Abstract
Rationale
Mitogen-activated protein kinases (MAPKs) are activated in the heart by disease- and stress-inducing stimuli where they participate in hypertrophy, remodeling, contractility, and heart failure. A family of dual-specificity phosphatases (DUSPs) directly inactivates each of the MAPK terminal effectors, potentially being cardioprotective.
Objective
To determine the role of DUSP1 and DUSP4 in regulating p38 MAPK function in the heart and the effect on disease.
Methods and Results
Here we generated mice and mouse embryonic fibroblasts (MEFs) lacking both Dusp1 and Dusp4 genes. While single nulls showed no molecular effects, combined disruption of Dusp1/4 promoted unrestrained p38 MAPK activity in both MEFs and the heart, with no change in the phosphorylation of c-Jun N-terminal kinases (JNK) or extracellular signal-regulated kinases (ERK1/2), at baseline or with stress stimulation. Single disruption of either Dusp1 or Dusp4 did not result in cardiac pathology, although Dusp1/4 double null mice exhibited cardiomyopathy and increased mortality with aging. Pharmacological inhibition of p38 MAPK with SB731445 ameliorated cardiomyopathy in Dusp1/4 double null mice indicating that DUSP1/4 function primarily through p38 MAPK in affecting disease. At the cellular level, unrestrained p38 MAPK activity diminished cardiac contractility and Ca2+ handling, which was acutely reversed with a p38 inhibitory compound. Poor function in Dusp1/4 double null mice was also partially rescued by Pln (phospholamban) deletion.
Conclusions
Our data demonstrate that Dusp1 and Dusp4 are cardioprotective genes that play a critical role in the heart by dampening p38 MAPK signaling that would otherwise reduce contractility and induce cardiomyopathy.
Subject codes: [11] Other heart failure, [110] Congestive, [15] Hypertrophy, [143] Gene regulation, [148] Heart failure - basic studies, [130] Animal models of human disease, [108] Other myocardial biology, [155] Physiological and pathological control of gene expression
Keywords: Dilated cardiomyopathy, genetically altered mice, signaling, MAPK, DUSP, Mitogen-activated protein kinases, signal transduction
INTRODUCTION
The greater mitogen-activated protein kinase (MAPK) signaling cascade consists of a sequence of successively acting kinases that result in the dual phosphorylation and activation of terminal kinases p38, c-Jun N-terminal kinases (JNKs), and extracellular signal-regulated kinases (ERKs) 1. The major upstream activators of ERK1/2 are two MAP kinase kinases (MAPKK), MEK1 and MEK2, while p38 kinases are directly activated by MKK6 and MKK3, and JNKs are directly activated by MKK4 and MKK7 1. Upstream of the MAPKKs, multiple MAPKKKs and even MAPKKKKs form a complex network that either directly sense stress stimulation, or are directly regulated by effectors such as low molecular weight G-proteins (Ras, Rac, Rho, etc.) and G-protein coupled receptors 1,2. In the heart, MAPK signaling pathways regulate hypertrophic growth, dilated cardiomyopathy with ventricular remodeling, cellular apoptosis, fibrosis, contractility, and cellular proliferation 2,3. With respect to p38 MAPK, overexpression of an activated MKK3 mutant protein in the heart promoted dilated cardiomyopathy and early lethality in transgenic mice, while activated MKK6 induced restrictive cardiomyopathy with hypertrophy that also led to early lethality 4. However, deletion of the primary p38 gene in the heart, p38α, also predisposed to disease as mice subjected to transverse aortic constriction showed a worse phenotype 5. Indeed, cardiac-specific overexpression of dominant negative (dn) p38α or dnMKK3 rendered the heart more susceptible to cardiac hypertrophy and ventricular remodeling with pressure overload stimulation 6. Thus, p38 appears to have both pathologic and compensatory functions in the heart, although a non-overexpression approach is needed to better examine its gain-of-function effects and determine if p38 could really be a relevant target for treating heart disease.
The duration, extent and subcellular compartment for p38, JNK, and ERK1/2 phosphorylation are critical determinants of the physiological response to any given mitogenic or stress stimulation. The terminal MAPKs (ERK1/2, JNK and p38) are either activated or inactivated through the phosphorylation status of the threonine and adjacent tyrosine residue within the activation loop of these kinases 1–3. A specialized family of phosphatases have evolved that can dephosphorylate both serine/threonine and tyrosine residues within the activation loop, known as the dual-specificity protein phosphatases (DUSPs). There are 11 Dusp genes in the mouse genome that are specialized for the MAPKs and hence have been referred to as MAPK phosphatases (MKPs) 7. A unique feature of most MKP/DUSPs is their regulation at the level of transcription following stress or mitogen stimulation, providing a negative feedback loop to dampen the extent and duration of MAPK signaling with a typical lag of 15–45 minutes 7. Once expressed, DUSPs are constitutively active and capable of direct binding to the activation loop in MAPKs, resulting in dephosphorylation and their inactivation. Each of the 11 MKP/DUSP family members differs with respect to subcellular localization, tissue expression pattern, and exact specificity for ERK1/2 vs JNK1/2 vs p38 7. DUSP1 (MKP-1) and DUSP4 (MKP-2) are each induced by stress stimulation in the heart or cultured myocytes with agonist treatment where they then reside mostly within the nucleus (although some cytoplasmic localization is observed) and have the highest degree of action against p38 MAPK, followed by JNK, then ERK1/2 7–12. While the function of the Dusp6 gene has been investigated in the heart, where it serves as an exclusive regulator of ERK1/2 signaling, with effects on hypertrophic growth and myocyte proliferation 13,14, the function of the p38 inactivating DUSPs has not been evaluated, nor have their roles in heart disease been characterized.
METHODS
Dusp1 null mice were described previously 15. The Dusp4 gene was targeted in embryonic stem cell using homologous recombination, after which gene-deleted mice were created using standard techniques. Mouse embryonic fibroblasts (MEFs) were generated from Dusp1/4 double null embryos harvested at embryonic day 12.5 and cultured in 10% FBS containing DMEM. Echocardiography was performed with a Hewlett Packard SONOS 5500 with a 15 mHz probe and images were collected in M-mode. Cardiac pressure overload was induced by transverse aortic constriction (TAC) in young adult mice as described previously 16. Myocytes were isolated from adult hearts and cultured for either Western blot analysis of MAPK phosphorylation, or for assessment of cellular shortening and Ca2+ handling as described previously 17. Results are shown as mean +/− SEM and significance between groups was evaluated by ANOVA or t-test where appropriate.
