SUMMARY
Neuronal mechano-sensitivity relies on mechanogated ion channels, but pathways regulating their activity remain poorly understood. TMEM150C was proposed to mediate mechano-activated current in proprioceptive neurons. Here, we studied functional interaction of TMEM150C with mechano-gated ion channels from different classes (Piezo2, Piezo1, and the potassium channel TREK-1) using two independent methods of mechanical stimulation. We found that TMEM150C significantly prolongs the duration of the mechano-current produced by all three channels, decreases apparent activation threshold in Piezo2, and induces persistent current in Piezo1. We also show that TMEM150C is co-expressed with Piezo2 in trigeminal neurons, expanding its role beyond proprioceptors. Finally, we cloned TMEM150C from the trigeminal neurons of the tactile-foraging domestic duck and showed that it functions similarly to the mouse ortholog, demonstrating evolutionary conservation among vertebrates. Our studies reveal TMEM150C as a general regulator of mechano-gated ion channels from different classes.
In Brief
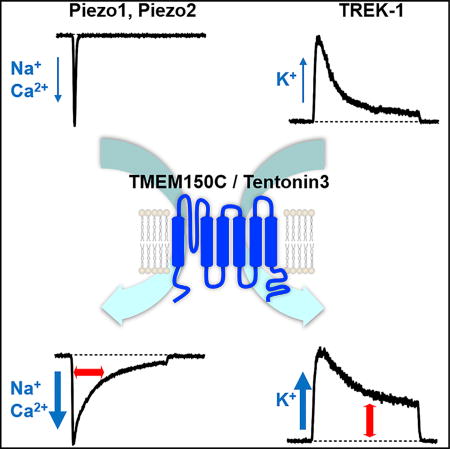
Mechano-gated ion channels are essential for somatosensation, proprioception, hearing, vasodilation, and axonal growth. Anderson et al. show that the transmembrane protein TMEM150C facilitates activity of mechano-gated ion channels from different classes: Piezo1/2 and the potassium-selective channel TREK-1. This study reveals a role for TMEM150C as an evolutionarily conserved regulator of mechano-sensitivity.
INTRODUCTION
Somatosensory ganglia of vertebrates contain various types of mechano-sensitive neurons, including low- and high-threshold mechano-receptors and proprioceptors. These neurons can convert mechanical stimulation into ionic current due to the presence of mechano-gated ion channels, including nonselective excitatory channels, such as by Piezo2, and potassium-selective inhibitory channels, such as TREK-1 (K2P2.1) (Alloui et al., 2006; Ranade et al., 2015). Activity of these channels is regulated through alternative splicing, interaction with the cytoskeleton, signaling pathways, and the lipid composition of the plasma membrane (Anderson et al., 2017; Borbiro and Rohacs, 2017; Murthy et al., 2017; Szczot et al., 2017). Despite recent advances, proteins and pathways involved in regulation of mechano-sensitivity remain poorly understood. Identification of novel mechano-gated ion channels and their modulators is essential for understanding mechano-sensitivity in somatic cells and neurons.
Piezo2 generates fast-inactivating mechano-evoked current (MA current) in neurons from trigeminal (TG) and dorsal root ganglia (DRG). The deletion of Piezo2 abrogates fast MA current in light touch mechano-receptors and proprioceptors without affecting mechano-sensitivity in neurons with intermediately and slowly inactivating MA currents (Ranade et al., 2014; Woo et al., 2015). TMEM150C/Tentonin3 was proposed to act as an ion channel mediating slowly inactivating MA current in proprioceptive neurons in mouse DRG (Hong et al., 2016). Recently, it was shown that heterologous expression of TMEM150C fails to generate MA current in cells with genomic ablation of the PIEZO1 gene (Dubin et al., 2017). Here, we tested the hypothesis that TMEM150C acts as a modulator of mechano-sensitivity, rather than an ion channel, by studying its effects on MA current in cells expressing bona fide mechano-gated channels from different classes: Piezo1, Piezo2, and the potassium-selective channel TREK-1.
RESULTS
TMEM150C Stimulates Piezo2 Mechano-current
TMEM150C has been reported to function in proprioceptors from mouse DRG (Hong et al., 2016), which also express Piezo2 (Florez-Paz et al., 2016; Woo et al., 2015). To test whether TMEM150C is co-expressed with Piezo2 in other types of somatosensory neurons, we analyzed distribution of TMEM150C in adult duck TG, a ganglion devoid of proprioceptors but rich in Piezo2-expressing mechano-receptors (84.5% of all neurons) (Schneider et al., 2017; Schneider et al., 2014). Using RNA in situ hybridization, we found that 83.3% ± 1.0% (19 TG sections, 2,808 total cells) of duck TG neurons express TMEM150C (Figures 1A and 1B). The abundance of TMEM150C and Piezo2-positive neurons in duck TG necessitates an overlap between the two neuronal populations, suggesting that TMEM150C is co-expressed with Piezo2 outside of proprioceptors.
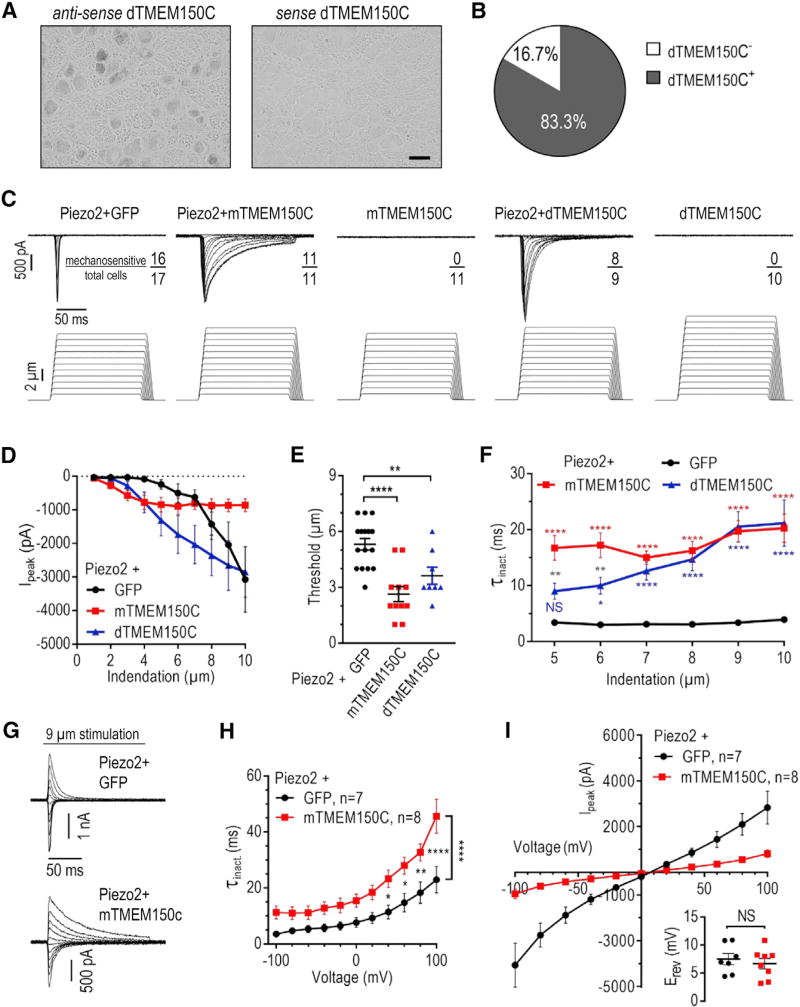
Figure 1. Mouse and Duck TMEM150C Potentiate Piezo2 Mechano-current in HEK293TΔP1 Cells.
