Abstract
After multiple decades of investigation, the precise mechanisms involved in fuel-stimulated insulin secretion are still being revealed. One avenue for gaining deeper knowledge is to apply emergent tools of “metabolomics”, involving mass spectrometry and nuclear magnetic resonance-based profiling of islet cells in their fuel-stimulated compared to basal states. The current article summarizes recent insights gained from application of metabolomics tools to the specific process of glucose-stimulated insulin secretion, revealing two new mechanisms that may provide targets for improving insulin secretion in diabetes.
Keywords: Metabolomics, insulin secretion, isocitrate, NADPH, adenylosuccinate (S-AMP)
Introduction
The pancreatic islets of Langerhans play a critical role in metabolic physiology by translating key markers of nutritional status into hormonal changes that orchestrate systemic energy and substrate balance. The secretion of the primary hormonal mediators, insulin and glucagon, are regulated in a reciprocal fashion such that the anabolic hormone insulin is induced and secreted in the fed condition, whereas the catabolic hormone glucagon is preferentially released in the fasted state. A key signal of the fasted to fed transition is a rise in circulating glucose levels that triggers insulin secretion from islet β-cells. The mechanism of glucose-stimulated insulin secretion (GSIS) has been investigated for decades, and a canonical view of the process has emerged to broad acceptance. The central model holds that glucose induces insulin secretion via its own glycolytic and oxidative metabolism, leading to an increase in ATP:ADP ratio, inhibition of ATP-sensitive K+ (KATP) channels, activation of voltage-gated Ca2+ channels, and influx of extracellular Ca2+ to stimulate insulin granule exocytosis.1 In more recent years, a modified version of the central model has emerged, in which the KATP channel-dependent pathway is viewed as the primary mediator of the “triggering” or first phase of insulin secretion, whereas other signals are likely to be key drivers of the more prolonged “amplifying” or second phase of secretion2. Consistent with these ideas, several studies have demonstrated a significant residual insulin secretion response to glucose in islets with pharmacologic or molecular suppression of KATP channels,2,3 strongly implicating KATP-channel independent signaling mechanisms in mediating the full response. Our laboratory and others have been focused on identifying the signaling pathways and mediators involved in the sustained second phase of insulin secretion.4–6
In type 1 diabetes (T1D), β-cells are destroyed by the immune system, creating complete insulin deficiency. The long-practiced clinical intervention of insulin injection is life-saving, but can lead to hyperglycemic and hypoglycemic episodes due to the lack of regulation of insulin delivery by normal physiologic signals. Cell-based approaches to insulin replacement involving human islets or stem-cell derived β-cells have been actively investigated. Strategies for inducing differentiation of human stem cells to insulin-producing cells have advanced steadily, including recent reports of cells that exhibit some level of GSIS.7,8 However, it is unlikely that these cells fully mimic the glucose response mechanisms of normal human islets, and further engineering is likely to be required. In type 2 diabetes (T2D), the transition from the obese, insulin resistant, pre-diabetic state to full blown diabetes is triggered by β-cell failure, a general term that encompasses loss of normal nutrient signaling, an inability to maintain a high rate of insulin secretion to compensate for impaired insulin action, and eventual loss of β-cell mass.9 A clearer understanding of the complete mechanism of GSIS is essential for ultimate success in creation of surrogate cells for insulin replacement therapy in T1D, as well as development of more efficacious drugs for treatment of T2D.
