Abstract
Purpose of review
To summarize recent evidence on the global epidemiology of adolescents (age 10–19 years) living with HIV (ALHIV), the burden of HIV on the health of adolescents and HIV-associated mortality.
Recent findings
In 2016, there were an estimated 2.1 million (uncertainty bound 1.4–2.7 million) ALHIV; 770,000 younger (age 10–14 years) and 1.03 million older (age 15–19 years) ALHIV, 84% living in sub-Saharan Africa. The population of ALHIV is increasing as more peri/postnatally infected ALHIV survive into older ages; an estimated 35% of older female ALHIV were peri/postnatally infected, compared to 57% of older male ALHIV. While the numbers of younger ALHIV deaths are declining, deaths among older ALHIV have remained static since peaking in 2012. In 2015, HIV-associated mortality was the 8th leading cause of adolescent death globally and the 4th leading cause in African low- and middle-income countries.
Summary
Needed investments into characterizing and improving adolescent HIV-related health outcomes include: strengthening systems for nationally and globally disaggregated data by age, sex and mode of infection; collecting more granular data within routine programs to identify structural, social and mental health challenges to accessing testing and care; and prioritizing viral load monitoring and adolescent-focused differentiated models of care.
Keywords: Adolescent, HIV, epidemiology, mortality, global
Introduction
It is estimated that in 2016 there were almost 2.1 million (uncertainty bounds (UB) 1.4 million–2.7 million) adolescents (age 10–19 years) living with HIV (1), including 770,000 (UB 520,000–1.01 million) younger adolescents, age 10–14 years, and 1.3 million (UB 870,000–1.68 million) older adolescents, age 15–19 years (2). Adolescents living with HIV (ALHIV) are a complex, heterogeneous population of children with peri/postnatally acquired HIV aging up into adolescence in combination with adolescents newly horizontally infected with HIV during adolescence (3). Peri/postnatally infected ALHIV (pALHIV) often have more advanced HIV disease with related co-morbidities and disabilities associated with delayed HIV treatment and lifelong infection (4). They may be highly antiretroviral therapy (ART)-experienced, having commenced ART during infancy or childhood, and are at higher risk of treatment failure and mortality during adolescence (5). However, horizontally infected ALHIV (hALHIV) may experience other social, economic and sexual health risk factors associated with acquiring HIV during adolescence (6).
In common, is that both pALHIV and hALHIV are navigating the developmental transition of adolescence while dealing with a chronic disease and sexually transmissible infection. They are a population at greater risk of being lost to follow-up and dying than younger children and adults living with HIV (7–11). This review summarizes the most recent evidence on the global epidemiology of adolescent HIV, the contribution of HIV to the burden of adolescent disease, underlying causes of HIV-associated mortality and discusses differentiated service delivery as a key investment to improve outcomes for ALHIV.
Global distribution of adolescents living with, newly infected by and dying with HIV
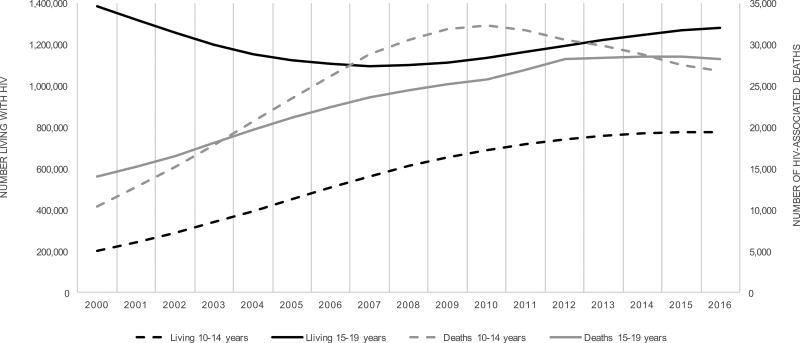
According to the most recent UNAIDS estimates (see Box 1), 71% of the estimated 2.1 million ALHIV in 2016 lived in just 10 high-burden countries, including nine in sub-Saharan Africa and India (2). Amongst the 770,000 younger ALHIV, 90% lived in sub-Saharan Africa (2). The estimated number of younger ALHIV has stabilized since 2014, likely corresponding with prevention of mother-to-child HIV transmission (PMTCT) program expansion that has led to fewer new peri/postnatally infected children annually, while ALHIV are aging up into older adolescence and young adulthood (Figure 1). There were initial declines in the number of older ALHIV up to 2008 that may have been related to a combination of fewer new older adolescent HIV infections and poor survival of pALHIV. New infections in older adolescents have declined globally by 45% from 470,000 (UB 290,000 – 600,000) in 2000 to 260,000 (UB 150,000–340,000) in 2016, with regional variation (2). While decreasing in other regions, annual infections have remained static in Central and South America with 17,000 (UB 8,500–32,000) since 2010, and in West and Central Africa with 62,000 (UB 15,000–130,000) since 2009 (2, 15). Overall, the population of older ALHIV is now increasing with an expanding cohort of pALHIV surviving (Figure 1) (2).
Box 1: UNAIDS-supported national and global adolescent HIV indicators.