(A and B) Representative images of RNA in situ hybridization (A) and quantification of TMEM150C-expressing neurons (B) in adult duck TG (2,808 cells from 19 TG sections from 2 animals). Scale bar, 50 µm.
(C) Exemplar whole-cell MA current traces recorded in HEK293TΔP1 cells expressing Piezo2 with or without mouse or duck TMEM150C in response to mechanical indentation with a glass probe to the indicated depth (Ehold = −80 mV).
(D) Peak MA current measured at different indentation depths in HEK293TΔP1 cells expressing indicated constructs (Ehold = −80 mV). Data shown as mean ± SEM.
(E) Quantification of MA current activation threshold (p < 0.0001, one-way ANOVA with Dunnett’s correction, **p < 0.001, ****p < 0.0001). Data shown as mean ± SEM.
(F) Quantification of MA current inactivation rate (τinact) measured at different indentation depths (ordinary two-way ANOVA with Bonferroni correction, p < 0.0001 for expression construct effect; NS, not significant; p > 0.05, *p < 0.05, **p < 0.01, ****p < 0.0001; red, blue, and gray asterisks indicate statistical comparisons between, respectively, Piezo2/mTMEM150C and Piezo2, Piezo2/dTMEM150C and Piezo2, and Piezo2/mTMEM150C and Piezo2/dTMEM150C). Data shown as mean ± SEM.
(G) Representative traces of whole-cell MA currents evoked in response to 9 µm mechanical indentation at different voltages from −100 mV to 100 mV, in 20 mV increments.
(H) Quantification of MA current τinact at different voltages (two-way ANOVA with Sidak’s correction, *p < 0.05, **p < 0.01, ****p < 0.0001). Data shown as mean ± SEM.
(I) Peak MA current-voltage plots in response to mechanical indentation of 5–10 µm for Piezo2/GFP and 4-9 µm for Piezo2/mTMEM150C. The inset shows quantification of the reversal potential Erev (unpaired t test; NS, not significant; p > 0.05). Data shown as mean ± SEM.
See also Figures S1, S3A, and S3B.
We and others have hypothesized that TMEM150C could act as a modulator of Piezo channel function (Dubin et al., 2017). To test this, we measured whole-cell MA current in cells co-expressing mouse TMEM150C (mTMEM150C) and mouse Piezo2 in response to mechanical stimulation with a glass probe (Hao and Delmas, 2011). To avoid potential confounding effects of endogenous MA current, we used HEK293T cells carrying a genomic ablation of the PIEZO1 gene (HEK293TΔP1) (Dubin et al., 2017; Lukacs et al., 2015). Co-expression of Piezo2 with GFP yielded typical fast-inactivating MA current, while the expression of mouse TMEM150C alone produced no mechanical response (Figures 1C, 1D, and S1A). However, co-expression of Piezo2 with TMEM150C produced a twofold decrease in the onset of detectable MA current (apparent activation threshold) from 5.3 ± 0.3 µm to 2.6 ± 0.4 µm (p = 0.0001, Dunnett’s test) (Figure 1E). Additionally, TMEM150C co-expression led to a fivefold prolongation in the rate of MA current inactivation (τinact) at all indentation depths that consistently produced MA current in both groups (5–10 µm), from 3.4–3.9 ms to 16.7–20.3 ms, respectively (two-way ANOVA, p < 0.0001 for expression construct effect) (Figure 1F). The effect did not depend on the amount of channel molecules on the surface, as we observed an increase in τinact independently of peak MA current amplitude (Figure S1B).
We also tested a TMEM150C ortholog cloned from TG of tactile specialist duck. Duck TMEM150C (dTMEM150C) is 87% identical to the mouse protein (Figure S1C). When expressed alone, duck TMEM150C did not confer mechano-sensitivity to HEK293TΔP1 cells (Figures 1C, 1D, and S1A). Upon co-expression with Piezo2, duck TMEM150C significantly decreased the apparent threshold of mechanical activation from 5.3 ± 0.3 µm to 3.6 ± 0.5 µm (p = 0.0098, Dunnett’s test) and prolonged τinact in the 5–10 µm indentation range from 3.4–3.9 ms to 9.0–21.2 ms (two-way ANOVA, p < 0.0001 for expression construct and voltage effects) (Figures 1E and 1F).
To understand whether the resultant MA current retained properties characteristic of Piezo2 alone, we analyzed voltage dependence of τinact. The inactivation rate of Piezo2 MA current increases with depolarization 6.4-fold, from 3.6 ± 0.6 ms at −100 mV to 23.0 ± 4.7 ms at 100 mV (two-way ANOVA, p < 0.0001 for voltage effect) (Coste et al., 2010; Wu et al., 2017b). We found a similarly significant fourfold increase in τinact in cells co-expressing Piezo2 and mouse TMEM150C, from 11.3 ± 2.3 ms at −100 mV to 45.7 ± 6.1 ms at 100 mV (two-way ANOVA, p < 0.0001 for voltage effect) (Figures 1G and 1H). Additionally, co-expression with TMEM150C did not change the reversal potential of the resultant MA current (Figures 1I and S1D).