Metabolomics Technology
The approach taken by our group to gain a deeper understanding of pancreatic islet cell nutrient signaling is to apply evolving tools of comprehensive metabolic analysis, or “metabolomics”. With the advent of more sensitive mass spectrometers and accompanying chromatographic strategies, researchers are able to profile an increasingly broad set of metabolic intermediates in response to controlled systems perturbations. These technologies, when coupled to nuclear magnetic resonance (NMR)- or mass spectrometry (MS)-based metabolic flux analysis, provide a comprehensive view of the metabolic fates of glucose and other nutrient secretagogues in islet cells. These technologies can be applied to relatively simple and well-controlled in vitro experiments in which cultured pancreatic islets or insulinoma cell lines are exposed to basal or stimulatory levels of glucose and other fuels for varying time periods, followed by comprehensive profiling of metabolites that differ in the basal compared to stimulated states. Inclusion of stable isotope-labeled secretagogues (e.g. 13C-labeled glucose) allows analysis of enrichment of downstream metabolites and calculation of metabolic flux rates.
Static metabolic profiling (“snap shots” at a single time point) with MS-based methods can be performed in a targeted or non-targeted fashion. Non-targeted metabolomics is used to compare two biological conditions with coverage of as many metabolites as possible regardless of the chemical class of the metabolites. It is best suited as a discovery tool for identifying metabolites that change in response to manipulation of a biological system rather than providing the specific concentration of a known metabolite. In contrast, targeted metabolomics provides more quantitative analysis by focusing on measurement of known metabolites in clusters with similar chemical structures (e.g. amino acids, organic acids, etc). The method often involves addition of stable isotope-labeled standards (usually 2H or 13C-labeled) to samples, and therefore provides precise quantification of a targeted analyte relative to known concentration of the added standard.10–13 Identification and measurement of metabolites by MS is typically coupled to a separation technique such as gas chromatography (GC-MS), liquid chromatography (LC-MS), ultra-performance liquid chromatography (UPLC-MS), or capillary electrophoresis (CE-MS).14,15 MS ionizes chemical species and sorts the ions based on their mass to charge ratio. Chromatography techniques help to separate metabolites based on their chemical properties. In contrast, NMR uses the magnetic properties of certain atomic nuclei, and detects spectral features originating from molecules that contain carbon or hydrogen.16 In general, MS methods have advantages of high sensitivity and small sample volumes, whereas NMR has little chemical bias and can be used directly on the sample with no need for extraction and derivatization. Metabolomics methods developed specifically for use in β-cell lines include an extraction protocol determined by statistical design prior to GC-MS analysis,17 and an extraction procedure developed for adherent cells compatible with LC-MS.18 In terms of analytical techniques, a 2D capillary LC-MS strategy has been devised to improve metabolite coverage in small tissue samples,19 and direct matrix-assisted laser desorption/ionization (MALDI)-TOF analysis of single islets has also been reported.20 More complete discussion of metabolomics methods and their use in disease research can be found in several recent reviews. 6,12,14–16,21
Our laboratory has used a combination of NMR- and MS-based metabolic flux analysis coupled with targeted MS-based static profiling of key intermediates of glucose, lipid, and amino acid metabolic pathways to gain insights into mechanisms of GSIS. Here we focus on our experiences in use of metabolomics for advancing our understanding of islet biology, and the implications of this new information for translational applications.