UNAIDS assists countries to generate national estimates of key HIV epidemic indicators for monitoring and understanding the HIV epidemic at national and global levels (12). As it is not possible to directly measure the epidemic indicators at a population level in most countries, available national surveillance and complementary research data are fed into the SPECTRUM model to generate estimates, a method used by UNAIDS since the early 2000s (13). Under the guidance of the UNAIDS Reference Group on Estimates, Modelling, and Projections, the model is continuously refined to incorporate the most up to date understanding of the HIV epidemic based on demographic, HIV epidemiologic and programmatic data (http://www.epidem.org/) (14). Estimates are generated on an annual basis and updated for the current and all historic years (15). The UNAIDS modeled estimates for the numbers of adolescents newly HIV-infected, living with HIV and HIV-associated deaths provide a critical global-level resource that can be used to guide policy and program planning, as well as prioritizing surveillance needs and research agendas. For a detailed description of the methods for the 2017 estimates see reference (12). Current national, regional and global indicator estimates are available at http://aidsinfo.unaids.org.
Fig 1. Trends in numbers of younger (age 10–14 years) and older (age 15–19 years) adolescents living with HIV and HIV-associated deaths between 2000 and 2016 (UNAIDS 2017 Spectrum Model estimates).
Legend: The primary y-axis (left) displays number of younger (black dashed line) and older (black solid line) adolescents living with HIV. The secondary left axis (right) displays the number of younger (grey dashed line) and older (grey solid line) adolescent deaths.
While there is little difference in the numbers of younger adolescent males compared to females living with HIV, important sex-based differences are apparent in older ALHIV. In 2016, females disproportionately accounted for 61% of older ALHIV, and 67% of new infections among older ALHIV (2). Furthermore, where 65% of older female ALHIV were estimated to have been horizontally infected, only 43% of older male ALHIV were horizontally infected (Figure 2) (16). These sex differences are particularly prominent in Eastern and Southern Africa, where 62% of older female ALHIV were estimated to be horizontally infected, compared with 28% of older male ALHIV (Figure 3) (16). Although the numbers of aging pALHIV are steadily increasing with successful ART, their relative contribution to the global epidemic will depend in large part on our ability to prevent new horizontal adolescent infections.
Fig 2. Numbers of older adolescents (age 15–19 years) living with HIV globally by sex and mode of transmission: 2000–2016 (Additional analysis of UNAIDS 2017 estimates by John Stover).
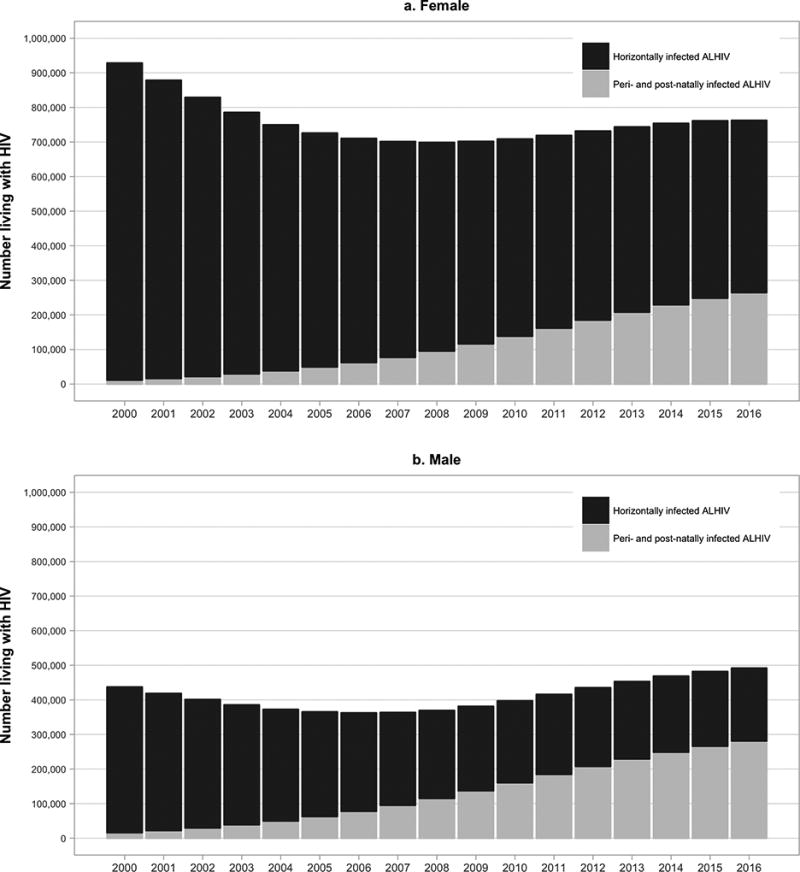
Legend: This figure displays the number of female (panel a) and male (panel b) older adolescents (age 15–19 years) globally living with HIV by mode of transmission (peri/postnatally infected in grey and horizontally infected in black)
Fig 3. Numbers of older adolescents (age 15–19 years) living with HIV in Eastern & Southern Africa by sex and mode of transmission: 2000–2016 (Additional analysis of UNAIDS 2017 estimates by John Stover).
