Introduction
The skin barrier is an important component of human health. Subsequently, impaired healing of the skin is a notable cause of patient morbidity and even mortality. In the United States, chronic wounds affect more than 6.5 million patients and 1–2% of the US population will experience a chronic wound in their lifetime.1 Restoring functional wound healing or influencing the wound-healing process to act in a regenerative fashion therefore has multiple benefits relating to quality of life and health care cost.
Although not completely understood, the importance of the immune system in wound healing has been well established. Wound healing is divided into 4 phases: hemostasis, inflammation, proliferation, and remodeling. These phases, the wound-healing cascade, are controlled by a complex set of signals from the wound and its microenvironment. Hemostasis is controlled by reflex vasoconstriction and the components of the coagulation cascade, resulting in a clot and the release of pro-inflammatory cytokines. Inflammation, also triggered directly by tissue injury, is important in readying the wound for closure. It is regulated by cell signaling molecules and is characterized by classic clinical observations—rubor, tumor, calor, and dolor—as well as a predictable pattern of infiltration from different immune cell types.2 The cell signaling molecules released, namely, cytokines, chemokines, and growth factors, are released by both resident cells as well as newly arrived leukocytes. 3 The proliferative phase results in wound closure and is followed by remodeling, during which the components of the closed wound are turned over and the tensile strength of the scar is increased. 4 The events of the inflammatory phase have a profound effect on final wound outcome, such that an abnormal inflammatory response will result in abnormal wound healing 3,5 Therefore, molecules that regulate the inflammatory phase of the wound-healing cascade are potential therapeutic targets for improved wound healing. One of these molecules is Interleukin-1 Receptor Antagonist (IL-1Ra).
IL-1Ra is a potent regulator of Interleukin-1 (IL-1) and therefore modulates a variety of inflammatory events. In fact, IL-1Ra (Anakinra; Amgen Corp., Thousand Oaks, CA) has been approved by the FDA for treatment of Rheumatoid Arthritis and Neonatal-Onset Multisystem Inflammatory Disease. The role of IL-1Ra as a regulator of inflammation suggests that it may be useful in therapies to improve wound healing. In addition, a few studies have shown that the disruption of IL-1 signaling can improve wound healing and reduce scaring.6,7 Little is known, however, about the local use of IL-1Ra in cutaneous wound healing. Therefore, the aim of this study was to determine the effect of locally administered IL-1Ra on wound healing in a diabetic mouse model.
Methods
Excisional Wound Model
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Southern California (protocol ID 20168). A splinted excisional wound model was used to more closely simulate human wound healing, specifically, to promote healing by re-epithelialization and granulation tissue formation.9 Male diabetic mice (db/db; BKS.Cg-m +/+ Leprdb; Jackson Laboratories, Bar Harbor, ME) were used. Mice were 14–17 weeks old at time of experiment and weighed 51–60 grams. Mice were anesthetized using continuous nasal administration of Isoflurane (1–4% in Oxygen) and kept on a heating pad for the duration of the procedure. The dorsum of each mouse was shaved with an electric clipper, rinsed with alcohol, and sterilely prepped with providone-iodine and draped. A sterile 6 mm punch biopsy was used to create two full thickness wounds on each experimental animal (n=7). Wounds were stented with 7 mm circular Silicone Wound Splints (Grace Bio-Labs, Bend, Oregon) and the splints were secured with Krazy Glue® (Elmer’s Inc., Columbus, OH) and interrupted 6-0 prolene sutures (Ethicon, Inc., Somerville, NJ). One-hour post wounding, wound margins were subcutaneously injected in a circumferential fashion with 70μl of either (1) low dose IL-1Ra (5μl anakinra, 0.75mg IL-1Ra) in a 3% gelatin-transglutaminase (TGase) gel vehicle or (2) TGase gel only. On each animal, one wound was treated with IL-1Ra and the other with the gel vehicle to serve as the control carrier. An optimal dose for localized application of IL1-Ra for wound healing has not yet been well described. The dose used in this study was chosen based on unpublished work from a collaborator studying the effect of IL-1Ra on non-diabetic wound healing. The TGase gel vehicle was used to maximize the local half-time of IL-1Ra.8 Wounds were then digitally imaged and dressed with Telfa (Covidien, Minneapolis, MN), Tegaderm (3M, St. Paul, MN), and Coban (3M, St. Paul, MN) to deter gnawing/scratching of wounds. Dressings were changed every 7 days or if they were found to be soiled or not intact on daily examination. Animals were allowed to recover, then housed individually to prevent wound disturbance. Animal care was performed by the institutional animal care facility. Biopsies were taken of the wound area and the surrounding skin on day 21, at which point the animals were euthanized with intraperitoneal pentobarbital (100–200 mg/kg) followed by cervical dislocation. Tissue specimens were fixed in 4% formaldehyde for 24 hours and embedded in paraffin. Paraffin sections were stained with hematoxylin and eosin or used for immunohistochemical analysis.