Metabolic Flux Analysis Implicates Anaplerotic Metabolism of Glucose in Stimulus-Secretion Coupling
Our early work focused on subclones of the rat insulinoma cell line INS-1 with varying capacity for GSIS.22 We reasoned that understanding of metabolic differences between robustly versus poorly glucose responsive cell lines might unveil novel metabolic pathways or factors that regulate glucose sensing. We first applied NMR-based mass isotopomer analysis to measure relative fluxes through the oxidative enzyme pyruvate dehydrogenase (PDH) and the anaplerotic enzyme pyruvate carboxylase (PC), using U-13C glucose as substrate. The studies showed that variable GSIS across a panel of INS-1-derived cell lines was tightly correlated to pyruvate anaplerosis rather than pyruvate oxidation.23 Flux through PC-catalyzed anaplerosis actually exceeds flux through PDH in isolated islets,24 and the strong correlation of anaplerotic metabolism of pyruvate with GSIS that we found with NMR-based flux analysis in INS-1-derived cell lines was subsequently confirmed by MS-based methods applied to primary pancreatic islets.16 During active glucose stimulation of β-cells, anaplerotic metabolism of pyruvate through PC fills the TCA cycle, allowing excess intermediates to exit the mitochondria to engage with cytosolic enzymes for use in various biosynthetic pathways. This includes possible diversion of TCA cycle intermediates into one of the three “pyruvate cycling” pathways that involve extramitchondrial citrate, isocitrate or malate.4,25 Systematic study of each of these pathways with recombinant adenoviruses containing shRNAs specific to key enzymes in each of the pathways implicated the cytosolic NADP-dependent isocitrate dehydrogenase (ICDc or IDH1) as a key extra-mitochondrial step (Figure 1).26 Supporting this idea, shRNA-mediated suppression of the citrate/isocitrate carrier (responsible for export of citrate and isocitrate from the mitochondria) or IDH1 results in significant impairment of GSIS.4,26,27 IDH1 knockdown also impairs secretion in response to glutamine in the presence of the glutamate dehydrogenase activator BCH.28
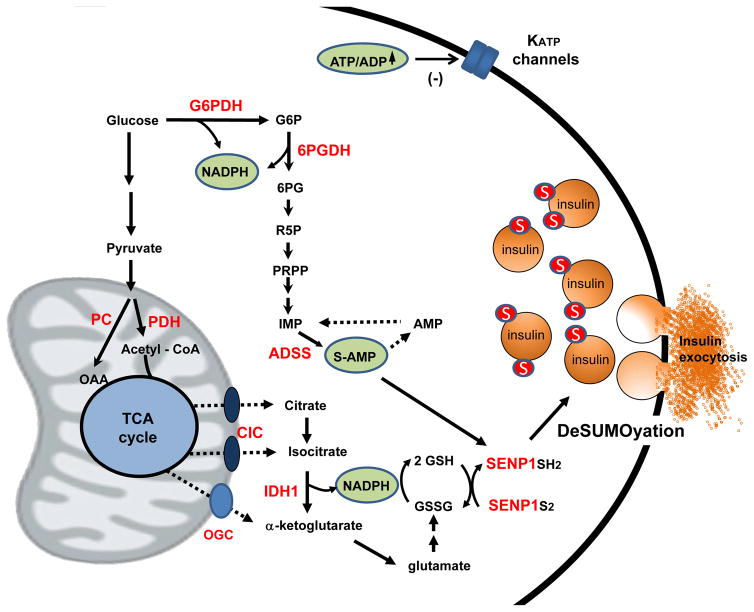
Figure 1. New mechanisms of glucose-stimulated insulin secretion revealed by metabolomics.
In addition to the classical pathway involving glycolytic and oxidative metabolism of glucose to increase ATP:ADP ratio and inhibit KATP channels, our recent metabolomics studies identify two pathways that play complementary roles. First, anaplerotic metabolism of glucose via pyruvate carboxylase (PC) rather than pyruvate dehydrogenase (PDH) creates excess TCA cycle intermediates that leave mitochondria in the form of citrate and isocitrate via the citrate/isocitrate carrier (CIC). Isocitrate engages with the cytosolic, NADP-dependent isoform of isocitrate dehydrogenase (IDH1) to form NADPH and reduce glutathione, leading to reduction and activation of the deSUMOylase enzyme SENP1. In addition, metabolism of glucose via the pentose shunt enzymes glucose-6-phosphate dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase (6PGDH) contributes to nucleotide synthesis, particularly the conversion of IMP to S-AMP via adenylosuccinate synthase (ADSS). S-AMP also stimulates insulin secretion in a SENP1-dependent manner. Figure modified from reference 37 and used with permission.