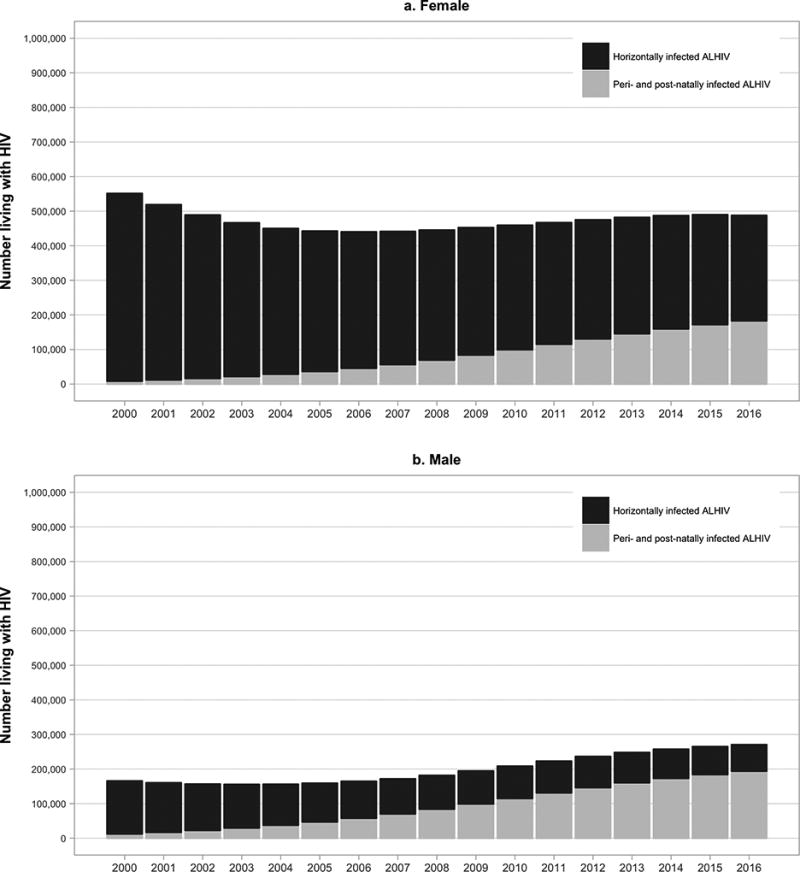
Legend: This figure displays the number of female (panel a) and male (panel b) older adolescents (age 15–19 years) in Eastern & Southern Africa living with HIV by mode of transmission (peri/postnatally infected in grey and horizontally infected in black)
Absolute numbers of deaths in younger ALHIV appear to be in decline, having peaked in 2010 at 32,000 (24,000–44,000) HIV-associated deaths and reduced to 27,000 (UB 19,000–37,000) in 2016 (Figure 1) (2). Corresponding with the distribution of younger ALHIV, more than half of younger adolescent HIV-associated deaths in 2016 occurred in Eastern and Southern Africa (16,000 [UB 12,000–22,000]) and almost 90% in sub-Saharan Africa overall. However, unlike the reduction in absolute numbers of deaths that has been observed in children (0–14 years) and adults (≥15 years), the absolute number of deaths in older adolescents remained static at 28,000 (UB 20,000–39,000) per year since peaking in 2012 (Figure 1). With the steadily increasing size of the older ALHIV population, mortality rates, rather than absolute numbers, are required to accurately monitor survival trends of older ALHIV. Empiric studies following cohorts of ALHIV in high-income countries have been used to complement and inform the UNAIDS-supported modelled mortality estimates in ALHIV. Comparable studies in high-burden low- and middle-income countries (LMICs) will be necessary to provide mortality rate trends to validate the UNAIDS estimates in these regions going forward (3). Strengthening data disaggregated by age, sex and mode of HIV transmission would facilitate more granular monitoring of sub-populations of ALHIV who have differing risks for newly acquiring HIV and HIV-associated mortality – evidence required to develop effective interventions to mitigate these risks.
The contribution of HIV to the global burden of adolescent disease
HIV-associated mortality is an important contributor to global adolescent mortality. The WHO Global Health Estimates for 2015, published in 2016, reported that HIV was the eighth leading cause of death among all adolescents globally (17). In African LMICs, HIV was the fourth leading cause of all adolescent deaths, following lower respiratory tract infections, diarrheal disease and meningitis. Globally, in younger adolescent females, HIV was the 4th leading cause of death, following lower respiratory tract infections, diarrheal disease and meningitis. In older adolescent females, HIV was the 8th leading cause of death, with maternal causes of death leading in this group. In both younger and older adolescent males, traumatic deaths related to road injury, drowning or interpersonal violence predominated, with HIV being the 7th and 10th leading cause of death in younger and older adolescent males, respectively (17).
This heterogeneity in causes of adolescent death by age and sex highlights that the need for disaggregated adolescent data is not exclusive to HIV. Reorientation of health system monitoring and service provision that is sensitive to the rapidly changing needs of adolescents will have benefits for the well-being of all adolescents, including ALHIV (18). Adolescent-specific health system policy implementation is starting to occur, the recently released South African National Adolescent and Youth Health Policy being one example (19). However, such efforts need to be more widespread to improve the quality of care and outcomes for adolescents, particularly in the global pursuit of Sustainable Development Goal 3 to achieve health for all at all ages (20).
