Abstract
OBJECTIVES
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors were approved by the U.S. Food and Drug Administration (FDA) as cholesterol-lowering therapies for patients with familial hypercholesterolemia or atherosclerotic cardiovascular disease. This study estimates the long-term health and economic value of PCSK9 inhibitors for older Americans (aged 51 and older).
METHODS
We conducted simulations using the Future Elderly Model (FEM), an established dynamic microsimulation model, to project the lifetime outcomes for the U.S. population aged 51 or older. Health effects estimates and confidence intervals from published meta-analysis studies were used to project changes in life expectancy, quality-adjusted life-years, and lifetime medical spending resulting from use of PCSK9 inhibitors. We considered two treatment scenarios: 1) current FDA eligibility; and 2) an extended eligibility scenario which includes patients with no pre-existing cardiovascular disease (CVD) but at high-risk. We assumed the price of PCSK9 inhibitors was discounted by 35% in the first 12 years and by 57% thereafter, with gradual uptake of the drug in eligible populations.
RESULTS
Utilization of PCSK9 inhibitors by individuals covered by current FDA approval would extend life-expectancy at age 51 by an estimated 1.1 years and would yield a lifetime net value of $5,800 per person. If utilization were extended to those at high-risk for CVD, PCSK9 inhibitors would generate a lifetime net benefit of $14,100 per person.
CONCLUSION
Expanded access to PCSK9 inhibitors would offer positive long-term net value for patients and the U.S. healthcare system at the current discounted prices.
Keywords: PCSK9, Evolocumab, Alirocumab, cholesterol, familial hypercholesterolemia, cardiovascular disease, microsimulation
1 INTRODUCTION
Despite advances in medical technologies, cardiovascular disease (CVD) remains the leading cause of death—nearly 7.2 million deaths annually—and a major cause of disability in the United States.(1, 2) From 2011 to 2012, the estimated annual direct and indirect costs of CVD and stroke were more than $310 billion, (3) with annual costs projected to nearly triple from 2010 to 2030, from $273 billion to $818 billion. (4, 5) Additional interventions are needed for people at risk of CVD, focusing both on patient lifestyle changes and managing modifiable cardiovascular risk factors successfully.
A large body of evidence demonstrates that low-density lipoprotein cholesterol (LDL-C) is a principal driver of atherosclerotic vascular disease (ASCVD)—the underlying cause of the majority of clinical manifestations of CVD—and thus the primary target for CVD risk reduction interventions.(6–9) For more than a decade, guidelines have indicated that patients with elevated LDL should use HMG-CoA reductase inhibitors, also known as statins, adjunct to diet to reduce cholesterol. Despite the use of statins, previous research has shown that a substantial proportion of treated high-risk patients fail to achieve target LDL-C levels.(10, 11) Moreover, statin intolerance is a common concern in clinical practice, with wide variation of individual lipid-lowering and risk reduction.(12–15) A substantial proportion of patients not meeting conventional LDL-C goals—more than 73 million U.S. adults (32%) experience elevated LDL-C(16)—suggests substantial benefits in reducing the burden of hypercholesterolemia.
Recently, the FDA approved two monoclonal antibodies targeting proprotein convertase subtilisin/kexin type 9 (PCSK9), alirocumab and evolocumab, which are novel lipid-lowering approaches that inhibit the binding of PCSK9 to the LDL receptor, resulting in powerful LDL-C lowering potency.(17–19) Both agents are administered via subcutaneous injection and were approved as an adjunct to diet and maximally tolerated statin therapy for treatment of adults with familial hypercholesterolemia (FH) or clinical ASCVD who require additional lowering of LDL-C.(20)
Preliminary Phase III clinical trials, though not powered to assess long-term cardiovascular outcomes because of the short study windows, showed approximately a 50% risk reduction in cardiovascular events and all-cause mortality while maintaining a favorable safety profile.(21–23) If clinical benefits observed in trials are sustained long term, PCSK9 inhibitors could become an important option for patients at high risk of ASCVD and potentially create substantial health benefit by preventing CVD events.
Despite health benefits suggested by the literature, payers and policymakers are concerned this new class of expensive specialty medications poses a substantial economic burden given the drugs’ current prices, which range from $14,100–$14,600 per patient per year. A recent study suggested that even if the drug price of PCSK9 inhibitors could be covered by an annual $245 billion savings in prevented CVD events, the high price of PCSK9 inhibitors still poses a substantial economic burden to the U.S. healthcare system if only accounting for direct medical costs from avoided CVD. (24) Another study suggested these agents may not be cost-effective in patients with FH or ASCVD at current U.S. prices.(25) Answers are needed about whether PCSK9 inhibitors will significantly improve mortality and reduce CVD events in Americans beyond currently available diet-statin therapy and prove cost-effective over time.(26) Given uncertainty in long-term efficacy, the FDA approved use of PCSK9 inhibitors under strict criteria, and because of the drugs’ high price, payers have suggested that PCSK9 inhibitors should be targeted to a narrow population. (20, 27, 28) However, a larger population with elevated LDL-C could benefit from PCSK9 inhibitors because clinical trials have shown substantial LDL-C lowering effects in persons who failed to receive adequate health benefits from statins regardless of their history of ASCVD.(29–31) Therefore, it is essential to evaluate the value of PCSK9 inhibitors under broader indications.
This article’s primary objective is to estimate the health benefits of PCSK9 inhibitors in the U.S. population with familial hypercholesterolemia or cardiovascular disease and to quantify the value of these gains, while taking into account the uncertainty surrounding the drugs’ clinical effectiveness. Secondarily, we estimate the long-term value of PCSK9 inhibitors if their utilization were extended to persons with no pre-existing cardiovascular disease but with high-CVD risks.
2 METHODS
2.1 The Future Elderly Model
We estimated potential health benefits and costs by using the Future Elderly Model (FEM), a dynamic microsimulation model that tracks cohorts older than age 50 to project their health and economic outcomes. Rather than aggregating health characteristics of a cohort, the FEM follows the evolution of individual-level health trajectories in a microsimulation framework. Initially, the FEM was developed to forecast long-term health and healthcare costs under different scenarios for medical technology and utilization.(32) In recent years, the FEM has been used to estimate the value of statin therapy in the obese population,(33) the value of aspirin,(34) and the value of delayed biological aging.(35, 36) The FEM also has been used to estimate the impacts of other health policy changes, such as the introduction of dietary sodium reduction policies,(37) tobacco control policies, (38) and U.S. pharmaceutical policy.(39) We describe the model and methods briefly here; complete technical information is available in the Appendix (FEM technical document).