Together, our observations show that MA current produced by co-expression of TMEM150C and Piezo2 has similar voltage dependence of inactivation and ion selectivity but significantly augmented activation threshold and inactivation kinetics compared to MA current produced by Piezo2 alone. Although these data do not exclude the possibility that TMEM150C is an ion channel active in the presence of another mechano-transducer (Hong et al., 2017), they suggest more strongly that TMEM150C is a positive modulator of Piezo2-mediated mechano-current. The similarity between the effects of duck and mouse TMEM150C suggest that the function of this protein is conserved among vertebrates.
TMEM150C Potentiates Piezo1 Mechano-current Independently of Stimulation Method
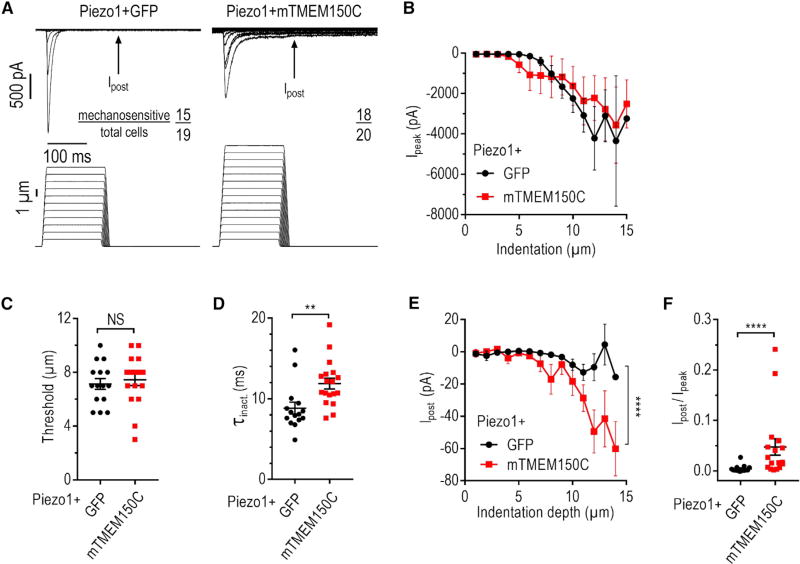
To test whether the observed effects are specific to Piezo2, we co-expressed TMEM150C with Piezo1, a homolog of Piezo2 that mediates mechano-responses in somatic cells (Murthy et al., 2017) and neural stem cells (Pathak et al., 2014) and guides axon growth in the developing brain (Koser et al., 2016). When expressed alone in HEK293TΔP1 cells, mouse Piezo1 produces fast-inactivating MA current (Figures 2A, 2B, and S2A). Co-expression with mouse TMEM150C did not change the apparent activation threshold (7.1 ± 0.4 µm and 7.4 ± 0.4 µm for Piezo1 alone and with TMEM150C, respectively, p = 0.61, t test) (Figure 2C) but significantly prolonged the average τinact, from 8.1 ± 0.7 ms to 11.9 ± 0.7 ms (p = 0.0012, Mann-Whitney U test) (Figure 2D). Importantly, in the presence of TMEM150C, MA current persisted beyond the removal of mechanical stimulation (Ipost; Figures 2E and 2F). This is consistent with an earlier observation documenting the presence of the persistent post-stimulus current upon expression of TMEM150C in wild-type HEK293T cells, which also express a small amount of endogenous Piezo1 (Dubin et al., 2017; Hong et al., 2016).
Figure 2. TMEM150C Potentiates MA Current Produced by Piezo1.
(A) Exemplar whole-cell MA current traces recorded in HEK293TΔP1 cells in response to mechanical indentation with a glass probe at Ehold = −80 mV. Arrow indicates the position of persistent post-stimulus MA current measurement.
(B) Peak MA current measured at different indentation depths in HEK293TΔP1 cells expressing indicated constructs (Ehold = −80 mV). Data shown as mean ± SEM.
(C and D) Quantification of MA current activation threshold (C) and inactivation rate τinact (D). NS, not significant; p > 0.05, **p < 0.01; unpaired t test (C) and Mann-Whitney U-test (D). Data shown as mean ± SEM.
(E) Post-stimulus MA current amplitude at different indentation depths. ****p < 0.0001 for expression construct effect, two-way ANOVA. Data shown as mean ± SEM.
(F) Quantification of peak-normalized amplitude of persistent post-stimulus MA current. ****p < 0.0001, Mann-Whitney U test. Data shown as mean ± SEM.
See also Figure S2A.