HIV-associated mortality and morbidity during adolescence
To reduce HIV-associated mortality during adolescence, an understanding of the underlying causes of HIV-associated mortality and morbidity is needed to complement estimates of the numbers of deaths and where they are occurring. Evidence from multiple regions and research teams is converging around common findings of poorer outcomes among ALHIV in general, and for older youth in particular.
In a study combining 1446 perinatally HIV-infected youth aged 13–30 years in the Pediatric HIV/AIDS Cohort Study (PHACS) Adolescent Master Protocol and International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1074 multicenter cohort studies in the United States (US) between 2007–2015, CDC-C and WHO-4 events were more frequent in older than younger youth (0.7, 0.9 and 2.1 events per 100 person-years in those aged 7–12, 13–17 and 18–30 years respectively, p <0.001 for trend) (21). Mental health and neurodevelopmental conditions were amongst the most frequent morbidity events (4 events per 100 person-years). Compared to youth of the same age in the general US population, mortality rates in pALHIV were 5.6-fold (95% CI 2.8–11.1) higher in those aged 15–19 years and 12.3-fold (95% CI 8.0–18.9) higher in those aged 20–29 years (21).
In the European Pregnancy and Paediatric HIV Cohort Collaboration (EPPICC), mortality was described in 3,526 children and adolescents after initiating ART in Europe and Thailand up until 2013 (22). By the end of the study period 3% (N=94) of children had died. Despite these ALHIV having received ART, 64% of deaths were due to HIV-related infections, predominantly bacterial infection and/or sepsis and 27% were due to other HIV-related causes. Two suicides, recorded amongst other non-HIV-related deaths, may be an unrecognized consequence of the mental health strains of growing up with HIV (23, 24).
The International Epidemiology Databases to Evaluate AIDS (IeDEA) Global consortium conducted a multiregional analysis of 164,218 adolescents and youth aged 10–24 years across the Caribbean and Central and South America, sub-Saharan Africa, and Asia, and observed that those over 15 years of age had substantially higher incidence of WHO stage 4 events following ART initiation compared with younger ALHIV 10–14 years of age (1.5 [95% CI 1.3–1.7] vs. 0.21 [95% CI 0.9–0.23] per 100 person-years) (25).
In the TREAT Asia Pediatric HIV Observational Database (TApHOD) of IeDEA Asia-Pacific, there were 60 deaths (0.80 [95% confidence interval 0.62–1.04] per 100 person-years) among 2416 ALHIV over 7458 person-years of follow-up reported between 2008–12 (26). The incidence of mortality was 1.12 (95% CI 0.75–1.67) and 0.68 (95% CI 0.49–0.94) per 100 person-years in older and younger adolescents, respectively (incidence rate ratio 1.65 [95% CI 0.94, 2.84]). Through a standardized cause of death reporting process, 58% of deaths were determined to have been due to underlying infectious causes, of which 46% were AIDS-related opportunistic infections, despite 95% of ALHIV being on ART at the time of death (26).
Even with access to ART, ALHIV across the globe are dying from AIDS-related causes and experiencing substantial mental health and neurodevelopmental morbidity, including in settings where second- and third-line ART are available. Furthermore, multi-regional collaborations have identified that despite expansion of HIV services and access to ART, inequality in survival remains. Mortality of ALHIV on ART living in LMICs remains higher compared to ALHIV on ART in high-income countries (22, 27, 28). Geographic inequalities in combination with the burden of AIDS-related morbidity and mortality, clearly indicate that survival for ALHIV is impacted by factors beyond access to ART (29–31). These may include social and mental health factors such as adherence challenges, treatment fatigue and depression (30, 32). To better understand mortality in ALHIV in LMICs, we need to collect more detailed data that go beyond the biomedical causes of death to understand contributing structural, social, emotional and mental health factors.
Burden of undiagnosed and un-retained ALHIV
To realize the UNAIDS 90-90-90 targets for ALHIV, adolescents must first be diagnosed as HIV-infected to achieve the first target (90% know their HIV status), and must be retained in care to achieve the second (90% on ART) and third (90% virologically suppressed) targets. However, there is a considerable population of ALHIV unaware of their HIV infection and, once diagnosed, ALHIV experience high rates of loss to follow-up (LTFU). In a community survey conducted in 2013–2015, following implementation of optimized opt-out provider-initiated counselling and testing at primary health care clinics in Harare, Zimbabwe, 38% (95% CI 30%–46%) of children and adolescents aged 8–17 years with positive HIV tests had not previously been diagnosed (33). Children were less likely to have been diagnosed if they were over 13 years of age, in good health and with both parents alive. These findings are consistent with population-level estimates from the Population Health Impact Assessment (PHIA) surveys in Zimbabwe and Swaziland that found youth aged 15–24 years to be less likely to know their HIV status and less likely to be on ART than older adults (34, 35). Late diagnosis of HIV has major implications for the health and survival of these ALHIV (36).
Once diagnosed, ALHIV struggle to remain in care. Older adolescents in Zimbabwe had higher LTFU rates than older children and younger adolescents (adjusted rate ratio 1.6, 95% CI 1.2–2.1) and starting ART during older adolescence was associated with a 1.7 (95% CI 1.1–2.8) times greater risk of LTFU compared to starting in any younger age group (11). Unlike for adults living with HIV, there are still evidence gaps to understand what proportion of LTFU in ALHIV is due to mortality as compared to other reasons for LTFU, such as undocumented transfer to care elsewhere (37–39). Thus, the accuracy of survival estimates in ALHIV are limited where LTFU is high and mortality may be underestimated as unascertained deaths in those LTFU are often not adjusted for (27). A priority for ALHIV must be earlier diagnosis and successful linkage to and retention in care so that they can initiate treatment and achieve sustained viral suppression to prevent avoidable morbidity and mortality.