See supplemental materials on-line for full listing of Materials and Methods.
RESULTS
Generation of Dusp1/4 double null mice
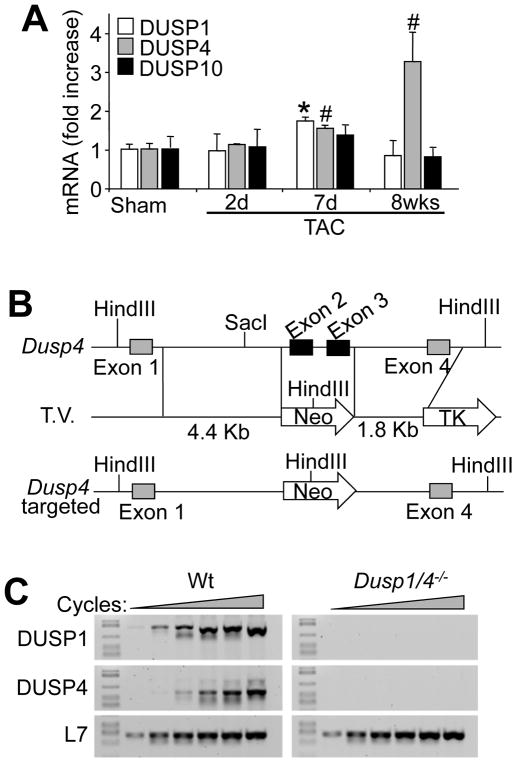
DUSP1, DUSP4 and DUSP10 are the major regulators of p38 MAPK dephosphorylation to allow inactivation and recycling of this kinase. Both DUSP1 and DUSP4 are induced by hypertrophic agonists in cultured cardiomyocytes or during heart failure, where they contribute to MAPK inactivation 10,11,18. Consistent with these previous observations we observed an increase in DUSP1 and DUSP4 mRNA in the mouse heart after 7 days of hypertrophic pressure overload stimulation, however by 8 weeks of stimulation when the heart is transitioning into failure only DUSP4 remained high (Figure 1A). DUSP10, while expressed in the heart, was constitutively present and not subject to induction with hypertrophy (Figure 1A). To begin to address the physiologic relevance of DUSP function in the heart in regulating p38 MAPK during disease, we inactivated the Dusp4 gene by targeting this locus in embryonic stem cells for the generation of gene-deleted mice (Figure 1B). RT-PCR confirmed the deletion of the gene product in the heart (Figure 1C). Given redundancy in function of Dusp4 with Dusp1, we crossed these 2 gene-deleted mice together, and for control purposes also analyzed DUSP1 mRNA from these hearts, which confirmed gene deletion as previously shown (Figure 1C) 15.
Figure 1. Gene-targeting of stress-responsive Dusp4 in the heart.
A, Normalized SYBR green quantitative PCR for DUSP1, DUSP4, and DUSP10 encoding mRNA from heart of mice subjected to Sham or TAC procedure for the indicated period of time in days or weeks (*P < 0.01 vs. sham; #P < 0.05 vs. sham; n=3–6 per time point). B, Schematic of the Dusp4 genetic locus and the targeting vector (T.V.) used to create Dusp4 gene-deleted mice. C, RT-PCR for DUSP1, DUSP4, and L7 (control) from hearts of control (Wt) and Dusp1/4 double null (Dusp1/4−/−) mice at 1 month of age.
Loss of Dusp1/4 only alters p38 MAPK activity
Part of the rationale for generating Dusp1/4 double null mice was based on our inability to consistently measure a difference in p38, JNK, or ERK1/2 phosphorylation in single Dusp1 or Dusp4 gene-targeted mouse hearts or mouse embryonic fibroblasts (MEFs) in culture (data not shown). Again, this is likely due to redundancy and compensation amongst the Dusp gene family members, of which Dusp1 and Dusp4 appear to function most similarly with respect to specificity for p38 7. Dusp1/4 double null mice were viable and overtly normal, and fibroblasts from these mice proliferated normally (data not shown). Interestingly, Dusp1/4−/− MEFs cultured in serum-containing medium showed a remarkable and specific defect in p38 MAPK dephosphorylation at baseline, while none of the other MAPK family members were affected (Figure 2A). Specifically, phospho-p38 was dramatically increased at all times in Dusp1/4−/− MEFs, with a corresponding downregulation of MKK6, its upstream activator (Figure 2A). As previously reported, activated p38 MAPK negatively regulates MKK6 mRNA stability, and thus decreases MKK6 expression in various cell types.19 Downstream of p38, there was a constitutive increase in phosphorylation of MAPK-activated protein kinase 2 (MK2) at T222 and T334 in the absence of Dusp1/4, which are direct p38 MAPK target sites not affected by JNK or ERK1/2 (Figure 2A). Dusp1/4−/− MEFs cultured in serum showed no baseline changes in the phosphorylation status of ERK1/2 or JNK or their upstream activating kinases (Figure 2A).
Figure 2. Combined deletion of Dusp1 and Dusp4 results in unrestrained p38 MAPK phosphorylation.
A, Western blot analysis of key MAPK pathway enzymes from serum-cultured MEFs derived from Wt or Dusp1/4−/− embryos. Arrowheads indicate changed band intensities detected from Dusp1/4−/− cells. B, Western blot analysis of MAPK phosphorylation from Wt or Dusp1/4−/− MEFs stimulated with anisomycin (1 μg/ml) for the indicated period of time under serum-starved conditions. Asterisk shows expanded p38 phosphorylation. C, Western blot analysis of the indicated proteins and phospho-proteins from hearts of adult mice subjected to sham or TAC procedure (15 minutes of stimulation) in Wt or Dusp1/4−/− mice previously fed vehicle or SB731445-formulated chow. Asterisks and arrow heads in the gel picture or bottom of it show bands that are differentially regulated by loss of Dusp1/4, while the 2 arrowheads on the right side show the migration of MK2. D, Western blot analysis of phosphorylated and total p38 MAPK from the hearts of 2 week-old Wt or Dusp1/4−/− mice injected with anisomycin systemically (10 mg/kg) for the indicated period of time. Asterisks show expanded p38 phosphorylation. E, Western blot analysis of phosphorylated and total p38 MAPK from the hearts of 2 month-old Wt or Dusp1/4−/− mice subjected to Sham or TAC procedure for the indicated periods of time in minutes or hours.