The FEM simulates the lives of older Americans based on the Health and Retirement Survey (HRS), a nationally representative biennial survey of Americans aged 51 and older. The FEM has three core components. The first is the Health Transition module, which consists of a series of health and functional status transition equations and mortality equations to model the health of the 51+ population over their lifetimes. Health is described by the presence of certain chronic conditions, and functional status is measured by limitations in activities of daily living, instrumental activities of daily living, and nursing home residency, reported by the HRS data. All health conditions, functional states, and risk factors were modeled with first-order Markov processes that controlled for a set of baseline variables, including age, gender, education, race, body-mass index, smoking status, and health at the time of entry into the study.
For the purpose of this study, we added biomarkers (specifically cholesterol and HbA1c levels), blood pressure measurements, and treatment status (including respondents’ current therapies for cholesterol, blood pressure control, and diabetes) in the health transition module. These variables were added to better identify the target population eligible for PCSK9 inhibitors and their CVD risk as the model moves forward. These variables were obtained from the HRS biomarker data available from 2006 to 2012. We added biomarker and blood pressure levels to the list of covariates predicting cardiovascular disease. In the simulations, biomarker and blood pressure transitions were modelled as a function of respondents’ social demographics, health status, and treatment status.
We computed quality-adjusted life-year (QALY) measures based on the EQ-5D, a standardized health-related quality-of-life instrument measuring a respondent’s general health status on five dimensions: mobility, daily activities, self-care, anxiety, depression, and pain.(40) EQ-5D scores are estimated with an ordinary least squares regression as a function of the chronic conditions and FEM-specified functional status, using Medical Expenditure Panel Survey (MEPS) data.
The second FEM component is the Policy Outcomes module, which examines fiscal outcomes, including the costs of health entitlement programs—specifically federal and state spending for Medicare and Medicaid. The FEM predicts expected enrollment for Medicare and Medicaid, as well as expenditures for both entitlement programs and private medical expenditures, given a set of health, economic, and demographic states and characteristics. The predictions are based on MEPS data prior to age 65 and the Medicare Current Beneficiary Survey after age 65.
The third component is a Replenishing Cohort module, which introduces new cohorts of 51-year-olds in each simulated year as the model progresses. The FEM predicts the demographic and health characteristics for these younger populations based on data from the National Health Interview Survey, the Current Population Survey, and the National Health Nutrition and Examination Survey.
2.2 Simulations
First, we conducted “cohort simulations,” tracking a 2016 cohort of Americans aged 51 to 52 until their death under alternative PCSK9 inhibitor scenarios. In addition, we conducted “population simulations” to investigate the population-wide trends implied by observing a representative cross-section of the older U.S. population in each period. We used the full FEM population to project outcomes (including the replenishing cohort) for the entire population of Americans aged 51 and older from year 2016 to year 2056. Population health outcomes were calculated for each time period by aggregating individual health measures.
2.2.1 Scenarios
We considered three scenarios—one representing the status quo and the other two representing scenarios introducing use of PCSK9 inhibitors. In PCSK9 inhibitor scenarios, we modified the status quo scenario by applying the health benefits and additional healthcare spending from PCSK9 inhibitor use to the current standard of care. First, a status quo scenario establishes a baseline assuming treatment strategies for managing hypercholesterolemia in the U.S prior to the introduction of PCSK9 inhibitors.
We next generated two scenarios to evaluate the benefits and costs of introducing PCSK9 inhibitors. Besides meeting the criteria described below, the patients are required to meet additional criteria to become eligible for PCSK9 inhibitors, including being aged 80 and younger, currently being on a cholesterol-lowering therapy, and having failed to reduce LDL-C to ≤ 70 mg/dl:
Current Eligibility: In this scenario, the populations eligible for PCSK9 inhibitors were defined by current FDA-approved indications(20) and the first two groups of statin benefit groups as outlined by American College of Cardiology/American Heart Association (ACC/AHA) guidelines,(8) which included those with familial hypercholesterolemia (defined as LDL-C level higher than 190 mg/dL)(41, 42) and preexisting CVD.
Extended Eligibility: This scenario extends access to patients without a history of CVD but with high-risk equivalents are also eligible for treatment with PCSK9 inhibitors. The CVD high-risk equivalents were defined as persons with diabetes aged 40 to 75 years, or with an estimated 10-year ASCVD risk >7.5%. This group corresponded with the statin benefit groups 3 and 4 in ACC/AHA guidelines.(8)
Uncertainty surrounding long-term effectiveness and pricing concerns have served as barriers to widespread adoption of PCSK9 inhibitors, and their adoption has been gradual.(27, 43) Therefore, among the PCSK9 inhibitor-eligible population, we assumed a zero probability of actual PCSK9 inhibitor assignment in year 2014, and the probability linearly increases to one through year 2020. The process to identify PCSK9 inhibitor eligibility in FEM simulations is detailed in Appendix A.
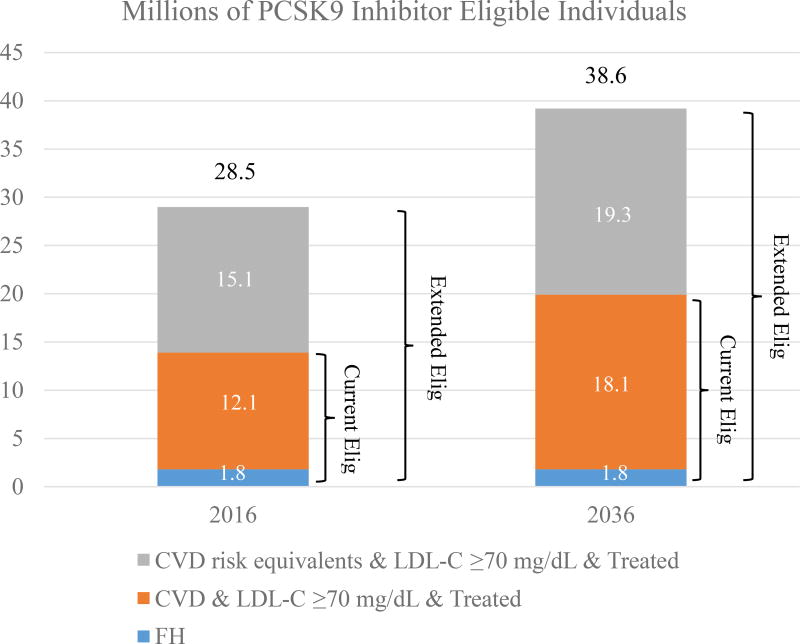
We estimated that 13.8 million individuals were eligible for PCSK9 inhibitors under current FDA approval in 2016; eligible individuals increased to 28.5 million under extended eligibility. (Figure 1). The actual PCSK9 inhibitor assignment after phasing in adoption in a gradual linear manner is displayed in Figure A1. There were about 4.6 million and 9.5 million individuals assigned to use PCSK9 inhibitors under current and extended eligibilities, respectively, in 2016.