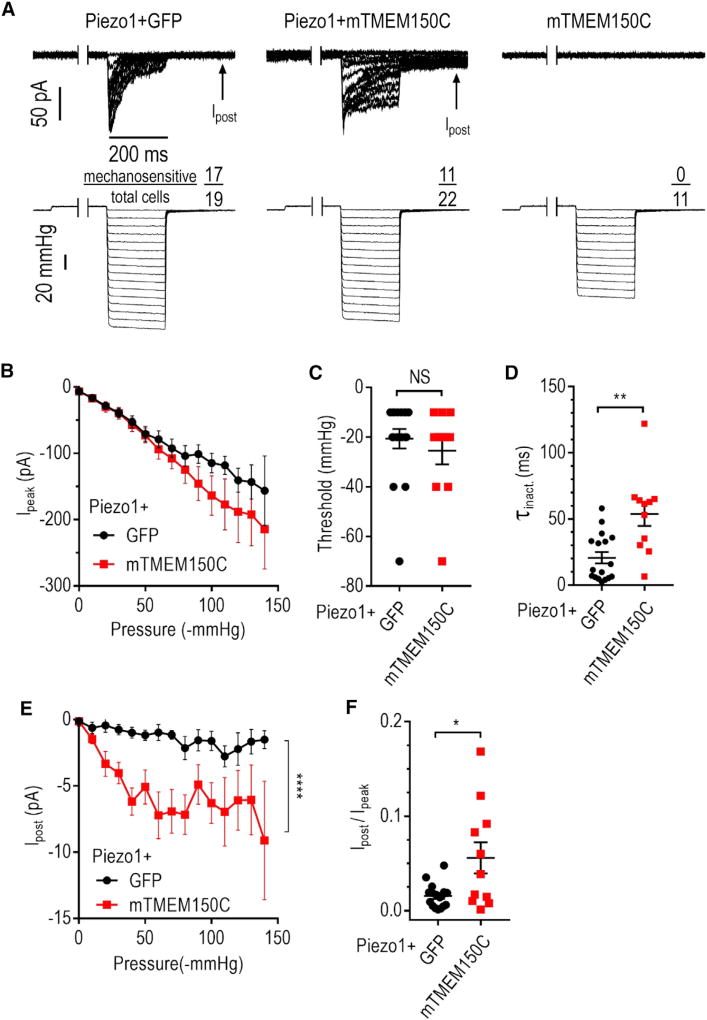
We wondered if the prolongation of inactivation and the induction of persistent post-stimulus current depend on the method of mechanical stimulation. Unlike Piezo2, Piezo1 can be activated by the application of mechanical force in a cell-attached patch using high-speed pressure clamp (HSPC) (Besch et al., 2002; Gottlieb et al., 2012). While we did not detect mechano-evoked activity in cells expressing TMEM150C alone, Piezo1-expressing cells produced robust MA current (Figures 3A and 3B). Co-expression of the two proteins did not change the apparent activation threshold (−20.6 ± 3.9 mmHg and −25.5 ± 5.5 mmHg for Piezo1 alone and with mTMEM150C, respectively, p = 0.37, Mann-Whitney U-test) (Figure 3C), but produced more than 2.6 fold increase in average τinact from 20.7 ± 4.3 ms to 53.9 ± 9.1 ms (p = 0.0011, t test) (Figures 3D and S2B). The HSPC approach produced a larger increase in τinact than stimulation with a glass probe, possibly due to perturbations in the lipid composition, basal tension, and mechanics of the plasma membrane caused by the formation of the gigaseal (Gottlieb et al., 2012; Suchyna et al., 2009). Additionally, co-expression with TMEM150C generated persistent post-stimulus current (Figures 3E and 3F). Overall, our findings with HSPC-mediated stimulation are consistent with those obtained through indentation of HEK293TΔP1 cells with a glass probe, demonstrating that the observed effects are independent of the method of mechanical stimulation.
Figure 3. TMEM150C Potentiates Piezo1 MA Current Evoked by High-Speed Pressure Clamp.
(A) Exemplar cell-attached MA current traces recorded in HEK293TΔP1 cells in response to application of a negative pressure in the pipette at Ehold = −60 mV. Each pressure step was preceded by a 500-ms step at 5 mmHg to remove inactivation. Arrow indicates the position of persistent post-stimulus MA current measurement.
(B) Quantification of peak MA current amplitude measured at −60 mV at different pressures. Data shown as mean ± SEM.
(C and D) Quantification of MA current activation threshold (C) and inactivation rate τinact (D). NS, not significant; p > 0.05, **p < 0.01; Mann-Whitney U-test (C) and unpaired t test (D). Data shown as mean ± SEM.
(E) Post-stimulus MA current amplitude at different pressures. ****p < 0.0001 for expression construct effect, two-way ANOVA. Data shown as mean ± SEM.
(F) Quantification of peak-normalized amplitude of persistent post-stimulus MA current. *p < 0.05, Welch’s test. Data shown as mean ± SEM.
See also Figure S2B.
Together, our data show that co-expression with TMEM150C prolongs inactivation kinetics of Piezo2 and Piezo1. However, we only detected a decrease in threshold with Piezo2 and the induction of persistent post-stimulus current with Piezo1. The absence of the persistent post-stimulus current at negative potentials in any condition other than upon co-expression of Piezo1 with TMEM150C (also see below) suggests that it is specific to this combination of proteins. The presence of channel-specific effects further supports the idea that TMEM150C is a modulator of Piezo channels.
TMEM150C Enhances Mechano-current Produced by TREK-1
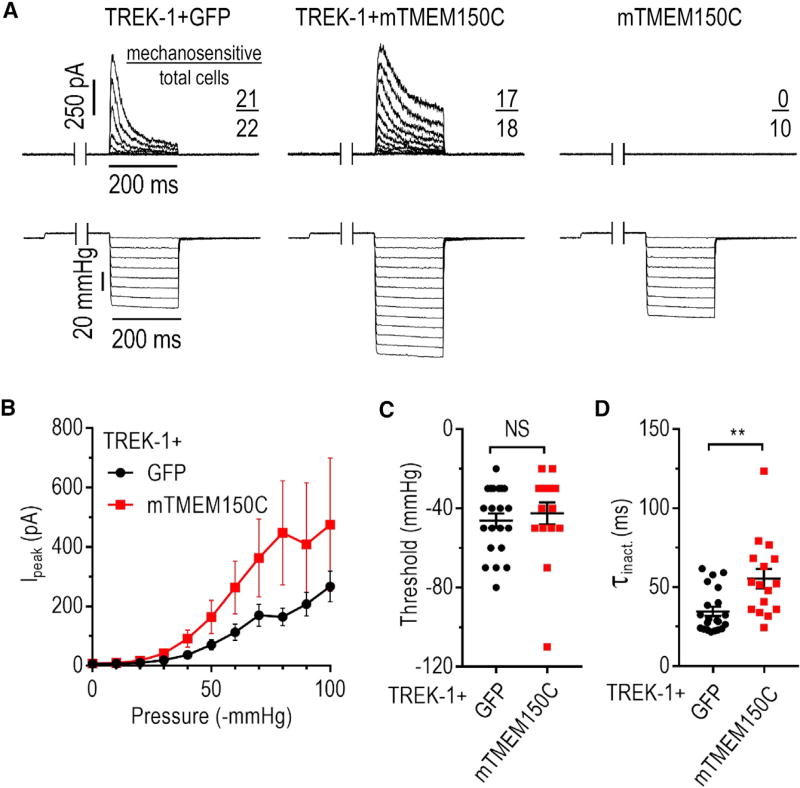
To determine whether TMEM150C expression modulates other types of mechano-transducers, we tested the mechano-gated potassium-selective channel TREK-1. Like Piezo2, TREK-1 is abundantly expressed in somatosensory neurons and is physiologically important for mechano-sensitivity (Alloui et al., 2006; Noël et al., 2009). Mechanical stimulation of HEK293TΔP1 cells expressing mouse TREK-1 by HSPC at 0 mV produced outward potassium-selective MA current with slow inactivation, whereas cells expressing mouse TMEM150C alone did not exhibit mechano-activity (Figures 4A and 4B). Co-expression of TREK-1 with TMEM150C did not change apparent activation threshold (−46.2 ± 3.7 mmHg and −40.6 ± 5.1 mmHg for TREK-1 alone TREK-1 with TMEM150C, respectively, p = 0.15, Mann-Whitney U test) (Figure 4C) but significantly prolonged the average τinact, from 34.7 ± 3.0 ms to 55.4 ± 6.1 ms (p = 0.0013, Mann-Whitney U test) (Figures 4D and S2C). Importantly, we measured MA current at a holding potential of 0 mV, which permits the efflux of potassium via TREK-1 but restricts the net current from a nonselective cation channel, a role originally proposed for TMEM150C (Hong et al., 2016, 2017). Thus, similar to Piezo2 and Piezo1, TREK-1 MA current is enhanced by co-expression with TMEM150C.