Wound Imaging and analysis
Wounds were digitally imaged directly after stent placement and then every 7 days until cessation of the experiment on day 21. The 7 day time interval between wound imaging was chosen to minimize manipulation of the wound upon dressing removal. Wound healing studies with shorter intervals between wound area quantification have already been reported.6 A Nikon D3000 with a Canfield Imaging System was used to capture standardized photographs. Wound areas were traced manually in an image analysis program (ImageJ v10.2, NIH) and splint diameter was used to calibrate measurements. Percent of original size of each wound was calculated and data were analyzed and plotted in Prism 6 (Graphpad Software Inc.).
Immunohistochemistry
Anti-mMPO antibodies (AF3667, R&D Systems, Minneapolis, MN) were used to stain for neutrophils (diluted 1:500). Secondary anti-goat antibodies were used from the VECTASTAIN Elite ABC kit (Goat IgG). Anti-F4/80 Antibodies (ab6640, Abcam) were used to assess for macrophages (diluted 1:500). Anti-Rat IgG (H+L) (Vector Laboratories) was used as the secondary antibody (diluted 1:500). The VECTASTAIN Elite ABC kit was used to control background stain in all IHC preparations. Dilutions were made with 5% mouse serum in PBS for Anti-MPO and Secondary Anti-goat IgG and with 1% mouse serum in PBS for Anti-F4/80 and Anti-Rat IgG. Prepared tissue specimens were imaged with a Carl Zeiss Axio Scope and an Olympus DP72 camera. Five photographs were taken of each specimen and the number of macrophages and neutrophils in each image were independently scored by two blinded reviewers. Data was analyzed and plotted in Prism 6 (Graphpad Software Inc.).
Statistical Analysis
Statistical analysis was performed in Prism 6. Wound closure data were analyzed by multiple t-tests and multiple comparisons were corrected for with the Holm-Sidak method. IHC data were analyzed with unpaired-t tests. A probability (p) value <0.05 was considered significant. All values are expressed as mean + SEM.
Results
IL-1Ra therapy decreases time to closure in splinted diabetic wounds
Diabetes leads to abnormal wound healing in part due to immune cell dysfunction. As such, we wished to determine the effect of local administration of the immunomodulatory agent IL1-Ra on diabetic wounds using the well established db/db mouse model. IL-1Ra treated wounds healed at a significantly greater rate than placebo treated wounds. Specifically, IL1-Ra treated wounds had significantly reduced wound area compared to placebo treated wounds, at 7 days and 14 days (p<0.02 and p<0.03, respectively). Although this trend continued at 21 days, the difference between IL-1Ra and placebo treated wounds did not reach statistical significance (p=0.13). (Figures 1 and 2)
Figure 1.
IL-1Ra treated wounds healed faster than placebo treated wounds. Photographs of full-thickness skin wounds treated with either IL-1Ra or the gel vehicle. Two wounds were created in each animal, one wound was treated with IL-1Ra and the other with the placebo. Wounds were then photographed every seven days until wound closure and compared. Inside diameter of the splint is 7mm. Images of one representative experiment is shown.
Figure 2.