Typically MAPKs are activated within seconds to minutes of stress or mitogen stimulation, and thereafter the DUSPs are transcriptionally induced within 15–45 minutes to mediate inactivation of the MAPKs 7. To analyze the kinetics of p38 MAPK inactivation over time we used serum-free cultures of Wt versus Dusp1/4−/− MEFs treated with the stress agent anisomycin (Figure 2B). This agent induced a transient activation of p38 MAPK in Wt MEFs at 10 and 30 minutes, but levels were back to baseline by 180 minutes. By comparison, Dusp1/4−/− MEFs showed no inactivation at 180 minutes, with even slightly greater total activation at all time points analyzed (Figure 2B). No effect was observed in JNK or ERK1/2 phosphorylation status, again suggesting that loss of Dusp1/4 genes only impacted p38 MAPK in MEFs (Figure 2B). We next investigated MAPK responsiveness in the heart at baseline or with acute transverse aortic constriction (TAC) stimulation for 15 minutes, in the presence or absence of the p38 inhibitor SB731445 given to the mice starting 2 days prior to surgery (Figure 2C). The data show greater p38 and MK2 activation at baseline in the hearts of Dusp1/4−/− mice (asterisks), with even greater activation after TAC stimulation compared with Wt controls (Figure 2C, arrowheads). Hearts from Dusp1/4−/− mice showed no changes in ERK1/2 or JNK1/2 phosphorylation at baseline or with TAC stimulation (Figure 2C). We also analyzed hearts from mice subjected to sham or TAC procedures for p38 and MK activation in either the cytoplasm or nucleus (Online Figure I). The data again show greater p38 and MK2 phosphorylation in hearts from Dusp1/4−/− mice, both at baseline and with TAC stimulation, as well as greater MK2 nuclear content in the absence of Dusp1/4, again suggesting greater activation (Online Figure I). We also injected 2-week old Wt and Dusp1/4−/− mice with anisomycin to induce a systemic stress response, and at select times thereafter, mice were sacrificed and the hearts removed for analysis of p38 MAPK phosphorylation (Figure 2D). Similar to the results observed in MEFs, anisomycin induced a transient increase in p38 MAPK phosphorylation in the hearts of Wt mice at 5–60 minutes, which was inactivated by 180 minutes. In contrast, hearts from Dusp1/4−/− mice showed no inactivation up to 180 minutes, and in fact, activation attained a higher level as time progressed (Figure 2D). We also repeated this same type of analysis in Wt versus Dusp1/4−/− mice subjected to pressure overload by TAC for varying lengths of time to gauge the temporal aspects of regulation within the heart more thoroughly (Figure 2E). Hearts from Wt mice showed variable and intermittent activation of p38 at different time periods of pressure overload, as shown previously 20. In contrast, hearts from Dusp1/4−/− mice subjected to TAC showed uniform and maximal p38 phosphorylation at every time point analyzed, with no periods of inactivation (Figure 2E). Taken together, these results suggest that loss of Dusp1/4 from the heart or cultured MEFs removes a break on p38 MAPK signaling, rendering this pathway unrestrained with greater net activity.
Dusp1/4−/− mice show cardiomyopathy
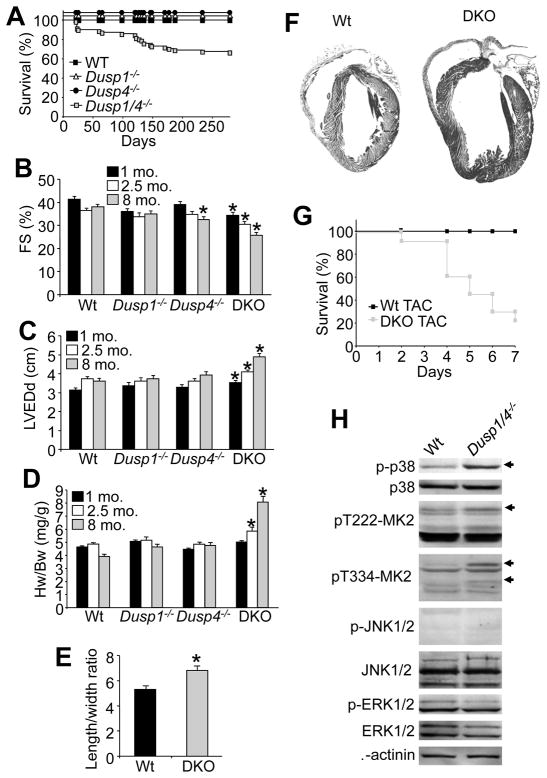
While Dusp1/4−/− mice were overtly normal at birth and young adulthood, we did observe a significant decrease in survival with aging, such that by 8 months 30% of the double null mice had perished compared with no lethality in Wt or single Dusp1 or Dusp4 null mice (Figure 3A). We suspected that some of these mice may have succumbed to heart failure as a cause of their lethality, as careful analysis by echocardiography showed progressive dilation of the ventricular chambers in the double knock out (DKO) mice, reductions in fractional shortening and secondary increases in heart weight normalized to body weight (Figure 3B–D), although no increase in TUNEL was observed suggesting the failure was not associated with apoptosis (Online Figure II). As a whole, Dusp1 and Dusp4 single nulls showed no cardiac abnormalities up to 8 months of age, except for a small, albeit significant reduction in fractional shortening in Dusp4−/− mice at 8 months of age (Figure 3B–D). The large increase in heart weight observed in Dusp1/4−/− mice at 8 months of age was characterized mostly by myocyte thinning, as measured from isolated adult myocytes from these hearts (Figure 3E), as well as frank dilation of the left ventricle at the whole organ level (Figure 3F). Dusp1/4 double knock-out (DKO) mice were also highly susceptible to lethality after TAC stimulation over 7 days, showing their propensity towards rapid failure (Figure 3G), and the few DKO mice that survived this procedure to 14 days showed much greater cardiac hypertrophy than Wt controls (Online Figure III). These phenotypic characteristics were likely due to unrestrained p38 MAPK activity, at least partially consistent with the cardiac phenotype described previously for activated-MKK3/6 overexpressing transgenic mice 4. Indeed, isolation and culturing (serum free) of adult myocytes from hearts of Wt versus Dusp1/4−/− mice showed again that only p38 MAPK was hyper-phosphorylated in double null cells, with activation of the p38-specific downstream kinase MK2 (Figure 3H). No changes were observed in JNK or ERK1/2 phosphorylation, again suggesting that DUSP1/4 are the primary regulators of p38 MAPK dephosphorylation in the heart, and their absence permits unrestrained activity that is associated with cardiomyopathy.
