Abstract
Mesenchymal stromal cells (MSC) can promote tissue protection following injury, in part by modulating inflammatory cell responses. The aim of this study was to investigate the potential tissue protective properties of encapsulated MSCs (eMSC) in an organotypic injury model induced by fibronectin culture. MSC were encapsulated in alginate beads containing a network of nanopores, which segregate the cells from the extracapsular milieu, while still permitting diffusion into and out of the capsule. An increase in blood brain barrier permeability during pathological conditions permits the influx of blood plasma constituents that can be quite harmful to surrounding tissues. In particular, increased concentrations of fibronectin have been shown in a number of diseases and CNS traumas, co-localizing in areas of activated microglia. We observed over a 14-day period, a consistent increase in OHC degradation in the presence of fibronectin measured by a significant decrease in slice area, the breakdown in OHC pyramidal layers, and consistent cell death over the culture period. Microglial ionized calcium-binding adapter molecule 1 (IBA-1) expression remained elevated throughout the culture period with the majority found within the pyramidal layers. When eMSC were added to the cultures, a significant decrease in OHC degradation was observed as reflected by a reduction in OHC area shrinkage and in the amount of cell death. In the presence of eMSC, pyramidal layer structure was maintained and axonal extension from the periphery of the OHCs was observed. Therefore, MSC, delivered in a nanoporous alginate matrix, can modulate responses to injury by reversing fibronectin-induced OHC degradation.
Keywords: Mesenchymal stromal cells, organotypic hippocampal slices, fibronection, tissue protection
1. Introduction
Central nervous system (CNS) associated pathologies are difficult to resolve, in part because they are induced by a wide range of biological processes and therefore represent a significant challenge to our healthcare system. Regenerative, immune-modulatory and neuro-protective interventions have been used to improve clinical outcomes. However, a treatment regimen encompassing all these approaches simultaneously will most likely provide the best outcomes. Mesenchymal stromal cells (MSC) secrete a plethora of immunomodulatory factors1 in addition to facilitating tissue regeneration and preservation.2 MSC transplantation has been reported to provide benefit in animal models of CNS pathologies.3 However, general transplantation modalities do not ensure long-term MSC persistence4 and would require repeated injections to sustain benefits for patients with chronic CNS diseases.
In order to circumvent limitations with current transplantation modalities, approaches to provide sustainable extracorporeal MSC paracrine support have been developed.5 Engineered nanoporous polymer-based systems represent an intriguing option as MSC or other cells can be encapsulated and delivered to CNS intrathecal spaces,5,6 where within the cerebral spinal fluid (CSF) the encapsulated cells respond to pathological cues by providing immuno-modulatory and trophic support. This approach has been shown to sustain MSC paracrine function and be feasibly transplanted to the CNS without any adverse effects.7 However, so far there is no evidence that encapsulated MSC (eMSC) can alleviate CNS tissue pathology either in vitro or in vivo. The studies described here utilized organotypic hippocampal slice cultures (OHC) as an in vitro model to evaluate the ability of eMSC to prevent CNS degeneration induced in the OHC by the presence of fibronectin. The results suggest that fibronectin is not a permissive CNS substrate and induces significant tissue degeneration over time. eMSC were able to reverse fibronectin-induced degeneration and sustain overall OHC health.
2. Results
2.1. Using fibronectin to induce injury-like conditions in OHC
Given the influx of fibronectin post CNS injury, an in vitro hippocampal slice culture system was established to investigate the effect of plasma fibronectin on health and survival of CNS tissue. Hippocampal slices were obtained from 4–6 day old rat pups, sectioned to 400 μm, and then plated on fibronectin or poly-d-lysine (PDL) and laminin (referred to as PDL+Laminin), a known permissive substrate. Slices were cultured for 14-days and phase contrast images were acquired every other day to observe temporal changes in slice morphology. Day 3 slices on fibronectin and PDL+Laminin exhibited sustained hippocampal superstructure with visible CA1-CA3 and dentate gyrus regions [see Figs. 1(a)i, iv]. By day 13 there was considerable tissue degradation within slices plated on fibronectin, in which hippocampal superstructures were essentially unidentifiable [see Fig. 1(a)iii]. At the same time point slices plated on PDL+Laminin exhibited less dramatic decreases in tissue area compared to fibronectin and hippocampal superstructures were still identifiable [see Fig. 1(a)vi]. To quantify the extent of tissue degradation, slice areas were measured every other day from day 3 until day 13. Areas were normalized to the initial slice area (acquired on day 0) at each time point. On day 5 there was statistically significant degradation on fibronectin-coated surfaces compared to PDL+Laminin conditions [see Fig. 1(b)]. By day 13 there was a 45% decrease in slice area compared to 20% on PDL+Laminin surfaces [see Fig. 1(b)]. The decrease in tissue area on PDL+Laminin was mainly due to cellular outgrowth. However, on fibronectin there was drastic alteration in cellular morphology suggestive of necrosis. Overall, fibronectin is deleterious to hippocampal in vitro tissue survival.
Fig. 1.