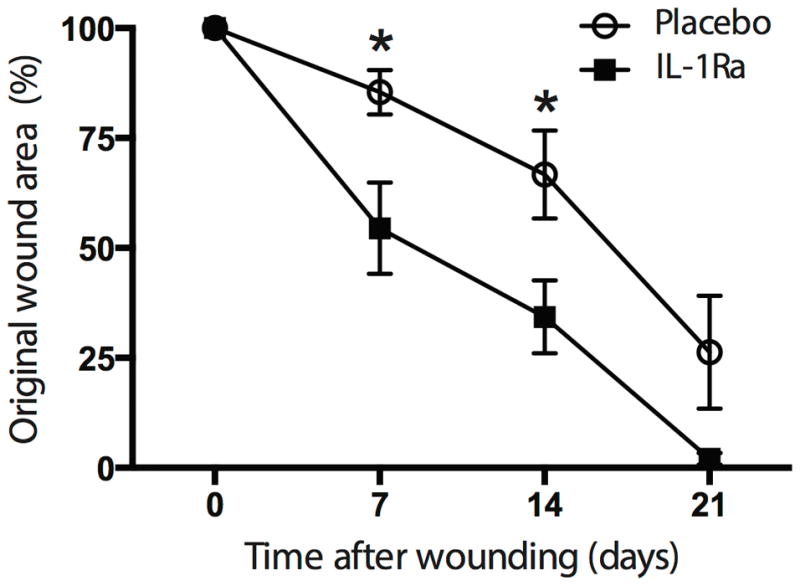
Wound area of IL-1Ra treated wounds (n=7) was significantly reduced compared to placebo treated wounds on days 7 (p<0.02) and 14 (p<0.03). There reduction in wound area on day 21 was not significantly reduced (p=0.13). Photographs of wound areas were quantified in image J. Data reported as mean ± SEM
IL-1Ra therapy is associated with significantly less inflammation in diabetic wounds
Given the role of IL-1Ra in regulating local inflammation via IL-1, we were interested in characterizing changes in the inflammatory infiltrate after treatment. Neutrophils were identified by staining for myeloperoxidase (MPO) and macrophages were identified by staining for anti-F4/80. There was significantly less neutrophil (p<0.001) and macrophage infiltration (p<0.02) in the IL-1Ra treated wounds compared to the placebo treated wounds. (Figures 3, 4, 5, and 6)
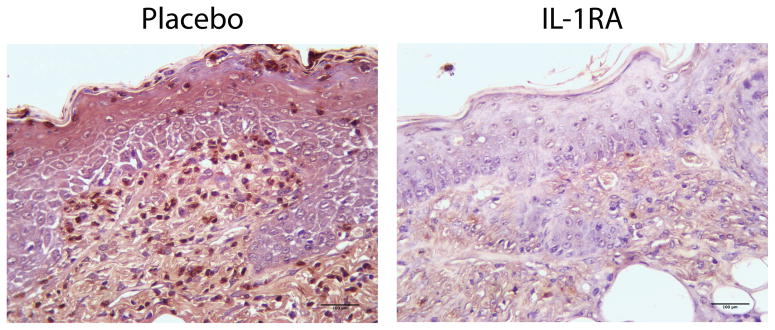
Figure 3.
IL-1Ra treated wounds showed less neutrophil infiltration on day 21 post wounding. Tissue specimens were collected on day 21 post wounding, formalin fixed, paraffin embedded, and immunohistochemically stained for myeloperoxidase, a neutrophil cell marker.
Figure 4.