Figure 4. TMEM150C Potentiates MA Current of Potassium-Selective TREK-1 Channel.
(A) Exemplar cell-attached MA current traces recorded in HEK293TΔP1 cells in response to application of a negative pressure in the pipette using a high-speed pressure clamp (Ehold = 0 mV). Each pressure step was preceded by a 500-ms step at 5 mmHg to remove inactivation.
(B) Quantification of peak MA potassium current amplitude measured at Ehold = 0 mV at different pressures. Data shown as mean ± SEM.
(C and D) Quantification of cell-attached MA current activation threshold (C) and average inactivation rate (D). NS, not significant; p > 0.05, **p < 0.01, Mann-Whitney U test. Data shown as mean ± SEM.
See also Figures S2C and S3C.
In agreement with the functional data, we found that TMEM150C co-immunoprecipitates with TREK-1 and Piezo2, suggesting that TMEM150C can form a complex with the ion channels or exist in the same lipid domain (Figure S3). To test TMEM150C function more broadly, we used mouse Kv1.1, a voltage-gated potassium channel with a role in neuronal mechano-sensitivity (Hao et al., 2013). However, co-expression with TMEM150C did not affect voltage dependence of activation of Kv1.1, and the channel did not co-immunoprecipitate with TMEM150C (Figure S4). Together, our data show that TMEM150C is a modulator of different classes of mechano-gated ion channels.
DISCUSSION
Here, we sought to understand the role of TMEM150C in the generation of mechano-activated current. We show, in agreement with previous observations, that TMEM150C expression does not produce MA current when expressed alone in HEK293TΔP1 cells (Dubin et al., 2017). Additionally, we demonstrate that TMEM150C expression prolongs the kinetics of MA current inactivation when co-expressed with Piezo1, Piezo2, or TREK-1, independently of the method of mechanical stimulation. When TMEM150C and TREK-1 are co-expressed, the resultant current is mediated by potassium, as expected if it was solely produced by TREK-1. Thus, our data strongly suggest that TMEM150C is a regulator of mechano-gated ion channels rather than a nonselective ion channel itself, as initially suggested (Hong et al., 2016). Inactivation rate is a key element of mechano-gated channel function that determines the amount of excitatory (or inhibitory in the case of TREK-1) flux of ions and ultimately influences generation of the action potential. Prolongation of Piezo1 and Piezo2 τinact has been linked to several pathological conditions, including hereditary xerocytosis (Bae et al., 2013; Glogowska et al., 2017), dehydrated hereditary stomatocytosis (Albuisson et al., 2013), and distal arthrogryposis (Coste et al., 2013; Murthy et al., 2017). What could be the mechanism of TMEM150C-mediated prolongation of τinact in such different ion channel classes? The Piezos and TREK-1 share no similarity in terms of amino acid sequence or overall structure (Guo and MacKinnon, 2017; Lolicato et al., 2014, 2017; Saotome et al., 2017; Zhao et al., 2018). Piezo1 is directly activated by stretch in membrane blebs (Cox et al., 2016) and in a lipid bilayer (Syeda et al., 2016), and so is TREK-1 (Brohawn et al., 2014; Clausen et al., 2017). Interestingly, single-channel activity of reconstituted Piezo1 does not substantially decay after mechanical stimulation, suggesting that inactivation in cells may require additional components not present in the tested bilayer (Syeda et al., 2016). Aside from post-translational modifications, these components could include lipids and transmembrane or intracellular proteins, such as components of the cytoskeleton (Anderson et al., 2017; Borbiro and Rohacs, 2017; Murthy et al., 2017; Wu et al., 2017a). We propose that TMEM150C influences at least one such component, leading to changes in mechano-evoked responses in the Piezos and TREK-1.
We show that co-expression with TMEM150C significantly decreases the apparent activation threshold of Piezo2. The effect is not caused by increased channel expression, because at higher indentations, the magnitudes of Piezo2 current with and without mouse TMEM150C become similar. We hypothesize that the change in threshold stems, at least partially, from an increase in the channel’s sensitivity to mechanical stimulation. This threshold change could in part be driven by the large prolongation of Piezo2 inactivation kinetics upon co-transfection with TMEM150C, giving rise to current that is detectable at lower stimulation. In this case, the absence of an effect of TMEM150C on Piezo1 and TREK-1 threshold could stem from a milder increase in τinact compared to Piezo2, and larger initial τinact. We therefore do not exclude the possibility that TMEM150C also produces a small decrease in activation threshold in these channels.
A TMEM150C homolog TMEM150A influences phospholipid homeostasis via interaction with phosphotydilinositol 4-kinase type IIIα, a key enzyme generating phosphatidylinositol 4-phosphate on the plasma membrane (Chung et al., 2015). Conceivably, TMEM150C could alter chemical composition of the plasma membrane, changing its mechanical properties, such as rigidity and tension. Tension alone, however, is unlikely to be the major factor regulating τinact, as stepwise application of a negative pressure increases membrane tension proportionally, yet τinact remains steady across a wide range of pressures (Lewis and Grandl, 2015; Wu et al., 2017b). Lipid content, on the other hand, can have a profound effect on ion channel function either directly, or via redistribution of the channels into membrane subdomains, and this was shown for the Piezos and TREK-1 (Bae et al., 2013; Borbiro et al., 2015; Comoglio et al., 2014; Qi et al., 2015; Sandoz et al., 2011). If a lipid-modulating role is confirmed for TMEM150C, then we should expect to see an effect of this protein on other ion channels and membrane proteins, even though we did not detect an effect on voltage dependence of Kv1.1 activation.