Figure 3. Dusp1/4−/− mice spontaneously develop cardiomyopathy with aging.
A, Kaplan-Meier survival curve of Wt (black square, n=10), Dusp1−/− (triangle, n=10), Dusp4−/− (circle, n=10), and Dusp1/4−/− (grey square, n=71) mice. (*p<0.01 vs. other genotypes). B and C, Assessment of fractional shortening (FS) and left ventricular end dimension in diastole (LVEDd) by echocardiography in Wt (n=8), Dusp1−/− (n=8), Dusp4−/− (n=8), and Dusp1/4−/− (DKO, n=17) mice at 1, 2, and 8 months of age. (*p<0.01 vs. Wt). D, Heart weight (Hw) normalized to body weight (Bw) in Wt, Dusp1−/−, Dusp4−/−, and Dusp1/4−/− (DKO) mice at 1, 2, and 8 months of age (n=10 per genotype/age). (*p<0.01 vs. Wt). E, Length/width ratio from Wt and Dusp1/4−/− (DKO) isolated adult cardiomyocytes (*p<0.01 vs. Wt; n=200 cells/genotype). F, Gross H&E-stained histological section in longitudinal section through hearts of the indicated mice at 8 months of age. G, Kaplan-Meier survival curve over 7 days after TAC stimulation in adult Wt or DKO mice (N=12 or greater mice per group at onset). H, Western blot analysis of MAPK pathway enzymes from Wt or Dusp1/4−/− isolated adult cardiomyocytes cultured in the absence of serum for 16h. Arrows indicate increased phospho-specific bands detected from Dusp1/4−/− cells.
Inhibition of p38 protects Dusp1/4−/− mice from cardiomyopathy
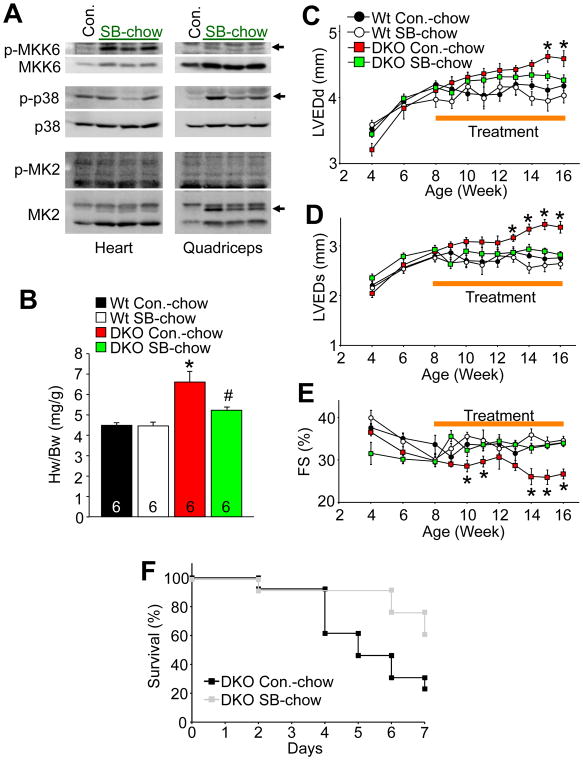
DUSP1 and DUSP4 can also dephosphorylate JNK, albeit less efficiently than p38 MAPK. Moreover, there are other DUSPs with greater specificity for JNK over p38 that would likely compensate when Dusp1/4 are deleted. Indeed, our analysis of Dusp1/4−/− MEFs and hearts failed to show any effect on JNK phosphorylation status, suggesting that the phenotype observed in the DKO mice was due solely to unrestrained p38 activity. However, to better establish specificity for p38 MAPK we treated Dusp1/4−/− mice with a selective, orally active p38 inhibitor, SB731445, which was formulated in mouse chow at a dosage of 50 mg/kg/day. Mice achieved a blood and heart tissue level of approximately 350 ng/ml (or per g for heart). In Wt control mice administered SB731445, we observed an increase in MKK6 phosphorylation in both heart and skeletal muscle, as well as an increase in total MKK6 protein, suggesting that p38 was inhibited because it normally reduces MKK6 expression through feedback as discussed earlier (Figure 4A). SB731445 binds to the ATP pocket in p38 and reduces its ability to phosphorylate substrates, but it does not prevent p38 itself from being phosphorylated, hence in skeletal muscle p38 was hyperphosphorylated due to greater MKK6 activity (Figure 4A). MK2 was hypo-phosphorylated (faster migration on a gel) in skeletal muscle, and to a lesser extent in cardiac muscle, again suggesting that the inhibitor was blocking p38 activity (Figure 4A). Wt and Dusp1/4−/− mice were placed on SB731445 chow beginning at 8 weeks of age for 2 months of treatment (6 mice per group). Mice were subjected to echocardiography every 2 weeks and sacrificed at 4 months of age for measurement of heart weights (echo started 4 weeks before treatment began). p38 inhibition with SB731445 prevented the increase in heart weight, prevented ventricular dilation, and prevented loss of fractional shortening in Dusp1/4−/− mice compared with vehicle treated DKO mice over the next 2 months of treatment (Figure 4B–E). Moreover, the known lethality in DKO mice with acute TAC stimulation was partially protected by SB731445 (Figure 4F). Inhibitor treatment of Wt mice had no effect in any of the parameters evaluated here. These results suggest that unrestrained p38 activity is the primary reason for dilated cardiomyopathy and hypertrophy in Dusp1/4−/− mice, again suggesting that other MAPKs, such as JNK and ERK1/2, are not appreciably involved.
Figure 4. Pharmacological inhibition of p38 MAPK prevents cardiomyopathy in Dusp1/4−/− mice.