The promise and potential of differentiated service delivery for ALHIV
Differentiated care is being widely promoted as a way to allow patients greater freedoms in managing their own care, shifting the locus of care into the community, where peer- and healthcare worker-led adherence clubs offer a more efficient and effective alternative to busy institutional settings (40, 41). It also has the potential to substantially reduce national HIV program costs and provider burden by decreasing the frequency of clinic visits for stable patients with evidence of viral suppression, when there are reliable supply chains to sustain multi-month medication refills (42, 43). In Rwanda, program implementers specifically created a visit schedule for virologically suppressed adolescents up to age 19 years that align 3-monthly clinical and medication pick-up visits with school breaks (44). However, full implementation of these models requires routine viral load monitoring, which is infrequently available in much of sub-Saharan Africa (45). There is a strong case to be made for prioritizing routine viral load monitoring for ALHIV, who are in a life stage where they are at high risk for poor outcomes (46).
Optimal approaches for differentiated care for adolescents who struggle with daily adherence, and are more often on second-line regimens, are less clear. Such youth are often given more frequent clinic visits, which can be perceived as punishment and include stern counseling that may engender guilt rather than encouragement (47). Although the vast majority could benefit from differentiated care and maintain stable HIV disease, greater attention should be focused on studying how to modify care models for the minority of adolescents who need more proactive engagement (48). While recent achievements in expanding treatment to 21 million people with HIV are to be celebrated, policies and programs that do not directly address ALHIV struggles with long-term care will limit their chances for future treatment success and sustained health benefits (49).
Conclusion
Adolescence is a turbulent, but temporary phase. Considering that ALHIV, whether peri/postnatally or horizontally infected, are the population that will be living with HIV for the longest span of their lives, strategic targeted investments during this transitional phase are warranted. Priority investments for monitoring ALHIV outcomes include: 1) strengthening systems for nationally and globally disaggregated adolescent data by age, sex and mode of transmission to monitor progress in reducing new infections and mortality in high-risk sub-populations of ALHIV; 2) collection of more granular data within the context of routine care settings to identify structural, social and mental health challenges to being diagnosed with HIV, retained in care and maintaining viral suppression; 3) prioritizing viral load monitoring for earlier identification of those with viral non-suppression and treatment failure; and 4) differentiated models of care to allow flexibility, freedom and independence for the majority of ALHIV that are retained in care and on stable ART.
Key Points.
Among the 2.1 million (uncertainty bound 1.4–2.7 million) adolescents living with HIV (ALHIV) globally in 2016, females disproportionately account for 61% of older ALHIV and 66% of new infections among older ALHIV. Sixty-five percent of older female ALHIV were estimated to have been horizontally infected compared to 43% of older male ALHIV.
HIV is the 8th leading cause of adolescent deaths globally, the 4th leading cause in younger adolescent females globally and the 4th leading cause of all adolescent mortality in African low- and middle-income countries
Despite receiving antiretroviral therapy ALHIV still experience substantial morbidity and AIDS-related mortality. To better understand mortality in ALHIV in LMICs, we need to collect more detailed data that go beyond the biomedical cause of death to understand contributing structural, social, emotional and mental health factors.
There is a considerable population of ALHIV unaware of their HIV infection and, once diagnosed, ALHIV experience high rates of loss to follow-up. A priority for ALHIV must be earlier diagnosis and successful linkage to and retention in care so that they can initiate treatment and achieve sustained viral suppression to prevent avoidable morbidity and mortality.
Key investments during the turbulent but temporary phase of adolescence that would enhance our ability to track their outcomes so we can develop interventions to address them include systems for nationally and globally disaggregated adolescent data; collection of more granular data within the context of routine care settings to identify structural, social and mental health challenges during adolescence; and prioritizing viral load monitoring and differentiated models of care for ALHIV.
Acknowledgments
The authors gratefully thank Mary Mahy, UNAIDS Strategic Information Department, for providing additional UNAIDS 2017 data; and Azar Kariminia, Kirby Institute, University of New South Wales, Sophie Desmonde, University of Toulouse, and Anne Neilan, Harvard University, for their assistance with clarifying data cited in the paper.
Financial support and sponsorship:
Amy L. Slogrove is supported by the Fogarty International Center of the National Institutes of Health under Award Number K43TW010683. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AHS has received program grants and travel support to her institution from ViiV Healthcare.
Footnotes
Conflicts of interest:
ALS has no conflicts of interest to declare.
References and recommended reading
Papers of particular interest, published within the annual period of review (last 2 years), have been highlighted as:
* of special interest
** of outstanding interest
- 1.Health for the world's adolescents: a second chance in the second decade. Geneva, Switzerland: World Health Organization; 2014. [cited 2017 January 06]. Available from: http://apps.who.int/adolescent/second-decade/files/1612_MNCAH_HWA_Executive_Summary.pdf. [Google Scholar]
- 2**.UNAIDS. [cited 2017 November 17];UNAIDS 2017 Estimates. 2017 Available from: http://aidsinfo.unaids.org/. Publicly available resource of UNAIDS estimates for key HIV indicators at regional, national and global levels.