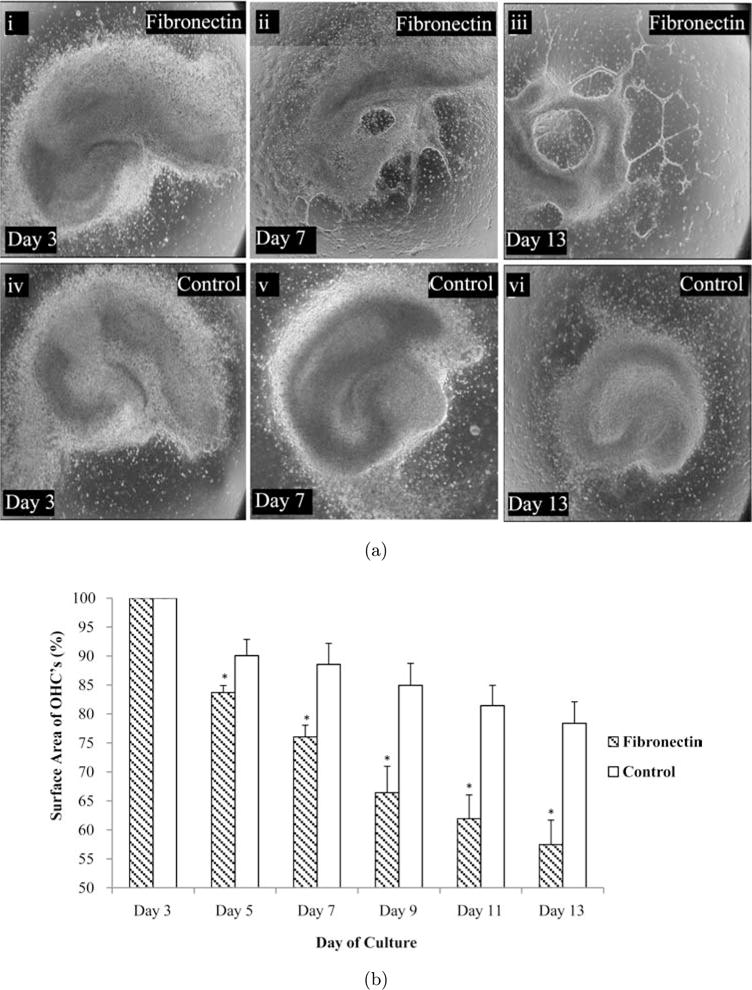
Fibronectin to induce injury-like conditions in OHC. (a) Representative images of an OHC placed on fibronectin or PDL+Laminin-coated surfaces on days 3, 7 and 13. OHC degenerate significantly by day 13 when cultured on a fibronectin-coated surface, however slices cultured on a surface coated with PDL+Laminin did not. (b) The surface areas of the OHCs were quantified and approximately a 45% loss in tissue was observed when slices were cultured on fibronectin. Asterisks represent statistical significance (p < 0.01) and error bars represent standard error, N = 7.
2.2. Cell death and damaged hippocampal regions
To better evaluate the degree of cell death with respect to the different surface coatings in the hippocampal cultures, slices were stained with propidium iodide (PI) exclusion dye. PI will only be incorporated into cells with damaged nuclear membranes and is used extensively to assess cell death in hippocampal slice cultures.8 OHCs were cultured on fibronectin or PDL+Laminin for 14 days and PI staining was performed immediately after isolation (day 0) and then at days 3, 7 and 14. Immediately after isolation there is a considerable amount of PI staining, due primarily to the isolation procedure. This baseline level of cell death remains 3 days after culture [see Fig. 2(a)i, iv]. By day 14, PI staining has diminished significantly on PDL+Laminin conditions, but on fibronectin PI staining is considerably elevated [see Fig. 2(a)iii, vi]. The staining was localized to the CA1-CA3 and DG regions of the hippocampal slice where the majority of neuronal cell bodies are found [see Fig. 2(a)iii]). In order to quantify the degree of PI staining, image thresholds were adjusted to levels acquired from images of paraformaldehyde (PF) fixed slices. Average fluorescent intensities were calculated from regions of interest created around the CA1-CA3 and DG regions. The data demonstrate that initially there are elevated PI levels on both coated surfaces, which proceeds to subside by day 7 [see Fig. 2(b)]. By day 14 there is a significant increase in PI levels in fibronectin-coated slice cultures, which remained diminished for PDL+Laminin cultured surfaces [see Fig. 2(b)]. These results suggest that PDL+Laminin is a favorable culture surface to maintain OHC survival. There is no increase in PI staining after the initial isolation-mediated elevation. However, fibronectin-coated surfaces induced an additional spike in PI staining at day 14, suggesting that fibronectin has deleterious effects on OHC survival.
Fig. 2.
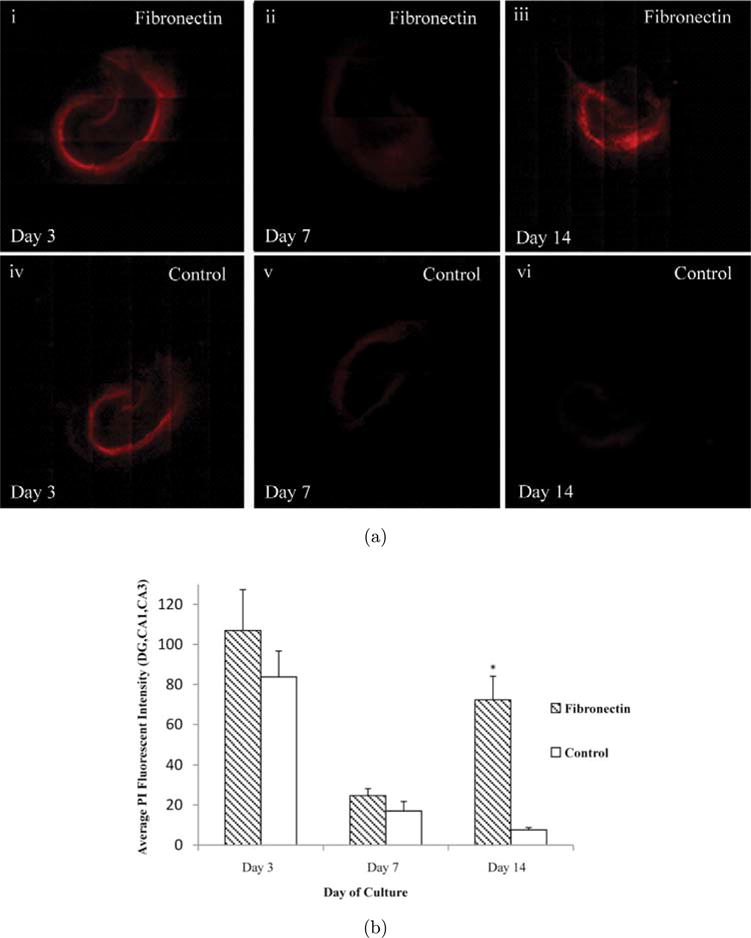
Cell death in damaged hippocampal regions. (a) Representative images of PI stained OHCs placed on fibronectin or PDL+Laminin on days 3, 7 and 14. On day 3 an initial cell death period is observed, probably due to the isolation of the hippocampal slice. By day 14 OHC PI levels decrease on PDL+Laminin conditions; however, they remain elevated when cultured on fibronectin. (b) The staining in the OHC was quantified by creating ROIs around the DG, CA1 and CA3 regions and then calculating the average PI fluorescence intensity. All images were thresholded to PI stained PF fixed OHCs before quantification. The intensity values diminished by day 14 in healthy control conditions; however they were drastically higher in the fibronectin conditions. Asterisks represent statistical significance (p < 0.01) and error bars represent standard error, N = 5.