A, Western blot analysis of key p38 MAPK pathway enzymes from heart or quadriceps of 2 month-old Wt mice fed with control (Con.) or p38 inhibitor SB731445-formulated chow for 1 week (50 mg/kg/day; SB-chow). Arrowheads indicate accelerated migration of MK2 characteristic of its unphosphorylated form, as well as the increase in MKK6 and phospho-p38. B, Heart weight (Hw) normalized to body weight (Bw) in 16 week-old Wt and Dusp1/4−/− (DKO) mice fed with either control chow (Con.-chow) or SB-chow from 8 to 16 weeks of age (*p<0.01 vs. Wt Con.-chow; #p<0.01 vs. DKO Con.-chow). The number of animals is indicated in the bars. C–E, Assessment of left ventricular end dimension in diastole (LVEDd), left ventricular end dimension in systole (LVEDs), and fractional shortening (FS) by echocardiography in Wt and Dusp1/4−/− (DKO) mice fed with either control chow (Con.-chow) or SB-chow from 8 to 16 weeks of age, although echo assessment began 4 weeks prior to treatment (*p<0.01 vs. other genotypes). F, Kaplan-Meier survival curve over 7 days after TAC stimulation in adult DKO mice with prior and continuous vehicle or SB731445-containing chow treatment (N=12 or greater mice per group at onset).
p38 negatively regulates cardiac contractility in Dusp1/4−/− mice
To probe more deeply into the mechanism of cardiomyopathy associated with unrestrained p38 activity in Dusp1/4−/− mice we assessed cardiac contractility at the whole organ and cellular level. p38 activity was previously suggested to reduce the sensitivity of myofilaments to Ca2+, thus acting as a negative inotrope 21. Consistent with these results, 2 month-old Dusp1/4−/− mice showed reduced systolic contractile performance with a Millar pressure-transducing catheter (Figure 5A). Dusp1/4−/− mice also showed depressed relaxation, collectively suggesting that increased p38 activity affected systolic and diastolic performance (Figure 5B). However, a parallel group of mice was also acutely treated with SB731445 chow for 2 weeks and assessed for contractile changes, which showed no rescue in systolic performance, but a significant rescue in relaxation and diastolic performance (Figure 5A and 5B). These results suggest that p38 activity is acutely involved in cardiomyocyte contractility.
Figure 5. Inhibition of p38 MAPK restores depressed contractility in Dusp1/4−/− mice and isolated cardiomyocytes.
A and B, Invasive hemodynamic assessment of contraction (Max dP/dt) and relaxation (Min dP/dt) velocities in anesthetized, close-chested Wt and Dusp1/4−/− (DKO) mice fed for 2 weeks with either control chow (Con.-chow) or p38 inhibitor SB731445-formulated chow (50 mg/kg/day; SB-chow). (N=5 or more mice per group) (*p<0.01 vs. Wt Con.-chow; #p<0.01 vs. DKO Con.-chow). C, Representative traces of fura-2 F340/F380 fluorescence ratio (black trace) and myocytes shortening (red trace) recordings from Wt or Dusp1/4−/− (DKO) isolated adult cardiomyocytes in the absence or presence of p38 inhibitor SB239063 (50 μM; SB). D–G, Average maximal peak amplitude of electrically evoked Ca2+ transients, time constant of Ca2+ decay, average maximal Ca2+ response to a 10 mM caffeine bolus, and percentage of shortening in isolated adult cardiomyocytes from the indicated genotypes in absence (Control) or presence of p38 inhibitor SB239063 (50 μM; SB). At least 4 animals were used, and the total number of cells analyzed is indicated in the bars (*p<0.05 vs. Wt Control; #p<0.05 vs. DKO Control).
Isolation of adult myocytes from 2 month-old Wt control and Dusp1/4−/− hearts also revealed a reduction in cellular contractile performance from DKO mice (Figure 5C, and 5G). Associated with this reduction in myocyte contractility was a reduction in peak Ca2+ release, a reduction in sarcoplasmic reticulum Ca2+ content, and prolonged relaxation times (Figure 5C–F). Acute administration of the p38 inhibitor SB239063 for 10 minutes reversed all these effects in the DKO adult cardiomyocytes, suggesting that acute phosphorylation events by p38 directly dampened contractility, although it is not clear if such effects are through phosphorylation of myofilament proteins, Ca2+ handling proteins, or both (see discussion). These results provide additional mechanistic insight into how enhanced p38 activity negatively impacts the heart, and how DUSP1/4 are protective by maintaining contractile performance.
To gain greater insight into the mechanism of how p38 affects contractility and secondary propensity to cardiomypathy we crossed Dusp1/4−/− mice with mice lacking Pln (phospholamban). The rationale here is that if deletion of Pln complemented (rescued) the contractile deficits in Dusp1/4−/− mice, it would suggest a mechanism primarily involving sarcoplasmic reticulum Ca2+ handling, and not myofilament Ca2+ sensitivity. The mice were analyzed at 4 months of age and show that DKO/Pln mice had a restoration in fractional shortening and prevention of ventricular dilation compared to Dusp1/4 DKO only mice (Figure 6A–C). These results suggest that loss of Pln, which enhances sarcoplasmic reticulum Ca2+ cycling, can partially restore function and prevent aspects of cardiomyopathy in DKO hearts, further suggesting that p38 regulates Ca2+ cycling as a mechanism for mediating depressed contractility when activated.
Figure 6. Deletion of Pln prevents cardiomyopathy in Dusp1/4 DKO mice.
A, Assessment of fractional shortening (FS %), B, left ventricular end dimension in diastole (LVEDd), and C, left ventricular end dimension in systole (LVEDs) in the indicated groups of mice at 4 months of age. Number of mice analyzed is shown in the bars. *P<0.05 versus Wt; #P<0.05 versus Dusp1/4 DKO mice
DISCUSSION
Of the 11 Dusp genes, 5 have been reported to prefer p38 and JNK for dephosphorylation over ERK1/2, including Dusp1, Dusp4, Dusp8, Dusp10, and Dusp16 7. However, some literature is in disagreement, and differences in substrate specificity have been reported. For example, DUSP4 (MKP-2) was suggested to primarily regulate ERK1/2, then JNK, but not p38 in cultured cells, although in vivo regulation of ERK1/2 was not observed 22. Prior to the generation of gene-deleted mice (and null MEFs), it was often difficult to ascertain the true specificity of a given DUSP protein for a MAPK subfamily member in vitro because of promiscuity associated with reconstitution assays that fail to mimic true physiologic concentrations and subcompartmentation, as it would occur in a cell. Analysis in cultured cells was also confusing as it often relied on overexpression or partial gene knock-down approaches, which again did not mimic known physiologic concentrations of the DUSPs. Hence the potentially only reliable means of assessing DUSP “physiologic” specificity is using gene-deleted cells or tissues to then probe for upreguation in selected MAPK members. Moreover, regulation even varies by tissue or cell type, depending on the levels of each DUSP expressed, the levels of other interacting proteins that support a complex between DUSP-MAPKs, and the levels of the individual MAPKs themselves and even their nuclear versus cytoplasmic localization. Thus, every tissue or cell type could have a unique profile of MAPK subfamily member regulation by an individual DUSP depending on what other DUSPs are expressed and the relative content of each MAPK subfamily member. We have generated or obtained gene-deleted mice for 7 of the 11 Dusp genes, and only DUSP1/4/10 appear to dominantly regulate p38 MAPK dephosphorylation in the heart and MEFs (results shown here and data not shown). DUSP8 appears to be mostly specific for JNK in the heart (data not shown), and DUSP6 is entirely specific for ERK1/2 in the heart and MEFs 13.