- 3*.Slogrove AL, Mahy M, Armstrong A, Davies MA. Living and dying to be counted: What we know about the epidemiology of the global adolescent HIV epidemic. J Int AIDS Soc. 2017;20(Suppl 3):4–15. doi: 10.7448/IAS.20.4.21520. This review summarized nationally-representative population-based survey prevalence data on adolescents living with HIV in the 15 highest adolescent HIV burden countries in 2016 and describes helpful examples of national case-based surveillance systems including adolescents living with HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sohn AH, Hazra R. Old Problems for New Providers: Managing the Postpediatric HIV Generation. Clin Infect Dis. 2017;64(8):1113–4. doi: 10.1093/cid/cix068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Judd A, Lodwick R, Noguera-Julian A, Gibb DM, Butler K, Costagliola D, et al. Higher rates of triple-class virological failure in perinatally HIV-infected teenagers compared with heterosexually infected young adults in Europe. HIV Med. 2016;18:171–80. doi: 10.1111/hiv.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bekker LG, Johnson L, Wallace M, Hosek S. Building our youth for the future. J Int AIDS Soc. 2015;18(2 Suppl 1):20027. doi: 10.7448/IAS.18.2.20027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009;51(1):65–71. doi: 10.1097/QAI.0b013e318199072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Judd A, Chappell E, Doerholt K, Galli L, Giaquinto C, Gibb D, et al. Long-term trends in mortality and AIDS-defining events among perinatally HIV-infeced children across Europe and Thailand. International AIDS Conference; 19 July 2016; Durban, South Africa. 2016. [Google Scholar]
- 9.Lamb MR, Fayorsey R, Nuwagaba-Biribonwoha H, Viola V, Mutabazi V, Alwar T, et al. High attrition before and after ART initiation among youth (15–24 years of age) enrolled in HIV care. AIDS. 2014;28(4):559–68. doi: 10.1097/QAD.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shroufi A, Ndebele W, Nyathi M, Gunguwo H, Dixon M, Saint-Sauveur JF, et al. Risk of death among those awaiting treatment for HIV infection in Zimbabwe: adolescents are at particular risk. J Int AIDS Soc. 2015;18:19247. doi: 10.7448/IAS.18.1.19247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Kranzer K, Bradley J, Musaazi J, Nyathi M, Gunguwo H, Ndebele W, et al. Loss to follow-up among children and adolescents growing up with HIV infection: age really matters. J Int AIDS Soc. 2017;20(1):21737. doi: 10.7448/IAS.20.1.21737. This retrospective analysis of 2273 older children and adolescents living with HIV in Zimbabwe between 2005–2009, identified a greater risk for being lost to follow-up in older adolescents than older children and younger adolescents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.UNAIDS. [cited 2017 November 20];Annex on Methods. 2017 Available from: http://aidsinfo.unaids.org/documents/estimates_methods_2017.pdf.
- 13.Stover J. Projecting the demographic consequences of adult HIV prevalence trends: the Spectrum Projection Package. Sexually Transmitted Infections. 2004;80(Suppl 1):i14–i8. doi: 10.1136/sti.2004.010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Mahy M, Penazzato M, Ciaranello A, Mofenson L, Yianoutsos C, Davies M-A, et al. Improving estimates of children living with HIV from the Spectrum AIDS Impact Model. AIDS. 2016;31(Suppl 1):S13–S22. doi: 10.1097/QAD.0000000000001306. This is a key paper to understand the methods behind and challenges of modelling and estimating HIV indicators for children living with HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UNAIDS. UNAIDS Data 2017. Geneva: 2017. [cited 2017 November 22]. Available from: http://www.unaids.org/en/resources/documents/2017/2017_data_book. [Google Scholar]
- 16.UNAIDS. [cited 2017 November 17];UNAIDS 2017 Estimates, further analysis by John Stover. 2017 Available from: http://aidsinfo.unaids.org/
- 17**.World Health Organization. Global Health Estimates 2015: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2015. 2016 [Available from: http://www.who.int/healthinfo/global_burden_disease/en/. This report details the most recent estimates of the gblobal burden of adolescent disease.
- 18**.World Health Organization. Global accelerated action for the health of adolescents (AA-HA!): guidance to support country implementation. 2017 [Available from: http://apps.who.int/iris/bitstream/10665/255415/1/9789241512343-eng.pdf?ua=1. This guidance document provides countries with a systematic approach to planning, implementing, monitoring and evaluating adolescent health programmes.
- 19**.South African National Department of Health. [cited 2017 November 27];National Adolescent and Youth Health Policy. 2017 Available from: https://www.idealclinic.org.za/docs/policies/National%20Adolescent%20and%20Youth%20Health%20Policy%202017.pdf. This is one of the first national policy documents for adolescent and youth health in a high HIV burden country. It outlines 6 key objectives with clear, feasible interventions to achieve each objective and ultimately improve the quality of care and outcomes for adolescents in South Africa.