2.3. Fibronectin affects microglia function
Microglia/macrophages in the CNS have been implicated in the pathological consequences of traumatic brain injury (TBI).9 Therefore, to characterize microglia/macrophages within OHCs slices at days 3, 7 and 14 of culture, slices were immunostained with IBA-1. IBA-1 is a calcium-binding protein that is specific to macrophages/microglia and is elevated when these cells are compromised. In contrast to the baseline level of IBA-1, which was greatest at day 3 in OHC fibronectin cultures [see Fig. 3(a)], on day 7 the levels of IBA-1 dropped significantly on PDL+Laminin conditions [see Fig. 3(a)iv]. OHC fibronectin cultures also displayed a drop in IBA-1 levels by day 7 [see Fig. 3(a)ii], however, they remained significantly elevated relative to PDL+Laminin conditions. IBA-1 levels remained significantly elevated compared to PDL+Laminin conditions on fibronectin cultures on day 14 as well, with the staining showing an even distribution throughout the three hippocampal regions, CA1, CA3 and DG [see Fig. 3(b)].
Fig. 3.
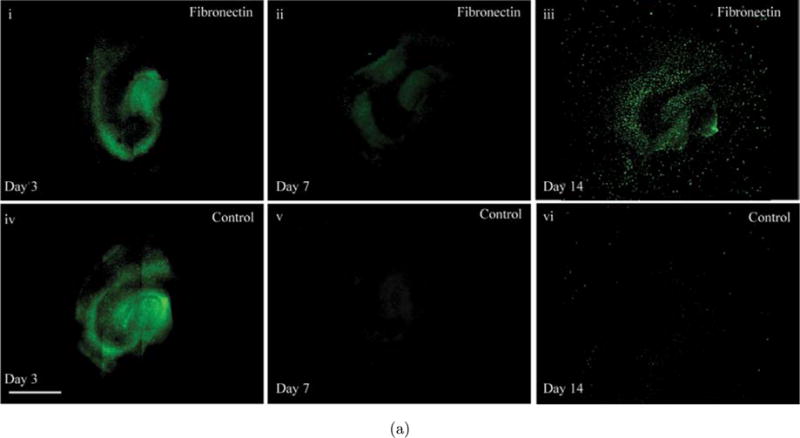
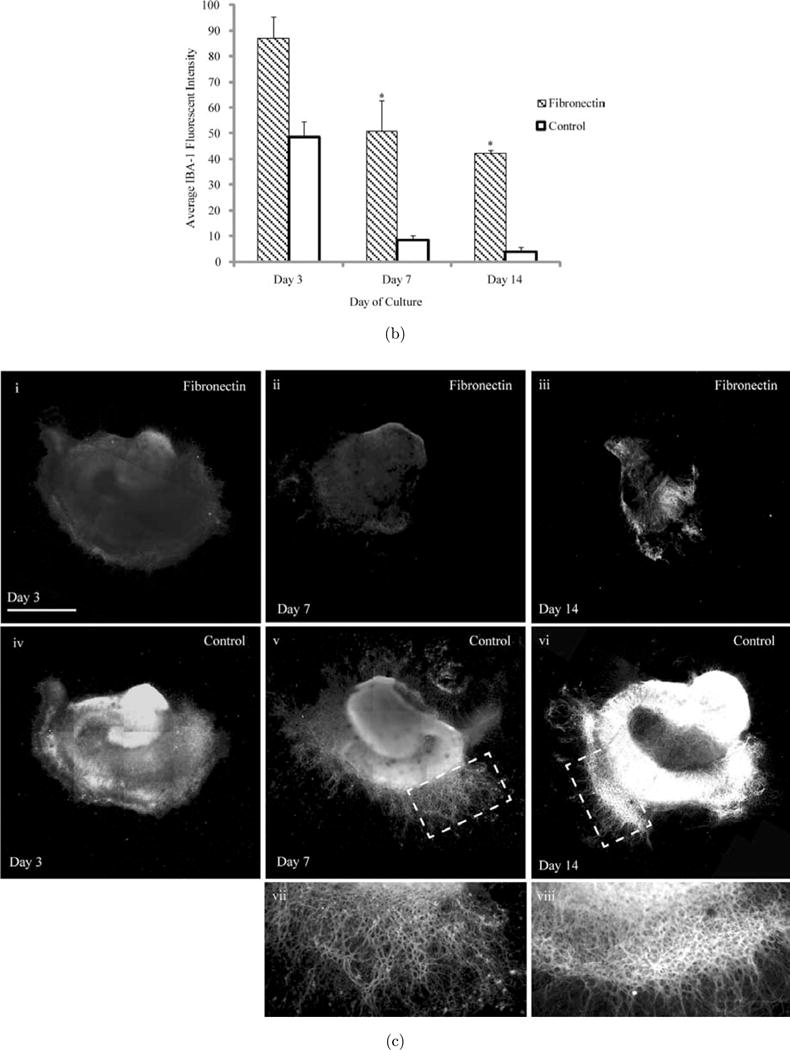
Effect of fibronectin on OHC microglia and neurons. (a) Images depicting IBA-1 microglial staining of OHCs cultured on fibronectin or PDL+Laminin. Initially, a considerable number of intensely fluorescing IBA-1 regions can be observed. By day 14 on PDL+Laminin this intensity significantly diminishes; however on fibronectin IBA-1 flourescence can still be observed. (b) Average fluorescent intensity of IBA-1 was quantified over the entire slice area. While IBA-1 intensity is reduced between day 3 and 7, the average fluorescent intensity of IBA-1 was always higher in slices cultured on fibronectin surfaces. This is evident throughout the 14-day culture period. (c) Representative images of OHCs stained with Tau. (i) OHC on Fibronectin on day 3, (iv) OHC on PDL+Laminin on day 3, (ii) OHC on Fibronectin on day 7, (v) OHC on PDL+Laminin day 7, (iii) OHC on Fibronectin on Day 14, (vi) OHC on PDL+Laminin day 14, (vii) Enlarged section of (v), (viii) Enlarged section of (vi.) Scale bar=1mm. Asterisks represent statistical significance (p < 0.05) and error bars represent standard error, N=3.