Single disruption of Dusp1, Dusp4 or Dusp10 did not appreciably impact baseline or inducible p38 MAPK phosphorylation status due to compensation, although Dusp1/4 double null MEFs and hearts from these DKO mice showed a dramatic and specific effect on only p38 MAPK phosphorylation, suggesting that these 2 DUSPs are the primary regulators of p38 in vivo, or that no remaining DUSPs are present in the heart with sufficient activity or specificity for p38. However, our loss-of-function results do not prove that DUSP1/4 cannot additionally affect JNK, as other DUSPs are still present that could potentially compensate for the loss of DUSP1/4, especially if they had preference for JNK over p38 (i.e. DUSP8). The critical aspect underlying our results is that loss of DUSP1/4 appears to account for the majority of DUSP activity towards p38 MAPK in fibroblasts and the heart, as the remaining 9 Dusp genes are not sufficient to fully inactivate p38 MAPK.
Another issue to consider is how MAPK activity is assessed in interpreting the effects of DUSP deletion. While the field relies heavily on direct assessment of ERK1/2, JNK, and p38 phosphorylation using phospho-specific antibodies, it is still arguably better to examine direct downstream targets of each MAPK. However, we have determined that this is not a trivial order, as MK2 appears to be the only truly specific target of any of the MAPK family members in our hands (at least for p38 activity in heart). We surveyed a number of reported ERK-specific phosphorylation sites in different target proteins, such as Elk-1, but none were affected in hearts of our DUSP1/4 DKO mice, nor was it affected in Erk1/2 double heart-specific deleted mice that we generated previously 23. c-Jun is also thought to be a specific target of JNK1/2, but p38 can also phosphorylate this target. However, c-Jun phosphorylation levels were also not changed in the hearts of our DUSP1/4 DKO mice, which is still consistent with DUSP1/4 only dominantly regulating p38 MAPK.
DUSP1 and DUSP4 are both thought to be primarily localized to the nucleus, thus it is difficult to envision how a specific alteration in nuclear p38 phosphorylation would lead to acute changes in myocyte contractility. However, DUSP1/4 both show some degree of localization and function within the cytosol. For example, some DUSP1 was observed within the cytosol of arterial smooth muscle cells, and some DUSP4 was similarly observed in the cytosol of cultured endothelial cells 24,25. Another issue related to subcellular compartments was suggested by the observation that p38 within the nucleus can activate MK2 and mediate its nuclear extrusion into the cytosol 26. Wang and colleagues showed that the failure and hypertrophic cardiomyopathic phenotype observed in activated MKK3 transgenic mice was reduced in mice lacking the gene encoding MK2 27. However, in our hands activated p38 promoted greater MK2 nuclear occupancy, not its extrusion, suggesting that in the adult heart of Dusp1/4−/− mice MK2 might have deleterious functions in the nucleus that are p38-dependent.
Dusp1/4−/− mice develop cardiomyopathy with aging, such that by 8 months the ventricular chambers have dilated and function is significantly diminished, the start of which can be observed as early as 2 months of age. In addition, 2 month-old Dusp1/4−/− mice show exaggerated hypertrophic enlargement following 2 weeks of pressure overload stimulation compared with a normal hypertrophic response in the single nulls and Wt mice. These results clearly indicate that Dusp1/4 are cardioprotective genes, and because the only identifiable molecular effect consistent with the known function of the Dusp gene family is an increase in p38 MAPK activity, we assume that unrestrained p38 activity underlies the cardiomyopathic phenotype in these DKO mice. Proof for this assumption came from the use of SB731445 over 2 months in Dusp1/4−/− mice, which prevented cardiac disease. These results suggest that prolonged p38 MAPK activity in the heart induces pathology, consistent with the phenotype of transgenic mice expressing an activated mutant of MKK6 specifically in cardiomyocytes of the heart, which showed restrictive hypertrophy and lethality, while activated MKK3 TG mice showed dilated cardiomyopathy with lethality 4. However, unlike MKK3/6 transgenic mice that presumably show near maximal p38 activity at all times, combined loss Dusp1/4 does not induce p38 activity, it merely removes a necessary brake, thus allowing prolongation in activation as long as a “physiologic” stimulus is present. Hence, our results suggest that increased p38 activity in the heart is profoundly pathologic and could be a novel target for inhibition in treating patients with heart failure, or select disease states that lead to hypertrophy and fibrosis, such as muscular dystrophy. Indeed, a hamster model of muscular dystrophy and associated heart disease showed less fibrosis and better cardiac function with systemic p38 MAPK inhibitor treatment 28. Similar cardioprotection was also observed in diabetic mice treated with a p38 MAPK inhibitor 29, or in mice post myocardial infarction injury 30. In contrast, we also performed a study in 8 month-old Dusp1/4−/− mice treated with SB731445 for 8 weeks and failed to observe any reversal in pathology, suggesting that once cardiomypathy is established, inhibition of p38 may not be effective (data not shown).
One potential mechanism whereby prolonged p38 MAPK activity could lead to cardiac pathology is through a reduction in contractile performance. Previously, p38 MAPK activation with adenoviral-based overexpression of an activated MKK3 mutant reduced contractile performance of adult rat cardiac myocytes, which was reversed with a p38 inhibitory compound 4. While Liao et al. never directly identified a mechanism of action whereby p38 negatively impacted contractility, they failed to observe a change in Ca2+ handling, suggesting that the effect was at the level of the myofilament 4. Indeed, detergent skinned papillary muscle strips from transgenic mice expressing an activated MKK6 mutant protein in the heart showed a reduction in maximum tension and ATPase activity, suggesting that p38 was dampening contractility at the level of myofilament proteins 31. We similarly observed a reduction in myocyte contractility with enhanced p38 activity due to Dusp1/4 deletion, which was reversed with SB731445 in minutes, suggesting that regulation of contractility was due to immediate phosphorylation of one or more proteins. Crossing into the Pln null background, which partially prevented disease and enhanced cardiac contractility in Dusp1/4 DKO mice suggests that part of the regulation by p38 is through affecting one or more Ca2+ handling protein/s, presumably at the level of sarcoplasmic reticulum.