- 20.United Nations. [cited 2017 January 18];Transforming our world: the 2030 agenda for sustainable development. 2015 Available from: https://sustainabledevelopment.un.org/post2015/transformingourworld/publication.
- 21**.Neilan AM, Karalius B, Patel K, Van Dyke RB, Abzug MJ, Agwu AL, et al. Association of Risk of Viremia, Immunosuppression, Serious Clinical Events, and Mortality With Increasing Age in Perinatally Human Immunodeficiency Virus-Infected Youth. JAMA Pediatr. 2017;171(5):450–60. doi: 10.1001/jamapediatrics.2017.0141. This is one of few papers that provides detailed data on morbidity and underlying causes of mortality in perinatally HIV-infected youth (US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.The European Pregnancy and Paediatric HIV cohort Collaboration (EPPICC) study group in EuroCoord. Long term trends in mortality and AIDS-defining events after combination ART initiation among children and adolescents with perinatal HIV in Europe and Thailand: cohort study. PLoS Med. 2017 doi: 10.1371/journal.pmed.1002491. Accepted. This is one of few papers to describe underlying causes of mortality in children with perinatally-acquired HIV initiating therapy prior to age 18 years (Europe and Thailand). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong M, Myer L, Zerbe A, Phillips T, Petro G, Mellins CA, et al. Depression, alcohol use, and stigma in younger versus older HIV-infected pregnant women initiating antiretroviral therapy in Cape Town, South Africa. Arch Womens Ment Health. 2017;20(1):149–59. doi: 10.1007/s00737-016-0688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermetet-Lindsay KD, Correia KF, Williams PL, Smith R, Malee KM, Mellins CA, et al. Contributions of Disease Severity, Psychosocial Factors, and Cognition to Behavioral Functioning in US Youth Perinatally Exposed to HIV. AIDS Behav. 2017;21:2703–2715. doi: 10.1007/s10461-016-1508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desmonde S, Neilan AM, Malateste K, Yiannoutsos C, Musick B, Patten GB, et al. Age-stratified rates of mortality and key clinical events in youth ages 0–24 years in the multiregional IeDEA network. 9th International HIV Pediatrics Workshop; 21 July 2017; Paris, France. 2017. [Google Scholar]
- 26.Sohn AH, Kariminia A, Lumbiganon P, Kurniati N, Lapphra K, Nguyen VL, et al. Standardized determinations of causes of death among children and adolescents in the TREAT Asia Pediatric HIV Observational Database (TApHOD). International HIV Pediatrics Workshop; 18–19 July 2015; Melbourne, Australia. 2014. [Google Scholar]
- 27.Slogrove AL, Judd A, Leroy V. The epidemiology of perinatally HIV-infected adolescents: a CIPHER cohort collaboration global analysis. International AIDS Conference; 20 July 2016; Durban, South Africa. 2016. [Google Scholar]
- 28.Slogrove AL, Judd A, Leroy V On behalf of the CIPHER Cohort Collaboration Adolescent Project Team. Inequality in mortality and access to antiretroviral therapy in adolescents living with perinatally-acquired HIV in sub-Saharan Africa: a Collaborative Initiative for Paediatric HIV Education and Research (CIPHER) Cohort Collaboration analysis. 9th International AIDS Society Conference on HIV Science; 2017 July 224; Paris, France. 2017. [Google Scholar]
- 29.Nichols SL, Brummel SS, Smith RA, Garvie PA, Hunter SJ, Malee KM, et al. Executive Functioning in Children and Adolescents With Perinatal HIV Infection. Pediatr Infect Dis J. 2015;34(9):969–75. doi: 10.1097/INF.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garvie PA, Brummel SS, Allison SM, Malee KM, Mellins CA, Wilkins ML, et al. Roles of Medication Responsibility, Executive and Adaptive Functioning in Adherence for Children and Adolescents With Perinatally Acquired HIV. Pediatr Infect Dis J. 2017;36(8):751–7. doi: 10.1097/INF.0000000000001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fish R, Judd A, Jungmann E, O'Leary C, Foster C Network HIVYP. Mortality in perinatally HIV-infected young people in England following transition to adult care: an HIV Young Persons Network (HYPNet) audit. HIV Med. 2014;15(4):239–44. doi: 10.1111/hiv.12091. [DOI] [PubMed] [Google Scholar]
- 32.Mutumba M, Bauermeister JA, Elkington KS, Bucek A, Dolezal C, Leu CS, et al. A Prospective Longitudinal Study of Mental Health Symptoms Among Perinatally HIV-Infected and HIV-Exposed but Uninfected Urban Youths. J Adolesc Health. 2016;58(4):460–6. doi: 10.1016/j.jadohealth.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Simms V, Dauya E, Dakshina S, Bandason T, McHugh G, Munyati S, et al. Community burden of undiagnosed HIV infection among adolescents in Zimbabwe following primary healthcare-based provider-initiated HIV testing and counselling: A cross-sectional survey. PLoS Med. 2017;14(7):e1002360. doi: 10.1371/journal.pmed.1002360. This paper highlights the population of perinatally infected adolescents who are diagnosed and enter care very late and well into adolescence, which results in substantial morbidity by this time. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hakim A, Radin E, Ruangtragool L, Herman-Roloff A, Ahmed N, Musuka G, et al. Correlates of being outside of the 90-90-90 cascade among adults aged 15–64 years in Zimbabwe: Results from the 2015–2016 Zimbabwe Population-based HIV Impact Assessment (ZIMPHIA). 9th IAS Conference on HIV Science 2017; July 2017; Paris, France. 2017. [Google Scholar]