Though important for physiology, the mechanism of activation and inactivation in Piezos and K2Ps is poorly understood. In Piezos, the magnitude of τinact is dictated by the C-terminal extracellular domain (CED), which forms a cap-like structure above the extracellular pore (Guo and MacKinnon, 2017; Saotome et al., 2017; Zhao et al., 2018). Reciprocal transposition of CED between Piezo1 and Piezo2 changes their τinact accordingly (Wu et al., 2017b). The apparent absence of inactivation in reconstituted Piezo1 suggests that even though CED is a key element of the inactivation mechanism, it requires other components. Indeed, inactivation of the Piezos can be influenced pharmacologically, by mutations, and via destruction of the cytoskeleton (Coste et al., 2013; Cox et al., 2016; Syeda et al., 2015). Interestingly, TREK channels also possess an extracellular cap-like structure (Brohawn et al., 2012; Dong et al., 2015; Lolicato et al., 2014, 2017), but its role in mechano-gating remains obscure. Studies identified the intracellular C terminus as a major modulator of gating via allosteric communication with the selectivity filter-based gate (Bagriantsev et al., 2011, 2012; Lolicato et al., 2014; Schewe et al., 2016). Even though the architecture of the channels appear strikingly different, the similarity between the effects of TMEM150C on Piezo1, Piezo2, and TREK-1 suggests the existence of common principles governing mechanogated channel opening and inactivation. Understanding how TMEM150C works could help reveal such mechanism.
TMEM150C was shown to have a role in proprioceptors, which also express Piezo2 (Hong et al., 2016). We now show that TMEM150C is expressed in 83.3% of neurons from TG of tactile specialist ducks, where proprioceptors are absent and the majority of cells are Piezo2-expressing touch receptors. The overlap between TMEM150C and Piezo2-expressing neurons may explain the prevalence of MA current with intermediate and slow inactivating kinetics in duck TG compared to mice or visually foraging chicken (Schneider et al., 2014, 2017). TMEM150C is also present in peptidergic and non-peptidergic nociceptors expressing Piezo2 (Borbiro et al., 2015; Prato et al., 2017). Since, as we show here, TMEM150C is a positive regulator of Piezo2 function, it is possible that in addition to proprioception, TMEM150C could also have a regulatory effect on light touch detection, heat, and mechano-nociception. However, behavioral tests using Tmem150C knockout mice only revealed deficits in motor coordination, consistent with TMEM150C expression in proprioceptors, while light touch and mechanical pain responses remained normal (Hong et al., 2016). Possibly, TMEM150C activity varies between different types of somatosensory neurons, such that the knockout may not have an equally significant impact on the function of light touch receptors and nociceptors as on proprioceptors. It is also possible that TMEM150’s effects are heterogeneous with regard to different splicing isoforms of Piezo2 (Szczot et al., 2017), which could have preferential expression in specific types of somatosensory neurons. TREK-1 is broadly expressed in the somatosensory system, where it is thought to counterbalance excitation by generating mechanically induced potassium efflux (Alloui et al., 2006; Noël et al., 2009). Since TMEM150C prolongs TREK-1 MA current, the effect of Tmem150C knockout on light touch via suppression of Piezo2 could be mitigated by simultaneous suppression of the inhibitory activity of TREK-1 (Brohawn et al., 2014). Importantly, TMEM150C is also present in the CNS (Hong et al., 2016), suggesting that the observed effects of Tmem150C knockout on behavior could have a more complex explanation, involving alterations in signal processing or brain development (Koser et al., 2016). Cell-type-specific knockouts are needed to reveal a role for TMEM150C in mechano-sensitivity.
EXPERIMENTAL PROCEDURES
Further details and outlines of resources can be found in Supplemental Experimental Procedures.
Animals
Tissues from adult domestic ducks, which were raised and slaughtered for the purpose of human consumption and not for this study, were purchased postmortem at MarWin Farm.
RNA In Situ Hybridization
TGs were fixed in paraformaldehyde, sectioned at 12–15 µm, probed with digoxigenin-labeled cRNA, and developed with alkaline-phosphatase-conjugated anti-digoxigenin Fab fragments.
Immunoprecipitation
HEK293ΔP1 cells were lysed in a buffer containing 1% CHAPS. Proteins were captured by antibodies immobilized on magnetic beads and analyzed by western blotting.
Electrophysiology
Electrophysiology data were collected from HEK293TΔP1 cells. For whole-cell recordings of MA current, cells held at −80 mV were stimulated with a glass probe in 1-µm, 150-ms steps with a velocity of 1,000 µm/s with 5 s between sweeps. For cell-attached recordings, membrane patches held at −60 mV for Piezo1 or 0 mV for TREK-1 were subjected to stepwise, 200-ms negative pressure steps (Δ10 mmHg) preceded by a 500-ms pre-pulse at 5 mmHg, with 3 s between stimuli. For Kv1.1 recordings, cells were stepped from −70 mV to 30 mV (Δ10 mV) from −70 mV holding potential. For details on electrophysiology and solution compositions, see Supplemental Experimental Procedures.
Statistical Analysis
Data were obtained from at least two independent experiments and are reported as mean ± SEM. Statistical tests were chosen based on normality of distributions and variance equality, or lack thereof, and the number of samples, are reported in figure legends.
Supplementary Material
Highlights.
TMEM150C is co-expressed with Piezo2 in somatosensory neurons
TMEM150C prolongs the duration of mechano-current produced by Piezo1/2 and TREK-1
TMEM150C is a general regulator of mechano-gated ion channels
TMEM150C function is conserved among vertebrates
Acknowledgments
We thank Pietro De Camilli and members of the Gracheva and Bagriantsev laboratories for their contributions throughout the project and Ardem Patapoutian for the gift of mPiezo2 plasmid and HEK293ΔP1 cells. E.O.A. is an Edward L. Tatum fellow and was supported by the Gruber Foundation. E.R.S. is a postdoctoral fellow of the Arnold and Mabel Beckman Foundation. This study was partly funded by NIH grant 1R01NS091300-01A1 (to E.O.G.) and by NSF CAREER grant 1453167 and NIH NINDS grant 1R01NS097547-01A1 (to S.N.B.).
Footnotes
DATA AND SOFTWARE AVAILABILITY
The accession number for the domestic duck (mallard) TMEM150C is GenBank: MG697237.
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.03.094.