FIGURE 1.
Projected Populations Eligible for PCSK9 Inhibitors by Statin-benefit Groups (SBGs), Year 2016 and 2036*
*Individuals in statin-benefit groups (recommended by ACC/AHA guidelines) who failed to achieve a goal LDL-C level (≥ 70 mg/dL) and who regularly take lipid-lowering therapy are potentially eligible for PCSK9 inhibitor use until age 80. Current Elig refers to the current eligibility criteria for PCSK9 inhibitors, corresponding to FDA approval. Extended Elig refers to the extended eligibility for PCSK9 inhibitors, using PCSK9 inhibitors as primary prevention therapy for those without clinical CVD but who possess CVD high-risk equivalents. CVD risk equivalents refer to individuals with a clinical diagnosis of diabetes and estimated 10-year CVD risk higher than 7.5%. CVD: Cardiovascular disease, defined as any diagnosis of congestive heart failure, coronary heart disease, angina, heart attack, and any other heart diseases. FH: familial hyperlipidemia, defined as those with LDL-C levels ≥190 mg/dl.
2.2.2 The impact of PCSK9 inhibitors on health and costs
To reflect the health impacts of PCSK inhibitors reported in the literature, we modified health transitions and outcomes of eligible individuals in the PCSK9 inhibitor scenarios, specifically by reducing the risk of having the first cardiovascular disease and all-cause mortality, as well as applying additional drug costs and disutility weights for PCSK9 inhibitors. The key parameters and their ranges for sensitivity analysis are listed in Table 1. For PCSK9 inhibitor-eligible individuals and for those without prior heart disease, we decreased the probabilities of heart disease incidence by factors with a mean of 0.54, and for those receiving PCSK9 inhibitors, we decreased their probabilities of mortality by factors with a mean of 0.45, (21, 22), which correspond to the risk-ratios reported by published meta-analyses. The risk reduction in mortality was further adjusted to account for the interaction between heart disease prevention and mortality. (Appendix A). Since the effectiveness of PCSK9 inhibitors among subgroups with different characteristics are not yet well established, we assumed the same effect for populations taking PCSK9 inhibitors across their life-years covered by the drugs.
Table 1.
Key Input Parameters Used In the Model
| Parameters | Base-case | Reference | Range for sensitivity Analysis |
Type of sensitivity analysis |
|---|---|---|---|---|
| Effect size | ||||
| Relative risk of events for PCSK9 inhibitor versus standard of care based on the Phase III clinical trials1 | Probabilistic sensitivity analysis performed by drawing random values from the confidence intervals for relative-risk estimates of the base-case estimate for each repetition of simulations. | |||
| Overall mortality | 0.45 | Navarese EP et al., 2015 | [0.23 – 0.86] | |
| Major cardiovascular events | 0.54 | Lipinski MJ et al., 2016 | [0.38 – 0.77] | |
| Costs (in 2015 dollars) | ||||
| Annual drug costs for PCSK9 inhibitors | ||||
| Original cost | $14,350 | Red Book Online | [10,763, 17,938] | |
| Discounted cost due to rebate and the entry of competitors2 | $9,328 | Tirrell M et al., 2015 | [6,996, 11,660] | One-way sensitivity analysis based on +/− 25% of the base-case estimates. |
| Cost without patent protection (after year 2028)3 | $6,249 | Conti RM et a., 2014 | [4,686, 7,811] | |
| Utility weights | ||||
| Disutility due to injection, per patient | 0.004 | Matza LS et al., 2013 | [0.003, 0.005] | One-way sensitivity analysis |
| Discount rate | 3% | [1%, 5%] | One-way sensitivity analysis |
The base-case assumed a constant relative reduction in the risk of overall mortality and major cardiovascular event, independent of how long the patients were treated by PCSK9 inhibitors. This was estimated from the most recent meta-analysis of PCSK9 trials that reported the end point. There were too few strokes in the short-term PCSK9 trials, so we didn’t consider the benefit in stroke with PCSK9 inhibitors.
The cost is calculated as $14,350 multiplied by 0.65 (a 35% discount).
The cost is calculated as $14,350 multiplied by 0.65, then multiplied by 0.67 (an additional 33% discount).
Although research has found an elevated risk of a neurocognitive adverse event suspected to be associated with use of PCSK9 inhibitors, the FDA concluded that the association was insignificant. (44) Evidence has shown utility differences between different treatment modalities, with a subcutaneous injection leading to lower health utility compared with an oral therapy; therefore, we imposed a disutility of 0.004 per person receiving PCSK9 inhibitors.(45)
Annual costs of PCSK9 inhibitors were assumed to be equal to their wholesale acquisition costs; assumed to be the mean of the 2015 annual costs of alirocumab ($14,600) and evolocumab ($14,100).(46) The price for a new branded drug usually falls by a discount rate ranging from 23% to 46%, after the entry of other branded competing drugs; and an additional discount of 33% will be applied due to the expiration of patent protection. (47, 48) Therefore, our analysis assumed that branded PCSK9 inhibitor drugs will be discounted by 35% after their first year on the market; after 2028, PCSK9 inhibitor drugs are discounted by an additional 33%, resulting in a 57% total discount.(47, 49) All costs outcomes were adjusted to 2015 US dollars.
2.3 Uncertainty
Three different approaches of sensitivity analysis were performed to evaluate the robustness of our results. Probability sensitivity analysis was used to adjust the parameter uncertainty surrounding clinical effectiveness, which was estimated with the wide confidence intervals of the meta-analyses results shown in Table 1. This uncertainty will likely diminish as more clinical trials are conducted and samples increase but needs to be accounted for in our analyses. To do so, we drew random values from the distributions of relative-risk estimates of effectiveness reported in the literature for each repetition of simulations. In detail, we first drew 200 sets of risk-ratio estimates from log-normal distributions and conducted separate simulations for each set of estimates; then we computed the results from the 200 simulations and sorted them for all outcomes of interest. As a result, the point estimates of our results correspond to the mean of each variable of interest across the 200 simulations. The bounds of the 95% confidence intervals correspond to the fifth lowest and highest results from the sorted estimates of the 200 simulations. These intervals can be interpreted as the 95% confidence intervals with regard to the clinical uncertainty of the effectiveness of PCSK9 inhibitors. Additional scenarios were generated to adjust the structural uncertainty of the model assumptions, in terms of eligibility criteria and effect size of PCSK9 inhibitors. One-way sensitivity analysis was performed to examine how the results would differ with changes in drug cost, disutility weights, and discount rate parameters. Details can be found in Appendix A.