Our more recent work suggests that the IDH1 reaction plays two key roles in mediating insulin release. First, it produces NADPH needed for the glutathione reductase reaction, which converts oxidized glutathione (GSSG) to its reduced form (GSH).29,30 Reduced GSH in turn reduces glutaredoxin, which interacts with various cellular proteins to influence their activity via reduction of disfulfide (cysteine-cysteine) bridges. Second, it generates α-ketoglutarate that can be transaminated to glutamate, a building block of glutathione. The latter finding emerged through targeted MS/MS profiling of amino acids during glucose stimulation of β-cells, demonstrating significant changes in levels of only 3 of 15 amino acids profiled. Among these, we observed a clear decrease in aspartate and a rise in glutamate in response to stimulatory glucose.29 Treatment of INS-1-derived with the general aminotransferase inhibitor AOA blocked the glucose-induced changes in both amino acids and partially blocked GSIS.29 These findings suggest that α-ketoglutarate generated by IDH1 is transaminated to glutamate by aspartate aminotransferase, providing a key substrate for de novo glutathione synthesis. Consistent with this idea, glucose stimulation of 832/13 cells increases both the total (GSH + GSSG) and reduced (GSH) pools of glutathione, an effect blocked by shRNA-mediated suppression of IDH1. The increase in reduced GSH was also demonstrated by cytosolic expression of a GSH biosensor (Grx1-roGFP) in intact cells.29
A key remaining element is to define how and why maintenance of robust pools of reduced glutathione (GSH) and glutaredoxin stimulate insulin secretion. These biochemical changes appear to be linked to insulin granule exocytosis via reduction of the desumoylation enzyme SENP1. MacDonald and co-workers have demonstrated that SENP1 triggers insulin granule exocytosis via deSUMOylation of secretory granule proteins, including tomosyn1.29,31,32 Glucose-dependent deSUMOylation of tomysyn1 influences its interactions with the granule trafficking proteins syntaxin1A and secretagogin. Glutaredoxin acts to reduce cysteine residues in SENP1, leading to activation of the enzyme. A direct connection between intermediates in the emergent glucose/isocitrate signaling pathway and SENP1 was demonstrated in studies of patch-clamped rodent and human β-cells perfused with isocitrate, NADPH or GSH. These intermediates, but not NADH or α-ketoglutarate, were as effective as glucose for stimulation of exocytosis,29,30 an effect lost in mouse islets with transgenic deletion of SENP1 or human islets with shRNA-mediated suppression of SENP1 expression.29 (See Figure 1 for overall schematic summary).
Do these findings have translational implications for rescue of GSIS in T2D? Importantly, the ability of glucose to drive insulin granule exocytosis in patch-clamped normal human β-cells is lost in β-cells from human donors with T2D. However, infusion of isocitrate or NAPDH rescues the exocytotic response in these cells.29 These findings suggest that islets from humans with T2D may be defective at a step (or steps) of anaplerotic metabolism of glucose upstream of the IDH1 reaction. Further studies will be required to define the specific molecular lesions in anaplerotic metabolism of glucose in islets from T2D subjects.