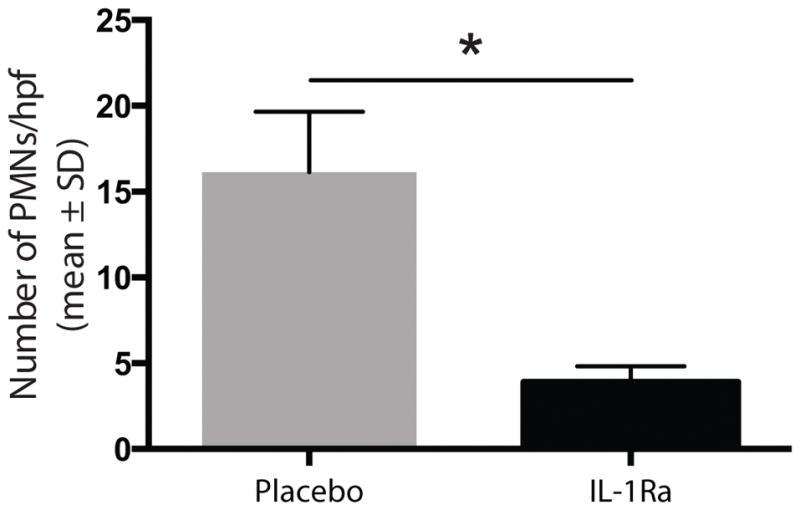
IL-1Ra treated wounds showed significantly less neutrophil (p<0.001) infiltration. Five high power fields for each specimen were scored by hand for neutrophil number.
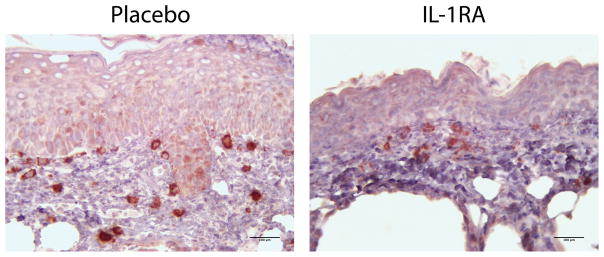
Figure 5.
IL-1Ra treated wounds showed less macrophage infiltration on day 21 post wounding. Tissue specimens were collected on day 21 post wounding, formalin fixed, paraffin embedded, and immunohistochemically stained for F4/80, a macrophage cell marker.
Figure 6.
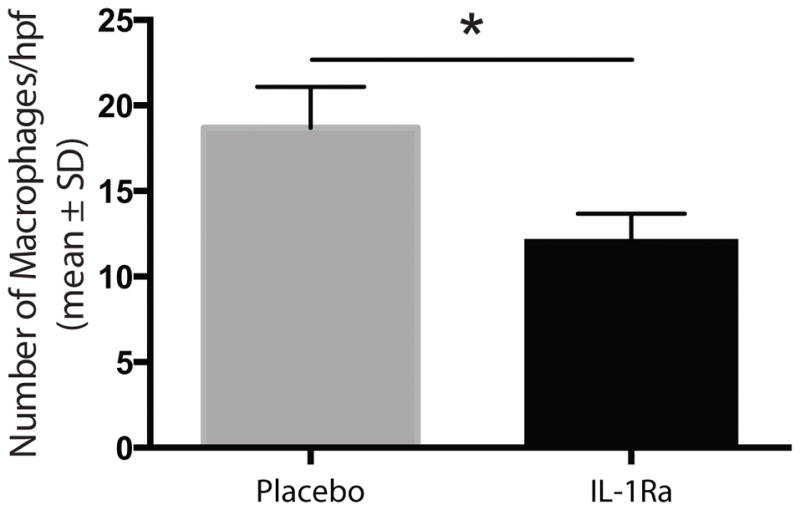
Treated wounds showed significantly less macrophage (p<0.02) infiltration. Five high power fields for each specimen were scored by hand for macrophages number.
Discussion
Wound Model Rationale
A diabetic mouse model was chosen for two reasons. Firstly, diabetes is common and affects more than 23 million people in the US and 25% of all diabetics will develop foot ulcers. More so, 67% of all lower extremity amputations are seen in patients with diabetes. 1 Secondly, diabetic wounds are a prime example of impaired healing resulting from aberrations in multiple processes. Examples include vascular disease, impaired granulocytic function, prolonged inflammation, defective neovascularization, decreased collagen synthesis, increased proteinases, and impaired macrophage function.10
IL-1Ra and Inflammation
IL-1Ra is a naturally occurring protein that regulates inflammation by inhibiting receptors of IL-1α and IL-1β, collectively referred to as IL-1. IL-1 is a cytokine involved in a range of activities important in immunity, injury, and inflammation.11 These include induction of cytokine and growth factor release, expression of endothelial adhesion molecules, PMN and Macrophage chemotaxis, and keratinocyte mitogenesis, migration, and secretion of pro-inflammatory cytokines.12 The integral involvement of IL-1 in immune system function suggests that IL-1Ra has a similarly important role. This is clinically evident by the use of IL-1Ra for treatment of Rheumatoid Arthritis and Neonatal-Onset Multisystem Inflammatory Disease, both disorders of dysfunctional inflammation. In addition, IL-1Ra is currently being studied in a range of other inflammatory conditions including the neuroinflammatory response in traumatic brain injury, hypersensitivity reactions, and tissue remodeling after myocardial infarction.13,14,15 Ultimately, the role of IL-1Ra in inflammation is good cause to consider it as a possible therapy for non-healing wounds in addition to the other mentioned diseases.