3 RESULTS
3.1 LIFE EXPECTANCY, QUALITY OF LIFE, AND FUNCTIONAL STATUS
Our cohort simulations revealed that PCSK9 inhibitor use would significantly increase life expectancy, disability-free life expectancy and quality of life, and reduce the incidence of heart disease. Table 1 summarizes how the scenarios would affect key health indicators over the life course of nationally representative 51-year-olds in comparison to the current standard of care (the status quo scenario).Compared to the status quo scenario, the cohort is expected to live on average 1.1 years longer under the FDA-approved PCSK9 inhibitor scenario and 1.9 years longer under the extended PCSK9 inhibitor eligibility criteria (Table 2).
TABLE 2.
The Impact of PCSK9 Inhibitor Use on Health Outcome at Age 51
| Status quo | PCSK9 inhibitor Scenario I - Current Eligibility |
PCSK9 inhibitor Scenario II - Extended Eligibility |
|||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | Mean | Difference* | 95% CI | Mean | Difference* | 95% CI | |
| Health outcomes, at age 51** | |||||||
| Life expectancy, years | 30.9 | 32.0 | 1.1 | [0.9, 1.4] | 32.7 | 1.9 | [1.5, 2.3] |
| Disability-free life expectancy, years | 22.9 | 23.2 | 0.4 | [0.3, 0.5] | 23.6 | 0.8 | [0.6, 1.0] |
| Quality-adjusted life years (QALYs) | 25.5 | 26.1 | 0.6 | [0.4, 0.8] | 26.7 | 1.2 | [0.9, 1.5] |
| Cumulative disease incidence at age 79 (per thousand)† | |||||||
| Heart Disease | 419.3 | 411.6 | −7.7 | [−0.9, −14.7] | 379.0 | −40.3 | [−35.5, −69.8] |
| Stroke | 207.2 | 219.0 | 11.8 | [5.0, 18.9] | 222.3 | 15.0 | [6.3, 23.4] |
| Diabetes | 473.8 | 479.5 | 5.7 | [1.7, 9.9] | 483.0 | 9.2 | [2.4, 14.5] |
| Hypertension | 732.4 | 737.6 | 5.2 | [0.3, 11.8] | 742.2 | 9.8 | [1.7, 14.9] |
| Cancer | 285.5 | 294.8 | 9.4 | [4.0, 16.3] | 299.9 | 14.4 | [7.0, 21.3] |
Current Eligibility refers to the current FDA-approved eligibility criteria for PCSK9 inhibitors. Extended Eligibility refers to using PCSK9 inhibitors as primary prevention therapy for those without clinical CVD but who possess CVD high-risk equivalents.
The result differences compared with the status quo scenario.
The results were calculated from age 51 until death.
Total incidence before age 79 for a 1,000 individuals without the disease at age 51. Disability-free life expectancy refers to reporting no instrumental activity of daily living or activity of daily living limitations and not living in a nursing home. Quality-adjusted life-years adjust length of life for quality based on a person’s chronic conditions and functional status. 95% confidence intervals of the difference estimates are presented in brackets, indicating the uncertainty of the effectiveness of PCSK9 inhibitors. The 95% confidence intervals for the mean values in each scenario can be found in the Supplemental Appendix.
The cumulative incident cases of cardiovascular disease by age 79 were predicted for 1,000 individuals without prior cardiovascular disease at age 51. Because of the efficacy of PCSK9 inhibitors on mortality reduction, individuals are estimated to live longer and develop chronic diseases. Given that the majority of people under FDA-approved eligibility have existing heart disease, the risk reduction of PCSK9 inhibitor on the first heart disease was diluted by the mortality effect, which resulted in only 7.7 less cases in every 1,000 people as compared to the status quo scenario, which was attributable to the population with FH or stroke but without existing heart disease. When the eligibility is extended to those without prior heart disease but are high-risk equivalent, PCSK9 inhibitors would lead to 40.3 fewer cases of heart disease. We would expect a greater incidence of other chronic conditions, including diabetes, hypertension, stroke, and cancer. Consequently, under the current eligibility scenario, among the 1.1 additional life years attributable to PCSK9 inhibitors, people live only 0.4-year in a healthy state.
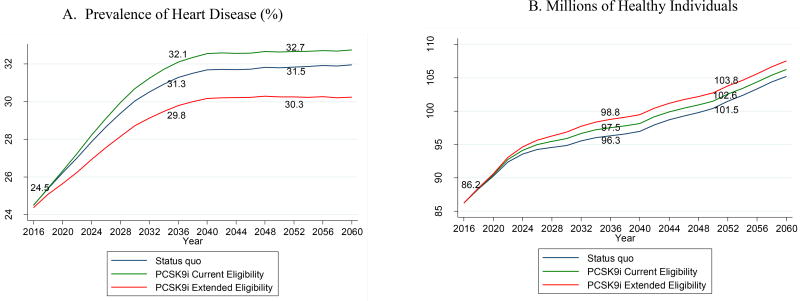
Figure 2 displays the results of population simulation analyses, where we predicted the effects of PCSK9 inhibitor use on the prevalence of heart disease (Figure 2A) and disability (Figure 2B) from year 2016 through the next four decades and compared the forecasted results in the status quo scenario with the PCSK9 inhibitor scenarios. Due to longer life expectancy of individuals with heart disease, in 2036, the prevalence of heart disease is projected to increase by 0.8 percentage points under current eligibility, compared to the status quo. In contrast, prevalence of heart disease is projected to fall by 1.5 percentage points relative to the status quo under extended eligibility, in which individuals use PCSK9 inhibitors to prevent the onset of heart disease. Due to the extension of life expectancy and the reduced risk of heart disease, we estimated 1.2 million to 2.5 million more members of the healthy population in each PCSK9 inhibitor scenario than those in the status quo scenario in 2036.
FIGURE 2.