AUTHOR CONTRIBUTIONS
E.O.A. designed and performed the majority of electrophysiological experiments and data analysis, with contributions from E.R.S. E.O.G. performed RNA in situ hybridization. E.O.G. and J.D.M. performed cloning. S.N.B. performed immunoprecipitation. E.O.A., E.O.G., and S.N.B. wrote the manuscript. E.O.G. and S.N.B. conceived the study and provided supervision throughout the project.
DECLARATION OF INTERESTS
The authors declare no competing financial interests.
References
- Albuisson J, Murthy SE, Bandell M, Coste B, Louis-Dit-Picard H, Mathur J, Fénéant-Thibault M, Tertian G, de Jaureguiberry JP, Syfuss PY, et al. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat. Commun. 2013;4:1884. doi: 10.1038/ncomms2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloui A, Zimmermann K, Mamet J, Duprat F, Noël J, Chemin J, Guy N, Blondeau N, Voilley N, Rubat-Coudert C, et al. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 2006;25:2368–2376. doi: 10.1038/sj.emboj.7601116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EO, Schneider ER, Bagriantsev SN. Piezo2 in cutaneous and proprioceptive mechanotransduction in vertebrates. Curr. Top. Membr. 2017;79:197–217. doi: 10.1016/bs.ctm.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae C, Gnanasambandam R, Nicolai C, Sachs F, Gottlieb PA. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc. Natl. Acad. Sci. USA. 2013;110:E1162–E1168. doi: 10.1073/pnas.1219777110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagriantsev SN, Peyronnet R, Clark KA, Honoré E, Minor DL., Jr Multiple modalities converge on a common gate to control K2P channel function. EMBO J. 2011;30:3594–3606. doi: 10.1038/emboj.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagriantsev SN, Clark KA, Minor DL., Jr Metabolic and thermal stimuli control K(2P)2.1 (TREK-1) through modular sensory and gating domains. EMBO J. 2012;31:3297–3308. doi: 10.1038/emboj.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besch SR, Suchyna T, Sachs F. High-speed pressure clamp. Pflugers Arch. 2002;445:161–166. doi: 10.1007/s00424-002-0903-0. [DOI] [PubMed] [Google Scholar]
- Borbiro I, Rohacs T. Regulation of Piezo channels by cellular signaling pathways. Curr. Top. Membr. 2017;79:245–261. doi: 10.1016/bs.ctm.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbiro I, Badheka D, Rohacs T. Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Sci. Signal. 2015;8:ra15. doi: 10.1126/scisignal.2005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn SG, del Mármol J, MacKinnon R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science. 2012;335:436–441. doi: 10.1126/science.1213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn SG, Su Z, MacKinnon R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc. Natl. Acad. Sci. USA. 2014;111:3614–3619. doi: 10.1073/pnas.1320768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Nakatsu F, Baskin JM, De Camilli P. Plasticity of PI4KIIIα interactions at the plasma membrane. EMBO Rep. 2015;16:312–320. doi: 10.15252/embr.201439151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen MV, Jarerattanachat V, Carpenter EP, Sansom MSP, Tucker SJ. Asymmetric mechanosensitivity in a eukaryotic ion channel. Proc. Natl. Acad. Sci. USA. 2017;114:E8343–E8351. doi: 10.1073/pnas.1708990114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoglio Y, Levitz J, Kienzler MA, Lesage F, Isacoff EY, Sandoz G. Phospholipase D2 specifically regulates TREK potassium channels via direct interaction and local production of phosphatidic acid. Proc. Natl. Acad. Sci. USA. 2014;111:13547–13552. doi: 10.1073/pnas.1407160111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Houge G, Murray MF, Stitziel N, Bandell M, Giovanni MA, Philippakis A, Hoischen A, Riemer G, Steen U, et al. Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of Distal Arthrogryposis. Proc. Natl. Acad. Sci. USA. 2013;110:4667–4672. doi: 10.1073/pnas.1221400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CD, Bae C, Ziegler L, Hartley S, Nikolova-Krstevski V, Rohde PR, Ng CA, Sachs F, Gottlieb PA, Martinac B. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat. Commun. 2016;7:10366. doi: 10.1038/ncomms10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong YY, Pike AC, Mackenzie A, McClenaghan C, Aryal P, Dong L, Quigley A, Grieben M, Goubin S, Mukhopadhyay S, et al. K2P channel gating mechanisms revealed by structures of TREK-2 and a complex with Prozac. Science. 2015;347:1256–1259. doi: 10.1126/science.1261512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin AE, Murthy S, Lewis AH, Brosse L, Cahalan SM, Grandl J, Coste B, Patapoutian A. Endogenous Piezo1 can confound mechanically activated channel identification and characterization. Neuron. 2017;94:266–270. doi: 10.1016/j.neuron.2017.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez-Paz D, Bali KK, Kuner R, Gomis A. A critical role for Piezo2 channels in the mechanotransduction of mouse proprioceptive neurons. Sci. Rep. 2016;6:25923. doi: 10.1038/srep25923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glogowska E, Schneider ER, Maksimova Y, Schulz VP, Lezon-Geyda K, Wu J, Radhakrishnan K, Keel SB, Mahoney D, Freidmann AM, et al. Novel mechanisms of PIEZO1 dysfunction in hereditary xerocytosis. Blood. 2017;130:1845–1856. doi: 10.1182/blood-2017-05-786004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb PA, Bae C, Sachs F. Gating the mechanical channel Piezo1: a comparison between whole-cell and patch recording. Channels (Austin) 2012;6:282–289. doi: 10.4161/chan.21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YR, MacKinnon R. Structure-based membrane dome mechanism for Piezo mechanosensitivity. eLife. 2017;6:e33660. doi: 10.7554/eLife.33660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Delmas P. Recording of mechanosensitive currents using piezoelectrically driven mechanostimulator. Nat. Protoc. 2011;6:979–990. doi: 10.1038/nprot.2011.343. [DOI] [PubMed] [Google Scholar]
- Hao J, Padilla F, Dandonneau M, Lavebratt C, Lesage F, Noël J, Delmas P. Kv1.1 channels act as mechanical brake in the senses of touch and pain. Neuron. 2013;77:899–914. doi: 10.1016/j.neuron.2012.12.035. [DOI] [PubMed] [Google Scholar]