2.4. Hippocampal slice health assessed through tau staining
In addition to assessing slice health through changes in hippocampal slice area and cell death, slices were stained for Tau to evaluate changes in neurons and axonal extension. By day 14 we observed maintenance of the distinct hippocampal regions within PDL+Laminin conditions, as compared to fibronectin [see Fig. 3(c)iii, vi]. PDL+Laminin also facilitated axonal extension from the periphery of the slice, as seen by the extensive axonal extension network that originates from the periphery of the CA1 region [see Fig. 3(c)vii, viii – enlarged sections of 3(c)v, vi respectively].
2.5. eMSC preserve tissue in OHCs on fibronectin
OHC culture on fibronectin led to significant tissue degradation and substantial cell death over time, events which are hallmarks of CNS pathology. MSC-secreted factors result in cyto-protection and have been found to be efficacious in ameliorating CNS pathologies.10 Alginate encapsulation of MSCs has been previously evaluated as an effective means of sustaining MSC tissue protective properties and persistence, even when transplanted at a site distant from the injury.5 Therefore, experiments were designed to determine if MSCs could mitigate the deleterious effects of fibronectin on OHCs. Slices were co-cultured with 5 × 105 eMSC on fibronectin or PDL+Laminin for 14 days. Phase contrast images were acquired every other day to observe and quantify slice degradation. Slices cultured on fibronectin-coated surfaces in the presence of eMSCs did not demonstrate substantial OHC degradation and the superstructure remained intact [see Fig. 4(a)]. The necrotic areas observed with fibronectin-cultured conditions were absent when MSCs were introduced into the environment [see Fig. 4(a)]. Next, we quantified tissue degradation. There was no difference between the PDL+Laminin conditions +/− eMSC [see Fig. 4(b)]. However, as early as day 5 there were significant tissue degradation differences between cultures placed on fibronectin alone and those treated with eMSCs [see Fig. 4(b)]. In fact, in the presence of eMSC, fibronectin did not demonstrate significant differences in slice area at any time point when compared to PDL+Laminin cultures [see Fig. 4(b)].
Fig. 4.
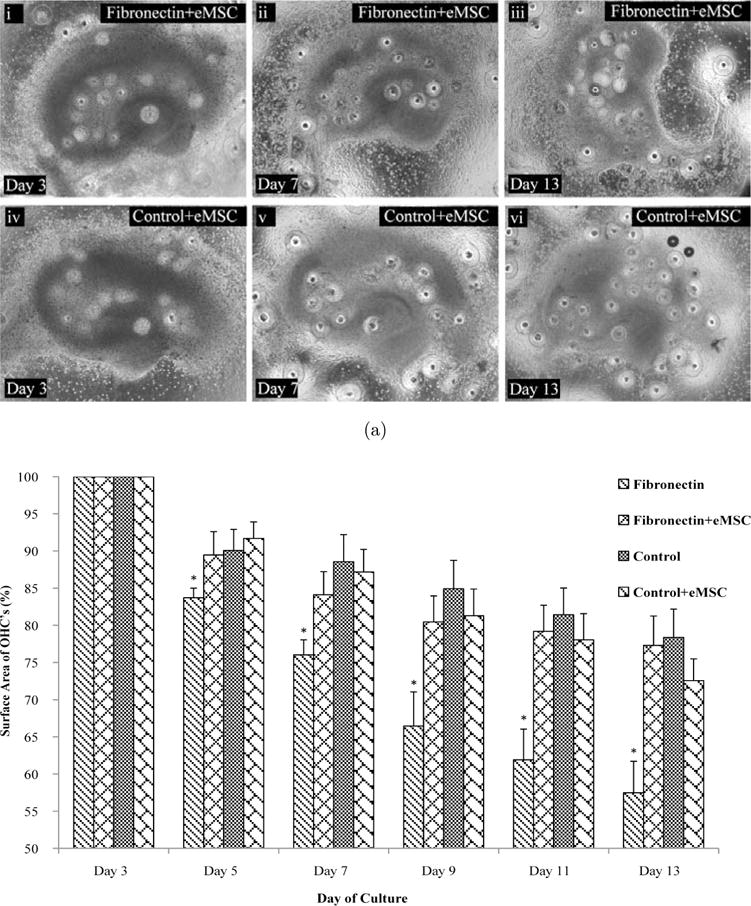
eMSC Preserve Tissue in OHCs on Fibronectin. Encapsulated MSC were cultured with OHC plated on either fibronectin or PDL+Laminin-coated surfaces for up to 14 days. (a) Representative images of an OHC placed on fibronectin or PDL+Laminin-coated surface cultured with eMSC on days 3, 7 and 13. The data indicate that eMSC prevent OHC degeneration induced by fibronectin-coated surfaces. (b) Quantitation of OHC degradation on fibronectin and PDL+Laminan-coated surfaces when eMSC are present in the culture, further supporting the efficacy of eMSC in preserving tissue architecture. Consistently, fibronectin-coated surfaces induced significant tissue degradation over time relative to PDL+Laminin conditions. When co-cultured with eMSC, OHC fibronectin cultures did not degrade significantly relative to PDL+Laminin. Asterisks represent statistical significance (p < 0.05) and error bars represent standard error, N = 3.