The Long-term Health Impacts of PCSK9 Inhibitor Use on U.S. Population Aged over 50*
Figure 3A compared the projected prevalence of heart disease, and Figure 3B compared the projected millions of healthy individuals with the status quo and two PCSK9 inhibitor scenarios. * Individuals in statin-benefit groups (recommended by ACC/AHA guidelines) who failed to achieve a goal LDL-C level (≥ 70 mg/dL) and who regularly take lipid-lowering therapy are potentially eligible for PCSK9 inhibitor use until age 80. Among the PCSK9 inhibitor-eligible population, the actual assignment of PCSK9 inhibitor use was phased-in with a probability of zero starting from year 2014 and linearly increased to 1 through year 2020. PCSK9 inhibitor Current Eligibility refers to current FDA-approved eligibility criteria for PCSK9 inhibitors. Extended Eligibility refers to using PCSK9 inhibitors as primary prevention therapy for those without clinical CVD but who possess CVD high-risk equivalents. Healthy individuals refers to those reporting no instrumental activity of daily living or activity of daily living limitations and not living in a nursing home. Heart disease includes any diagnosis of congestive heart failure, coronary heart disease, angina, heart attack, and any other heart diseases.
3.2 Cost-effectiveness
We projected the expected lifetime medical costs for our cohorts at age 51 and estimated the value of health benefits against medical costs. We used a 3% discount rate as base-case to compute present values from the age of 50 for both costs and benefits. We considered the value per QALY gained as $150,000, which is an acceptable threshold of three times the U.S. gross domestic product (GDP) per capita, as recommended by the World Health Organization.(50) Compared with the status quo, the values associated with QALYs gained are estimated to be $45,900 higher in the FDA-approved eligibility scenario and $83,900 higher in the extended eligibility scenario. Under the FDA-approved indications, the additional healthcare costs, including the drug cost of PCSK9 inhibitors are estimated to be $40,100 compared to the status quo. In the extended eligibility scenario, we estimated a larger additional incremental healthcare cost ($69,800) due to the effect on longevity from more people receiving PCSK9 inhibitors. Compared to the status quo, PCSK9 inhibitors generated an average $132,000 and $125,900 per additional QALY gained in the FDA-approved eligibility and extended eligibility scenarios, respectively. The net value of PCSK9 inhibitors is estimated to be $5,800 in the FDA-approved eligibility scenario but is significant at a 0.10 level, while the value in extended eligibility scenario is $14,100 and significant at a 0.05 level. (Table 3). Incremental QALYs gained under the extended eligibility scenario relative to the FDA-approved eligibility scenario would cost on average $120,400 per QALY and provide $8,200 of net value per capita (Appendix Table A4).
TABLE 3.
The Net Benefits of PCSK9 Inhibitor Use at Age 51 ($2015 thousands)
| Status quo | PCSK9 Inhibitor Scenario I – Current Eligibility |
PCSK9 Inhibitor Scenario II – Extended Eligibility |
|||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | Mean | Difference* | 95% CI | Mean | Difference* | 95% CI | |
| Value of expected quality-adjusted life-years gained (Discounted) | 2465.6 | 2511.5 | 45.9 | [33.5, 58.9] | 2549.5 | 83.9 | [66.5, 102.5] |
| Per Capita Life-time healthcare and medication costs (Discounted) | |||||||
| Healthcare costs | 538.7 | 567.9 | 29.3 | [21.4, 39.6] | 587.4 | 48.6 | [36.7, 59.4] |
| PCSK9i medication costs† | 0.0 | 10.8 | 10.8 | [10.1, 11.4] | 21.3 | 21.3 | [20.4, 22.3] |
| Total | 538.7 | 578.7 | 40.1 | [32.3, 50.7] | 608.5 | 69.8 | [59.3, 82.3] |
| Incremental Cost-Effectiveness Ratio (ICER) | 132.2 | [110.8, 153.9] | 125.9 | [111.4, 140.1] | |||
| Net value per capita (in thousands)** | 5.8 | [−1.1, 13.7] | 14.1 | [4.2, 25.3] | |||
Current Eligibility refers to current FDA-approved eligibility criteria for PCSK9 inhibitors. Extended Eligibility refers to using PCSK9 inhibitors as primary prevention therapy for those without clinical CVD but who possess CVD high-risk equivalents.
The result differences compared with the status quo scenario.
Calculated by multiplying QALY-gains with a willingness-to-pay of $150,000.
PCSK9 inhibitor medication costs were calculated based on a discount rate of 35% from 2016 to 2027, with an additional 33% discount since year 2028. All amounts are in present value at age 51, computed with a 3% discount rate. 95% confidence intervals of the mean differences are presented in brackets, indicating the uncertainty of the effectiveness of PCSK9 inhibitors. The 95% confidence intervals for the mean values in each scenario can be found in the Supplemental Appendix. All the costs were adjusted to year 2015 US thousand dollars.
At the population level, the aggregate incremental medical costs in both PCSK9 inhibitor scenarios were estimated to outweigh the total value of QALYs gained in the first eight years (Figure A2 in the Appendix A). However, the net values become positive since year 2026 in both scenarios and are projected to grow after the drugs go off patent. The FEM projected a cumulative net present value of $0.54 trillion in the PCSK9 inhibitor current eligibility scenario and $0.90 trillion in the extended eligibility scenario by 2036.
3.3 Sensitivity analysis
Five other scenarios were created to account for additional sources of uncertainty surrounding our simulations. The results of these analyses are presented in Appendix A. Most scenarios resulted in similar or less favorable net values for PCSK9 inhibitors, as compared with our baseline results. The net values of PCSK9 inhibitors become negative if only 10% of the population eligible for PCSK9 inhibitors was statin-intolerant and used PCSK9 inhibitors. For the FDA-approved scenario, higher positive net values and favorable ICERs were observed when using the clinical effectiveness estimated from the relationship between the LDL-C reduction and the risks of event, instead of using estimates from the PCSK9 inhibitor meta-analysis. In general, despite that, the base-case estimate is sensitive to assumptions in eligibility criteria and the effect size of the drugs, with the average net value per capita ranging from -$3,600 to $11,400 under the FDA-approved eligibility and from $-900 to $12,900 under extended eligibility; the mean ICER estimates in the scenarios were all below or equal to $150,000 per QALY gained (Table A9).
In one-way sensitivity analyses varying drug cost, utility weight, and discount rate, estimates are most sensitive to the cost of PCSK9 inhibitors (Table A11). If patients have no access to the discount for the PCSK9 inhibitors during the patent protection period, the net value of PCSK9 inhibitors would be small under the FDA-approved eligibility.
