1097/00008469-200206000-00012. [DOI] [PubMed] [Google Scholar]
- Yaron Y, Lehavi O, Orr-Urtreger A, Gull I, Lessing JB, Amit A, Ben-Yosef D. Maternal serum HCG is higher in the presence of a female fetus as early as week 3 post-fertilization. Hum. Reprod. 2002;17:485–489. doi: 10.1093/humrep/17.2.485. [DOI] [PubMed] [Google Scholar]
- Zelkowitz P, Gold I, Feeley N, Hayton B, Carter CS, Tulandi T, Abenhaim HA, Levin P. Psychosocial stress moderates the relationships between oxytocin, perinatal depression, and maternal behavior. Horm. Behav. 2014;66:351–360. doi: 10.1016/j.yhbeh.2014.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.