IL-1Ra and Wound Healing
An abnormal inflammatory response results in abnormal wound healing. Although inflammation is a necessary component of healing, persistent inflammation will result in delayed wound closure. 4 Therefore, pro-inflammatory cytokines play an important role in wound repair, one example being they are greatly upregulated during the inflammatory phase of wound healing.16 Of the pro-inflammatory cytokines, IL-1 is the first detectable upregulated cytokine after wound creation.17 Neutrophils, macrophages, and resident cells all contribute to the expression of IL-1.16 A variety of evidence already exists supporting the role of IL-1 signaling in wound healing. For example, impaired IL-1 signaling has been shown to accelerate wound healing in a mouse model.6,7 In contrast, unopposed IL-1 signaling in mice deficient in IL-1Ra have delayed wound healing.18 IL-1Ra, therefore, appears necessary to successful healing in vivo. More so, IL-1Ra has a role in the improved healing observed in human oral mucosa and the beneficial effect of mesenchymal skin cells (MSCs) on cutaneous wounds. IL-1Ra is upregulated in the MSCs derived from human oral mucosa, a tissue that heals faster and with less scaring than skin (results not published). For these reasons, IL-1Ra is of particular interest regarding inhibition of IL-1 signaling to improve healing.
In the context of chronic wounds, IL-1Ra may be beneficial across a range of chronic wound types. Chronic wounds can result from any disruption in the normal wound healing cascade. Therefore, a wide variety of conditions are associated with abnormal wound healing. In general, poorly healing states are propagated by a combination of multiple physiologic and biochemical insults to the wound healing process which tend to be specific to the particular condition. Common features seen across the range of chronic wound types would be the ideal targets for improving wound healing, however, they have been difficult to identify.19 It has been suggested that persistent inflammation is a hallmark of chronic wounds, therefore, modulation of the inflammatory process may be an ideal target for multiple chronic wound types.19 For example, with a drug such as IL-1Ra. However, given the diversity of conditions associated with chronic wounds, a disease specific approach to therapy would clearly be the most efficacious option. Consistent with this concept, as will be described in greater detail below, we believe that in addition to being a potential therapy for multiple chronic wound types, IL-1Ra may be particularly effective for diabetic wounds given the specific role of macrophage dysfunction and the IL-1 pathway in these wounds.
Results and Significance
The experiments reported were performed to assess the possible role of locally delivered IL-1Ra in the treatment of non-healing wounds. In these experiments, wounds treated with IL-1Ra healed at a faster rate than the wounds treated with placebo. Wounds treated with IL-1Ra also demonstrated less neutrophil and macrophage infiltrate on biopsy, 21 days after wounding. These results are consistent with the previously mentioned studies regarding wound healing. In addition, these findings suggest that a disruption in IL-1 signaling shortly after wounding can have significant effects on final wound outcome.