4 DISCUSSION
Our study estimates the economic value of PCSK9 inhibitors for the United States over the next 40 years and demonstrates that use as indicated could substantially improve health outcomes among Americans over age 50, including reducing the incidence of cardiovascular disease and extending life expectancy. Assuming current discounted prices, treatment with PCSK9 inhibitors for older Americans under current indications yield substantial net benefits. When analyzing a cohort of 51-year-olds living in 2016, PCSK9 inhibitors used under FDA-approved indications are estimated to yield a lifetime net value of $5,800 per capita (which is significant at a 0.10 level). Interestingly, PCSK9 inhibitors are estimated to generate a higher lifetime net benefit, up to $14,100 per capita, when use is extended to people without a history of CVD but who are at risk of developing the disease. Given our comparator—the status quo scenario of treatment strategies to manage hypercholesterolemia, which include not only statins but also other interventions such as ezetimide, diet, or exercise under current clinical guidelines—our study informs potential values of PCSK9 inhibitors to the healthcare system in addition to the current standard of care.
Adding PCSK9 inhibitors to the treatment arsenal is also cost-effective, even with broad eligibility criteria. We estimate an incremental cost-effectiveness ratio (ICER) of $132,200 under current FDA-approved indications and $125,900 under extended eligibility, which fall well within the accepted range for well-known recent innovative therapies. In the case of the new oral anticoagulant dibigatran for stroke prevention, the ICER was reported as $143,000 versus traditional warfarin therapy in elderly Americans. In another example, the novel hepatitis C treatment, sofosbuvir, showed ICERs ranging from $9,700 to $284,300 depending on the patient's status with respect to treatment history, HCV genotype, and presence of cirrhosis. (51–53)
Although evidence of the effectiveness of PCSK9 inhibitors continues to accumulate, the drug poses a challenge for payers and policymakers who have raised concerns about price and short-term affordability.(26, 27, 54) Our results suggest that PCSK9 inhibitors would increase lifetime medical spending because of increased drug costs and the impact of PCSK9 inhibitors on extending life, ultimately increasing economic burdens from higher risk of comorbidities accompanying natural aging. We also find that the value of PCSK9 inhibitors is sensitive to the price of this new drug. Therefore, if consumers have access to a discounted price for the drug, the value of health gained could outweigh costs over the long term and more generous coverage criteria for PCSK9 inhibitors would potentially generate a higher net value, under the assumption that the efficacy of these drugs in CVD incidence and overall mortality are consistent with the current evidence among PCSK9 inhibitor users.
In a recent study, Kazi et al. estimated an incremental cost of about $150,000 per QALY gained for PCSK9 inhibitors compared with adding ezetimibe to statins (25) when the price of PCSK9 inhibitor is $6,810 per year, which is close to the price level we assumed when the drug goes generic. The discrepancy in results also can be explained by the model parameters and assumptions. First, their model contains only CVD-associated health and health spending outcomes; they did not capture disability or costs attributable to hypertensive heart disease, peripheral arterial disease, or other non-cardiovascular outcomes associated with atherosclerotic cardiovascular disease. Therefore, they likely underestimated the cost savings and QALY gains associated with PCSK9 inhibitors, while the FEM medical spending module covers a wider range of spending from all associated comorbidities, mortality, and disabilities. Second, their study assumed perfect long-term compliance to therapy, potentially overestimating the outcomes of their control group and resulting in lower effectiveness in the PCSK9 inhibitor group. In contrast, our status quo scenario represents the current strategies and treatment for hypercholesterolemia in the United States, which is more representative of real-world practice. Our study also considers a gradual take-up of this new class of drug, which might cause lower incremental costs from PCSK9 inhibitors but is closer to reality when adopting a novel medical technology.
Our study has several limitations. First, as reflected by the wide confidence intervals around our results, there remains uncertainty regarding the clinical effectiveness of PCSK9 inhibitors. Our results reflect the uncertainty from existing meta-analyses that aggregate results across several trials, but the evidence is still deficient. For instance, a recently published trial, focusing on long-term cardiovascular outcomes (FOURIER study) raised questions about the validity of some of our parameters.(55) This new study found risk reductions on stroke, which we did not include, but did not find an all-cause mortality reduction among patients with clinically evident cardiovascular disease, even though significant reductions in aggregated major cardiovascular events were achieved. While the study adds important new information about the effectiveness of PCSK9 inhibitors, it was not sufficiently powered to estimate overall mortality within a short follow-up period (on average 2.5 years). In addition, the trial enrolled healthier and younger patients than the real world, who are at lower risk of CVD events and might benefit less from aggressive secondary prevention. Similar with other randomized controlled trials literature we used to estimate the health effects of PCSK9 inhibitors, the effectiveness might not be generalizable to the real world and might differ from clinical trial settings due to lower adherence with drug therapy. A recently published commentary also highlighted the importance of using real-world data to estimate the efficacy of the new technologies when performing cost-effectiveness analyses, to provide more valuable results for a comprehensive population of people who could benefit from the interventions.(56) Thus, more evidence is needed to clarify the long-term health benefits of PCSK9 inhibitors. In addition, since the FEM did not model second CVD events, we might underestimate health benefits of PCSK9 inhibitors for those with prior CVD. The potential cost reductions and quality of life improvements due to avoided secondary CVD events are ignored in a secondary prevention setting, while the additional costs we find are likely overestimated. In addition, in the FEM we didn’t estimate the medical spending due to a specific diagnosis or event; therefore, a limitation of our study is the inability to estimate costs of cardiovascular events avoided by the treatment. Lastly, we assumed that the relevant treatment population for PCSK9 inhibitors included persons on cholesterol-lowering therapies who were not at goal LDL-C. In reality, many patients are either not at maximally tolerated doses or are non-adherent with their current therapies.(57) Optimizing therapies may reduce the size of the population who could potentially benefit from PCSK9 inhibitors, and our estimates may be an upper bound of the value of PCSK9 inhibitors in these populations. Also, other interventions besides PCSK9 inhibitors could be considered as comparators for cholesterol management, such as more aggressive lifestyle changes relevant to risk factors.
In sum, CVD imposes an enormous health burden on the older population in the United States. Although PCSK9 inhibitors are expected to increase healthcare spending, our study estimates the value of health gains outweighs the costs, based on the best available evidence of the drugs’ health impact. In addition to current FDA-approved indications, our study also suggested potential greater value if more people gain access to this new class of drugs.
Supplementary Material
Highlights.
- What is already known about the topic?
- Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have been approved by the U.S. Food and Drug Administration (FDA) as cholesterol-lowering therapies in a secondary prevention setting for persons with familial hypercholesterolemia or atherosclerotic cardiovascular disease.
- Although evidence has shown an effect on mortality and primary cardiovascular disease prevention for PCSK9 inhibitors, the cost-effectiveness of these novel therapies has been debated due to the drugs’ high price.
- What does the paper add to existing knowledge?
- We add to the literature regarding the uncertain long-term economic value of PCSK9 inhibitors by estimating the benefits and costs of their utilization by the FDA-approved patient population, accounting for the uncertainty of the clinical effectiveness of PCSK9 inhibitors.