Metabolomics Identifies Novel Nucleotide Secretagogues
The classical KATP-channel dependent pathway of GSIS invokes an increase in ATP:ADP ratio as a key signal for suppression of KATP channel activity, leading to membrane depolarization, activation of voltage-gated Ca2+ channels, and triggering of insulin granule release by Ca2+ signaling. However, recent non-targeted metabolomics studies suggested that glucose also alters the levels of a variety of other purine and nucleotide pathway intermediates in islet cells,33–36 although the functional significance of these changes was not defined. To investigate this further, our group developed a targeted, quantitative LC-MS/MS method for profiling of 37 different nucleotides and purine pathway intermediates.37 The method was applied to the robustly glucose responsive INS-1-derived cell line 832/13 22 exposed to basal or stimulatory glucose concentrations. Glucose stimulation caused a clear rise in NADPH and NADH levels, consistent with operation of the glucose/isocitrate pathway of GSIS discussed earlier. Stimulatory glucose also caused a clear decrease in inosine monophosphate (IMP), and an equally striking increase in adenylosuccinate (S-AMP).37 IMP and S-AMP are the substrate and product of a single enzymatic reaction in which IMP is converted to S-AMP by adenylosuccinate synthase (ADSS), suggesting that this enzyme could play a regulatory role in β-cell glucose sensing (Figure 1). Indeed, pharmacologic suppression of ADSS with alanosine, or molecular suppression of enzyme function by delivery of an ADSS-specific shRNA, caused impairment of GSIS with an attendant decrease in S-AMP, effects that could be overcome by addition of adenine. Moreover, shRNA-mediated suppression of adenylosuccinate lyase (ADSL), a more proximal enzyme in the S-AMP biosynthetic pathway, impaired GSIS and lowered S-AMP levels. The effect of ADSL suppression to lower S-AMP was independent of decreases in other adenine nucleotides or GTP, implying a specific role for S-AMP in regulation of insulin secretion. Indeed, infusion of S-AMP into patch-clamped β-cells from normal (non-diabetic) human donors at low glucose (1 mM) revealed that it is equipotent to stimulatory glucose (12 mM) for activation of exocytosis. Moreover, similar to intermediates in the glucose/isocitrate pathway, S-AMP rescues exocytosis in glucose-unresponsive β-cells from humans with T2D. Also, analogous to the glucose/isocitrate pathway, the stimulatory effect of S-AMP on exocytosis in patch-clamped human β-cells is impaired by shRNA-mediated suppression of SENP1.37
The new glucose/isocitrate and S-AMP pathways of GSIS summarized above are viewed as complementary to the classical KATP-channel-dependent initiating pathway. It remains to be determined if the newly described pathways are additive or synergistic with each other and/or the KATP channel-dependent mechanism in control of GSIS. Our studies on the glucose/isocitrate pathway also imply a particular impact of NADPH generated by the IDH1 reaction, relative to other NADPH producing enzymes such as malic enzyme or the pentose monophosphate shunt enzymes glucose-6-phosphate dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase (6PGDH). With regard to the pentose pathway enzymes, inhibition of G6PDH with dehydroepiandrosterone (DHEA),35 or 6PGDH by siRNA or the chemical inhibitor 6-AN,38 were both reported to result in impaired GSIS. The effects of 6PGDH inhibition were ascribed to accumulation of early PPP intermediates and activation of p-ERK,38 whereas DHEA treatment caused a decrease in GSH levels, although effects on NADPH were not reported.35 These findings appear inconsistent with our findings that shRNA-mediated suppression of IDH1 is sufficient to ablate the glucose-induced increment in GSH and GSH:GSSG ratio in islet cells.29 In addition, the finding that S-AMP has a direct effect to activate exocytosis in normal islets, and is able to rescue dysfunctional exocytosis in human T2D islets seems more aligned with the concept that S-AMP is the key regulator of GSIS produced by the pentose shunt.37 However, these issues will require further investigation.
Future studies should include attempts to estimate the relative contributions of different pathways for NADPH formation, using recently described methods.39 In this approach, pentose pathway flux is measured by the classical method of the difference between 14CO2 production from [1-14C] and [6-14C] glucose. The contribution of the pentose shunt to NADPH synthesis is then measured by fractional enrichment (FE) of the NADPH and G6P pools in cells incubated with [3-D] glucose.39 Using these methods, a surprisingly large contribution of folate metabolism to total NAPDH production was recently reported in HEK293T cells.39 Application of similar methods, possibly including use of different substrates with strategic deuterium labeling, to pancreatic islet cells should allow the fractional contributions of various NADPH producing pathways to be quantified. Performance of such flux experiments in the presence and absence of strategic suppression of contributory enzymes such as G6PDH and IDH1 will add to our knowledge of key operative pathways for control of GSIS.