IL-1Ra treated wounds demonstrated accelerated healing and decreased neutrophil infiltration. Therefore, we propose that IL-1Ra accelerates the healing process by limiting the initial inflammatory burden in the wound. IL-1Ra does this by inhibiting the IL-1 mediated expression of Intercellular Adhesion Molecules (ICAMs) on vascular endothelium, thereby inhibiting neutrophil chemotaxis.20 Although neutrophils are an important defense against microbial infection, the toxic products that are produced by the neutrophil to kill phagocytosed microorganisms can escape the cell and damage surrounding tissue.20 This would exacerbate the disease process. Therefore, less neutrophilic infiltrate translates to less tissue damage and improved healing. Note that it has been previously shown that neutrophil depletion accelerates healing, likewise, that neutrophils are deleterious to wound closure.21
Decreasing the inflammatory burden in the wound area does more than just prevent further tissue damage from neutrophil products; it will also decrease the phagocytic load for macrophages. In normal wound healing, neutrophils are the first cells to arrive to the site of injury. After performing their function, the cells will subsequently undergo apoptosis. Macrophages will then infiltrate the tissue and peak in number around 3–5 days after tissue injury. A primary function of these macrophages is to remove apoptotic neutrophils and necrotic debris. If this debris is not removed, pro-inflammatory cytokines remain elevated, inflammation is prolonged, and healing is delayed. 22 Therefore, by decreasing the number apoptotic neutrophils needed to be cleared, inflammation should resolve more quickly and healing should be accelerated. It should also be noted that one of the characteristics of non-healing wounds in diabetic individuals is the presence of increased apoptotic cell load. This is a result of impaired apoptotic cell clearance by macrophages.20 Therefore, it is reasonable to conclude that in diabetic mice, treatment with IL-1Ra would be especially therapeutic, as observed in our experiments.
IL-1Ra treated wounds also demonstrated less macrophage infiltration on day 21 post wounding. We propose that this observation is directly related to the how far the wound has progressed in the wound-healing cascade. In other words, that treated wounds are at a more mature stage of healing and, resultantly, display fewer macrophages. We do not believe that the decreased number of macrophages is a direct effect IL-1Ra on macrophage infiltration. In contrast to the almost instant response of neutrophils to injury, macrophages are not a predominant cell type until 3–5 days after injury. The effects of IL-1Ra on macrophage chemotaxis could be limited by the half-life of locally administered IL-1Ra. It should be noted, however, that IL-1β signaling has been shown to contribute to a persistent pro-inflammatory wound macrophage phenotype in diabetic wounds.7 Furthermore, these authors showed that inhibition of IL-1β signaling was sufficient to induce a macrophage phenotype switch from a pro-inflammatory to a healing-associated state, which was associated with improved healing in diabetic mice. This suggests that IL-1Ra mediated IL-1 inhibition may likewise have a positive impact on macrophage phenotype, and therefore be a prime topic for further study.
It is of interest that a single dose of IL-1Ra one-hour post wounding resulted in improved wound closure and decreased inflammation 21 days after wound creation and treatment. This highlights the importance of the events in the inflammatory phase of healing for final wound outcome. The large impact of a single dose may be explained by the previously described mechanism. Specifically, that by decreasing the initial burden of inflammatory cells there is less neutrophil mediated tissue damage and less apoptotic neutrophils left for macrophages to clear.
The limitations of this study include a small sample size and the relatively wide interval between wound photography. No change in behavior or cutaneous side effects of IL-1Ra were noted in our animals. In humans, systemic use of the medication does increase the risk of injection site reaction, infection, headache, nausea, and diarrhea.23
In conclusion, our findings support the hypothesis that IL-1Ra may have an important role in cutaneous wound healing, by promoting successful resolution of acute inflammation leading to accelerated wound closure. Since IL-1Ra is already an FDA approved drug, our results have significant impact since it could easily be repurposed to treat non-healing wounds in patients with diabetes or other known co-morbid risk factors. Our future work includes confirmation of our findings in a non-rodent wound healing model such as mini-pig. Although more data on minimal effective dose, optimal timing, and mechanism of action will be required prior to human clinical trials, IL-1Ra has the potential to be translated into a therapy that will augment our current arsenal of agents against refractory wounds.
Acknowledgments
We thank Lillian Young for histology advice and Bo Han for providing transglutaminase gel.