- In addition to FDA-approved use, our study estimates the potential value of PCSK9 inhibitors as a primary prevention therapy for cardiovascular disease.
- What insights does the paper provide for informing healthcare-related decision making?
- This study estimates the potential gains to society if all patients currently indicated to use PCSK9 inhibitors had access to them at discounted prices. Our estimates suggest greater access would lead to substantial net benefits to society.
Acknowledgments
The authors are grateful to the National Institute on Aging for its support through the Roybal Center for Health Policy Simulation (grant no. P30AG024968). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors acknowledge support from Alwyn Cassil of Policy Translation LLC in drafting this article.
References
- 1.The global burden of disease: 2004 update. [Accessed December 15, 2016];2006 Available from: http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf?ua=1.
- 2.WHO. Prevention of cardiovascular disease: guidelines for assessment and management of cardivascular risk. Geneva: World Health Organization 2007; [Accessed December 01, 2016]. Available from: http://www.who.int/cardiovascular_diseases/guidelines/PocketGL.ENGLISH.AFR-D-E.rev1.pdf. [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 4.Weintraub WS, Daniels SR, Burke LE, et al. Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation. 2011;124:967–90. doi: 10.1161/CIR.0b013e3182285a81. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–44. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 6.Boekholdt SM, Arsenault BJ, Mora S, et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. Jama. 2012;307:1302–9. doi: 10.1001/jama.2012.366. [DOI] [PubMed] [Google Scholar]
- 7.Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circulation Cardiovascular quality and outcomes. 2011;4:337–45. doi: 10.1161/CIRCOUTCOMES.110.959247. [DOI] [PubMed] [Google Scholar]
- 8.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63:2889–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Guideline N. [Accessed December 15, 2016];Cardiovascular disease: risk assessment and reduction, including lipid modification. Available from: https://www.nice.org.uk/guidance/CG181.
- 10.Rodriguez F, Olufade T, Heithoff K, et al. Frequency of high-risk patients not receiving high-potency statin (from a large managed care database) The American journal of cardiology. 2015;115:190–5. doi: 10.1016/j.amjcard.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Mann D, Reynolds K, Smith D, et al. Trends in statin use and low-density lipoprotein cholesterol levels among US adults: impact of the 2001 National Cholesterol Education Program guidelines. The Annals of pharmacotherapy. 2008;42:1208–15. doi: 10.1345/aph.1L181. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Annals of internal medicine. 2013;158:526–34. doi: 10.7326/0003-4819-158-7-201304020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glueck CJ, Budhani SB, Masineni SS, et al. Vitamin D deficiency, myositis-myalgia, and reversible statin intolerance. Current medical research and opinion. 2011;27:1683–90. doi: 10.1185/03007995.2011.598144. [DOI] [PubMed] [Google Scholar]
- 14.Hsia SH, Desnoyers ML, Lee ML. Differences in cholesterol management among states in relation to health insurance and race/ethnicity across the United States. Journal of clinical lipidology. 2013;7:675–82. doi: 10.1016/j.jacl.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waters DD, Brotons C, Chiang CW, et al. Lipid treatment assessment project 2: a multinational survey to evaluate the proportion of patients achieving low-density lipoprotein cholesterol goals. Circulation. 2009;120:28–34. doi: 10.1161/CIRCULATIONAHA.108.838466. [DOI] [PubMed] [Google Scholar]
- 16.Mozaffarian D, Benjamin EJ, Go AS, et al. Executive Summary: Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:447–54. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 17.Agabiti Rosei E, Salvetti M. Management of Hypercholesterolemia, Appropriateness of Therapeutic Approaches and New Drugs in Patients with High Cardiovascular Risk. High blood pressure & cardiovascular prevention : the official journal of the Italian Society of Hypertension. 2016;23:217–30. doi: 10.1007/s40292-016-0155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gouni-Berthold I. PCSK9 antibodies: A new class of lipid-lowering drugs. Atherosclerosis Supplements. 2015;18:21–7. doi: 10.1016/j.atherosclerosissup.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Lin L, Zhang W, et al. Efficiency and safety of proprotein convertase subtilisin/kexin 9 monoclonal antibody on hypercholesterolemia: a meta-analysis of 20 randomized controlled trials. Journal of the American Heart Association. 2015;4:e001937. doi: 10.1161/JAHA.115.001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. [Accessed September 15 2016];Amgen. Repatha (evolocumab) Package Insert. Available from: http://pi.amgen.com/united_states/repatha/repatha_pi_hcp_english.pdf.
- 21.Navarese EP, Kolodziejczak M, Schulze V, et al. Effects of Proprotein Convertase Subtilisin/Kexin Type 9 Antibodies in Adults With Hypercholesterolemia: A Systematic Review and Meta-analysis. Annals of internal medicine. 2015;163:40–51. doi: 10.7326/M14-2957. [DOI] [PubMed] [Google Scholar]
- 22.Lipinski MJ, Benedetto U, Escarcega RO, et al. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. European heart journal. 2016;37:536–45. doi: 10.1093/eurheartj/ehv563. [DOI] [PubMed] [Google Scholar]
- 23.Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. The New England journal of medicine. 2015;372:1489–99. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 24.Glueck CJ, Shah P, Goldenberg N, et al. Eligibility for PCSK9 treatment in 734 Hypercholesterolemic patients referred to a regional cholesterol treatment center with LDL cholesterol >/= 70 mg/dl despite maximal tolerated cholesterol lowering therapy. Lipids in health and disease. 2016;15:55. doi: 10.1186/s12944-016-0227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 Inhibitor Therapy in Patients With Heterozygous Familial Hypercholesterolemia or Atherosclerotic Cardiovascular Disease. Jama. 2016;316:743–53. doi: 10.1001/jama.2016.11004. [DOI] [PubMed] [Google Scholar]
- 26.Weintraub WS, Gidding SS. PCSK9 Inhibitors: A Technology Worth Paying For? PharmacoEconomics. 2016;34:217–20. doi: 10.1007/s40273-015-0355-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehr SR. Will the PCSK9 Inhibitors Be Employers' "Line in the Sand"? American health & drug benefits. 2016;9:171–4. [PMC free article] [PubMed] [Google Scholar]
- 28.Lyons K. [Accessed October 30, 2016];High Cholesterol PCSK9 Drugs Could be Next To Bust Budgets, Stretch Pocketbooks. 2015 Available from: http://www.prnewswire.com/news-releases/high-cholesterol-pcsk9-drugs-could-be-next-to-bust-budgets-stretch-pocketbooks-300096091.html.