- 35.Nkambule R, Nuwagaba-Biribonwoha H, Mnisi Z, Ao T, Ginindza C, Duong Y, et al. Substantial Progess in Confrontine the HIV Epidemic in Swaziland: First Evidence of National Impact. 9th IAS Conference on HIV Science; July 2017; Paris, France. 2017. [Google Scholar]
- 36*.McHugh G, Simms V, Dauya E, Bandason T, Chonzi P, Metaxa D, et al. Clinical outcomes in children and adolescents initiating antiretroviral therapy in decentralized healthcare settings in Zimbabwe. J Int AIDS Soc. 2017;20(1):21843. doi: 10.7448/IAS.20.1.21843. This prospective cohort study describes hospitalization and mortality rates as well as causes of mortality in children and adolescents receiving HIV care in public clinics in Zimbabwe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geng EH, Glidden DV, Emenyonu N, Musinguzi N, Bwana MB, Neilands TB, et al. Tracking a sample of patients lost to follow-up has a major impact on understanding determinants of survival in HIV-infected patients on antiretroviral therapy in Africa. Trop Med Int Health. 2010;15(Suppl 1):63–9. doi: 10.1111/j.1365-3156.2010.02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egger M, Spycher BD, Sidle J, Weigel R, Geng EH, Fox MP, et al. Correcting mortality for loss to follow-up: a nomogram applied to antiretroviral treatment programmes in sub-Saharan Africa. PLoS Med. 2011;8(1):e1000390. doi: 10.1371/journal.pmed.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geng EH, Odeny TA, Lyamuya R, Nakiwogga-Muwanga A, Diero L, Bwana M, et al. Retention in Care and Patient-Reported Reasons for Undocumented Transfer or Stopping Care Among HIV-Infected Patients on Antiretroviral Therapy in Eastern Africa: Application of a Sampling-Based Approach. Clin Infect Dis. 2016;62(7):935–44. doi: 10.1093/cid/civ1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myer L, Iyun V, Zerbe A, Phillips TK, Brittain K, Mukonda E, et al. Differentiated models of care for postpartum women on antiretroviral therapy in Cape Town, South Africa: a cohort study. Journal of the International AIDS Society. 2017;20(Suppl 4):21636. doi: 10.7448/IAS.20.5.21636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsondai PR, Wilkinson LS, Grimsrud A, Mdlalo PT, Ullauri A, Boulle A. High rates of retention and viral suppression in the scale-up of antiretroviral therapy adherence clubs in Cape Town, South Africa. Journal of the International AIDS Society. 2017;20(Suppl 4):21649. doi: 10.7448/IAS.20.5.21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grimsrud A, Bygrave H, Doherty M, Ehrenkranz P, Ellman T, Ferris R, et al. Reimagining HIV service delivery: the role of differentiated care from prevention to suppression. Journal of the International AIDS Society. 2016;19(1):21484. doi: 10.7448/IAS.19.1.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips A, Shroufi A, Vojnov L, Cohn J, Roberts T, Ellman T, et al. Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature. 2015;528(7580):S68–76. doi: 10.1038/nature16046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nsanzimana S, Remera E, Ribakare M, Burns T, Dludlu S, Mills EJ, et al. Phased implementation of spaced clinic visits for stable HIV-positive patients in Rwanda to support Treat All. Journal of the International AIDS Society. 2017;20(Suppl 4):21635. doi: 10.7448/IAS.20.5.21635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joint United Nations Programme on HIV/AIDS (UNAIDS) Ending AIDS: Progress towards the 90-90-90 targets. Geneva: UNAIDS; 2017. Jul 20, [cited 30 November 2017]. Available from: http://www.unaids.org/en/resources/documents/2017/20170720_Global_AIDS_update_2017. [Google Scholar]
- 46**.Marcus R, Ferrand RA, Kranzer K, Bekker LG. The case for viral load testing in adolescents in resource-limited settings. J Int AIDS Soc. 2017;20(Suppl 7):37–42. doi: 10.1002/jia2.25002. In the context of scarce resources, this review argues for prioritization of viral load monitoring among adolescents who have disproportionately higher rates of virological failure than other age groups. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mark D, Armstrong A, Andrade C, Penazzato M, Hatane L, Taing L, et al. HIV treatment and care services for adolescents: a situational analysis of 218 facilities in 23 sub-Saharan African countries. Journal of the International AIDS Society. 2017;20(Suppl 3):21591. doi: 10.7448/IAS.20.4.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48*.Yang E, Mphele S, Moshashane N, Bula B, Chapman J, Okatch H, et al. Distinctive barriers to antiretroviral therapy adherence among non-adherent adolescents living with HIV in Botswana. AIDS care. 2017:1–8. doi: 10.1080/09540121.2017.1344767. This qualitative research study makes an important contribution to understanding the barriers experienced by non-adherent adolescents living with HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.UNAIDS. [cited 2017 November 2017];Right to Health. 2017 Available from: http://www.unaids.org/en/resources/documents/2017/20171120_right_to_health.