2.6. eMSC impart cytoprotective action
In order to qualify the cytoprotective action of eMSCs on OHC cultures, PI staining was performed. OHC fibronectin and PDL+Laminin cultures were incubated in the presence of eMSCs for two weeks and PI staining was performed immediately after isolation and at days 3, 7 and 14. Qualitative observations suggested that the initial cell death observed at day 3, due to the isolation procedure, was decreased significantly in eMSC conditions. This was evident in both fibronectin and PDL+Laminin conditions. By day 7 PI intensity diminished in all conditions, however the eMSC-treated conditions displayed the greatest drop in intensity (see Fig. 5). In contrast, day 14 untreated OHCs cultured on fibronectin displayed a considerable increase in PI staining, consistent with our previous observations (see Fig. 5). However, eMSC-treated slices displayed negligible PI staining, suggesting that the negative effects of fibronectin on OHC culture from day 7 to 14 were either diminished or prevented due to eMSC secreted factors. PI intensities remained diminished in PDL+Laminin conditions whether or not they were treated with eMSCs (see Fig. 5).
Fig. 5.
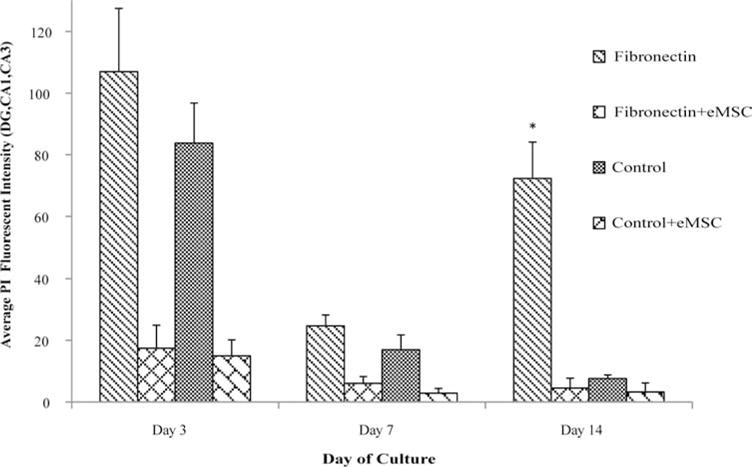
eMSC are cytoprotective. PI Staining of the OHC was quantified by defining a ROI around the DG, CA1 and CA3 regions and then calculating the average fluorescence intensity. Fluorescence intensity, which corresponds to the amount of dead cell within the OHC, was considerably higher in the fibronectin condition by day 14. The presence of eMSCs significantly mitigated cell death. This trend was also observed at days 3 and 7. Asterisk represent statistical significance (p < 0.05) and error bars represent standard error, N = 3.
We next assessed whether eMSCs had an effect on either IBA-1 or Tau expression changes induced by fibronectin. eMSCs attenuated microglia IBA1-expression on day 7 as well as day 14 to levels comparable to PDL+Laminin conditions [see Fig. 6(a)]. Lastly, in the presence of eMSC, even as early as day 3, we observed higher expression levels of Tau in both PDL+Laminin and Fibronectin conditions as compared to cultures without eMSC [see Fig. 6(b)i, v]. By day 14, not only did we observe a remarkable improvement of neuronal structure in fibronectin conditions with the addition of eMSC [see Fig. 6(b)iii, vii], but we also detected axonal extension from the periphery of the slice, similar to that observed in PDL+Laminin conditions [see Fig. 6(b)iv, viii]. The extension observed in eMSC added to PDL+Laminin conditions appears to be different from that observed without eMSC, where axonal extensions occurred sporadically along the periphery, most extensively in the subiculum area [see Fig. 6(b)vii, viii]
Fig. 6.