Footnotes
Conflict of Interest disclosure statement:
None
Abbreviations and Other Footnotes
None
References
- 1.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair and Regeneration : Official Publication of the Wound Healing Society [and] the European Tissue Repair Society. 2009;17(6):763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross R, Odland G. Human wound repair. II. inflammatory cells, epithelial-mesenchymal interrelations, and fibrogenesis. The Journal of Cell Biology. 1968;39(1):152–168. doi: 10.1083/jcb.39.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strbo N, Yin N, Stojadinovic O. Innate and adaptive immune responses in wound epithelialization. Advances in Wound Care. 2014;3(7):492–501. doi: 10.1089/wound.2012.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neligan Peter. Plastic surgery. London: Elsevier Saunders; 2013. [Google Scholar]
- 5.Koh TJ, DiPietro LA. Inflammation and wound healing: The role of the macrophage. Expert Reviews in Molecular Medicine. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomay AA, Daley JM, Sabo E, Worth PJ, Shelton LJ, Harty MW, et al. Disruption of interleukin-1 signaling improves the quality of wound healing. The American Journal of Pathology. 2009;174(6):2129–2136. doi: 10.2353/ajpath.2009.080765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirza RE, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin-1beta induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013;62(7):2579–2587. doi: 10.2337/db12-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang JY, Tan SJ, Yang Z, Tayag C, Han B. Tumor bioengineering using a transglutaminase crosslinked hydrogel. PloS one. 2014;9(8):e105616. doi: 10.1371/journal.pone.0105616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galiano RD, Michaels J5, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair and Regeneration : Official Publication of the Wound Healing Society [and] the European Tissue Repair Society. 2004;12(4):485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 10.Singer AJ, Clark RA. Cutaneous wound healing. The New England Journal of Medicine. 1999;341(10):738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 11.Krumm B, Xiang Y, Deng J. Structural biology of the IL-1 superfamily: Key cytokines in the regulation of immune and inflammatory responses. Protein Science : A Publication of the Protein Society. 2014;23(5):526–538. doi: 10.1002/pro.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bos JD, Luiten RM. Skin Immune System. In: Stockfleth E, Ulrich C, editors. Skin Cancer after Organ Transplantation. Boston, MA: Springer US; 2009. pp. 45–62. [Google Scholar]
- 13.Helmy A, Guilfoyle MR, Carpenter KL, Pickard JD, Menon DK, Hutchinson PJ. Recombinant human interleukin-1 receptor antagonist in severe traumatic brain injury: A phase II randomized control trial. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34(5):845–851. doi: 10.1038/jcbfm.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen MM, Lovato P, MacLeod AS, Witherden DA, Skov L, Dyring-Andersen B, et al. IL-1beta-dependent activation of dendritic epidermal T cells in contact hypersensitivity. Journal of Immunology (Baltimore, Md: 1950) 2014;192(7):2975–2983. doi: 10.4049/jimmunol.1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbate A, Salloum FN, Vecile E, Das A, Hoke NN, Straino S, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117(20):2670–2683. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- 16.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiological Reviews. 2003;83(3):835–870. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- 17.Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77(8):1627–1652. [PubMed] [Google Scholar]
- 18.Ishida Y, Kondo T, Kimura A, Matsushima K, Mukaida N. Absence of IL-1 receptor antagonist impaired wound healing along with aberrant NF-kappaB activation and a reciprocal suppression of TGF-beta signal pathway. Journal of Immunology (Baltimore, Md: 1950) 2006;176(9):5598–5606. doi: 10.4049/jimmunol.176.9.5598. 176/9/5598 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Nunan R, Harding KG, Martin P. Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Disease Models & Mechanisms. 2014;7(11):1205–1213. doi: 10.1242/dmm.016782. http://doi.org/10.1242/dmm.016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klebanoff SJ. Myeloperoxidase: Friend and foe. Journal of Leukocyte Biology. 2005;77(5):598–625. doi: 10.1189/jlb.1204697. jlb.1204697 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. Journal of Leukocyte Biology. 2003;73(4):448–455. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 22.Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PloS One. 2010;5(3):e9539. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quartier P, Allantaz F, Cimaz R, Pillet P, Messiaen C, Bardin C, … Pascual V. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial) Annals of the Rheumatic Diseases. 2011;70(5):747–754. doi: 10.1136/ard.2010.134254. http://doi.org/10.1136/ard.2010.134254. [DOI] [PMC free article] [PubMed] [Google Scholar]