Acknowledgments
Studies from the authors’ laboratories summarized in this paper were supported by National Institutes of Health grant DK42538 (to C.B.N.) and Canadian Institutes of Health Research grant MOP244739 (to P.E.M).
Footnotes
Conflict of Interest Statement. The authors have no conflicts to report pertaining to this research.
References
- 1.Newgard CB, Matschinsky FM. Substrate control of insulin release. In: Jefferson JAC, editor. Handbook of Physiology. Oxford University Press; 2001. pp. 125–152. [Google Scholar]
- 2.Henquin JC, Ravier MA, Nenquin M, Jonas JC, Gilon P. Hierarchy of the beta-cell signals controlling insulin secretion. Eur J Clin Invest. 2003;33:742–750. doi: 10.1046/j.1365-2362.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- 3.Gembal M, Gilon P, Henquin JC. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest. 1992;89:1288–1295. doi: 10.1172/JCI115714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2008;295:E1287–1297. doi: 10.1152/ajpendo.90604.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18:162–185. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Newgard CB. Metabolomics and Metabolic Diseases: Where Do We Stand? Cell Metab. 2017;25:43–56. doi: 10.1016/j.cmet.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagliuca FW, Millman JR, Gurtler M, et al. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nature biotechnology. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- 9.Muoio DM, Newgard CB. Mechanisms of disease:Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nature reviews Molecular cell biology. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 10.An J, Muoio DM, Shiota M, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nature medicine. 2004;10:268–274. doi: 10.1038/nm995. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara CT, Wang P, Neto EC, et al. Genetic networks of liver metabolism revealed by integration of metabolic and transcriptional profiling. PLoS genetics. 2008;4:e1000034. doi: 10.1371/journal.pgen.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bain JR, Stevens RD, Wenner BR, Ilkayeva O, Muoio DM, Newgard CB. Metabolomics applied to diabetes research: moving from information to knowledge. Diabetes. 2009;58:2429–2443. doi: 10.2337/db09-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamboni N, Saghatelian A, Patti GJ. Defining the metabolome: size, flux, and regulation. Molecular cell. 2015;58:699–706. doi: 10.1016/j.molcel.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patti GJ, Yanes O, Siuzdak G. Innovation: Metabolomics: the apogee of the omics trilogy. Nature reviews Molecular cell biology. 2012;13:263–269. doi: 10.1038/nrm3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alves TC, Pongratz RL, Zhao X, et al. Integrated, Step-Wise, Mass-Isotopomeric Flux Analysis of the TCA Cycle. Cell Metab. 2015;22:936–947. doi: 10.1016/j.cmet.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danielsson AP, Moritz T, Mulder H, Spegel P. Development and optimization of a metabolomic method for analysis of adherent cell cultures. Analytical biochemistry. 2010;404:30–39. doi: 10.1016/j.ab.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz MA, Burant CF, Kennedy RT. Reducing time and increasing sensitivity in sample preparation for adherent mammalian cell metabolomics. Analytical chemistry. 2011;83:3406–3414. doi: 10.1021/ac103313x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards JL, Edwards RL, Reid KR, Kennedy RT. Effect of decreasing column inner diameter and use of off-line two-dimensional chromatography on metabolite detection in complex mixtures. J Chromatogr A. 2007;1172:127–134. doi: 10.1016/j.chroma.2007.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards JL, Kennedy RT. Metabolomic analysis of eukaryotic tissue and prokaryotes using negative mode MALDI time-of-flight mass spectrometry. Analytical chemistry. 2005;77:2201–2209. doi: 10.1021/ac048323r. [DOI] [PubMed] [Google Scholar]