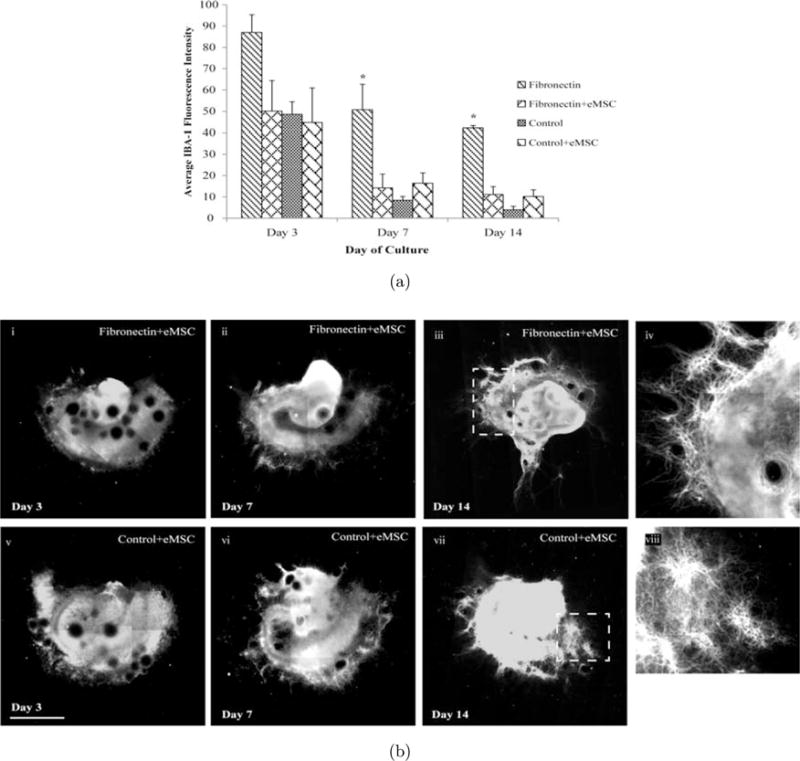
eMSC attenuate microglial activation and enhance Tau expression. eMSC’s were cultured with OHCs plated on fibronectin and PDL+Laminin for 14 days. (a) At days 3, 7 and 14 slices were immunostained for IBA-1, an activated microglia marker. Average fluorescence intensity of the entire slice area was calculated at each time point. IBA-1 levels on day 3 in eMSC-treated fibronectin conditions were reduced to levels comparable to that seen in PDL+Laminin conditions. Consistent with earlier observations, OHC IBA-1 levels remained elevated at days 7 and 14 of culture on Fibronectin conditions, however, eMSCs diminished the levels significantly at both time points. (b) Representative images of OHCs stained with Tau. (i) OHC on Fibronectin on day 3 in the presence of eMSC, (v) OHC on PDL+Laminin on day 3 in the presence of eMSC, (ii) OHC on fibronectin on day 7 in the presence of eMSC, (vi) OHC on PDL+Laminin day 7 in the presence of eMSC, (iii) OHC on fibronectin on Day 14 in the presence of eMSC, (vii) OHC on PDL+Laminin day 14 in the presence of eMSC, (iv) Enlarged section of (iii), (viii) Enlarged section of (vii). Scale bar = 1mm. Asterisk represent statistical significance (p < 0.05) and error bars represent standard error, N = 3.
3. Discussion
MSCs represent a unique stem cell therapeutic platform in that their main benefits in CNS disease models are attributed to secreted factors and not cell differentiation.5 This benefit has been further harnessed via an encapsulation strategy designed to immobilize MSCs locally near sites of injury.5 The efficacy of the platform was evaluated here, to determine if tissue degradation associated with fibronectin culture could be inhibited in the presence of eMSCs. EMSCs prevented the tissue degradation associated with OHC fibronectin culture to levels similar to PDL+Laminin cultures. Similar observations have been made in vivo where MSCs inhibited pathological events associated with experimental TBI.17 The amount of cell death in 14 day fibronectin OHC cultures was also reduced significantly with eMSC treatment, consistent with MSC benefits in glucose-deprivation and neuroinflammatory models.11,12 Additionally, eMSCs attenuated IBA-1 levels in fibronectin cultures to levels similar to PDL+Laminin.
There is literature evidence that MSCs mitigate microglia activation in vitro as lower microglial levels of iNOS and secreted TNF-α were observed in the presence of MSCs.13 Similarly, in vivo models of ischemia indicated that MSCs modulate microglial function to simultaneously mitigate pro-inflammatory and promote anti-inflammatory functions.14 Recently, a neuro-inflammatory OHC model demonstrated the MSC PGE2 secretion mediated tissue protective action by modulating microglial inflammatory function. However, while PGE2 may be an important mediator, MSCs secrete a plethora of neuroprotective15 and regenerative16 factors, which may also contribute to the MSC regenerative benefits described in these systems.
MSCs have been reported to be efficacious in several models of CNS pathology.10,17 However, it has been found that MSCs injected directly into CNS tissue do not survive 7 days post transplantation.14 Others have observed that 0.0005% of intravenously transplanted MSC reach the cerebral parenchyma and were not detected 2 weeks post infusion.18 An encapsulated platform could improve MSC persistence near the site of injury and potentially augment the benefits achieved with MSC transplantation. Our results, using an in vitro hippocampal slice culture degradation model, suggest that nanoporous alginate eMSCs can sustain their tissue protective properties. Future studies will be performed to determine the efficacy of such an approach relative to free MSC transplants in experimental models of CNS pathology.
In summary, we have demonstrated that MSC contained in nanoporous alginate capsules can attenuate fibronectin-induced organotypic hippocampus slice injury.
4. Experimental Procedures
4.1. Human MSC Culture
All cell cultures were incubated in a humidified 37° C, 5%CO2 environment. Human MSCs (hMSC) were purchased from Texas A&M at passage 1 and cultured as previously described.5 Briefly, hMSCs were cultured in MEM-α (Gibco) medium, containing no deoxy and ribo nucleosides, supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA), 1 ng/mL basic fibroblast growth factor (bFGF) (Gibco), 100 units/mL penicillin and 100 μg/mL streptomycin (Gibco). hMSC were plated at 5000 cells per cm2 and allowed to proliferate to 70% confluence (approximately 4 to 5 days). Only hMSCs at passages 2 through 5 were used to initiate subsequent experiments.