Hence, Dusp1/4−/− mice most likely have a chronic reduction in cardiac contractility due to attenuated Ca2+ cycling, which then likely requires neuroendocrine enhancement to maintain cardiac output, leading to a secondary hastening of disease. Sustained neuroendocrine drive is known to cause hypertrophy and/or augment heart failure 32. More importantly, because p38 MAPK is activated in a semi-sustained manner in failing human hearts 33, and since p38 inhibitors appear to slightly but significantly augment cardiac contractile performance in diseased myocytes, drugs that block p38 could be of further benefit in heart failure patients 32. Finally, in addition to alterations in contractility and associated Ca2+ handling, it is likely that unrestrained activation of p38 is pathologic and leads to dilated and/or hypertrophic cardiomyopathy for additional reasons, such as enhanced fibrosis and remodeling 4, 28,34,35.
Supplementary Material
Novelty and Significance.
What Is Known?
Constitutive activation of p38 in the heart using cardiac-specific transgenesis induces cardiac dysfunction.
DUSP proteins dephosphorylate of MAPK leading to their inactivation.
p38 activity is associated with depressed cardiac contractility.
What New Information Does This Article Contain?
DUSP1 and DUSP4 are cardioprotective factors.
DUSP1 and DUSP4 protect the heart by specifically inactivating p38, allowing this kinase to maintain a dynamic range of activity.
DUSP1 and DUSP4 are the primary regulators of p38 activity in the heart.
Unrestrained activation of p38 activity by removing the brake of DUSP1/4 leads to cardiomyopathy that can be prevented with a p38 pharmacologic inhibitor.
Deletion of Pln prevents loss of contractile performance and ventricular dilation in Dusp1/4 null mice, suggesting that p38 affects contractility by altering calcium handling at the level of the sarcoplasmic reticulum.
This study was designed to examine the function of Dusp genes as negative regulators of the MAPK superfamily to better understand which kinases might be pathologic and the best targets for pharmaco-therapy. We show that deletion of Dusp1 and Dusp4 together in gene-targeted mice leads to cardiomyopathy with aging, and enhances decompensation and disease with pressure overload stimulation, by leading to prolongation and maintenance of p38 MAPK activity. Our results suggest that p38 MAPK inhibitors may be useful in the treatment of heart failure patients.
Acknowledgments
SOURCES OF FUNDING
This work was supported by grants from the NIH (to J.D.M., and J.N.L). J.D.M was also supported by the Howard Hughes Medical Institute. J.H.vB. and F.A. were supported by local affiliate grants from the American Heart Association. M.A.M. was supported by a Heart and Stroke Foundation of Canada postdoctoral fellowship. J.H.v.B. was also supported by a Rubicon fellowship from the Netherlands Organization for Scientific Research, and F.A. was also supported by a 1-year fellowship from the Italian Intesa SanPaolo SpA.
We would like to thank Dr Evangelia G. Kranias for supplying the Pln gene-targeted mice (University of Cincinnati, Cincinnati OH, USA).
Non-standard Abbreviations
- Dn
dominant negative
- DUSP
dual-specificity phosphatase
- ERK
extracellular signal-regulated kinase
- FS
fractional shortening
- JNK
c-Jun N-terminal kinase
- LVEDd
left ventricular end diastolic dimension
- MAPK
mitogen-activated protein kinase
- MEF
mouse embryonic fibroblast
- MK2
MAPK-activated protein kinase 2
- MKK
mitogen-activated protein kinase kinase
- MKP
MAPK phosphatase
- TAC
transverse aortic constriction
- Wt
wild-type
Footnotes
DISCLOSURES
None.
References
- 1.Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 2.Baines CP, Molkentin JD. STRESS signaling pathways that modulate cardiac myocyte apoptosis. J Mol Cell Cardiol. 2005;38:47–62. doi: 10.1016/j.yjmcc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90:1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao P, Georgakopoulos D, Kovacs A, Zheng M, Lerner D, Pu H, Saffitz J, Chien K, Xiao RP, Kass DA, Wang Y. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc Natl Acad Sci USA. 2001;98:12283–12288. doi: 10.1073/pnas.211086598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishida K, Yamaguchi O, Hirotani S, Hikoso S, Higuchi Y, Watanabe T, Takeda T, Osuka S, Morita T, Kondoh G, Uno Y, Kashiwase K, Taniike M, Nakai A, Matsumura Y, Miyazaki J-I, Sudo T, Hongo K, Kusakari Y, Kurihara S, Chien KR, Takeda J, Hori M, Otsu K. p38α mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol Cell Biol. 2004;24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, Braunwart J, Glascock BJ, Klevitsky R, Kimball TF, Hewett TE, Molkentin JD. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest. 2003;111:1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickinson RJ, Keyse SM. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci. 2006;119:4607–4615. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- 8.Franklin CC, Kraft AS. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J Biol Chem. 1997;272:16917–16923. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- 9.Fischer TA, Singh K, O’Hara DS, Kaye DM, Kelly RA. Role of AT1 and AT2 receptors in regulation of MAPKs and MKP-1 by ANG II in adult cardiac myocytes. Am J Physiol. 1998;275:H906–H916. doi: 10.1152/ajpheart.1998.275.3.H906. [DOI] [PubMed] [Google Scholar]
- 10.Lim HW, New L, Han J, Molkentin JD. Calcineurin enhances MAPK phosphatase-1 expression and p38 MAPK inactivation in cardiac myocytes. J Biol Chem. 2001;276:15913–9. doi: 10.1074/jbc.M100452200. [DOI] [PubMed] [Google Scholar]
- 11.Communal C, Colucci WS, Remondino A, Sawyer DB, Port JD, Wichman SE, Bristow MR, Singh K. Reciprocal modulation of mitogen-activated protein kinases and mitogen-activated protein kinase phosphatase 1 and 2 in failing human myocardium. J Card Fail. 2002;8:86–92. doi: 10.1054/jcaf.2002.32755. [DOI] [PubMed] [Google Scholar]
- 12.Bueno OF, De Windt LJ, Lim HW, Tymitz KM, Witt SA, Kimball TR, Molkentin JD. The dual-specificity phosphatase MKP-1 limits the cardiac hypertrophic response in vitro and in vivo. Circ Res. 2001;88:88–96. doi: 10.1161/01.res.88.1.88. [DOI] [PubMed] [Google Scholar]