- 29.Colhoun HM, Robinson JG, Farnier M, et al. Efficacy and safety of alirocumab, a fully human PCSK9 monoclonal antibody, in high cardiovascular risk patients with poorly controlled hypercholesterolemia on maximally tolerated doses of statins: rationale and design of the ODYSSEY COMBO I and II trials. BMC cardiovascular disorders. 2014;14:121. doi: 10.1186/1471-2261-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kereiakes DJ, Robinson JG, Cannon CP, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: The ODYSSEY COMBO I study. American heart journal. 2015;169:906–15. e13. doi: 10.1016/j.ahj.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Roth EM, Taskinen MR, Ginsberg HN, et al. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized Phase 3 trial. International journal of cardiology. 2014;176:55–61. doi: 10.1016/j.ijcard.2014.06.049. [DOI] [PubMed] [Google Scholar]
- 32.Goldman DP, Shang B, Bhattacharya J, et al. Consequences of health trends and medical innovation for the future elderly. Health affairs (Project Hope) 2005;24(Suppl 2):W5R5–17. doi: 10.1377/hlthaff.w5.r5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaudette E, Goldman DP, Messali A, et al. Do Statins Reduce the Health and Health Care Costs of Obesity? PharmacoEconomics. 2015;33:723–34. doi: 10.1007/s40273-014-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agus D, Gaudette É, Messali A, et al. The long-term benefits of increased aspirin use by at-risk Americans aged 50 and older. PLOS ONE. 2016 doi: 10.1371/journal.pone.0166103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldman DP, Gaudette E, Cheng WH. Competing Risks: Investing in Sickness Rather Than Health. American journal of preventive medicine. 2016;50:S45–50. doi: 10.1016/j.amepre.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Goldman DP, Cutler D, Rowe JW, et al. Substantial health and economic returns from delayed aging may warrant a new focus for medical research. Health affairs (Project Hope) 2013;32:1698–705. doi: 10.1377/hlthaff.2013.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vidyanti IB-DR. Proceedings of the 2015 Winter Simulation Conference. Huntington Beach, CA: 2015. Projecting long-term impact of modest sodium reduction in Los Angeles County. [Google Scholar]
- 38.Hurd MZY, Girosi F, Goldman DP. In: The Effects of Tobacco Control Policy on the Social Security Trust Fund, in After tobacco : what would happen if Americans stopped smoking? Bearman KMN PS, Wright L, editors. Columbia University Press; New York: 2011. [Google Scholar]
- 39.Lakdawalla DN, Goldman DP, Michaud PC, et al. U.S. pharmaceutical policy in a global marketplace. Health affairs (Project Hope) 2009;28:w138–50. doi: 10.1377/hlthaff.28.1.w138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dolan P, Roberts J. Modelling valuations for Eq-5d health states: an alternative model using differences in valuations. Medical care. 2002;40:442–6. doi: 10.1097/00005650-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Gidding SS, Champagne MA, de Ferranti SD, et al. The Agenda for Familial Hypercholesterolemia: A Scientific Statement From the American Heart Association. Circulation. 2015;132:2167–92. doi: 10.1161/CIR.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 42.Singh S, Bittner V. Familial hypercholesterolemia--epidemiology, diagnosis, and screening. Current atherosclerosis reports. 2015;17:482. doi: 10.1007/s11883-014-0482-5. [DOI] [PubMed] [Google Scholar]
- 43.Lauer MS. PCSK9 Inhibitors: Lots of Work Done, Lots More to Do. Annals of internal medicine. 2016;164:624–5. doi: 10.7326/M16-0422. [DOI] [PubMed] [Google Scholar]
- 44.FDA. [Accessed October 10 2016];FDA Briefing Document: Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) 2015 Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM450072.pdf.
- 45.Matza LS, Cong Z, Chung K, et al. Utilities associated with subcutaneous injections and intravenous infusions for treatment of patients with bone metastases. Patient preference and adherence. 2013;7:855–65. doi: 10.2147/PPA.S44947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Truven Health Analytics. [Accessed September 15, 2016];Red Book Online. Available from: http://www.redbook.com/redbook/about.
- 47.Grabowski HGVJ. Brand loyalty, entry, and price competition in pharmaceuticals after the 1984 Drug Act. J Law Econ. 1992;35 [Google Scholar]
- 48.Tirrell M. [Accessed November 30, 2016];Pricing wars heat up over hepatitis C drugs. 2015 Available from: http://www.cnbc.com/id/102396903.
- 49.Conti RMBE. [Accessed September 15, 2016];Specialty drugs prices and utilization after loss of U.S. patent exclusivity, 2001–2007. 2014 Available from: http://www.nber.org/papers/w20016.
- 50.Edejer TTBR, Adam T. Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis. Geneva, Switzerland: World Health Organization; 2003. [Accessed August 10, 2016]. Available from: http://www.who.int/choice/publications/p_2003_generalised_cea.pdf. [Google Scholar]
- 51.Kasmeridis C, Apostolakis S, Ehlers L, et al. Cost effectiveness of treatments for stroke prevention in atrial fibrillation: focus on the novel oral anticoagulants. PharmacoEconomics. 2013;31:971–80. doi: 10.1007/s40273-013-0090-1. [DOI] [PubMed] [Google Scholar]
- 52.Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Annals of internal medicine. 2015;162:407–19. doi: 10.7326/M14-1152. [DOI] [PubMed] [Google Scholar]
- 53.Chhatwal J, Kanwal F, Roberts MS, et al. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162:397–406. doi: 10.7326/M14-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shrank WLA, Singh S, Brennan T. In the debate about cost and efficacy, PCSK9 inhibitors may be the biggest challenge yet. [Accessed November 01, 2016];Health Affairs Blog. 2015 Available from: http://healthaffairs.org/blog/2015/02/17/in-the-debate-about-cost-and-efficacy-pcsk9-inhibitors-may-be-the-biggest-challenge-yet/
- 55.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. The New England journal of medicine. 2017;376:1713–22. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 56.Toth PP, Stevens W, Chou JW. Why have published studies of the cost effectiveness of PCSK-9 inhibitors yielded such markedly different results. Journal of medical economics. 2017:1–6. doi: 10.1080/13696998.2017.1327440. [DOI] [PubMed] [Google Scholar]
- 57.Hirsh BJ, Smilowitz NR, Rosenson RS, et al. Utilization of and Adherence to Guideline-Recommended Lipid-Lowering Therapy After Acute Coronary Syndrome: Opportunities for Improvement. Journal of the American College of Cardiology. 2015;66:184–92. doi: 10.1016/j.jacc.2015.05.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.