4.2. Alginate Microencapsulation
Alginate Poly-L-Lysine microencapsulation of hMSCs was performed as previously described,19 with some modifications. A 2.2% (w/v) alginate (Sigma-Aldrich, MW: 100000–200000 g/mol, G-content 65–70%) solution was generated with Ca2+-free DMEM (GIBCO). The solution was filtered using a 25-μ syringe filter (Fisher Brand, Pittsburg, PA). A 70% confluent monolayer culture of MSC was removed with trypsin (Gibco) and counted. The cells were centrifuged at 400 g and resuspended at a concentration of 4 × 107 cells/mL with Ca2+-free DMEM. The cell solution was then diluted 10× with 2.2% alginate yielding a final cell concentration of 4 × 106 cells/mL and a 2% (w/v) alginate solution. The solution was transferred to a syringe pump (KD Scientific, Holliston, MA). Alginate beads were generated using an electrostatic bead generator (Nisco, Zürich, Switzerland) with an accelerating electrode at a flow rate of 2mL/h and an applied voltage of 6.4 kV. The resulting bead diameter was 225 ± 25 μm. The beads were extruded into a 100 mL bath of CaCl2 (100 mM) (Sigma Aldrich) containing 145 mM NaCl (Sigma-Aldrich) and 10 mM MOPS (Sigma-Aldrich). Microencapsulated cells were washed once with PBS and then treated for 2 min with poly-L-Lysine (PLL) (sigma–Aldrich, Mw: 68600 g/mol) (0.05% w/v). The capsules were washed one more time, resuspended in MSC culture medium and transferred to 25 mm2 tissue culture flasks.
4.3. Surgical procedures
The surgical procedures were all performed in accordance with protocols approved by the NIH guidelines for the care and use of laboratory animals. Sprague-Dawley female with litter (10 pups) were usually delivered (Taconic Farms Inc, NY) on postnatal day 2 allowing for a few days for acclimatization. On the day of dissection, the pups were removed from the litter, euthanized and their hippocampi removed, sliced and placed in culture. OHCs generated were counterbalanced across all treatment groups. A total of 20 (male/female) postnatal day 4–6 Sprague-Dawley pups (Taconic, NY) were used. First, the brains of the pups were removed and placed in ice cold Gey’s Balanced Salt Solution (Sigma-Aldrich Corp, MO) supplemented with 10 mM D-glucose (Sigma-Aldrich Corp, MO) and 3 μM Kynurenic Acid (Sigma-Aldrich Corp, MO). The hippocampi were separated from the surrounding cortex and sliced into 400 μm slices using a McIlwain Tissue Chopper (Vibratome, IL). The slices were then carefully placed onto fabricated strips of polydimethylsiloxane (PDMS) (Sylgard 184, Fischer Scientific, MA). The petri dish was filled with 750 μL of serum containing medium (1:1:2 of heat inactivated horse serum, Hanks Balanced Salt Solution, Basal Medium Eagle, supplemented with 0.5 mM L-Glutamine, 30 μg/ml gentamycin and 10 mM HEPES, all from Invitrogen, CA). After 24 h the medium was changed to a serum-free media (Neurobasal A, 1× B27, 0.5 mM L-glutamine, 30 μg/mL gentamycin and 10 mM HEPES, all from Invitrogen CA). Thereafter, half of the medium was changed every 48 h.
4.4. Condition preparation and tissue processing
Prefabricated strips of PDMS (150 μm thickness) were sterilized with 70% ethanol, and then placed on top of a round, sterile, microscope cover slip. The coverslips and PDMS were then placed within individual 35 mm petri dishes (Becton, Dickinson, NJ). Oxygen plasma treatment sterilized the entire assembly and irreversibly bonded the PDMS to the glass coverslip. After plasma treatment the petri dish assemblies were treated with 1.5 mL of sodium hydroxide for 1 h, followed by two 1 mL washes with deionized water. Fibronectin (Sigma-Aldrich Corp, MO) was applied at a concentration of 25 ug/mL for 3 h to some conditions while PDL (Sigma-Aldrich Corp, MO) and laminin (Sigma-Aldrich Corp, MO) were applied, to control culture conditions at a concentration of 80 μg/mL and 1 mg/mL, respectively. The PDL was applied first overnight, while the laminin was applied for 2 h the following day. Finally, each petri dish was filled with 750 uL of serum-free media after the initial coatings for 24 h before removal and organotypic hippocampal slice addition. Hippocampus slice explants were placed on top of the treated PDMS in the assembly to complete each OHC.
Approximately 750 μl of serum-containing medium was added to the cultures, a volume chosen to optimize the media-oxygen interface, and placed in a humidified 5% CO2 incubator at 37°C. After 24 h, the serum-containing media was exchanged for 750 μL of serum-free media. In later experiments, alginate capsules containing a total of 50000 hMSCs were distributed evenly into petri dishes of both the control and injury conditions in order to assess the models’ response to the cellular therapeutic.
Half the medium was changed every 48 h until day 14, the end of each experimental period. Exchanging half the media served to replenish essential nutrients without entirely removing secreted growth factors or cytokines, preserving each culture’s microenvironmental condition. Phase contrast images were taken of each condition to qualitatively assess slice degradation, axonal outgrowth and overall slice health using an Olympus CKX41 microscope (Olympus, PA).
4.5. Assessing cell viability
The organotypic slices were stained on days 3, 7 and 14 using 5 ug/mL PI, a DNA intercalating stain, diluted in 37°C serum-free media. The stain was allowed to permeate through the slice for 30 min by returning the cultures to the incubator and was subsequently removed before imaging. After washing the slices with 37°C serum-free media, they were imaged using the TRIT-C channel on an Olympus IX81 spinning disk confocal microscope (Olympus, PA) using Slidebook software (Intelligent Imaging Innovations; Denver, CO). Each slice was used as its own positive control for cell death by applying a combination of PF fixation and cell membrane permeabilization using Triton-X. Thus, a 0.1% Triton-X, TBS (TBS - 0.5 M Tris Base, 9% NaCl, pH 7.4) solution was applied to the slices for approximately 45 min. The solution was then removed, cultures washed and then exposed to PI for 48 h at 4°C. After a final wash, the slices were ready to be imaged. Since PI is excluded from viable cells, exposure to PF and Triton-X results in the whole culture taking up PI and becoming strongly fluorescent (Adamchick et al., 2000). This was confirmed by observing the slices at higher magnification (data not shown). This was done for each time point since OHCs thin out over the length of culture.