- Hong GS, Lee B, Wee J, Chun H, Kim H, Jung J, Cha JY, Riew TR, Kim GH, Kim IB, Oh U. Tentonin 3/TMEM150c Confers Distinct Mechanosensitive Currents in Dorsal-Root Ganglion Neurons with Proprioceptive Function. Neuron. 2016;91:708–710. doi: 10.1016/j.neuron.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Hong GS, Lee B, Oh U. Evidence for mechanosensitive channel activity of Tentonin 3/TMEM150C. Neuron. 2017;94:271–273. doi: 10.1016/j.neuron.2017.03.038. [DOI] [PubMed] [Google Scholar]
- Koser DE, Thompson AJ, Foster SK, Dwivedy A, Pillai EK, Sheridan GK, Svoboda H, Viana M, Costa LD, Guck J, et al. Mechanosensing is critical for axon growth in the developing brain. Nat. Neurosci. 2016;19:1592–1598. doi: 10.1038/nn.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AH, Grandl J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. eLife. 2015;4:e12088. doi: 10.7554/eLife.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolicato M, Riegelhaupt PM, Arrigoni C, Clark KA, Minor DL., Jr Transmembrane helix straightening and buckling underlies activation of mechanosensitive and thermosensitive K(2P) channels. Neuron. 2014;84:1198–1212. doi: 10.1016/j.neuron.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolicato M, Arrigoni C, Mori T, Sekioka Y, Bryant C, Clark KA, Minor DL., Jr K2P2.1 (TREK-1)-activator complexes reveal a cryptic selectivity filter binding site. Nature. 2017;547:364–368. doi: 10.1038/nature22988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs V, Mathur J, Mao R, Bayrak-Toydemir P, Procter M, Cahalan SM, Kim HJ, Bandell M, Longo N, Day RW, et al. Impaired PIEZO1 function in patients with a novel autosomal recessive congenital lymphatic dysplasia. Nat. Commun. 2015;6:8329. doi: 10.1038/ncomms9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy SE, Dubin AE, Patapoutian A. Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 2017;18:771–783. doi: 10.1038/nrm.2017.92. [DOI] [PubMed] [Google Scholar]
- Noël J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, Lazdunski M. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009;28:1308–1318. doi: 10.1038/emboj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DT, Bernardis E, Flanagan LA, Tombola F. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. USA. 2014;111:16148–16153. doi: 10.1073/pnas.1409802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prato V, Taberner FJ, Hockley JRF, Callejo G, Arcourt A, Tazir B, Hammer L, Schad P, Heppenstall PA, Smith ES, Lechner SG. Functional and molecular characterization of mechanoinsensitive “silent” nociceptors. Cell Rep. 2017;21:3102–3115. doi: 10.1016/j.celrep.2017.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Andolfi L, Frattini F, Mayer F, Lazzarino M, Hu J. Membrane stiffening by STOML3 facilitates mechanosensation in sensory neurons. Nat. Commun. 2015;6:8512. doi: 10.1038/ncomms9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Bégay V, Coste B, Mainquist J, et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature. 2014;516:121–125. doi: 10.1038/nature13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SS, Syeda R, Patapoutian A. Mechanically activated ion channels. Neuron. 2015;87:1162–1179. doi: 10.1016/j.neuron.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz G, Bell SC, Isacoff EY. Optical probing of a dynamic membrane interaction that regulates the TREK1 channel. Proc. Natl. Acad. Sci. USA. 2011;108:2605–2610. doi: 10.1073/pnas.1015788108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, Ward AB. Structure of the mechanically activated ion channel Piezo1. Nature. 2017;554:481–486. doi: 10.1038/nature25453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schewe M, Nematian-Ardestani E, Sun H, Musinszki M, Cordeiro S, Bucci G, de Groot BL, Tucker SJ, Rapedius M, Baukrowitz T. A Non-canonical voltage-sensing mechanism controls gating in K2P K(+) channels. Cell. 2016;164:937–949. doi: 10.1016/j.cell.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ER, Mastrotto M, Laursen WJ, Schulz VP, Goodman JB, Funk OH, Gallagher PG, Gracheva EO, Bagriantsev SN. Neuronal mechanism for acute mechanosensitivity in tactile-foraging waterfowl. Proc. Natl. Acad. Sci. USA. 2014;111:14941–14946. doi: 10.1073/pnas.1413656111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ER, Anderson EO, Mastrotto M, Matson JD, Schulz VP, Gallagher PG, LaMotte RH, Gracheva EO, Bagriantsev SN. Molecular basis of tactile specialization in the duck bill. Proc. Natl. Acad. Sci. USA. 2017;114:13036–13041. doi: 10.1073/pnas.1708793114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchyna TM, Markin VS, Sachs F. Biophysics and structure of the patch and the gigaseal. Biophys. J. 2009;97:738–747. doi: 10.1016/j.bpj.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syeda R, Xu J, Dubin AE, Coste B, Mathur J, Huynh T, Matzen J, Lao J, Tully DC, Engels IH, et al. Chemical activation of the mechanotransduction channel Piezo1. eLife. 2015;4:e07369. doi: 10.7554/eLife.07369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syeda R, Florendo MN, Cox CD, Kefauver JM, Santos JS, Martinac B, Patapoutian A. Piezo1 channels are inherently mechanosensitive. Cell Rep. 2016;17:1739–1746. doi: 10.1016/j.celrep.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczot M, Pogorzala LA, Solinski HJ, Young L, Yee P, Le Pichon CE, Chesler AT, Hoon MA. Cell-type-specific splicing of Piezo2 regulates mechanotransduction. Cell Rep. 2017;21:2760–2771. doi: 10.1016/j.celrep.2017.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, Jessell TM, Wilkinson KA, Patapoutian A. Piezo2 is the principal mechanotransduction channel for proprioception. Nat. Neurosci. 2015;18:1756–1762. doi: 10.1038/nn.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lewis AH, Grandl J. Touch, tension, and transduction: the function and regulation of Piezo ion channels. Trends Biochem. Sci. 2017a;42:57–71. doi: 10.1016/j.tibs.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Young M, Lewis AH, Martfeld AN, Kalmeta B, Grandl J. Inactivation of mechanically activated Piezo1 ion channels is determined by the C-terminal extracellular domain and the inner pore helix. Cell Rep. 2017b;21:2357–2366. doi: 10.1016/j.celrep.2017.10.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Zhou H, Chi S, Wang Y, Wang J, Geng J, Wu K, Liu W, Zhang T, Dong M-Q, et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature. 2018;554:487–492. doi: 10.1038/nature25743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.