- 13.Maillet M, Purcell NH, Sargent MA, York AJ, Bueno OF, Molkentin JD. DUSP6 (MKP3) null mice show enhanced ERK1/2 phosphorylation at baseline and increased myocyte proliferation in the heart affecting disease susceptibility. J Biol Chem. 2008;283:31246–31255. doi: 10.1074/jbc.M806085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purcell NH, Wilkins BJ, York A, Saba-El-Leil MK, Meloche S, Robbins J, Molkentin JD. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci U S A. 2007;104:14074–14079. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorfman K, Carrasco D, Gruda M, Ryan C, Lira SA, Bravo R. Disruption of the erp/mkp-1 gene does not affect mouse development: normal MAP kinase activity in ERP/MKP-1-deficient fibroblasts. Oncogene. 1996;13:925–931. [PubMed] [Google Scholar]
- 16.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 17.Goonasekera SA, Hammer K, Auger-Messier M, Bodi I, Chen X, Zhang H, Reiken S, Elrod JW, Correll RN, York AJ, Sargent MA, Hofmann F, Moosmang S, Marks AR, Houser SR, Bers DM, Molkentin JD. Decreased cardiac L-type Ca2+ channel activity induces hypertrophy and heart failure in mice. J Clin Invest. 2012;122:280–290. doi: 10.1172/JCI58227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer TA, Singh K, O’Hara DS, Kaye DM, Kelly RA. Role of AT1 and AT2 receptors in regulation of MAPKs and MKP-1 by ANG II in adult cardiac myocytes. Am J Physiol. 1998;275:H906–H916. doi: 10.1152/ajpheart.1998.275.3.H906. [DOI] [PubMed] [Google Scholar]
- 19.Ambrosino C, Mace G, Galban S, Fritsch C, Vintersten K, Black E, Gorospe M, Nebreda AR. Negative feedback regulation of MKK6 mRNA stability by p38alpha mitogen-activated protein kinase. Mol Cell Biol. 2003;23:370–381. doi: 10.1128/MCB.23.1.370-381.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoshijima M, Chien KR. Mixed signals in heart failure: cancer rules. J Clin Invest. 2002;109:849–855. doi: 10.1172/JCI15380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao P, Wang SQ, Wang S, Zheng M, Zheng M, Zhang SJ, Cheng H, Wang Y, Xiao RP. p38 Mitogen-activated protein kinase mediates a negative inotropic effect in cardiac myocytes. Circ Res. 2002;90:190–196. doi: 10.1161/hh0202.104220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondoh K, Nishida E. Regulation of MAP kinases by MAP kinase phosphatases. Biochim Biophys Acta. 2007;1773:1227–1237. doi: 10.1016/j.bbamcr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Kehat I, Davis J, Tiburcy M, Accornero F, Saba-El-Leil MK, Maillet M, York AJ, Lorenz JN, Zimmermann WH, Meloche S, Molkentin JD. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res. 2011;108:176–183. doi: 10.1161/CIRCRESAHA.110.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai K, Wang H, Lee WS, Jain MK, Lee ME, Haber E. Mitogen-activated protein kinase phosphatase-1 in rat arterial smooth muscle cell proliferation. J Clin Invest. 1996;98:1560–1567. doi: 10.1172/JCI118949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson CJ, Sloss CM, Plevin R. Inactivation of JNK activity by mitogen-activated protein kinase phosphatase-2 in EAhy926 endothelial cells is dependent upon agonist-specific JNK translocation to the nucleus. Cell Signal. 2001;13:29–41. doi: 10.1016/s0898-6568(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 26.Engel K, Kotlyarov A, Gaestel M. Leptomycin B-sensitive nuclear export of MAPKAP kinase 2 is regulated by phosphorylation. EMBO J. 1998;17:3363–3371. doi: 10.1093/emboj/17.12.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Streicher JM, Ren S, Herschman H, Wang Y. MAPK-activated protein kinase-2 in cardiac hypertrophy and cyclooxygenase-2 regulation in heart. Circ Res. 2010;106:1434–1443. doi: 10.1161/CIRCRESAHA.109.213199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyoi S, Otani H, Matsuhisa S, Akita Y, Tatsumi K, Enoki C, Fujiwara H, Imamura H, Kamihata H, Iwasaka T. Opposing effect of p38 MAP kinase and JNK inhibitors on the development of heart failure in the cardiomyopathic hamster. Cardiovasc Res. 2006;69:888–898. doi: 10.1016/j.cardiores.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Westermann D, Rutschow S, Van Linthout S, Linderer A, Bücker-Gärtner C, Sobirey M, Riad A, Pauschinger M, Schultheiss HP, Tschöpe C. Inhibition of p38 mitogen-activated protein kinase attenuates left ventricular dysfunction by mediating pro-inflammatory cardiac cytokine levels in a mouse model of diabetes mellitus. Diabetologia. 2006;49:2507–2513. doi: 10.1007/s00125-006-0385-2. [DOI] [PubMed] [Google Scholar]
- 30.Liu YH, Wang D, Rhaleb NE, Yang XP, Xu J, Sankey SS, Rudolph AE, Carretero OA. Inhibition of p38 mitogen-activated protein kinase protects the heart against cardiac remodeling in mice with heart failure resulting from myocardial infarction. J Card Fail. 2005;11:74–81. doi: 10.1016/j.cardfail.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Vahebi S, Ota A, Li M, Warren CM, de Tombe PP, Wang Y, Solaro RJ. p38-MAPK induced dephosphorylation of alpha-tropomyosin is associated with depression of myocardial sarcomeric tension and ATPase activity. Circ Res. 2007;100:408–415. doi: 10.1161/01.RES.0000258116.60404.ad. [DOI] [PubMed] [Google Scholar]
- 32.Dorn GW, 2nd, Molkentin JD. Manipulating cardiac contractility in heart failure: data from mice and men. Circulation. 2004;109:150–158. doi: 10.1161/01.CIR.0000111581.15521.F5. [DOI] [PubMed] [Google Scholar]
- 33.Haq S, Choukroun G, Lim H, Tymitz KM, del Monte F, Gwathmey J, Grazette L, Michael A, Hajjar R, Force T, Molkentin JD. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- 34.Bassi R, Heads R, Marber MS, Clark JE. Targeting p38-MAPK in the ischaemic heart: kill or cure? Curr Opin Pharmacol. 2008;8:141–146. doi: 10.1016/j.coph.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Xu L, Kappler CS, Menick DR. The role of p38 in the regulation of Na+-Ca2+ exchanger expression in adult cardiomyocytes. J Mol Cell Cardiol. 2005;38:735–743. doi: 10.1016/j.yjmcc.2005.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.