4.6. Histology and immunohistochemistry
Slices, not used in PI staining, were fixed on day 3, 7 and 14 in 4% PF to study temporal expressions. After being fixed, slices were blocked using a 10% goat serum, 0.1% Triton-X 100, 1% bovine serum albumin, TBS solution for 45 min at room temperature. Slices were stained with either a rabbit primary antibody (1:800) to IBA-1 (IgG) (Abcam, MA), or a rabbit primary antibody (1:400) to Tau (IgG) (Bectin, Dickinson, NJ) overnight at 4°C. IBA-1 staining was followed by an Alexa Fluor 488 goat anti-rabbit secondary (1:450) (Invitrogen, CA) for 2 h at room temperature, and Tau staining this was followed by an Alexa Fluor 647 goat anti-rabbit secondary (1:500) (Invitrogen, CA) for 2 h at room temperature. Slices were imaged on either the GFP channel (IBA-1) or Cy-5 channel (Tau) at all three time points. An isotype control was used to account for background staining using rabbit IgG (Zymed, CA) at the same concentration as the rabbit primary antibody, also overnight at 4°C. All sections were washed at room temperature with PBS after each antibody addition and before imaging.
4.7. Image analysis
Bright field and fluorescent images were taken of each condition to qualitatively assess slice degradation, axonal outgrowth and overall slice health during tissue processing. To obtain quantitative results concerning slice surface area, images were analyzed using ImageJ software (NIH). Regions of interest were drawn along the boundary of each organotypic slice at the beginning of each culture and superimposed on the slice at each subsequent time point to calculate changes in slice area. Fluorescence pixel intensities of PI stained images were obtained using ImageJ software, and specific tissue regions were scrutinized including the dentate gyrus, CA1 and CA3 hippocampal regions.20 Pixel intensities were averaged and normalized to surface area of the ROI of that particular condition. IBA-1images were captured using the same microscope. These images were also analyzed using ImageJ software following a similar protocol to the PI stain results.
4.8. Statistical analysis
A one-sided student t-test for unpaired data was used to compare the fluorescent signal of each of the stains for 5 experiments with 1–3 cultures per condition in each individual experiment. The areas analyzed were the dentate gyrus, CA1 and CA3 region of the hippocampus. Statistical significance was considered for differences with a p-value less than or equal to 0.05, unless stated otherwise.
Acknowledgments
Funding for this work was partially funded by the New Jersey Commission for Brain Injury Research (BIR-1.08.016), NIH Biotechnology Training Grant (2T32 GM008339-21), graduate fellowship programs IGERT on stem cell research NSF DGE 0801620 and the New Jersey commission on spinal cord injury 10-2947-SCR-R-0.
Abbreviations
- CNS
central nervous system
- BBB
blood brain barrier
- AD
Alzheimers disease
- MS
multiple sclerosis
- SCI
spinal cord injury
- TBI
traumatic brain injury
- MSC
mesenchymal stromal cells
- eMSC
encapsulated mesenchymal stromal cells
- hMSC
human mesenchymal stromal cells
- CSF
cerebro-spinal fluid
- PDL
poly-d-lysine
- OHC
organotypic hippocampal slice cultures
- PI
propidium iodide
- GFAP
glial fibrillary acidic protein
References
- 1.Shi Y, et al. Cell Res. 2010;20:510. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 2.Stappenbeck TS, Miyoshi H. Science. 2009;324:1666. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- 3.Quertainmont R, et al. PLoS One. 2012;7:e39500. doi: 10.1371/journal.pone.0039500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parekkadan B, Milwid JM. Annu Rev Biomed Eng. 2010;12:87. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barminko J, et al. Biotechnol Bioeng. 2011;108:2747. doi: 10.1002/bit.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kishima H, et al. Neurobiol Dis. 2004;16:428. doi: 10.1016/j.nbd.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Heile AM, et al. Neurosci Lett. 2009;463:176. doi: 10.1016/j.neulet.2009.07.071. [DOI] [PubMed] [Google Scholar]
- 8.Macklis JD, Madison RD. J Neurosci Methods. 1990;31:43. doi: 10.1016/0165-0270(90)90007-3. [DOI] [PubMed] [Google Scholar]
- 9.Gao HM, Hong JS. Trends Immunol. 2008;29:357. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HJ, Lee JH, Kim SH. J Neurotrauma. 2010;27:131. doi: 10.1089/neu.2008.0818. [DOI] [PubMed] [Google Scholar]
- 11.Zhong C, et al. Neurosci Lett. 2003;342:93. doi: 10.1016/s0304-3940(03)00255-6. [DOI] [PubMed] [Google Scholar]
- 12.Foraker J, et al. J Neurochem. 2011;119:1052. doi: 10.1111/j.1471-4159.2011.07511.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhou C, et al. Brain Res. 2009;1269:23. doi: 10.1016/j.brainres.2009.02.049. [DOI] [PubMed] [Google Scholar]
- 14.Ohtaki H, et al. Proc Natl Acad Sci USA. 2008;105:14638. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurozumi K, et al. Mol Ther. 2005;11:96. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Lee RH, et al. J Cell Biochem. 2011;112:3073. doi: 10.1002/jcb.23250. [DOI] [PubMed] [Google Scholar]
- 17.Walker PA, et al. Stem Cells Dev. 2010;19:867. doi: 10.1089/scd.2009.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harting MT, et al. J Neurosurg. 2009;110:1189. doi: 10.3171/2008.9.JNS08158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maguire T, et al. Biotechnol Bioeng. 2007;98:631. doi: 10.1002/bit.21435. [DOI] [PubMed] [Google Scholar]
- 20.Adamchick Y, et al. Brain Res Protoc. 2000;5:153. doi: 10.1016/s1385-299x(00)00007-6. [DOI] [PubMed] [Google Scholar]
- 21.Morrison B, et al. Br J Pharmacol. 2002;137:1255. doi: 10.1038/sj.bjp.0704986. [DOI] [PMC free article] [PubMed] [Google Scholar]