- 21.Gooding JR, Jensen MV, Newgard CB. Metabolomics applied to the pancreatic islet. Arch Biochem Biophys. 2016;589:120–130. doi: 10.1016/j.abb.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 23.Lu D, Mulder H, Zhao P, et al. 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS) Proc Natl Acad Sci U S A. 2002;99:2708–2713. doi: 10.1073/pnas.052005699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuit F, De Vos A, Farfari S, et al. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J Biol Chem. 1997;272:18572–18579. doi: 10.1074/jbc.272.30.18572. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab. 2005;288:E1–15. doi: 10.1152/ajpendo.00218.2004. [DOI] [PubMed] [Google Scholar]
- 26.Ronnebaum SM, Ilkayeva O, Burgess SC, et al. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem. 2006;281:30593–30602. doi: 10.1074/jbc.M511908200. [DOI] [PubMed] [Google Scholar]
- 27.Joseph JW, Jensen MV, Ilkayeva O, et al. The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J Biol Chem. 2006;281:35624–35632. doi: 10.1074/jbc.M602606200. [DOI] [PubMed] [Google Scholar]
- 28.Odegaard ML, Joseph JW, Jensen MV, et al. The mitochondrial 2-oxoglutarate carrier is part of a metabolic pathway that mediates glucose- and glutamine-stimulated insulin secretion. J Biol Chem. 2010;285:16530–16537. doi: 10.1074/jbc.M109.092593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferdaoussi M, Dai X, Jensen MV, et al. Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional beta cells. J Clin Invest. 2015;125:3847–3860. doi: 10.1172/JCI82498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivarsson R, Quintens R, Dejonghe S, et al. Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes. 2005;54:2132–2142. doi: 10.2337/diabetes.54.7.2132. [DOI] [PubMed] [Google Scholar]
- 31.Dai X-Q, Plummer G, Casimir M, et al. SUMOylation Regulates Insulin Exocytosis Downstream of Secretory Granule Docking in Rodents and Humans. Diabetes. 2011;60:838–847. doi: 10.2337/db10-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferdaoussi M, Fu J, Dai X, et al. SUMOylation and calcium control syntaxin-1A and secretagogin sequestration by tomosyn to regulate insulin exocytosis in human ss cells. Sci Rep. 2017;7:248. doi: 10.1038/s41598-017-00344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang M, Joseph JW. Assessment of the metabolic pathways associated with glucose-stimulated biphasic insulin secretion. Endocrinology. 2014;155:1653–1666. doi: 10.1210/en.2013-1805. [DOI] [PubMed] [Google Scholar]
- 34.Lorenz MA, El Azzouny MA, Kennedy RT, Burant CF. Metabolome response to glucose in the beta-cell line INS-1 832/13. J Biol Chem. 2013;288:10923–10935. doi: 10.1074/jbc.M112.414961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spegel P, Sharoyko VV, Goehring I, et al. Time-resolved metabolomics analysis of beta-cells implicates the pentose phosphate pathway in the control of insulin release. Biochem J. 2013;450:595–605. doi: 10.1042/BJ20121349. [DOI] [PubMed] [Google Scholar]
- 36.El-Azzouny M, Evans CR, Treutelaar MK, Kennedy RT, Burant CF. Increased glucose metabolism and glycerolipid formation by fatty acids and GPR40 receptor signaling underlies the fatty acid potentiation of insulin secretion. J Biol Chem. 2014;289:13575–13588. doi: 10.1074/jbc.M113.531970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gooding JR, Jensen MV, Dai X, et al. Adenylosuccinate Is an Insulin Secretagogue Derived from Glucose-Induced Purine Metabolism. Cell Rep. 2015;13:157–167. doi: 10.1016/j.celrep.2015.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goehring I, Sauter NS, Catchpole G, et al. Identification of an intracellular metabolic signature impairing beta cell function in the rat beta cell line INS-1E and human islets. Diabetologia. 2011;54:2584–2594. doi: 10.1007/s00125-011-2249-7. [DOI] [PubMed] [Google Scholar]
- 39.Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510:298–302. doi: 10.1038/nature13236. [DOI] [PMC free article] [PubMed] [Google Scholar]